1. Introduction
Plants have evolved many different mechanisms to survive soil moisture deficits and low-water stress (LWS). Growth and biomass production are often among the first traits to decline under LWS, due to the partial or complete stomatal closure and concomitant reductions in photosynthesis and the down-regulation of metabolic processes [
1]. In wild plants, reductions in growth and biomass production due to stomatal limitations in photosynthesis can be paired with early flowering and seed set to escape LWS, or it can be paired with dormancy to tolerate LWS [
2]. For some crops, these acclimation strategies (i.e., early flowering and early seed) may be harnessed by crop breeders to select for germplasm that produce similar yields under LWS and control or no-stress conditions, therefore leading to the production of crops that consistently produce high yields under a wide range of field conditions [
3]. However, for crops for which biomass is the primary product, such as lettuce and other leafy vegetables, the process of breeding for consistent yield across a range of soil moisture content is more difficult. The natural, evolved acclimation strategies of plants involving growth reduction and early flowering decreases yield in leafy vegetables experiencing LWS. Alternative physiological responses to improve yield consistency must be investigated and introgressed into commercial cultivars of leafy greens in order to improve lettuce water-use efficiency (WUE) and biomass production under a range of soil moisture conditions in irrigated agriculture.
Current commercial cultivars of lettuce (
Lactuca sativa L.) experience significant reductions in biomass during deficit soil moisture conditions, but some cultivars experience less severe reductions than others [
4]. These cultivars produce a more consistent yield under a range of field conditions, and can be considered water-use-efficient or tolerant of stomatal limitations; they are thus good candidates for breeding programs with goals to improve WUE in lettuce. The crop wild relatives of lettuce include a ruderal weed called
Lactuca serriola L. [
5], which is typically considered to have a greater WUE than its cultivated relative because of its ability to grow in waste areas. It has higher carbon assimilation under LWS conditions, at least in part due to higher mesophyll conductance [
6]. Mesophyll conductance has been implicated in improved carbon assimilation during stomatal limitations, and has also been shown to vary significantly among species and cultivars (reviewed in [
7]). Mesophyll conductance (
gm) describes the diffusion of carbon from the stomata through the leaf mesophyll, to the sites of carboxylation. Higher
gm drives a diffusion gradient between the stomata and the sites of carboxylation, which allows more CO
2 to enter the leaf through partially closed stomata, thereby allowing for both reduced transpiration and continued intake of CO
2.
There is considerable research on the mechanisms that affect
gm, which may be applicable to lettuce during stomatal limitations. Anatomical parameters of the leaf such as cell wall thickness and chloroplast arrangement have been shown in some cases to influence mesophyll conductance [
8,
9,
10,
11,
12]. Cell wall thickness accounts for over 50% of the total resistance to carbon movement [
9]; however, we chose to focus our attention in this study on the conductance through the liquid portion of the cell, because lettuce cells have a high relative water content. Biochemical factors appear to have a strong influence on carbon diffusion through the liquid portion of the cell, and thus a strong influence on short term acclimation to stomatal limitations [
8,
13]. There is evidence that the process is protein-facilitated [
13], and both aquaporins [
14,
15] and carbonic anhydrases (CA) [
15,
16] have emerged as important components of the process. Carbonic anhydrases catalyze a reversible CO
2 to HCO
3 reaction which facilitates the diffusion of carbon through the liquid portion of the cells. Aquaporins or plasma membrane intrinsic proteins (PIPs) affect the permeability of plasma membranes, and there is mixed evidence for their influence on
gm. Using aquaporin knockout lines, Huang et al. (2021) [
17] found reduced mesophyll conductance in rice in different growth environments and different growth stages; however, Kromdijk et al. (2020) [
18] found no influence of aquaporins on mesophyll conductance rates in
Arabidopsis thaliana.
Nitrogen content may also affect carbon assimilation because of its decreased uptake during stomatal limitations [
19] and its importance to the process of carbon assimilation. The majority of N used in carbon assimilation is invested in Rubisco [
20]. Nitrogen is taken up through the roots and translocated through the plant via transpiration flow; therefore, reduced transpiration due to stomatal limitations leads to reduced N uptake. If carbon assimilation is limited by N availability during stomatal limitations and decreased N uptake, plants that can uptake and store more N before stomatal limitations might maintain higher carbon assimilation rates during periods of stomatal closure.
In this study, we tested the hypothesis that higher biomass production in lettuce during stomatal limitations is due to higher carbon assimilation rates. We previously identified cultivars with minimal reductions in biomass during periods of stomatal limitations [
4], and labeled these as tolerant (T). We paired these cultivars with others that experienced severe reductions in biomass during periods of stomatal closure but had similar leaf shapes, and designated these as susceptible (S). We induced stomatal limitations to photosynthesis in these plants and measured carbon assimilation and other photosynthetic traits. Further, we tested the hypothesis that
gm rates and N content are higher in tolerant lettuce cultivars. We also used RNA-seq to understand expression patterns of genes involved in carbon assimilation, including carbonic anhydrases and aquaporins, which have been shown to influence mesophyll conductance in other plants. Our objectives were to understand the differences in carbon assimilation among cultivars during stomatal limitations in order to identify traits to improve LWS tolerance in leaf lettuce cultivars.
2. Materials and Methods
The cultivars chosen for this study included three cultivars described by Eriksen et al. [
4] as tolerant (T) to LWS induced stomatal limitations, and were paired with three cultivars with similar leaf shapes which we considered sensitive or susceptible (S) to stomatal limitations. Sensitive cultivars had large, statistically significant reductions in biomass under LWS treatments. There were a limited number of T cultivars, so it was not possible to use three T cultivars with the same leaf shape; by comparing T cultivars with S cultivars with the same leaf shape, we could partially control for the effect of leaf shape on LWS response.
Cv. Australian (T) is a leaf-type lettuce with a rosette of deeply lobed green leaves. In Eriksen et al. [
4], it weighed on average 146.9 g (SD = 44.8) under control irrigation treatments, and 124.6 g (SD = 22.8) under reduced irrigation treatments. It was paired with cv. Green Salad Bowl (S) which has a similar rosette of deeply lobed green leaves, but had significant reductions in biomass during stomatal closure; it weighed on average 378.2 g (SD = 170.0) under control irrigation treatments and 236.5 g (SD = 91.4) under reduced irrigation. Cv. Slobolt (T) is also a leaf type lettuce, with large, pale green smooth-edge leaves, and it weighed on average 220.6 g (SD = 34.1) under control irrigation treatments and 201.6 g (SD = 60.9) under reduced irrigation treatments. It was paired with cv. Black-Seeded Simpson (S), which weighed on average 404.2 g (SD = 135.8) under control irrigation treatments, and 257.9 g (SD = 13.2) under reduced irrigation treatments. Cv. Western Green (T) is a leaf-type lettuce with dark green leaves, toothed margins, and a slightly waxy appearance; it weighed on average 265.1 g (SD = 55.3) under control irrigation treatments, and 243.2 g (SD = 54.1) under reduced irrigation treatments. It was paired with cv. Xena (S), which weighed on average 434.4 g (SD = 160.7) under control irrigation treatments, and 289.6 g (SD = 114.8) under reduced irrigation. In some trials, we also grew plants of the weedy, wild relative of cultivated lettuce,
L. serriola accession US96UC23 in order to compare these cultivars to a related plant with high WUE during stomatal closure.
We conducted three trials to collect photosynthesis data (May 2016; July 2016, February 2017). For each trial, we direct seeded three plants of each cultivar in 3.78 L pots with a sterilized mix of 1:1 potting soil (SunGro Sunshine Mix #1 Professional Potting Mix, Agawam, MA, USA, containing sphagnum peat moss, perlite, and dolomite, and 0.25% calcium silicate) and sand. Trial sizes were limited by the number of plants that could be accurately measured during the peak of the photosynthetic diurnal cycle. The plants were grown for six weeks in a greenhouse under standard growing conditions, meaning the average soil volumetric water content [VWC] fluctuated around 0.2 m3/m3, and temperatures in the greenhouse were maintained between 15 and 25 °C. We used full-spectrum lights (Sun System 3, Vancouver, WA, USA) to supplement natural light and allow for a minimum of 12 h daily light during all trials. Light conditions with supplementation ranged between 162 and 1570 µmol m−2 s−1, and averaged 1030 µmol m−2 s−1 (±356 µmol m−2 s−1). The average saturating light for photosynthesis in lettuce was measured at approximately 800 µmol m−2 s−1 before this study. We monitored soil moisture with EC-5 soil moisture probes that were positioned approximately 3 cm from the plant, and moisture readings were recorded every two hours using an Em50 data logger (METER Group, Pullman, WA, USA). Plants were fertilized twice with 20N-20P-20K general purpose fertilizer (Jack’s Nutrients Professional, Allentown, PN, U.S.A., also containing calcium, magnesium, boron, copper, iron, manganese, molybdenum, and zinc) according to the manufacturer’s instructions at week one and week six.
At six weeks, soil was allowed to dry to approximately 0.01 m3/m3 VWC for two weeks to induce stomatal limitations, where stomatal limitations are the primary factor influencing carbon assimilation rates. Data were recorded at 7 days post the onset of treatment (dpt) and 14 dpt. Data were not recorded after 14 dpt because the plants were then 9 weeks old, past typical harvest maturity size, and beginning to experience the effects of root constriction in the pot.
2.1. Gas Exchange Measurements and Data Analysis
We measured carbon assimilation and stomatal conductance rates before treatment, 7 days post treatment (dpt), and 14 dpt using a LI-COR 6400XT Portable Photosynthesis System with a 6400-02 LED Source (LI-COR Biosciences, Lincoln, NE, USA). All measurements were taken between 1000 and 1400 h (adjusted to Pacific standard time in USA) on two middle leaves. Preliminary diurnal curves found no significant differences among measurements taken during these hours in L. sativa or L. serriola (F > 1.42 for both species, p > 0.25 for both species). We set the light intensity to 800 µmol m−2 s−1 based on preliminary work that suggested this range is saturating for cultivated lettuce. Flow rates were set to 500 µmol m−2 s−1, and a mixer was used to control CO2 concentrations at near atmospheric levels (400 ppm). We maintained leaf temperature at 20 °C, and infrared gas analyzers (IRGAs) were matched every 30 min. We recorded carbon assimilation (A) and stomatal conductance (gsw) rates until both parameters reached relative stability (~3–5 min).
Leaf intrinsic water use efficiency
iWUE was calculated according to the LI-COR 6400XT manual using the following formula:
where
E is transpiration.
The data were analyzed in R v.4.2.0 [
21]. The assumptions for parametric tests were tested using boxplots, Shapiro–Wilk tests for normality, and Levene tests for homoscedasticity. If the data failed to fit the assumptions for parametric tests, they were transformed, or we used a parametric ANOVA followed by post-hoc Tukey HSD tests and non-parametric Kruskal–Wallis H test followed by post-hoc multiple comparison tests to confirm the results of parametric tests. If the results were the same, we reported the results of parametric tests only in these cases.
2.2. Trial 3 and 4 Deviations in Methods
During the third and fourth trial, three additional plants of each cultivar were maintained at a higher soil VWC to prevent stomatal limitations (i.e., control water treatments). Stomatal conductance rates in these plants were measured at
gsw > 0.2 mol H
2O m
−2 s
−1, and stomatal conductance rates in plants with induced stomatal closure were measured at an average of
gsw = 0.06 mol H
2O m
−2 s
−1, the g
sw rate approximately at which plants begin to experience biochemical limitations to photosynthesis [
22]. During the third trial, 1–2 mid-aged leaves were removed from each plant at 14 dpt and dried for a total carbon and nitrogen analysis, and 3–4 of the youngest leaves were removed from each plant and flash-frozen in liquid nitrogen for transcriptome sequencing. During the fourth trial, mesophyll conductance rates were estimated on six plants of each lettuce cultivar.
2.3. Total N and C Measurement
Exactly 3 g of leaf tissue was dried in an oven for 24 h, and submitted to the University of California Davis Analytical Laboratory for a total N and C analysis using the combustion method. Protocols followed the methods described in the AOAC International official methods [
23]. The percentage of total N and C was measured using parametric statistics as described above.
2.4. Transcriptome Sequencing and Data Analysis
Total RNA was extracted and sequenced for three plants of each treatment of each cultivar on an Illumina platform at Novogene Co., Ltd. (Beijing, China). The raw reads were filtered to remove adapters and low-quality reads, such as reads containing >10% N. This resulted in 42 libraries with 41.8–62.9 million raw reads and 38.5–59.3 clean reads. The average library contained 47.5 +/− 4.5 million reads with a GC content of 44%.
Reads were mapped to the v5 lettuce genome [
24], the most recent version available at the time of analysis, using Tophat 2. An average of 83% of reads were mapped. Expression was quantified using FPKM (fragments per kilo bases per million reads), and differential expression was evaluated with DESeq [
25].
Transcript functions were hypothesized by homology using BLASTx against the TAIR10 protein database with a cutoff value of e = 1 × 10
−10. For heatmaps, we built gene lists from transcripts that were annotated as involved in the carbon fixation pathway by KEGG. Aquaporins were identified in the dataset by a reciprocal best BLASTx against the lettuce genome database (
https://www.lettucegdb.com/toolblast?dbindex=3, accessed on 15 March 2025) of 4 TIPs and PIPs from
N. tabacum, which Flexas et al. [
14] found to be influential on mesophyll conductance. Transcripts were included in the heatmaps based on significant differential expression patterns among groups, based on a B-H adjusted
p value of less than 0.05 and an absolute value of the log2-fold change greater than 2.
2.5. Estimation of Mesophyll Conductance and In Vivo Rubisco Activity by A/Ci Curves
We constructed A/Ci curves using the ACI Curve2 auto program on the LI-COR 6400XT on 3 plants of each cultivar with open stomata (gsw > 0.2 mol H2O m−2 s−1), and 3 plants of each cultivar with partially closed stomata (gsw < 0.2 mol H2O m−2 s−1). Carbon assimilation measurements was measured at different CO2 concentrations of 400, 300, 200, 100, 50, 10, 0, 400, 400, 500, 600, 800, 1000, and 1200 µmol CO2, and chamber light was set at saturating levels for cultivated lettuce at 800 µmols photons m−2 s−1.
To analyze A-C
i curves, we used the R package plant ecophys [
26] using the default non-linear regression fitmethod to calculate Jmax, Gamma*, and Rd in order to calculate mesophyll conductance using the formula devised by Harley et al. [
27].
3. Results
During trials 1 and 2, the average soil VWC was maintained at 0.03 m2/m2, and the relative water content of the leaf did not decline; therefore, all measurements were taken during a period that should induce stomatal limitations, and under which photosynthesis is affected primarily by stomatal limitations rather than biochemical limitations.
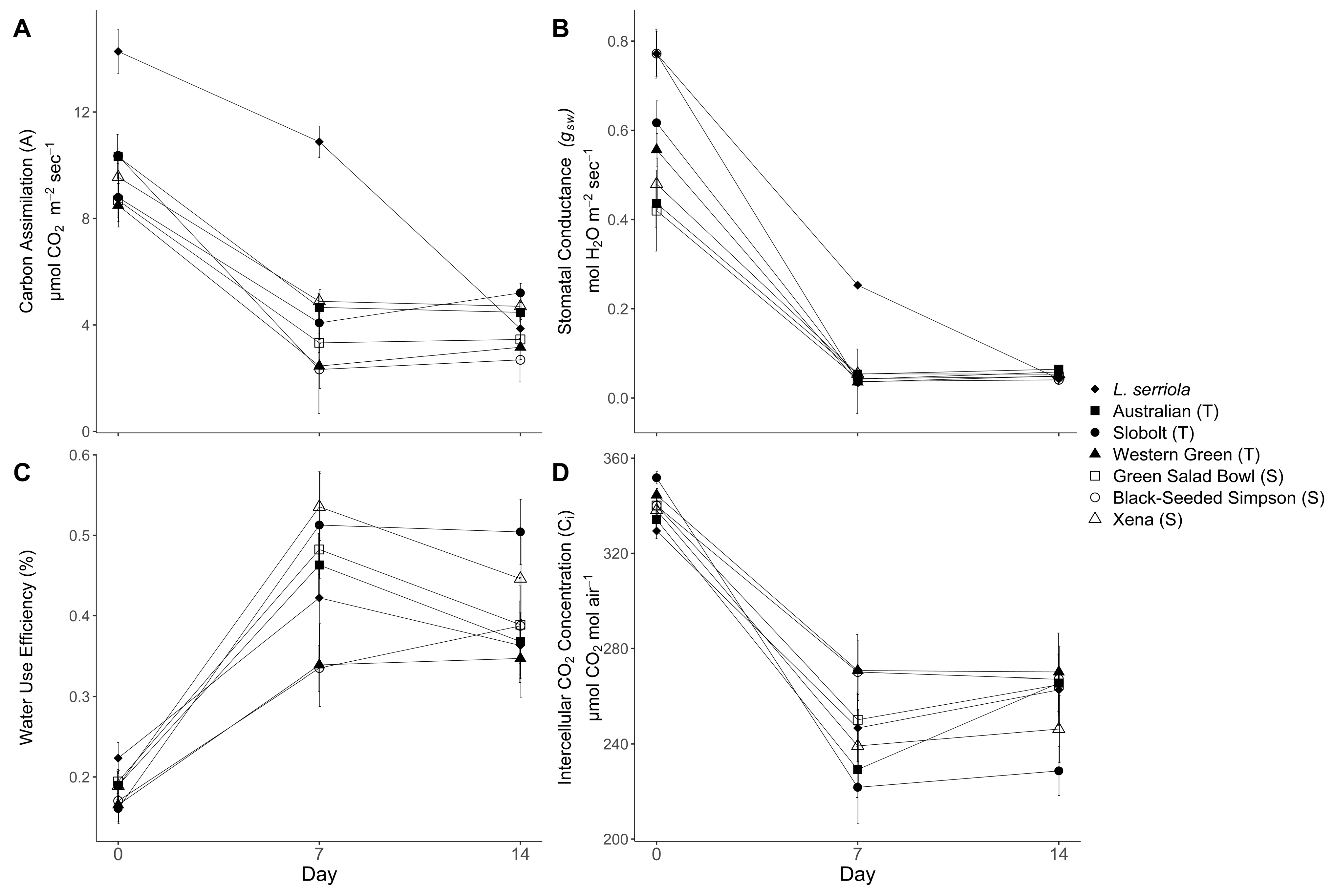
Stomatal conductance rates and, concomitantly, carbon assimilation rates declined with time and soil VWC in all cultivars by 7 and 14 days post onset of treatment (dpt) (
Figure 1A,B). Carbon assimilation and stomatal conductance decreased by 7 dpt, and for most plants, carbon assimilation and stomatal conductance stayed low by 14 dpt; there was no further decline between days 7 and 14 dpt. Prolonged exposure to LWS did not cause the carbon assimilation to decline further, except in
L. serriola. The wild relative
L. serriola had higher carbon assimilation and higher stomatal conductance 7 dpt, but these higher rates did not persist to 14 dpt, and
L. serriola plants did not have the highest calculated iWUE (
Figure 1C) or intercellular CO
2 concentration (C
i) at 7 or 14 dpt (
Figure 1D). Among the cultivated lettuce samples, there were no significant differences in stomatal conductance rates among T and S varieties at 7 or 14 dpt, because treatments were calibrated to maintain that rate of stomatal conductance indicative of stomatal limitations (
Figure 1B). Despite having similar stomatal conductance rates, however, there were significant differences among cultivars in carbon assimilation rates at 7 dpt (ANOVA F = 13.37, df = 6,
p < 0.001,
Figure 1A) that was driven by significantly higher carbon assimilations rates in
L. serriola. When
L. serriola was removed from the dataset, there was also a significant difference among cultivars tested at 14 dpt (ANOVA F = 3.18,
p = 0.006) resulting from differences among the cv.s: Slobolt (T) and Black-Seeded Simpson (S) (Slobolt = 5.21 µmol CO
2 m
−2 s
−1; Black-Seeded Simpson = 2.69 µmol CO
2 m
−2 s
−1; Tukey HSD
p = 0.02). The tolerant cv. Slobolt had the highest net carbon assimilation rate, as well as the highest iWUE, though the lowest C
i. Cv. Australian (T) had a higher carbon assimilation rate than most S cultivars except for cv. Xena, but it was not statistically significant.
There was no significant difference in percent C among cultivars with no stomatal closure (ANOVA F = 2.62, df = 6,
p = 0.06,
Figure 2A), though there was a significant difference in percent N among cultivars (ANOVA F = 6.11, df = 6,
p = 0.003) (
Figure 2B), driven by a slightly higher percent N in
L. serriola (Tukey HSD,
p < 0.05 for each pairwise comparison involving
L. serriola, though none were significant at a Bonferroni-corrected
p value of 0.002). There was a highly significant difference in the percent N among cultivars with stomatal limitations (ANOVA F = 19.22, df = 6,
p = 5.15 × 10
−6), again driven by a higher percent N in
L. serriola (Tukey HSD,
p < 0.002 for each comparison involving
L. serriola,
Figure 2).
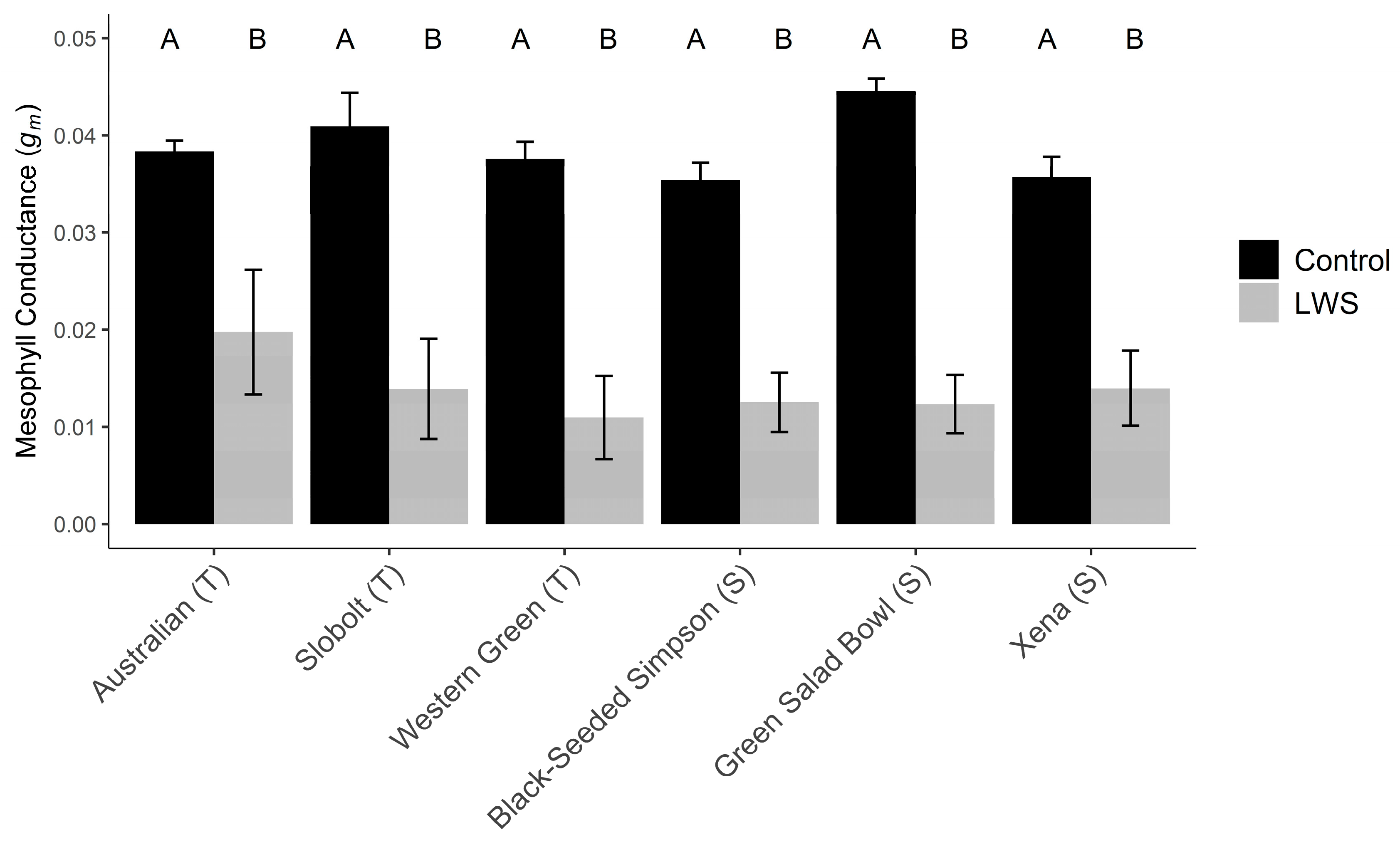
Mesophyll conductance calculated from AC
i curves was significantly lower in plants with stomatal limitations than in plants with open stomata (two-way ANOVA, Treatment F
(1,5) = 161.4,
p = 5.4 × 10
−5). ‘Australian’ had slightly higher mesophyll conductance rates during stomatal limitations than the other cultivars, though this was not statistically significant (two-way ANOVA, Cultivar F
(1,5) = 0.9,
p = 0.56) (
Figure 3).
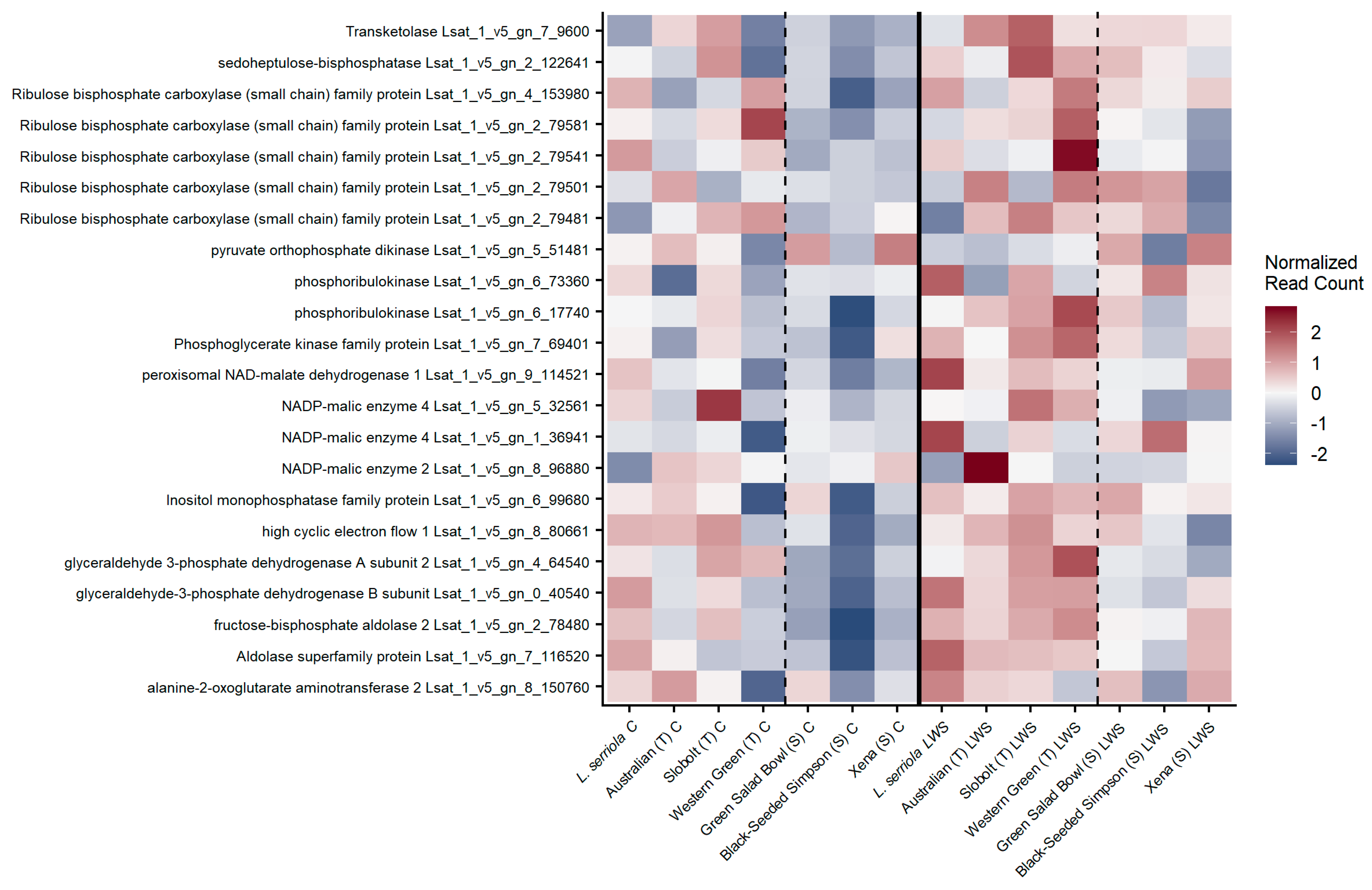
For transcripts annotated within the KEGG pathway for carbon assimilation, we considered only transcripts with high expression (total normalized read counts > 10,000 for all samples). There was up-regulation of genes within the carbon assimilation pathway within the T cultivars compared to S cultivars under both treatments; however, the up-regulation was stronger when stomata were partially closed (
Figure 4). Interestingly, cv. Australian had significant up-regulation of a putative NADP-malic enzyme 2 transcript during stomatal limitations, while another NADP-malic enzyme was significantly up-regulated in cv. Slobolt under control water treatments. All T cultivars accessions had consistent up-regulation of transcripts annotated as glyceraldehye-3 phosphate dehydrogenase A and B subunits, as well as two aldolase transcripts. We found significant enrichment of genes involved in antenna proteins in photosynthesis among the list of differentially expressed (DE) genes in cv.s Australian and Green Salad Bowl, and cv.s Australian and Western Green during stomatal limitations (
Table 1). Among the DE genes in cv.s Slobolt and Western Green, there was also a significant enrichment of antenna proteins during stomatal limitations. There was significant enrichment of genes involved in the KEGG pathway carbon fixation in photosynthetic organisms in the DE genes of cv. Slobolt and Black-Seeded Simpson when stomata were fully open. When comparing DE within each cultivar among treatments (diagonal line of
Table 1), there were no genes annotated to either KEGG pathway that were DE among treatments (cv.s Australian (T), Slobolt (T), Western Green (T), Xena (S)) and no significant enrichment of genes in these pathways among treatments (cv.s Black-Seeded Simpson (S), Green Salad Bowl (S),
L. serriola).
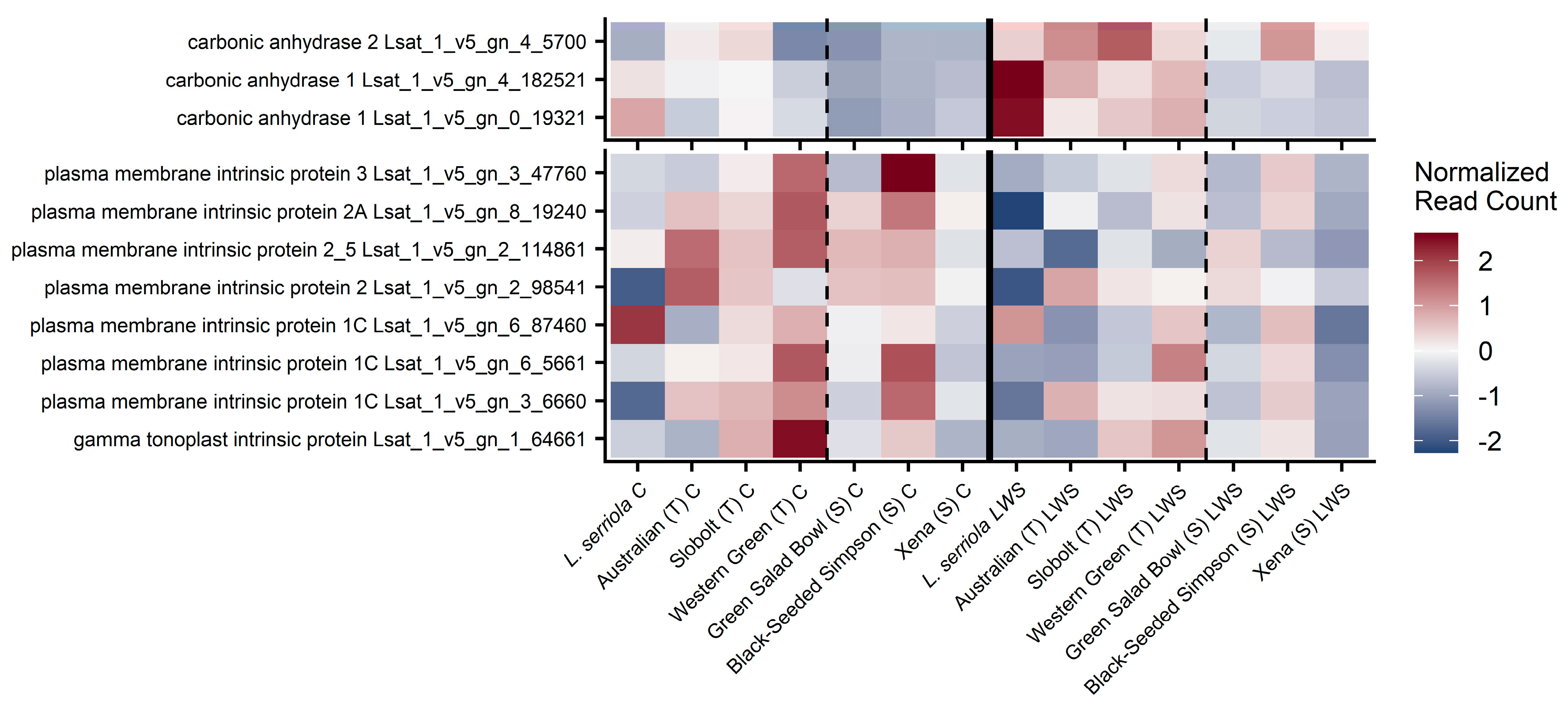
We found two transcripts with significant homology to published sequences of carbonic anhydrase 1 with high expression in our dataset (Lsat_1_v5_gn_4_182521, and Lsat_1_v5_gn_0_19321), and one transcript with significant homology to carbonic anhydrase 2 (Last_1_v5_gn_4_5700). There was an increase in the expression values of these transcripts during stomatal limitations in all T cultivars (
Figure 5). We found the expression of eight transcripts that were significant BLASTx matches against NtAQP1, a
Nicotiana tabacum gene involved in improving mesophyll conductance [
14]. Generally, these transcripts appear more up-regulated in T accessions than S accessions, but primarily when stomata are fully open (
Figure 5).
4. Discussion
California and Arizona produce the majority of the lettuce grown in the United States, with most of the production in the San Joaquin, Santa Maria, Imperial, and Salinas Valleys of California, and the Yuma region of Arizona; production is most often highest in Monterey County in the Salinas Valley [
28]. Lettuce production is irrigated in these regions; however, breeding for consistent yield among variable soil moisture conditions is a long-term goal. Harnessing traits that plants have evolved to survive deficit soil moisture in wild populations is not productive in lettuce because most of those traits that wild populations use to tolerate stomatal limitations and reduced carbon assimilation involve reductions in biomass. Because biomass is the primary product in lettuce, harnessing these traits would result in decreased productivity and yield in lettuce; thus, breeding for consistent yield is a significant challenge. Improved WUE that maximizes photosynthesis and biomass production during stomatal closure may be the most effective tactic to pursue.
In this study, we selected cultivars described in Eriksen et al. (2016) [
4] as being “tolerant” of stomatal limitations (cv. Australian, Slobolt, Western Green); that is, they had consistent yields, or statistically insignificant reductions in biomass during stomatal limitations in the field. We paired these with cultivars that were designated “susceptible” to stomatal limitations (cv. Green Salad Bowl, Black-Seeded Simpson, Xena) due to significant differences in biomass of plants with stomatal limitations. Our objective in this study was to test hypotheses about physiological differences among cultivars that allow for improved photosynthesis and carbon assimilation during stomatal limitations when stomatal limitations are the primary limiting factor. We hypothesized that the movement of C through the mesophyll cells of the leaf, and N content to continue growth under reduced transpiration should have effects on carbon assimilation and biomass production under these conditions.
We saw steady reductions in carbon assimilation as stomatal conductance declined, but even when stomatal conductance was similarly low in all cultivars, there were measurable differences in carbon assimilation rates. Carbon assimilation was higher in some T cultivars, but not all T cultivars. Cv. Slobolt (T) and Australian (T) had consistently higher carbon assimilation rates than similar S cultivars. In this study, cv. Xena (S) had higher carbon assimilation rates than cv. Western Green (T), in contrast to designations made in Eriksen et al. [
4]. The T and S designations were based on biomass during field trials; however, these studies took place in the greenhouse. These designations of T or S were then not consistent across time or studies.
Mesophyll conductance is affected by the structure of the plant cell, including the surface area of chloroplasts exposed to airspaces [
29,
30], cell wall thickness, and microfibril alignment [
31], but biochemical factors are important to move carbon through the aqueous portions of the cell. Adhikari et al. [
32] found increased concentrations of carbonic anhydrases in the wild relative
L. serriola compared to the cultivated lettuce cv. Salinas, suggesting that this biochemical factor may be at least partially responsible for higher measured mesophyll conductance rates in
L. serriola [
6]. We estimated
gm using carboxylating efficiency curves but found no significant differences among cultivars during stomatal limitations. The putatively T cultivar ‘Australian’ had slightly higher
gm; however, this rate was not statistically significant. We found eight putative aquaporins in our transcriptome database that might contribute to improved
gm; however, there was no strong pattern of increased expression of these in T and S plants. We identified three putative carbonic anhydrases that also might affect
gm that were expressed in our dataset, and there does appear to be a stronger expression of these in T varieties than in the S plants during stomatal limitations.
The importance of
gm in carbon assimilation during LWS is well established; however, not many studies have looked at the variation among cultivars or populations in
gm and the role it might have in influencing LWS tolerance among closely related groups. Ouyang et al. [
33] found significant differences among rice (
Oryza sativa) cultivars under moderate and severe drought treatments, and Momayyezi and Guy [
34] found variation in
gm along a latitudinal cline of black cottonwood (
Populus trichocarpa) that they link specifically to carbonic anhydrase (CA) activity. Similarly to the present study, however, Parri et al. [
35] found only small differences in
gm among cultivars of Italian olive trees (
Olea europea L.), but no statistically significant effect of cultivar on
gm rates. It may be that the anatomical characteristics that constrain
gm have a higher impact on
gm than CA activity, and that these anatomical characteristics are more highly conserved than the gene expression or activity of CA within a species. Because we found only small and statistically insignificant differences in
gm rates among cultivars, it appears that CA activity and
gm may only have small effects, if any, on the variation in LWS tolerance among lettuce cultivars.
Another factor influencing carbon assimilation and photosynthesis is nitrogen content. Given the importance of N to the photosynthetic system [
20], it could be postulated that a higher N uptake efficiency before stomatal limitations are induced could provide a plant additional stored resources to draw on during stomatal limitations when N uptake is reduced due to reduced transpiration. We measured the total N content and found a significantly higher percent N content in
L. serriola and cv. Australian (T) under both stomatal conditions. The S cultivar Black-Seeded Simpson also had a relatively high N content, though it was not significantly higher than the other tolerant cultivars Slobolt and Western Green.
Variation in N uptake efficiency among cultivars is well established. Ahmad et al. [
36] found significant differences in leaf N content among varieties of wheat, Koutroubas and Ntanos [
37] in rice, and Benincasa et al. [
38] in sweet pepper, tomato, and lettuce. Other studies have found that N improves LWS tolerance, including Song et al. [
39] in maize seedlings, and Iqbal et al. in cotton. [
40]. Macias-González et al. [
41] looked at the genetics of robustness or consistency of lettuce under different N and water deficient conditions, and found major and minor clusters of QTLs relating to trait consistency in different conditions They further suggest that selecting for plant fresh weight in suboptimal but not stressful water or N conditions might be the most efficient method to improve N use efficiency and WUE.
We also saw differences in transcript expression values that suggested other metabolic differences among accessions. We saw the consistent up-regulation of transcripts involved in glycolysis in T accessions, including fructose-bisphosphate aldolase and glyceraldehyde 3-phosphate dehydrogenase (G3PD). The up-regulation of glycolysis is not uncommon in plants during stomatal limitations [
42,
43,
44]. One of the products of G3PD’s activity on 3-carbon phosphorylated sugar is 3-phosphoglycerate, which is a precursor to glycine-betaine (via the amino acid serine). Glycine-betaine has been shown to act as a compatible solute and improve plant tolerance to deficit soil moisture conditions [
45,
46,
47].
We also found the significant up-regulation of NADP malic enzyme (NADP-ME) in the tolerant accessions
L. serriola, cv. Australian, and cv. Slobolt. NADP malic enzyme (NADP-ME) is localized to the chloroplasts or cytoplasm of bundle-sheath cells and catalyzes the reversible reactions involving the decarboxylation of malate to form pyruvate, the initial compound in glycolysis and polyphenol biosynthesis. Malate is critical in CAM and C4 photosynthesis as a carbon-concentrating mechanism, but it also regulates pH and functions as an osmolyte involved in stomatal closure [
48]. In transgenic
Nicotiana tabacum expressing
Zea mays NADP-ME, there was reduced stomatal aperture and increased carbohydrate export from the leaves, leading to increased carbon assimilation and therefore improved WUE [
49]. Engineering C4 photosynthetic mechanisms into C3 crops has been identified as a potential mechanism for improving WUE in crops [
50,
51], and the potential for natural variation among cultivars in NADP-ME expression levels under low-water stress in crops that leads to improved WUE should be investigated further.