Composting a Mixture of Cactus Pear Pruning Waste and Spent Coffee Grounds: The Chemical Evaluation of Organic Fertilizer in Response to Basil Quality and Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Campaigns
2.2. Basil Plant Growth Experimental Trial
2.3. Polyphenol Extraction
2.4. Total Polyphenol Content
2.5. Flavonoid Content
2.6. Total Antioxidant Activity
- Ac = Control absorbance (DPPH 60 μM);
- As = Absorbance of the sample;
- Ab = Absorbance of the blank.
2.7. Ferric Reducing Antioxidant Power (FRAP)
2.8. Statistical Elaborations
3. Results
3.1. Composting Campaings
3.2. Basil Growth
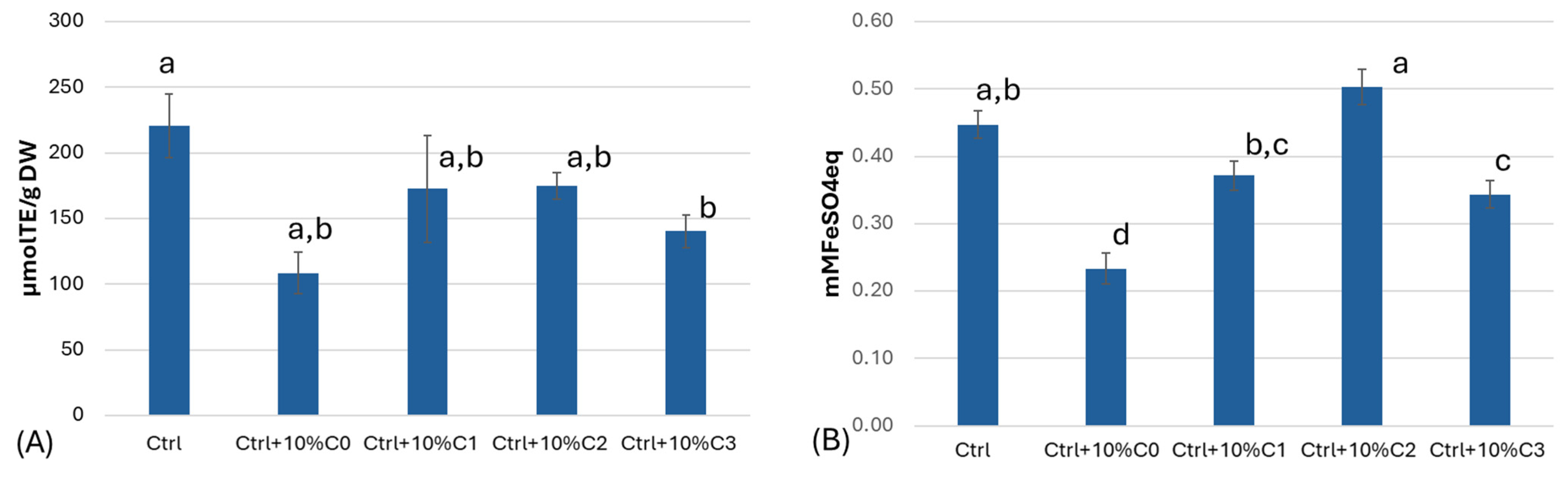
3.3. Total Polyphenol and Flavonoid Content and Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| C | Carbon |
| C0 | Mixture composted with only OFI |
| C1 | Mixture composted with OFI–SCG 10:1 |
| C2 | Mixture composted with OFI–SCG 3.3:1 |
| C3 | Mixture composted with OFI–SCG 2:1 |
| Ctrl | Commercial soil |
| DW | Dry weight |
| DM | Dry matter |
| FRAP | Ferric Reducing Antioxidant Power |
| FW | Fresh weight |
| GAE | Gallic acid equivalents |
| OFI | Opuntia ficus-indica |
| SCG | Spent coffee ground |
| T1-T3 | Months from sowing |
| WC | Water content |
References
- Di Bella, G.; Vecchio, G.L.; Albergamo, A.; Nava, V.; Bartolomeo, G.; Macrì, A.; Bacchetta, L.; Turco, V.L.; Potortì, A.G. Chemical characterization of Sicilian dried nopal [Opuntia ficus-indica (L.) Mill.]. J. Food Compos. Anal. 2022, 106, 104307. [Google Scholar] [CrossRef]
- Méndez, L.P.; Flores, F.T.; Martín, J.D.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Physicochemical characterization of cactus pads from Opuntia dillenii and Opuntia ficus-indica. Food Chem. 2015, 188, 393–398. [Google Scholar] [CrossRef]
- Mulas, M.; Dessena, L. Quantitative and qualitative analysis of cladodes from new selections of Opuntia ficus-indica. Acta Hortic. 2019, 1247, 155–161. [Google Scholar] [CrossRef]
- Ochoa, M.J.; Barbera, G. History and economic and agro-ecological importance. In Crop Ecology, Cultivation and Uses of Cactus Pear; FAO and ICARDA: Coquimbo, Chile, 2017; pp. 1–11. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/c7920e28-b849-40ae-b091-4d40d295a8e9/content (accessed on 3 June 2025).
- Procacci, S.; Bojórquez-Quintal, E.; Platamone, G.; Maccioni, O.; Lo Vecchio, V.; Morreale, V.; Alisi, C. Opuntia ficus-indica Pruning Waste Recycling: Recovery and Characterization of Mucilage from Cladodes. Nat. Resour. 2021, 12, 91–107. Available online: https://www.scirp.org/html/2-2001000_108538.htm (accessed on 3 June 2025).
- Aparicio-Ortuño, R.; Jiménez-González, O.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Cladodes of Opuntia ficus-indica as a functional ingredient in the production of cookies: Physical, antioxidant and sensory properties. Sustain. Food Technol. 2024, 2, 816–825. [Google Scholar] [CrossRef]
- Alisi, C.; Bacchetta, L.; Bojorquez, E.; Falconieri, M.; Gagliardi, S.; Insaurralde, M.; Martinez, M.F.F.; Orozco, A.M.; Persia, F.; Sprocati, A.R.; et al. Mucilages from Different Plant Species Affect the Characteristics of Bio-Mortars for Restoration. Coatings 2021, 11, 75. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- Martins, M.; Ribeiro, M.H.; Almeida, C.M.M. Physicochemical, Nutritional, and Medicinal Properties of Opuntia ficus-indica (L.) Mill. and Its Main Agro-Industrial Use: A Review. Plants 2023, 12, 1512–1557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saidi, R.; Ziadi, M.; Bouazizi, S.; Bouallagui, H.; Hamdi, M. Biohydrogen and volatile fatty acids production from prickly pear cladodes (Opuntia ficus-indica) as renewable feedstock. EuroMediterr. J. Environ. Integr. 2025, 1–12. [Google Scholar] [CrossRef]
- Bacchetta, L.; Canditelli, M.; Platamone, G.; Procacci, S.; Di Palma, P.R.; Maccioni, O.; Montereali, M.R.; Alisi, C.; Forni, C. Use of cactus pear pruning waste to improve soil properties and to produce high-quality compost. Org. Agric. 2024, 14, 263–275. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Inst. Chem. Eng. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, Z.; Yu, T.; Yan, F. Spent coffee grounds: Present and future of environmentally friendly applications on industries—A review. Trends Food Sci. Technol. 2024, 143, 104312. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M. A Review of Recent Advances in Spent Coffee Grounds Upcycle Technologies and Practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Singh, T.A.; Pal, N.; Sharma, P.; Passari, A.K. Spent coffee ground: Transformation from environmental burden into valuable bioactive metabolites. Rev. Environ. Sci. Biotechnol. 2023, 22, 887–898. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of Spent Coffee Ground Compost in Peat-Based Growing Media for the Production of Basil and Tomato Potting Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Afriliana, A.; Hidayat, E.; Yoshiharu, M.; Taizo, M.; Harada, H. Evaluation of potency spent coffee grounds for make black compost. E3S Web Conf. EDP Sci. 2020, 142, 04002. [Google Scholar] [CrossRef]
- De Bomfim, A.S.C.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2022, 1, 2–20. [Google Scholar] [CrossRef]
- Liu, K.; Price, G.W. Evaluation of three composting systems for the management of spent coffee grounds. Bioresour. Technol. 2011, 102, 7966–7974. [Google Scholar] [CrossRef]
- Reyes-Torres, M.; Oviedo-Ocaña, E.R.; Dominguez, I.; Komilis, D.; Sánchez, A. A systematic review on the composting of green waste: Feedstock quality and optimization strategie. Waste Manag. 2018, 77, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Using cow dung and spent coffee grounds to enhance the two-stage co-composting of green waste. Bioresour. Technol. 2017, 245, 152–161. [Google Scholar] [CrossRef]
- Cruz, S.; Marques dos Santos Cordovil, C.S. Espresso coffee residues as a nitrogen amendment for small-scale vegetable production. J. Sci. Food Agric. 2015, 95, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- ANPA Metodi di Analisi del Compost”-Manuali e Linee Guida 3/2001. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-di-analisi-del-compost (accessed on 3 June 2025).
- Bartlett, R.J.; James, B.R. Chromium. In Methods of Soil Analysis. Part 3–Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series; n. SSSA e ASA Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Haug, R.T.; Raton, B.; New, L.; Washington, Y. The Practical Handbook of Compost Engineering. In The Practical Handbook of Compost Engineering; Routledge: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Oshins, C.; Michel, F.; Louis, P.; Richard, T.L.; Rynk, R. The composting process. In The Composting Handbook: A How-to and Why Manual for Farm, Municipal, Institutional and Commercial Composters; Academic Press: Cambridge, MA, USA, 2021; pp. 51–101. [Google Scholar] [CrossRef]
- Decree, L. Decreto Legislativo n. 75, 29 Aprile 2010: Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell’articolo 13 della legge 7 Luglio 2009 n. 88. Gazz. Uff. Della Repubb. Italiana 2010, 106, 75. [Google Scholar]
- Sundberg, C.; Smårs, S.; Jönsson, H. Low pH as an inhibiting factor in the transition from mesophilic to thermophilic phase in composting. Bioresour. Technol. 2004, 95, 145–150. [Google Scholar] [CrossRef]
- Hsu, J.H.; Lo, S.L. Effect of composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environ. Pollut. 2001, 114, 9–127. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer Nature: Dordrecht, The Netherlands, 1988. [Google Scholar] [CrossRef]
- Mohd Noor Keeflee, S.N.K.; Wan Mohd Zain, W.N.A.; Mohd Nor, M.N.; Jamion, N.A.; Yong, S.K. Growth and Metal Uptake of Spinach with Application of Co-Compost of Cat Manure and Spent Coffee Ground. Heliyon 2020, 6, e05086. [Google Scholar] [CrossRef]
- Nocentini, M.; Mastrolonardo, G.; Michelozzi, M.; Cencetti, G.; Lenzi, A.; Panettieri, M.; Knickeret, H. Effects of biochar and compost addition in potting substrates on growth and volatile compounds profile of basil (Ocimum basilicum L.). J. Sci. Food Agric. 2024, 104, 1609–1620. [Google Scholar] [CrossRef]
- Corrado, G.; Chiaiese, P.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Rouphael, Y. Successive Harvests Affect Yield, Quality and Metabolic Profile of Sweet Basil (Ocimum basilicum L.). Agronomy 2020, 10, 830. [Google Scholar] [CrossRef]
- Di Lazzaro, P.; Metelli, G.; Lai, A. Pathogen Growth Inhibition in Ocimum basilicum (L.), Malus domestica (Borkh.), and Citrus limon (L.) by Low-Dose UV-C LED Exposure. Ann. Agric. Crop. Sci. 2023, 9, 1144. Available online: www.austinpublishinggroup.com (accessed on 3 June 2025). [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Wu, Y.; Zhang, D.; Qi, Z.; Yang, R. Spent Coffee Ground and Its Derivatives as Soil Amendments—Impact on Soil Health and Plant Production. Agronomy 2024, 15, 26. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Lv, X. Capability of leaf water content and its threshold values in reflection of soil–plant water status in maize during prolonged drought. Ecol. Indic. 2021, 124, 107395. Available online: https://www.sciencedirect.com/science/article/pii/S1470160X21000601 (accessed on 3 June 2025). [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J.A. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vela-Cano, M.; Cervera-Mata, A.; Purswani, J.; Pozo, C.; Delgado, G.; González-López, J. Bacterial community structure of two Mediterranean agricultural soils amended with spent coffee grounds. Appl. Soil Ecol. 2019, 137, 12–20. [Google Scholar] [CrossRef]
- Comino, F.; Cervera-Mata, A.; Aranda, V.; Martín-García, J.M.; Delgado, G. Short-term impact of spent coffee grounds over soil organic matter composition and stability in two contrasted Mediterranean agricultural soils. J. Soils Sediments 2020, 20, 1182–1198. [Google Scholar] [CrossRef]
- Petrik, S.; Obruča, S.; Benešová, P.; Márová, I. Bioconversion of spent coffee grounds into carotenoids and other valuable metabolites by selected red yeast strains. Biochem. Eng. J. 2014, 90, 307–315. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Aranda, V.; Ontiveros-Ortega, A.; Comino, F.; Martín-García, J.M.; Vela-Cano, M.; Delgado, G. Hydrophobicity and surface free energy to assess spent coffee grounds as soil amendment. Relationships with soil quality. Catena 2021, 196, 104826. [Google Scholar] [CrossRef]
- Hachicha, R.; Rekik, O.; Hachicha, S.; Ferchichi, M.; Woodward, S.; Moncef, N.; Cegarra, J. Co-composting of spent coffee ground with olive mill wastewater sludge and poultry manure and effect of Trametes versicolor inoculation on the compost. Chemosphere 2012, 88, 677–682. Available online: https://www.sciencedirect.com/science/article/pii/S0045653512003931 (accessed on 3 June 2025). [CrossRef]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. Available online: https://www.sciencedirect.com/science/article/pii/S096399691400708X (accessed on 3 June 2025). [CrossRef]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. Available online: https://www.mdpi.com/2076-3921/14/1/74 (accessed on 3 June 2025). [CrossRef]
- Verrillo, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in Basil leaves. Chem. Biol. Technol. Agric. 2021, 8, 28. [Google Scholar] [CrossRef]
- De la Portilla, N.; Vaca, R.; Mora-Herrera, M.; Salinas, L.; Del Aguila, P.; Yañez-Ocampo, G. Soil Amendment with Biosolids and Inorganic Fertilizers: Effects on Biochemical Properties and Oxidative Stress in Basil (Ocimum basilicum L.). Agronomy 2020, 10, 1117. Available online: https://www.mdpi.com/2073-4395/10/8/1117 (accessed on 3 June 2025). [CrossRef]
- Taie, H.A.A.; Salama, Z.A.-E.R.; Radwan, S. Potential Activity of Basil Plants as a Source of Antioxidants and Anticancer Agents as Affected by Organic and Bio-organic Fertilization. Not. Bot. Horti Agrobot. 2010, 38, 119–127. [Google Scholar]
- Solís-Oba, A.; Hernández-Rivadeneyra, J.I.; Castro-Rivera, R.; Manjarrez, N.; Solís-Oba, M. Effect of composts produced from vegetable waste and/or manure on lettuce crop and their antioxidants content. Mex. J. Biotechnol. 2020, 5, 86–105. [Google Scholar] [CrossRef]
- Coria-Cayupán, Y.S.; Sánchez de Pinto, M.I.; Nazareno, M.A. Variations in bioactive substance contents and crop yields of lettuce (Lactuca sativa L.) cultivated in soils with different fertilization treatments. J. Agric. Food Chem. 2009, 57, 10122–10129. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Milenković, L.; Stanojević, J.; Cvetković, D.; Stanojević, L.; Lalević, D.; Šunić, L.; Fallik, E.; Ilić, Z. New technology in basil production with high essential oil yield and quality. Ind. Crops Prod. 2019, 140, 111718. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Marzouki, M.; M’Rabet, Y.; Mezni, M.; Ait Ouazzou, A.; Hosni, K. Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities. Arab. J. Chem. 2020, 13, 6451–6460. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.Q.; Thi, N.Q.N.; Thi, C.Q.N.; Truc, T.T.; Nghi, P.T.B. Studies on chemical, polyphenol content, flavonoid content, and antioxidant activity of sweet basil leaves (Ocimum basilicum L.) IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012083. [Google Scholar] [CrossRef]
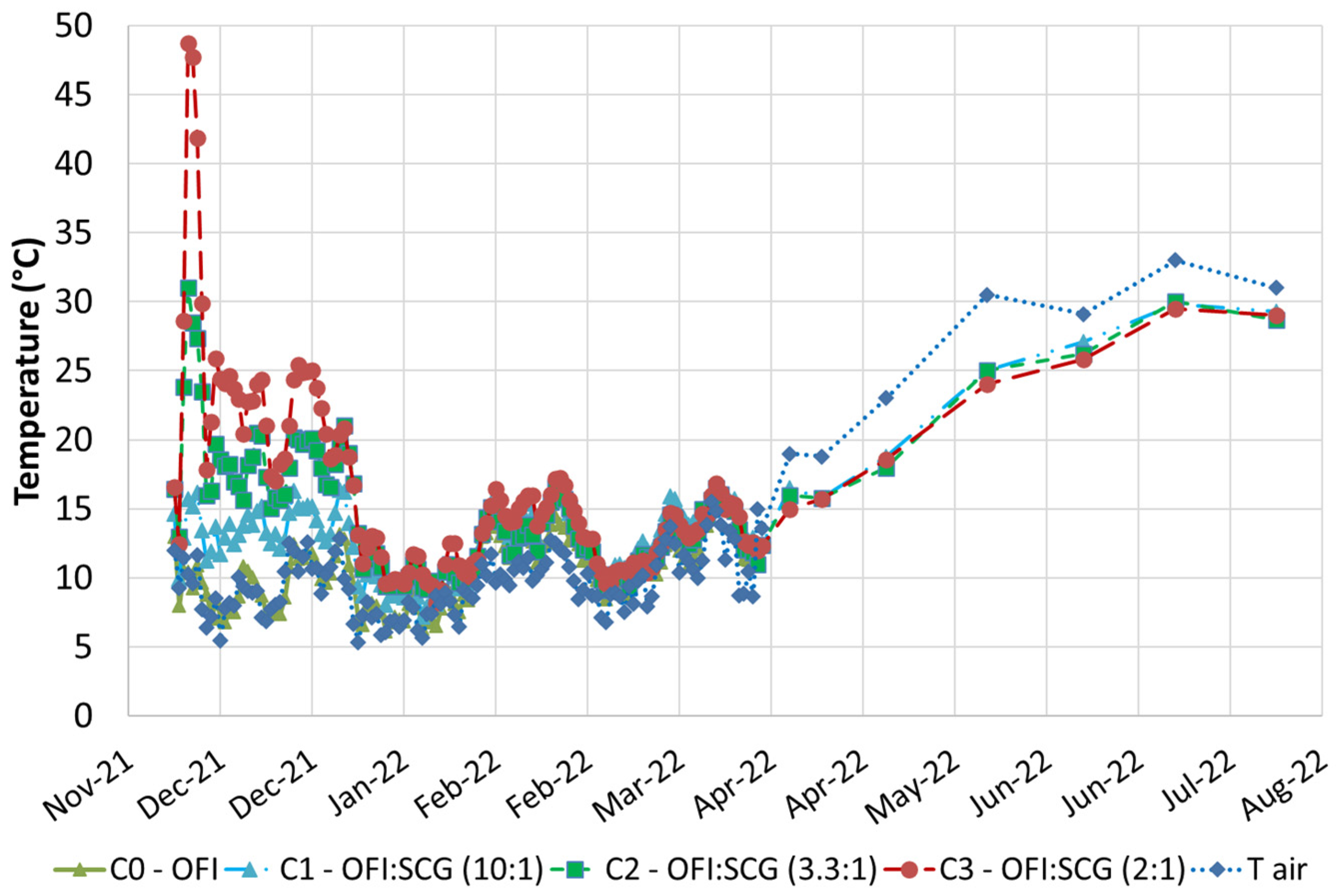

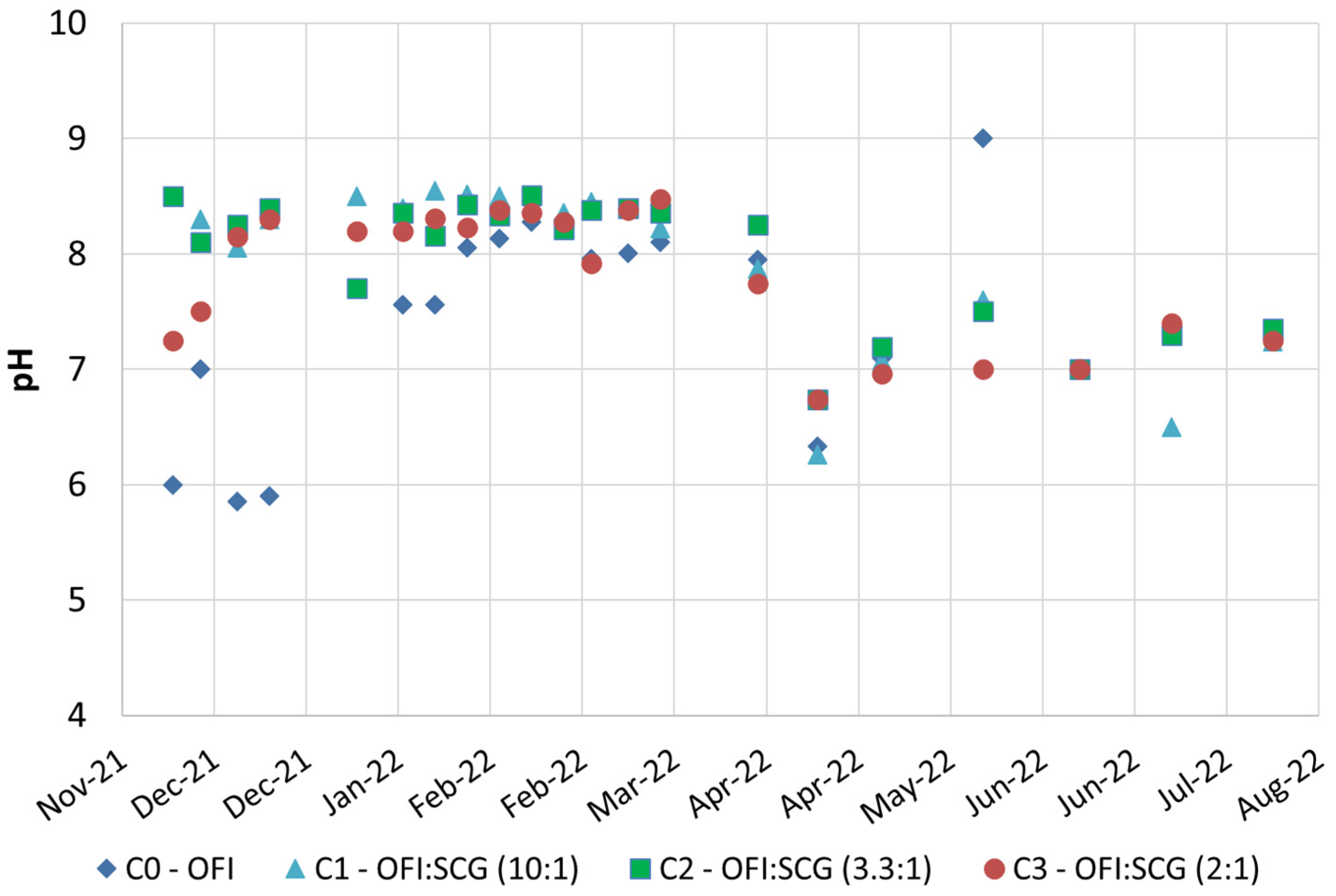
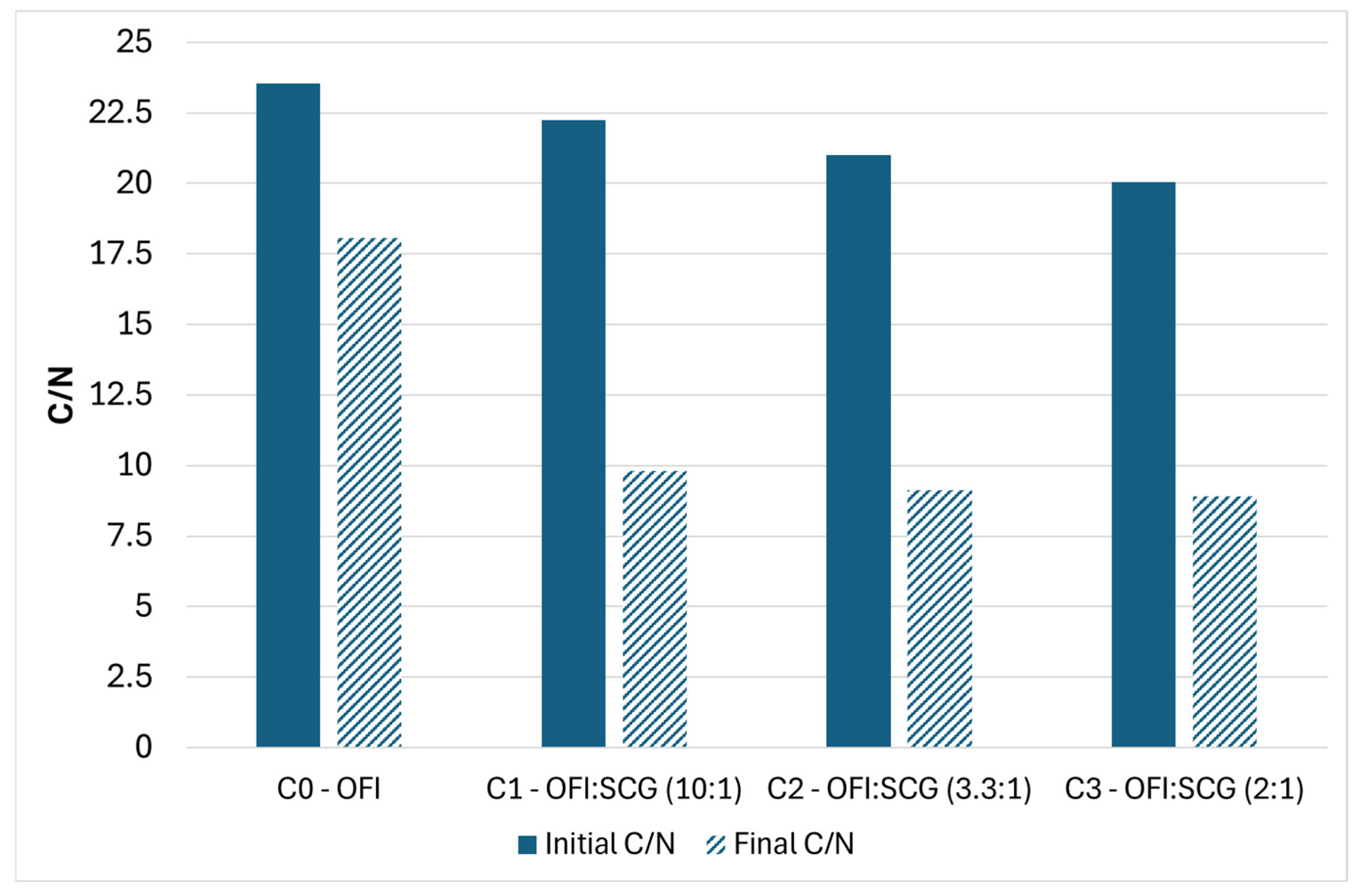


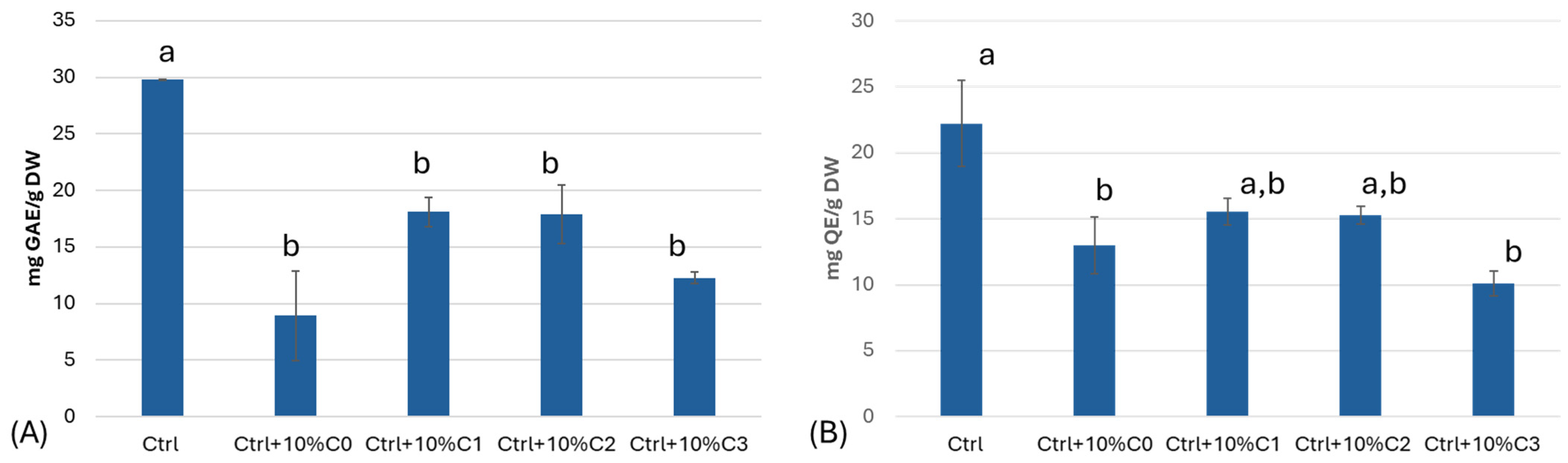
| Material (kg)/Sample | C0 Only OFI | C1 OFI–SCG 10:1 | C2 OFI–SCG 3.3:1 | C3 OFI–SCG 2:1 |
|---|---|---|---|---|
| Opuntia ficus-indica (OFI) cladodes | 61 | 56 | 47 | 41 |
| Spent coffee grounds (SCGs) | 0 | 6 | 14 | 20 |
| Bulking agent (BA) | 13 * | 13 * | 11 | 11 |
| Inoculum (cured compost) | 1 | 1 | 1 | 1 |
| Tot. (kg) | 75 | 75 | 73 | 73 |
| Soil Samples | Description |
|---|---|
| Ctrl | Control, only commercial soil |
| Ctrl + 10% C0 | Commercial soil supplemented by 10% of C0 compost (OFI only) |
| Ctrl + 10% C1 | Commercial soil supplemented by 10% of C1 compost (OFI–SCG 10:1) |
| Ctrl + 10% C2 | Commercial soil supplemented by 10% C2 compost (OFI–SCG 3.3:1) |
| Ctrl + 10% C3 | Commercial soil supplemented by 10% of C3 compost (OFI–SCG 2:1) |
| OFI | SCGs | C0 | C1 | C2 | C3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Concentration Limit Allowed by D. LGS 75/2010 | Units of Measure | Value | ±ue | Value | ±ue | Value | ±ue | Value | ±ue | Value | ±ue | Value | ±ue |
| Cd | 1.5 | mg kg−1 | 0.042 | 0.002 | <0.020 | 0.162 | 0.004 | 0.145 | 0.004 | 0.118 | 0.004 | 0.113 | 0.005 | |
| Cr (VI) | 0.5 | mg kg−1 | 0.078 | 0.005 | <0.012 | 0.104 | 0.004 | 0.065 | 0.005 | 0.043 | 0.005 | 0.045 | 0.004 | |
| Mn | mg kg−1 | 224 | 9 | 20.8 | 1.4 | 204 | 9 | 149 | 6 | 141 | 4 | 119 | 3 | |
| Hg | 1.5 | mg kg−1 | 0.029 | 0.001 | 0.033 | 0.001 | 0.038 | 0.005 | 0.028 | 0.002 | ||||
| Ni | 100 | mg kg−1 | 18.5 | 1.0 | 2.86 | 0.16 | 8.72 | 0.24 | 11.0 | 0.5 | 8.4 | 0.1 | 10.5 | 0.4 |
| Pb | 140 | mg kg−1 | 0.541 | 0.043 | <0.100 | 5.42 | 0.23 | 3.90 | 0.16 | 3.79 | 0.23 | 4.33 | 0.14 | |
| Cu | 230 | mg kg−1 | 20.6 | 0.9 | 22.4 | 1.6 | 21.1 | 0.6 | 30.8 | 0.9 | 32.5 | 0.7 | 34.8 | 0.6 |
| Zn | 500 | mg kg−1 | 48.2 | 3.3 | 9.03 | 0.88 | 261 | 18 | 264 | 7 | 207 | 4 | 200 | 12 |
| Ca | % | 5.78 | 0.16 | 0.138 | 0.006 | 4.05 | 0.11 | 3.86 | 0.11 | 3.19 | 0.03 | 2.78 | 0.02 | |
| Fe | % | 0.008 | 0.001 | 0.0048 | 0.0002 | 0.296 | 0.012 | 0.182 | 0.008 | 0.240 | 0.016 | 0.175 | 0.012 | |
| P | % | 0.175 | 0.007 | 0.185 | 0.010 | 0.394 | 0.016 | 0.691 | 0.029 | 0.701 | 0.030 | 0.6182 | 0.0001 | |
| Mg | % | 0.671 | 0.019 | 0.175 | 0.007 | 0.517 | 0.014 | 0.584 | 0.016 | 0.589 | 0.003 | 0.539 | 0.015 | |
| K | % | 4.92 | 0.14 | 0.873 | 0.036 | 2.63 | 0.07 | 3.23 | 0.09 | 3.09 | 0.09 | 2.85 | 0.08 | |
| Roots | Shoots | ||||
|---|---|---|---|---|---|
| Plants/Composts | Fresh Weight (g) | Length (cm) | Fresh Weight (g) | Height (cm) | Shoot Nodes (n°) |
| Ctrl | 1.58± 0.42 | 11.88 ± 0.90 a | 4.95 ± 0.88 ab | 23.72 ± 3.24 | 8.50 ± 0.37 a |
| Ctrl + 10% C0 | 1.28 ± 0.17 | 14.20 ± 1.47 a | 4.26 ± 0.71 ab | 19.80 ± 2.65 | 8.60 ± 0.61 a |
| Ctrl + 10% C1 | 2.13 ± 0.72 | 13.36 ± 1.51 a | 4.44 ± 1.08 ab | 24.57 ± 5,55 | 8.43 ± 0.62 a |
| Ctrl + 10% C2 | 3.58 ± 1.18 | 10.16 ± 1.09 a | 7.95 ± 1.57 a | 30.55 ± 5.68 | 7.67 ± 0.77 a |
| Ctrl + 10% C3 | 0.98 ± 0.37 | 6.11 ± 1.31 b | 2.49 ± 0.91 b | 13.35 ± 4.36 | 5.09 ± 0.88 b |
| F-value | 2.32 | 0.25 | 3.42 | 2.11 | 4.85 |
| Statistical Significance | 0.073 | 0.001 | 0.017 | 0.098 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Palma, P.R.; Gazzola, G.; Procacci, S.; Maccioni, O.; Montereali, M.R.; Tolaini, V.; Canditelli, M.; Bacchetta, L. Composting a Mixture of Cactus Pear Pruning Waste and Spent Coffee Grounds: The Chemical Evaluation of Organic Fertilizer in Response to Basil Quality and Growth. Horticulturae 2025, 11, 640. https://doi.org/10.3390/horticulturae11060640
Di Palma PR, Gazzola G, Procacci S, Maccioni O, Montereali MR, Tolaini V, Canditelli M, Bacchetta L. Composting a Mixture of Cactus Pear Pruning Waste and Spent Coffee Grounds: The Chemical Evaluation of Organic Fertilizer in Response to Basil Quality and Growth. Horticulturae. 2025; 11(6):640. https://doi.org/10.3390/horticulturae11060640
Chicago/Turabian StyleDi Palma, Paolo Roberto, Giulio Gazzola, Silvia Procacci, Oliviero Maccioni, Maria Rita Montereali, Valentina Tolaini, Margherita Canditelli, and Loretta Bacchetta. 2025. "Composting a Mixture of Cactus Pear Pruning Waste and Spent Coffee Grounds: The Chemical Evaluation of Organic Fertilizer in Response to Basil Quality and Growth" Horticulturae 11, no. 6: 640. https://doi.org/10.3390/horticulturae11060640
APA StyleDi Palma, P. R., Gazzola, G., Procacci, S., Maccioni, O., Montereali, M. R., Tolaini, V., Canditelli, M., & Bacchetta, L. (2025). Composting a Mixture of Cactus Pear Pruning Waste and Spent Coffee Grounds: The Chemical Evaluation of Organic Fertilizer in Response to Basil Quality and Growth. Horticulturae, 11(6), 640. https://doi.org/10.3390/horticulturae11060640







