Comprehensive Transcriptomics, Hormone Metabolomics, and Physiological Analysis Reveal the Mechanism of Exogenous GA4+7 Breaking the Seed Dormancy in Polygonatum cyrtonema Hua
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and Seed Treatment
2.2. Determination of Germination Rate and Physiological Indexes
2.3. Transcriptome Sequencing and Analysis
2.4. Hormone Metabolomics Measurement and Analysis
2.5. qRT-PCR Validation
2.6. Statistical Analysis
3. Results
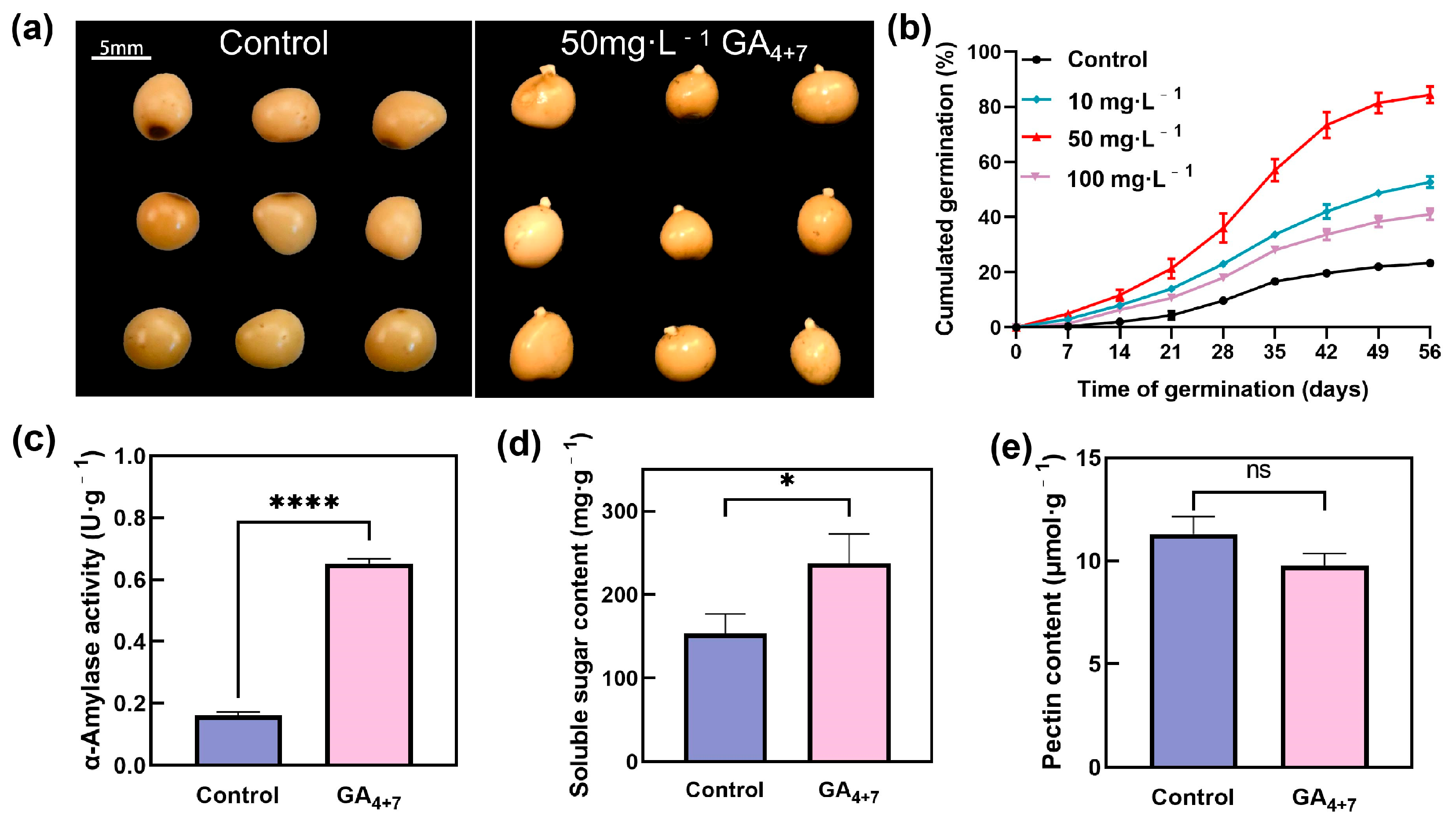
3.1. Effects of Exogenous GA4+7 Treatment on P. cyrtonema Seed Germination and Physiological Indexes
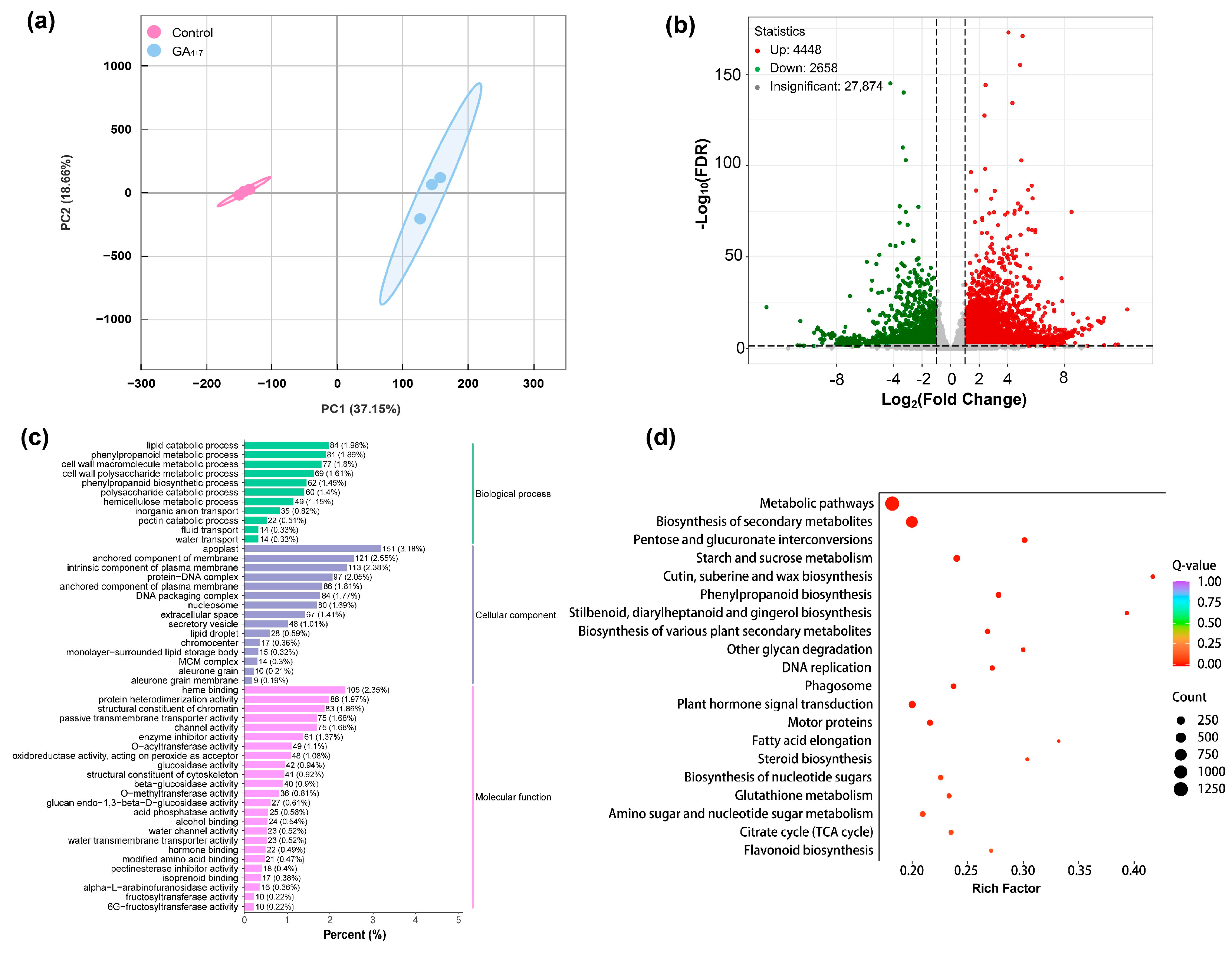
3.2. Transcriptome Sequencing and Differential Expression Genes Analysis
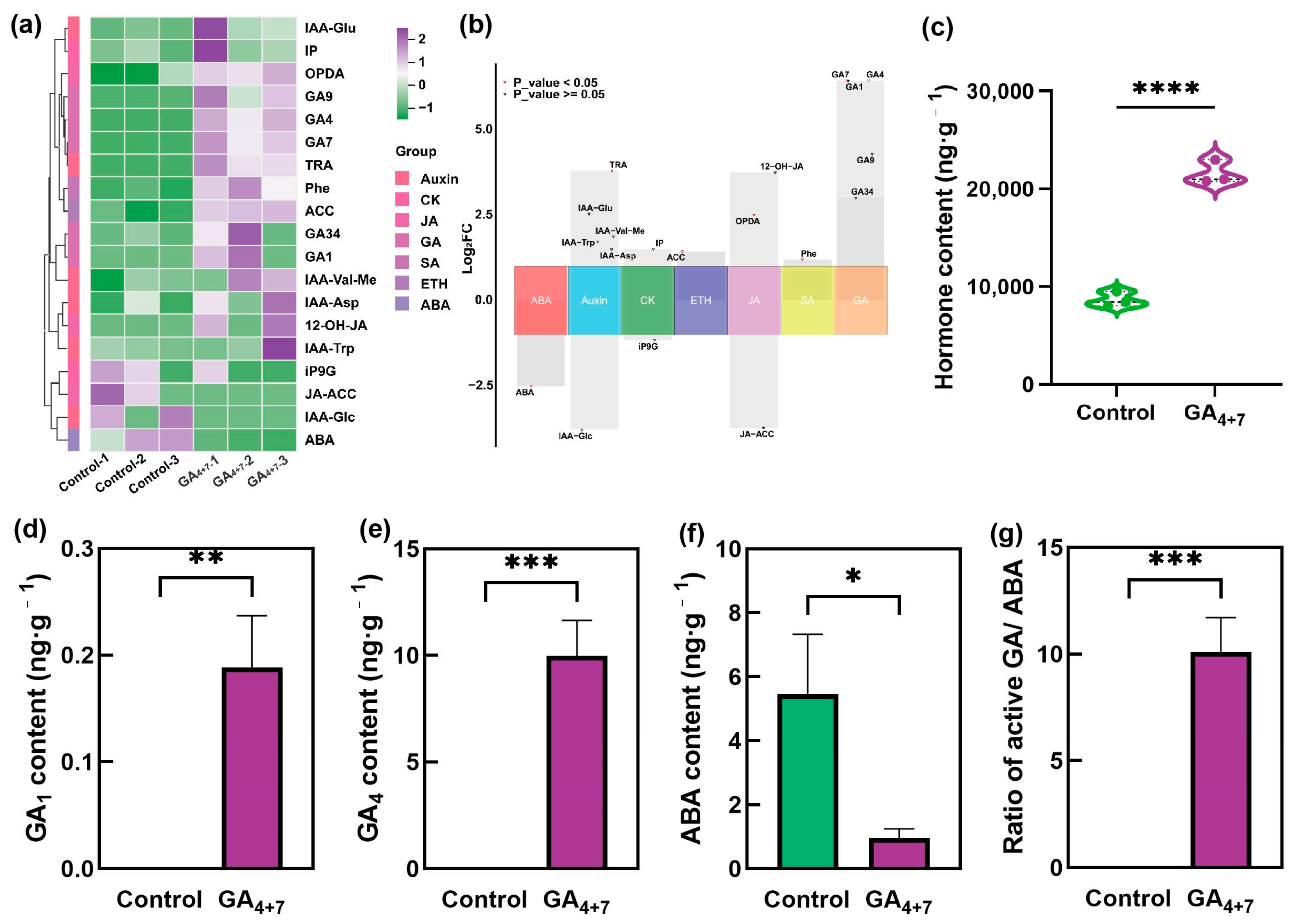
3.3. Hormone Metabolomic Analysis
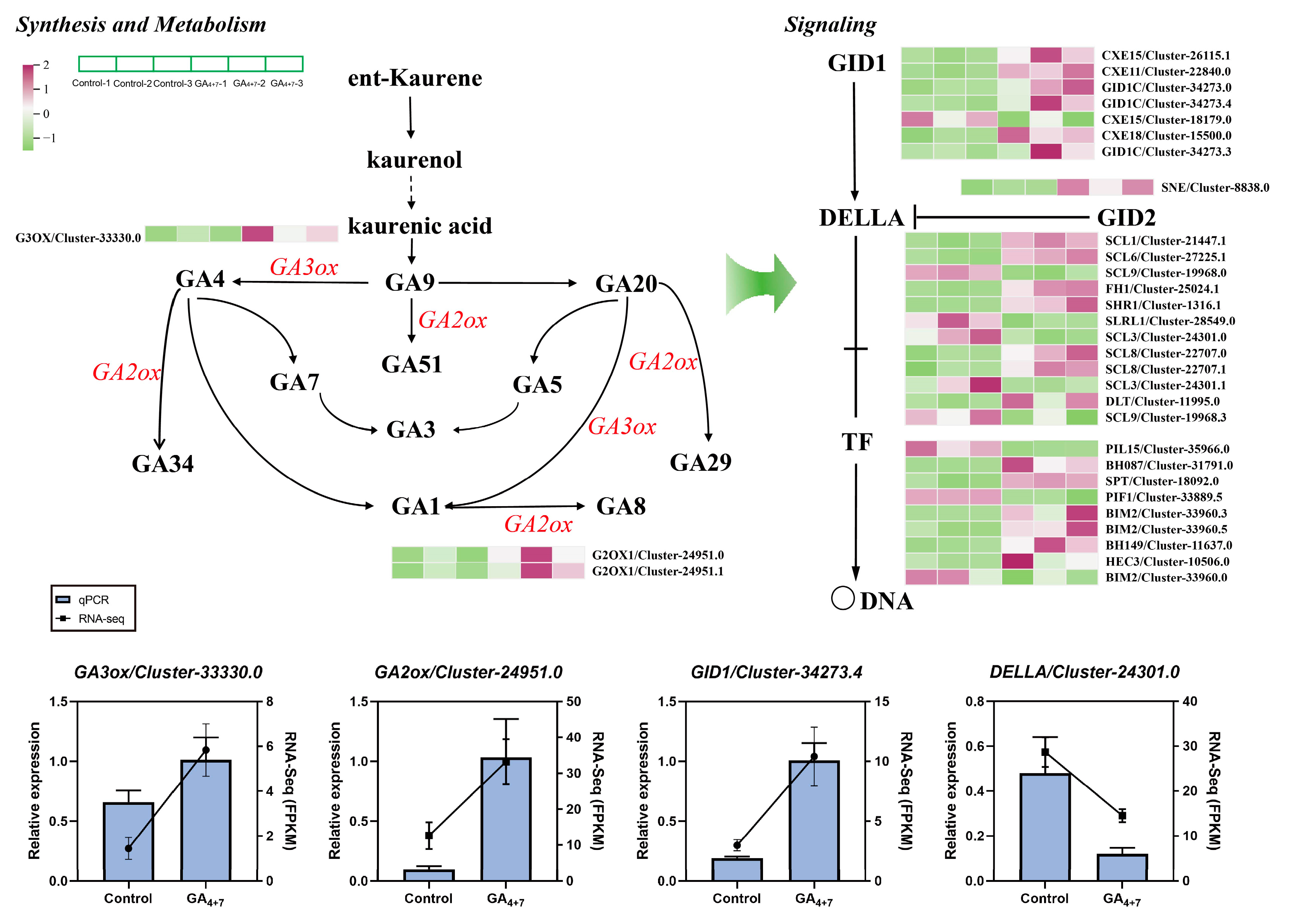
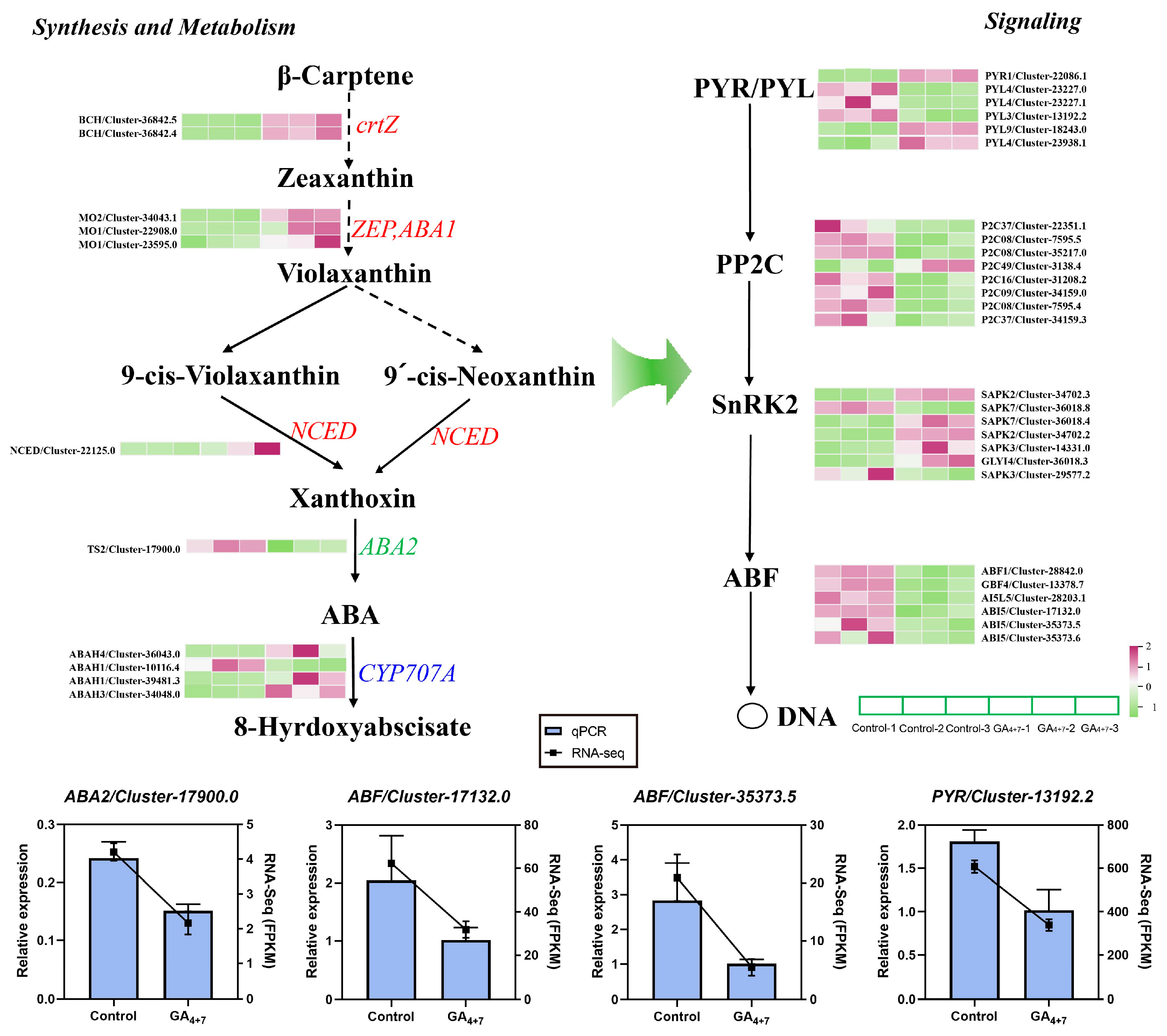
3.4. Differential Gene Expression in GA and ABA Biosynthesis and Signal Transduction Pathways
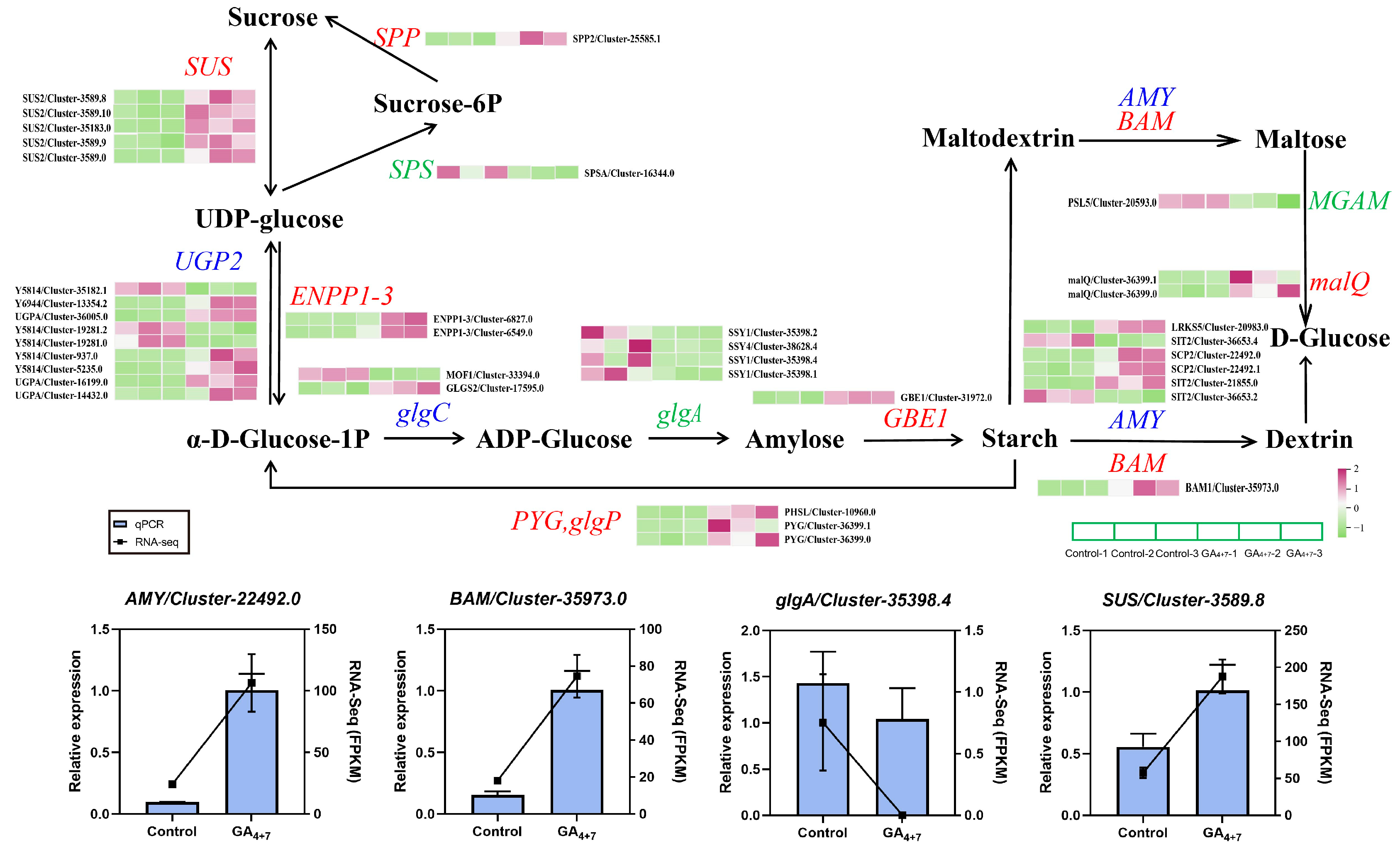
3.5. Differential Gene Expression in Sucrose and Starch Metabolism Pathways
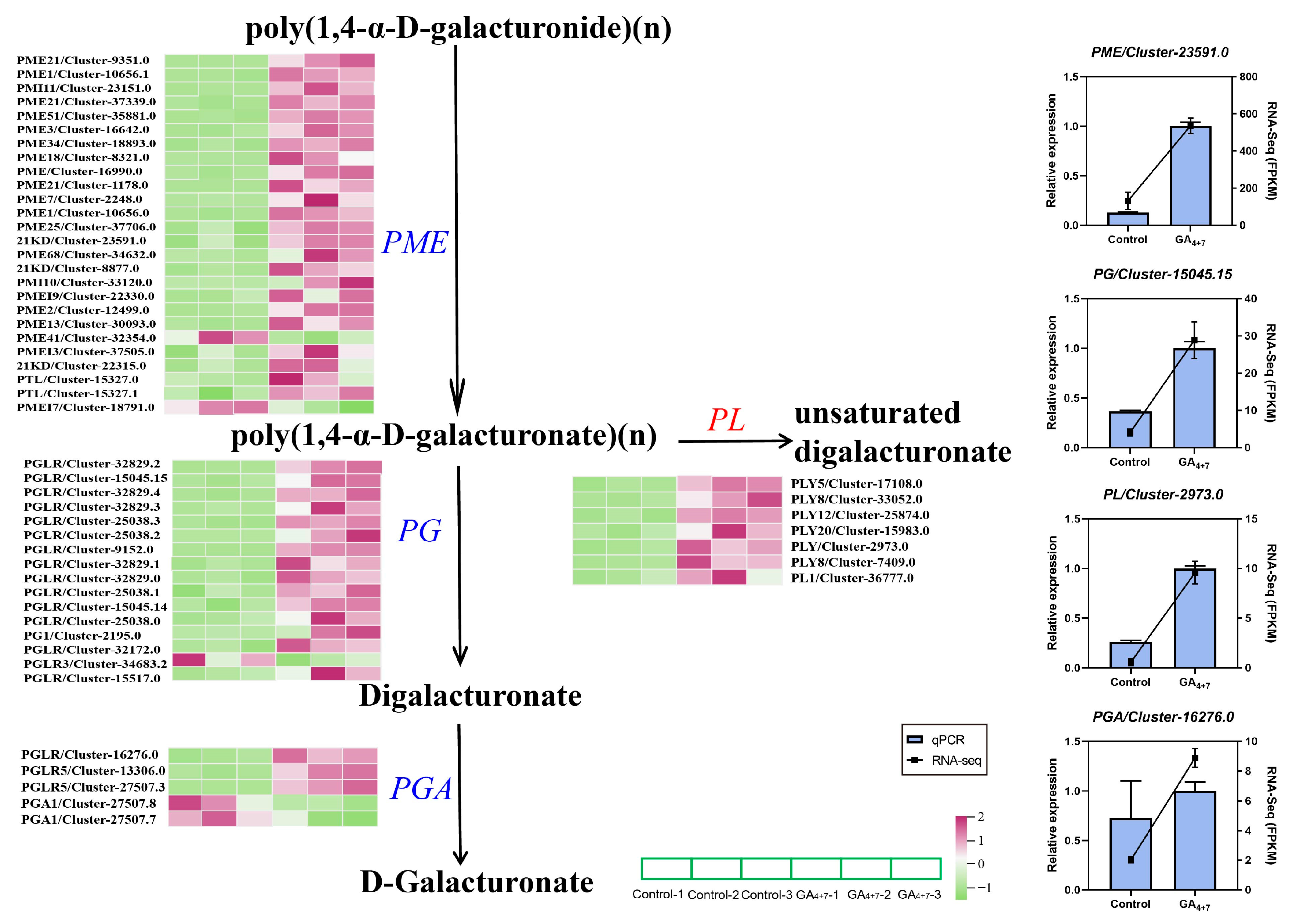
3.6. Effect of Exogenous GA4+7 Treatment on Genes Related to Pectin Catabolism
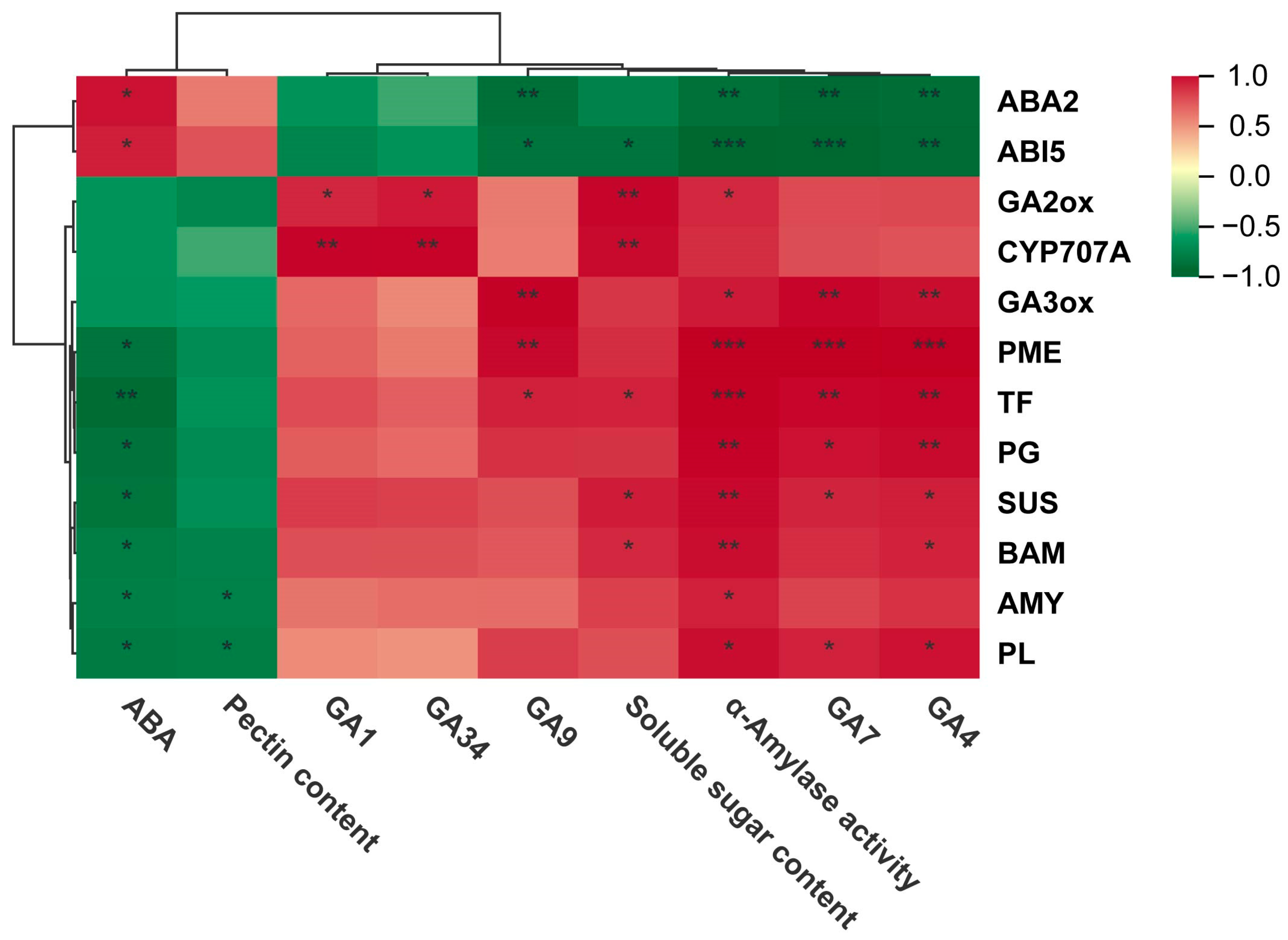
3.7. Correlation Analysis
4. Discussion
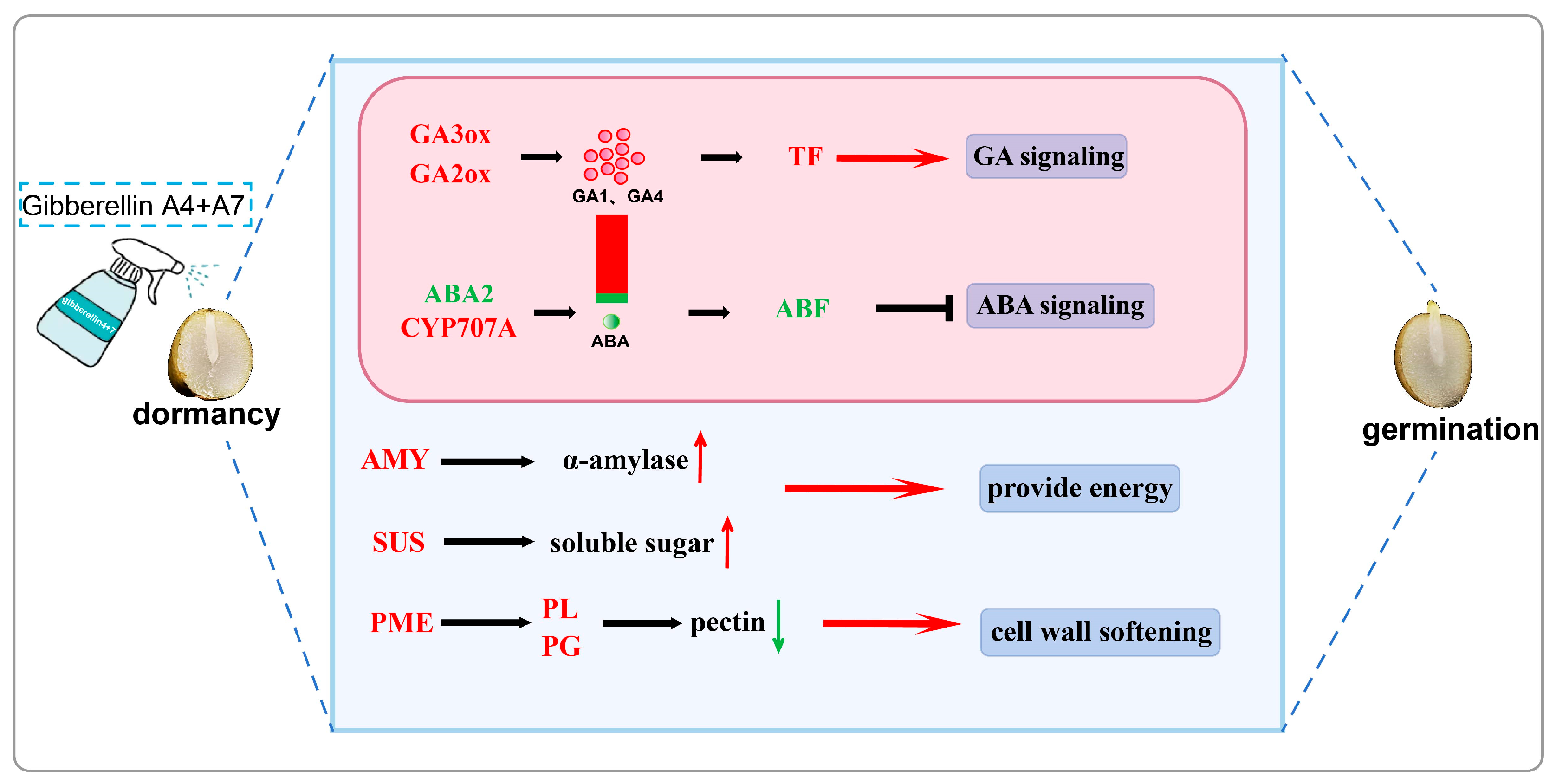
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Jiang, D.; Chen, B.; Huang, J.; Huang, L.-J.; Li, N. Biochemical Compounds and Pharmacological Functions of a Traditional Chinese Medicinal Herb Polygonati rhizoma. Med. Plant Biol. 2024, 3, e012. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Jia, M.; Chai, Y.; Bao, Y. Polygonatum sibiricum Saponin Exerts Beneficial Hypoglycemic Effects in Type 2 Diabetes Mice by Improving Hepatic Insulin Resistance and Glycogen Synthesis-Related Proteins. Nutrients 2022, 14, 5222. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, L.; Jiang, P.; Xu, G.; Sun, T. Immunological Regulation of the Active Fraction from Polygonatum sibiricum F. Delaroche Based on Improvement of Intestinal Microflora and Activation of RAW264.7 Cells. J. Ethnopharmacol. 2022, 293, 115240. [Google Scholar] [CrossRef]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G. Polygonatum sibiricum Polysaccharide Prevents Depression-like Behaviors by Reducing Oxidative Stress, Inflammation, and Cellular and Synaptic Damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Yang, J.-J.; Zhang, X.; Dai, J.-F.; Ma, Y.-G.; Jiang, J.-G. Effect of Fermentation Modification on the Physicochemical Characteristics and Anti-Aging Related Activities of Polygonatum kingianum Polysaccharides. Int. J. Biol. Macromol. 2023, 235, 123661. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Ma, Y.-L.; Thakur, K.; Hussain, S.S.; Wang, J.; Zhang, Q.; Zhang, J.-G.; Wei, Z.-J. Molecular Mechanism and Inhibitory Targets of Dioscin in HepG2 Cells. Food Chem. Toxicol. 2018, 120, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, Y. Seed Dormant Characteristics of Polygonatum sibiricum Red. Plant Res. 2010, 30, 753–757. [Google Scholar]
- Zhang, Y.; Li, Y.; Wang, Z. Study on the Reasons for Dormancy of Polygonatum sibiricum Seeds. Seeds 2011, 30, 58–61. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Chen, S. Morphological and Anatomical Studies During Seed Germination of Polygonatum cyrtonema Hua. Seeds 2020, 39, 5–10. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, W.; Wu, K.; Chen, J.; Li, Y.; Tao, Z. Integrating Transcriptomics and Hormones Dynamics Reveal Seed Germination and Emergence Process in Polygonatum cyrtonema Hua. Int. J. Mol. Sci. 2023, 24, 3792. [Google Scholar] [CrossRef]
- Zhang, W.F. Experiment on breaking down dormancy of Polygonatum cyrtonema Hua seed by different plant growth regulators. J. Tradit. Chin. Med. 2008, 36, 43–44. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Zhao, Y. Effect of Varied Treatments on Germination of Polygonatum cyrtonema Hua Seeds. J. Fujian Agric. 2015, 30, 469–472. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, N.; Shi, S.; Ge, P.; Xing, S. Study on the Relationship Between Seed Structure and Germination of Polygonatum cyrtonema Hua. Seeds 2020, 39, 7–12+19. [Google Scholar] [CrossRef]
- Xu, X.; Shu, Y.; Yu, C.; Jiang, J. Study on seeds production techniques of Polygonatum cyrtonema. J. Zhejiang Agric. Sci. 2022, 63, 1685–1688. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Lu, L.; Yi, X.; Yao, N.; Lai, Z.; Zhang, J. Comparative Transcriptome Study of the Elongating Internode in Elephant Grass (Cenchrus purpureus) Seedlings in Response to Exogenous Gibberellin Applications. Ind. Crops Prod. 2022, 178, 114653. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed Germination and Dormancy: The Classic Story, New Puzzles, and Evolution. J. Integr. Plant Biol. 2019, 61, 541–563. [Google Scholar] [CrossRef]
- Huang, X.; Wu, X.; Sun, G.; Jiang, Y.; Yan, H. Transcriptome Analysis Reveals Candidate Genes Involved in Gibberellin-Induced Fruit Development in Rosa Roxburghii. Plants 2023, 12, 3425. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Kieliszewska-Rokicka, B.; Vermeer, E.; Karssen, C.M. Gibberellin-Induced Hydrolysis of Endosperm Cell Walls in Gibberellin-Deficient Tomato Seeds Prior to Radicle Protrusion. Planta 1988, 174, 500–504. [Google Scholar] [CrossRef]
- Debeaujon, I.; Koornneef, M. Gibberellin Requirement for Arabidopsis Seed Germination Is Determined Both by Testa Characteristics and Embryonic Abscisic Acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef]
- Voegele, A.; Linkies, A.; Müller, K.; Leubner-Metzger, G. Members of the Gibberellin Receptor Gene Family GID1 (GIBBERELLIN INSENSITIVE DWARF1) Play Distinct Roles during Lepidium Sativum and Arabidopsis Thaliana Seed Germination. J. Exp. Bot. 2011, 62, 5131–5147. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Beach, R. Control of Transcription of A-Amylase and rRNA Genes in Barley Aleurone Protoplasts by Gibberellin and Abscisic Acid. Nature 1985, 316, 275–277. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The α-Amylase Induction in Endosperm during Rice Seed Germination Is Caused by Gibberellin Synthesized in Epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef]
- Pan, T.; Lin, L.; Wang, J.; Liu, Q.; Wei, C. Long Branch-Chains of Amylopectin with B-Type Crystallinity in Rice Seed with Inhibition of Starch Branching Enzyme I and IIb Resist in Situ Degradation and Inhibit Plant Growth during Seedling Development: Degradation of Rice Starch with Inhibition of SBEI/IIb during Seedling Development. BMC Plant Biol. 2018, 18, 9. [Google Scholar] [CrossRef]
- Kim, H.T.; Choi, U.-K.; Ryu, H.S.; Lee, S.J.; Kwon, O.-S. Mobilization of Storage Proteins in Soybean Seed (Glycine max L.) during Germination and Seedling Growth. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 1178–1187. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Lee, S.; Cheng, H.; King, K.E.; Wang, W.; He, Y.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J. Gibberellin Regulates Arabidopsis Seed Germination via RGL2, a GAI/RGA-like Gene Whose Expression Is up-Regulated Following Imbibition. Genes Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Cheng, H.; Wu, W.; Soo, H.M.; Peng, J. Gibberellin Mobilizes Distinct DELLA-Dependent Transcriptomes to Regulate Seed Germination and Floral Development in Arabidopsis. Plant Physiol. 2006, 142, 509–525. [Google Scholar] [CrossRef]
- Hussain, A.; Cao, D.; Peng, J. Identification of Conserved Tyrosine Residues Important for Gibberellin Sensitivity of Arabidopsis RGL2 Protein. Planta 2007, 226, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Zhao, L.; Chen, H.; Qian, W.; Wang, P.; Zhang, X.; Xia, T. Transcriptomic and Metabolic Insights into the Distinctive Effects of Exogenous Melatonin and Gibberellin on Terpenoid Synthesis and Plant Hormone Signal Transduction Pathway in Camellia Sinensis. J. Agric. Food Chem. 2019, 67, 4689–4699. [Google Scholar] [CrossRef]
- Feng, K.; Li, X.; Yan, Y.; Liu, R.; Li, Z.; Sun, N.; Yang, Z.; Zhao, S.; Wu, P.; Li, L. Integrated Morphological, Metabolome, and Transcriptome Analyses Revealed the Mechanism of Exogenous Gibberellin Promoting Petiole Elongation in Oenanthe javanica. Front. Plant Sci. 2023, 14, 1225635. [Google Scholar] [CrossRef]
- Ge, N.; Jia, J.-S.; Yang, L.; Huang, R.-M.; Wang, Q.-Y.; Chen, C.; Meng, Z.-G.; Li, L.-G.; Chen, J.-W. Exogenous Gibberellic Acid Shortening After-Ripening Process and Promoting Seed Germination in a Medicinal Plant Panax notoginseng. BMC Plant Biol. 2023, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Si, Q.; Yang, K.; Zhang, W.; Zhang, L.; Okita, T.W.; Yan, Y.; Tian, L. Transcriptome Analysis Reveals the Effects of Exogenous Gibberellin on the Germination of Solanum torvum Seeds. Agronomy 2024, 14, 1736. [Google Scholar] [CrossRef]
- Koornneef, M. Induction and Analysis of GibbereUin Sensitive Mutants in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 1980, 58, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain Flood Adaptation in Rice through a 14-3-3 Protein OsGF14h. Nat. Commun. 2022, 13, 5664. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, J.-L.; Chang, H.; Zhou, Z.-L. Developmental Anatomy of Polygonatum sibiricum Red.during the Seed Germination Process. Northwest J. Bot. 2013, 33, 1584–1588. [Google Scholar]
- Chen, Y.; Yang, F.-Q.; Chen, S.-S. Study on the Changes of Endogenous Hormones Contents in Polygonatum cyrtonema Seed Germination. Chin. Mater. Medica 2020, 43, 523–527. [Google Scholar] [CrossRef]
- Song, Q.; Cheng, S.; Chen, Z.; Nie, G.; Xu, F.; Zhang, J.; Zhou, M.; Zhang, W.; Liao, Y.; Ye, J. Comparative Transcriptome Analysis Revealing the Potential Mechanism of Seed Germination Stimulated by Exogenous Gibberellin in Fraxinus Hupehensis. BMC Plant Biol. 2019, 19, 199. [Google Scholar] [CrossRef]
- Yanxia, Z.; Jianping, J.; Yanfen, H.; Qingsong, D.; Kunhua, W. Comparative Transcriptome Analysis of the Effects of Friction and Exogenous Gibberellin on Germination in Abrus cantoniensis. Plant Signal. Behav. 2022, 17, 2149113. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and Seed Germination. In Annual Plant Reviews; Hedden, P., Thomas, S.G., Eds.; Wiley: Hoboken, NJ, USA, 2016; Volume 49, pp. 253–284. ISBN 978-1-119-21042-9. [Google Scholar]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated Role of ABA in Seed Maturation, Dormancy, and Germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The Current Status of Research on Gibberellin Biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, W.; Huang, J.; Wang, Y.; Hu, L.; Yuan, Y.; Lyu, M.; Wu, B. Role of Gibberellin and Its Three GID1 Receptors in Jasminum Sambac Stem Elongation and Flowering. Planta 2022, 255, 17. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional Analysis of Arabidopsis NCED6 and NCED9 Genes Indicates That ABA Synthesized in the Endosperm Is Involved in the Induction of Seed Dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The Short-Chain Alcohol Dehydrogenase ABA2 Catalyzes the Conversion of Xanthoxin to Abscisic Aldehyde[W]. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhao, X.Y.; Shao, X.X.; Wang, F.; Zhou, C.; Liu, Y.G.; Zhang, Y.; Zhang, X.S. Abscisic Acid Regulates Early Seed Development in Arabidopsis by ABI5-Mediated Transcription of SHORT HYPOCOTYL UNDER BLUE1. Plant Cell 2014, 26, 1053–1068. [Google Scholar] [CrossRef]
- Fu, D.; Wu, W.; Mustafa, G.; Yang, Y.; Yang, P. Molecular Mechanisms of Rice Seed Germination. New Crops 2025, 2, 100051. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Nai, G.; Feng, L.; Li, Y.; Zhou, Q.; Ma, Z.; Yue, Y.; Chen, B.; Mao, J. Genome-Wide Identification of BAM Genes in Grapevine (Vitis vinifera L.) and Ectopic Expression of VvBAM1 Modulating Soluble Sugar Levels to Improve Low-Temperature Tolerance in Tomato. BMC Plant Biol. 2021, 21, 156. [Google Scholar] [CrossRef]
- Chen, P.-W.; Chiang, C.-M.; Tseng, T.-H.; Yu, S.-M. Interaction between Rice MYBGA and the Gibberellin Response Element Controls Tissue-Specific Sugar Sensitivity of α-Amylase Genes. Plant Cell 2006, 18, 2326–2340. [Google Scholar] [CrossRef]
- Riffkin, H.L.; Duffus, C.M.; Bridges, I.C. Sucrose Metabolism during Endosperm Development in Wheat (Triticum aestivum). Physiol. Plant. 1995, 93, 123–131. [Google Scholar] [CrossRef]
- Lin, L.; Lin, J.; Zhou, M.; Yuan, Y.; Li, Z. Lipid Remodelling and the Conversion of Lipids into Sugars Associated with Tolerance to Cadmium Toxicity during White Clover Seed Germination. Physiol. Plant. 2024, 176, e14433. [Google Scholar] [CrossRef]
- Vorwerk, S.; Somerville, S.; Somerville, C. The Role of Plant Cell Wall Polysaccharide Composition in Disease Resistance. Trends Plant Sci. 2004, 9, 203–209. [Google Scholar] [CrossRef]
- Shrestha, S.; Rahman, M.S.; Qin, W. New Insights in Pectinase Production Development and Industrial Applications. Appl. Microbiol. Biotechnol. 2021, 105, 9069–9087. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Su, X.; Chen, L.; Zhu, Z. Transcriptome Analysis Reveals Key Metabolic Pathways and Gene Expression Involving in Cell Wall Polysaccharides-Disassembling and Postharvest Fruit Softening in Custard Apple (Annona squamosa L.). Int. J. Biol. Macromol. 2023, 240, 124356. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Li, Q.; Zhu, B. Pectinolytic Lyases: A Comprehensive Review of Sources, Category, Property, Structure, and Catalytic Mechanism of Pectate Lyases and Pectin Lyases. Bioresour. Bioprocess. 2021, 8, 79. [Google Scholar] [CrossRef]
- Vogel, J.P.; Raab, T.K.; Schiff, C.; Somerville, S.C. PMR6, a Pectate Lyase–Like Gene Required for Powdery Mildew Susceptibility in Arabidopsis. Plant Cell 2002, 14, 2095–2106. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell Wall Mechanics and Growth Control in Plants: The Role of Pectins Revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- Müller, K.; Levesque-Tremblay, G.; Bartels, S.; Weitbrecht, K.; Wormit, A.; Usadel, B.; Haughn, G.; Kermode, A.R. Demethylesterification of Cell Wall Pectins in Arabidopsis Plays a Role in Seed Germination. Plant Physiol. 2012, 161, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhao, C.; Li, Q.; Niu, Y.; Pan, Y.; Li, G.; Cheng, Y.; Zhang, A. Pectin Methylesterase 31 Is Transcriptionally Repressed by ABI5 to Negatively Regulate ABA-Mediated Inhibition of Seed Germination. Front. Plant Sci. 2024, 15, 1336689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Wan, J.; Hao, C.; Zeng, Z.; Hu, Y.; Yang, G.; Yang, H.; Zhou, X. Comprehensive Transcriptomics, Hormone Metabolomics, and Physiological Analysis Reveal the Mechanism of Exogenous GA4+7 Breaking the Seed Dormancy in Polygonatum cyrtonema Hua. Horticulturae 2025, 11, 627. https://doi.org/10.3390/horticulturae11060627
Qiu M, Wan J, Hao C, Zeng Z, Hu Y, Yang G, Yang H, Zhou X. Comprehensive Transcriptomics, Hormone Metabolomics, and Physiological Analysis Reveal the Mechanism of Exogenous GA4+7 Breaking the Seed Dormancy in Polygonatum cyrtonema Hua. Horticulturae. 2025; 11(6):627. https://doi.org/10.3390/horticulturae11060627
Chicago/Turabian StyleQiu, Mi, Jionglan Wan, Chunxiang Hao, Zixin Zeng, Yalong Hu, Guoqun Yang, Hua Yang, and Xiaoyun Zhou. 2025. "Comprehensive Transcriptomics, Hormone Metabolomics, and Physiological Analysis Reveal the Mechanism of Exogenous GA4+7 Breaking the Seed Dormancy in Polygonatum cyrtonema Hua" Horticulturae 11, no. 6: 627. https://doi.org/10.3390/horticulturae11060627
APA StyleQiu, M., Wan, J., Hao, C., Zeng, Z., Hu, Y., Yang, G., Yang, H., & Zhou, X. (2025). Comprehensive Transcriptomics, Hormone Metabolomics, and Physiological Analysis Reveal the Mechanism of Exogenous GA4+7 Breaking the Seed Dormancy in Polygonatum cyrtonema Hua. Horticulturae, 11(6), 627. https://doi.org/10.3390/horticulturae11060627





