Sensitivity of Leafy Vegetables to Simulated Mesotrione Residues in the Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Soil, and Herbicide
2.2. Experimental Procedures for Bioassay Under Controlled Conditions
2.3. Statistical Analyses
3. Results
3.1. Influence of Simulated Mesotrione Residues on the Shoot Fresh Weight
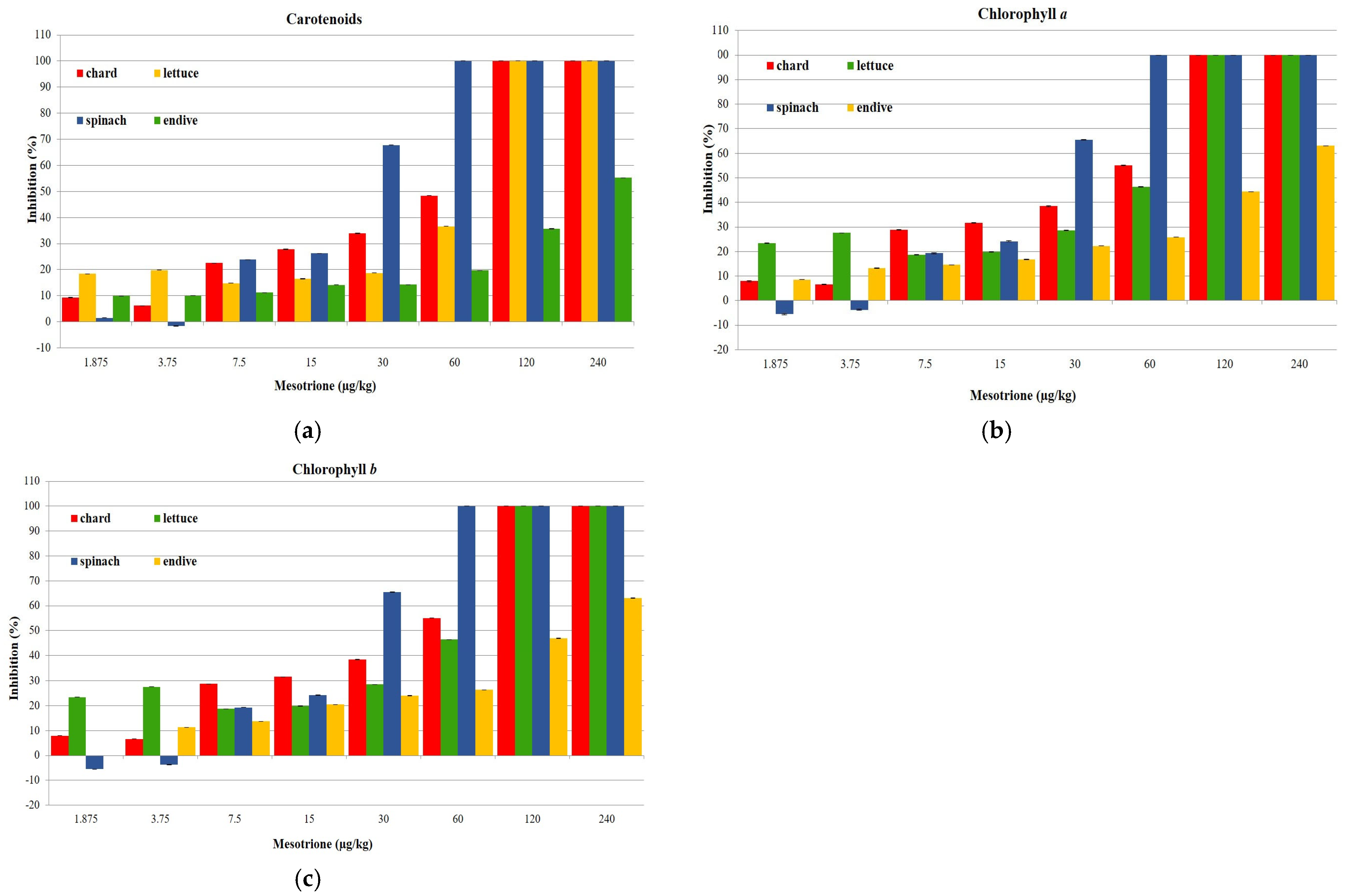
3.2. Influence of Simulated Mesotrione Residues on the Pigment Content (Carotenoids, Chlorophyll a and Chlorophyll b)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, G.; Bartlett, D.; Fraser, T.; Hawkes, T.; Holt, D.; Towson, J.; Wichert, R. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 5, 120–128. [Google Scholar] [CrossRef]
- Sutton, P.; Richards, C.; Buren, L.; Glasgow, L. Activity of mesotrione on resistant weeds in maize. Pest Manag. Sci. 2002, 58, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Creech, J.E.; Monaco, T.A.; Evans, J.O. Photosynthetic and growth responses of Zea mays L. and four weed species following post-emergence treatments with mesotrione and atrazine. Pest Manag. Sci. 2004, 60, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Riddle, R.N. Field and Greenhouse Bioassays to Determine Rotational Crop Response to Mesotrione Residues. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 2012. Available online: https://atrium.lib.uoguelph.ca/server/api/core/bitstreams/d4318c63-7def-42d1-a2dd-423b28433a46/content (accessed on 28 February 2025).
- Jhala, A.J.; Kumar, V.; Yadav, R.; Jha, P.; Jugulam, M.; Williams, M.M.; Hausman, N.E.; Dayan, F.E.; Burton, P.M.; Dale, R.P.; et al. 4-Hydroxyphenylpyruvate dioxygenase (HPPD)-inhibiting herbicides: Past, present, and future. Weed Technol. 2023, 37, 1–14. [Google Scholar] [CrossRef]
- Grossmann, K.; Ehrhardt, T. On the mechanism of action and selectivity of the corn herbicide topramezone: A new inhibitor of 4- hydroxyphenylpyruvate dioxygenase. Pest Manag. Sci. 2007, 63, 429–439. [Google Scholar] [CrossRef]
- Chaabane, H.; Vulliet, E.; Calvayrac, C.; Coste, C.M.; Cooper, J.F. Behaviour of sulcotrione and mesotrione in two soils. Pest Manag. Sci. 2008, 64, 86–93. [Google Scholar] [CrossRef]
- Beaudegnies, R.; Edmunds, A.J.F.; Fraser, T.E.M.; Hall, R.G.; Hawkes, T.M.; Mitchell, G.; Shaetzer, J.; Wendeborn, S.; Wibley, J. Herbicidal 4-hydroxy phenylpyruvate dioxygenase inhibitors—A review of the triketone chemistry story from a Syngente perspective. Bioorg. Med. Chem. 2009, 17, 4134–4152. [Google Scholar] [CrossRef]
- Ndikuryayo, F.; Moosavi, B.; Yang, W.; Yang, G. 4-Hidroxyphenylpyruvate Dioxigenase Inhibitors: From Chemical Biology to Agrochemicals. J. Agric. Food Chem. 2017, 65, 8523–8537. [Google Scholar] [CrossRef]
- Riddle, R.N.; O’ Sullivan, J.; Swanton, C.J.; Acker, R.C.V. Crop response to carryover of mesotrione residues in the field. Weed Technol. 2013, 27, 92–100. [Google Scholar] [CrossRef]
- Rouchaud, J.; Neus, O.; Eelen, H.; Bulcke, R. Dissipation and Mobility of the Herbicide Mesotrione in the Soil of Corn Crops; Mededelingen–Faculteit Landbouwkundige En Toegepaste Biologische Wetenschappen, Universiteit Gent: Gent, Belgium, 2000; Volume 65, pp. 51–58. [Google Scholar]
- Dyson, J.S.; Beulke, S.; Brown, C.D.; Lane, M.C.G. Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications. J. Environ. Qual. 2002, 31, 613–618. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance mesotrione. EFSA J. 2016, 14, 4419. Available online: https://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/4419.pdf (accessed on 28 February 2025).
- Felix, J.; Doohan, D.J.; Bruins, D. Differential vegetable crop responses to mesotrione soil residues a year after application. Crop Prot. 2007, 26, 1395–1403. [Google Scholar] [CrossRef]
- Su, W.; Hao, H.; Wu, R.; Xu, H.; Xue, F.; Lu, C. Degradation of Mesotrione Affected by Environmental Conditions. Bull. Environ. Contam. Toxicol. 2017, 98, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Sikkema, P.H.; Robinson, D.E. Response of four market classes of dry bean to mesotrione soil residues. Crop Prot. 2007, 26, 1655–1659. [Google Scholar] [CrossRef]
- Robinson, D.E. Atrazine Accentuates Carryover Injury from Mesotrione in Vegetable Crops. Weed Technol. 2008, 22, 641–645. [Google Scholar] [CrossRef]
- Riddle, R.N.; O’ Sullivan, J.; Swanton, C.J.; Acker, R.C.V. Field and Greenhouse Bioassays to Determine Mesotrione Residues in Soil. Weed Technol. 2013, 27, 565–572. [Google Scholar] [CrossRef]
- Pintar, A.; Stipičeić, S.; Lakić, J.; Barić, K. Phytotoxicity of Mesotrione Residues on Sugar Beet (Beta vulgaris L.) in Agricultural Soils Differing in Adsorption Affinity. Sugar Tech 2020, 22, 137–142. [Google Scholar] [CrossRef]
- Pintar, A.; Svečnjak, Z.; Lakić, J.; Magdić, I.; Brzoja, D.; Barić, K. The Susceptibility of Pea (Pisum sativum L.) to Simulated Mesotrione Residues as Affected by Soil pH Manipulation. Agriculture 2021, 11, 688. [Google Scholar] [CrossRef]
- Pismarović, L.; Milanović-Litre, A.; Kljak, K.; Lazarević, B.; Šćepanović, M. Soil solution pH can affect the response of the common bean (Phaseolus vulgaris L.) to mesotrione residues. Plant Soil Environ. 2022, 68, 237–244. [Google Scholar] [CrossRef]
- Young, B.G.; Young, J.M.; Matthews, J.L. Soybean (Glycine max) Response to Foliar Applications of Mesotrione. Weed Technol. 2003, 17, 651–654. [Google Scholar] [CrossRef]
- Melo, C.A.D.; Dias, R.C.; Mendes, K.F.; Assis, A.C.L.P.; Reis, R.M. Herbicides carryover in systems cultivated with vegetable crops. Rev. Bras. De Herbicidas 2016, 15, 67–78. [Google Scholar] [CrossRef]
- Maeghe, L.; Desmet, E.M.; Bulcke, R. Soil activity and persistence of sulcotrione and mesotrione. Commun. Agric. Appl. Biol. Sci. 2004, 69, 41–48. [Google Scholar]
- Brankov, M.; Vieira, B.C.; Rajković, M.; Simić, M.; Vukadinović, J.; Mandić, V.; Dragičević, V. Herbicide drift vs. crop resilience—The influence of micro-rates. Plant Soil Environ. 2023, 69, 161–169. [Google Scholar] [CrossRef]
- Jovanović-Radovanov, K.; Herbicidi-Praktikum, I. Izdanje; izdavač: Beograd, Srbija, 2020; pp. 57–68. [Google Scholar]
- Kadivar, M.R.; Muchhadiya, R.M.; Gohil, B.S.; Kumawat, P.D. Evaluating the safety of herbicide by bioassay techniques: A review. Int. J. Cult. Stud. 2023, 7, 84–91. [Google Scholar]
- Allemann, J.; Molomo, J.M. Sensitivity of selected dry bean (Phaseolus vulgaris L.) cultivars to mesotrione in a simulated carry-over trial. S. Afr. J. Plant Soil 2016, 33, 229–235. [Google Scholar] [CrossRef]
- Jovanović-Radovanov, K. Imazethapyr persistence in sandy loam detected using white mustard bioassay. J. Environ. Sci. Health Part B 2017, 52, 711–718. [Google Scholar] [CrossRef]
- Jovanović-Radovanov, K.; Rančić, D. Susceptibility of Selected Crops to Simulated Imazethapyr Carryover: A Morpho-Anatomical Analysis. Agronomy 2023, 13, 1857. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R Software Package for Dose-Response Studies: The Concept and Data Analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: http://www.R-project.org (accessed on 28 February 2025).
- Belz, R.G.; Carbonari, C.A.; Duke, S.O. The potential influence of hormesis on evolution of resistance to herbicides. Curr. Opin. Environ. Sci. Health 2022, 27, 100360. [Google Scholar] [CrossRef]
- Brito, I.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Pestemer, W.; Günther, P. No-observable Effect Level (NOEL). In Herbicide Bioassays; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 137–152. [Google Scholar]
- Belz, R.G.; Duke, S.O. Herbicides and plant hormesis. Pest Manag. Sci. 2014, 70, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Pintar, A.; Stipičevic, S.; Svecnjak, Z.; Baric, K.; Lakic, J.; Sraka, M. Crop sensitivity to mesotrione residues in two soils. Field and laboratory bioassays. Chil. J. Agric. Res. 2020, 80, 496–504. [Google Scholar] [CrossRef]
- Santín-Montanyá, I.; Alonso-Prados, J.L.; Villarroya, M.; García-Baudín, J.M. Bioassay for Determining Sensitivity to Sulfosulfuron on Seven Plant Species. J. Environ. Sci. Health Part B 2006, 41, 781–793. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Gholami Abadan, F. Negative Effects of Residual Herbicides on Sensitive Crops: Impact of Rimsulfuron Herbicide Soil Residue on Sugar beet. J. Res. Weed Sci. 2018, 1, 393–397. [Google Scholar]
- Torma, M.; Radvany, B.; Hodi, L. Effect of mesotrione residues on following crops. J. Plant Dis. Prot. 2004, 19, 801–805. [Google Scholar]
- Vrbničanin, S.; Božić, D.; Rančić, D.; Jovanović-Radovanov, K. Susceptibility of different varieties of Canada thistle (Cirsium arvense (L.) Scop.) to some herbicides. Acta Herbol. 2004, 13, 457–464. [Google Scholar]



| C vs. Applied Treatment (μg a.i./kg soil) | Chard | Lettuce | Spinach | Endive |
|---|---|---|---|---|
| Shoot Fresh Weight | ||||
| p | ||||
| C vs. 30 | 0.004 | <0.0005 | ||
| C vs. 60 | 0.009 | <0.0005 | ||
| C vs. 120 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
| C vs. 240 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
| F | 41.68 | 62.895 | 55.09 | 45.75 |
| Plant Species | Shoot Fresh Weight | |||||
|---|---|---|---|---|---|---|
| Regression Parameters (±SE) | EC50 | EC20 | EC10 | |||
| B | D | C | ||||
| Chard | −1.6 (0.4) | 104.4 (8.8) | −37.1 (7.3) | 23.9 (3.5) | 10.2 (2.3) | 6.2 (2.0) |
| Lettuce | −1.6 (0.5) | 108.9 (16.0) | −48.2 (9.2) | 34.3 (7.7) | 14.6 (3.9) | 8.9 (3.3) |
| Spinach | −1.4 (0.3) | 107.2 (6.8) | −41.3 (12.9) | 13.2 (2.4) | 4.8 (1.7) | 2.7 (1.3) |
| Endive | −0.5 (0.3) | 353.1 (498.4) | −51.6 (16.5) | 990.3 (3921.5) | 68.6 (186.4) | 14.4 (29.4) |
| C vs. Applied Treatment (μg a.i./kg soil) | Chard | Lettuce | Spinach | Endive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carot | Chl a | Chl b | Carot | Chl a | Chl b | Carot | Chl a | Chl b | Carot | Chl a | Chl b | |
| p | ||||||||||||
| C vs. 1.875 | <0.0005 | <0.0005 | 0.003 | |||||||||
| C vs. 3.75 | <0.0005 | <0.0005 | <0.0005 | 0.025 | 0.047 | |||||||
| C vs. 7.5 | 0.009 | <0.0005 | 0.008 | <0.0005 | 0.002 | 0.001 | 0.011 | 0.007 | ||||
| C vs. 15 | <0.0005 | <0.0005 | <0.0005 | 0.0001 | <0.0005 | <0.0005 | <0.0005 | 0.001 | 0.018 | 0.018 | 0.001 | 0.005 |
| C vs. 30 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | 0.01 | <0.0005 | 0.001 |
| C vs. 60 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
| C vs. 120 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
| C vs. 240 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
| F | 128.03 | 113.84 | 122.69 | 197.98 | 239.73 | 149.09 | 131.30 | 163.37 | 164.47 | 35.60 | 47.93 | 27.31 |
| Plant Species | Parameter Measured | Regression Parameters (±SE) | EC50 | EC20 | EC10 | ||
|---|---|---|---|---|---|---|---|
| B | D | C | |||||
| Chard | Carotenoids | −1.9 (1.7) | 113.3 (26) | 14.1 (10.2) | 66.9 (20.2) | 32.2 (19.3) | 21 (20.1) |
| Chlorophyll a | −1.1 (0.9) | 131.9 (60.7) | 7.8 (17.1) | 71.1 (63.2) | 19.4 (12.9) | 9.1 (10.6) | |
| Chlorophyll b | −1.2 (0.7) | 125.4 (36.1) | 8.3 (11.7) | 63.0 (34.5) | 19.1 (9.8) | 9.5 (8.0) | |
| Lettuce | Carotenoids | −10.4 (18.2) | 100.1 (1.9) | 17.7 (0.9) | 67.4 (13.9) | 59.0 (1.9) | 54.6 (9.1) |
| Chlorophyll a | −6.7 (5.2) | 100.9 (4.4) | 23.0 (2.0) | 68.2 (7.3) | 55.4 (4.4) | 49.1 (8.1) | |
| Chlorophyll b | −5.8 (5.4) | 101.5 (7.4) | 25.8 (3.4) | 69.9 (10.1) | 55.1 (7.4) | 48.0 (11.8) | |
| Spinach | Carotenoids | −2.3 (0.9) | 103.4 (6.6) | 3.5 (7.4) | 22.7 (3.7) | 12.5 (4.0) | 8.8 (3.9) |
| Chlorophyll a | −2.2 (0.8) | 104 (6.6) | −2.1 (7.5) | 22.4 (3.6) | 11.8 (3.7) | 8.1 (3.5) | |
| Chlorophyll b | −1.8 (0.8) | 107 (10.5) | −12.3 (12.3) | 23.9 (5.6) | 11.0 (5.3) | 7.0 (4.7) | |
| Endive | Carotenoids | −1.7 (0.4) | 81.8 (22.9) | 10.8 (0.9) | 176.1 (73.0) | 77.4 (16.8) | 47.9 (6.4) |
| Chlorophyll a | −0.9 (0.3) | 251.1 (392.3) | 10.0 (2.6) | 1043.1 (2975) | 206.8 (485.2) | 80.2 (165.3) | |
| Chlorophyll b | −0.6 (0.3) | 274.2 (377.6) | 1.0 (9.1) | 2194.4 (8262.7) | 183.8 (552.9) | 43.1 (11.6) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radivojević, M.; Nedeljković, D.; Jovanović-Radovanov, K. Sensitivity of Leafy Vegetables to Simulated Mesotrione Residues in the Soil. Horticulturae 2025, 11, 644. https://doi.org/10.3390/horticulturae11060644
Radivojević M, Nedeljković D, Jovanović-Radovanov K. Sensitivity of Leafy Vegetables to Simulated Mesotrione Residues in the Soil. Horticulturae. 2025; 11(6):644. https://doi.org/10.3390/horticulturae11060644
Chicago/Turabian StyleRadivojević, Milena, Dejan Nedeljković, and Katarina Jovanović-Radovanov. 2025. "Sensitivity of Leafy Vegetables to Simulated Mesotrione Residues in the Soil" Horticulturae 11, no. 6: 644. https://doi.org/10.3390/horticulturae11060644
APA StyleRadivojević, M., Nedeljković, D., & Jovanović-Radovanov, K. (2025). Sensitivity of Leafy Vegetables to Simulated Mesotrione Residues in the Soil. Horticulturae, 11(6), 644. https://doi.org/10.3390/horticulturae11060644






