Genetic Breeding to Improve Freeze Tolerance in Blueberries, a Review
Abstract
1. Introduction
2. Spring Freeze Causes Damage to Blueberries
3. Winter Freezing Brings Injuries to Blueberries
4. Advancement in Phenotyping Blueberry Phenological Development and Cold Hardiness
4.1. Phenological Development
4.2. Winter Hardiness
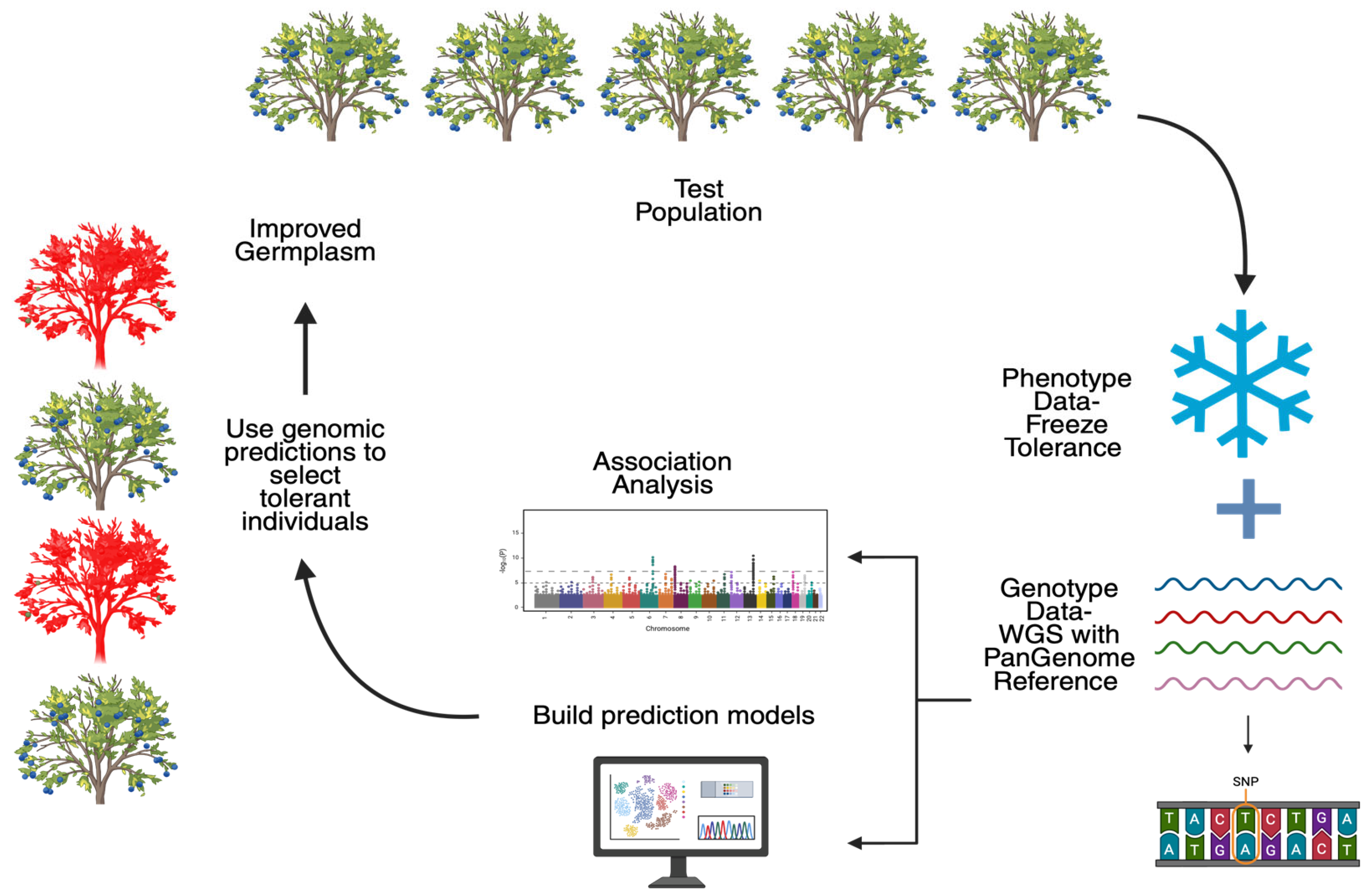
5. Genetic Analysis of Freeze Tolerance and Flowering Phenology in Blueberries
5.1. Gene Regulation Associated with Flowering and Chilling Requirements
5.2. Gene Regulation Associated with Dormancy and Freeze Tolerance
5.3. Marker Discovery of Chilling Requirement, Freeze Tolerance, and Phenology in Blueberries
| Traits | Populations | Population Size | Marker Types | Marker Number | Linage Map Size (cm) | QTLs | Associated Markers from GWAS | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chilling requirement | V. darrowii (2x) × V. corymbosum (2x) | 82 | EST-PCR, SSR, RAPD, SNP | 265 | 1740 | LG 6 and 8 | N/A | [112] |
| Chilling requirement | V. darrowii (2x) × V. corymbosum (2x) | 117 | SNP markers from capture Seq | 17,468 | 1539.4 | LG 5 | N/A | [160] |
| Chilling requirement | SHB diversity population | 95 | SNP markers from ddRADseq | 65,145 | N/A | N/A | 12 SNPs on chr. 4 and 12 | [157] |
| Cold hardiness | V. darrowii (2x) × V. corymbosum (2x) | 82 | EST-PCR, SSR, RAPD, SNP | 265 | 1740 | LG 4 | N/A | [112] |
| Cold hardiness | V. darrowii (2x) × V. corymbosum (2x) | 117 | SNP markers from Capture Seq | 17,468 | 1539.4 | LG 2 and 10 | N/A | [160] |
| Flowering time | V. darrowii (2x) × V. corymbosum (2x) | 117 | SNP markers from Capture Seq | 17,468 | 1539.4 | LG 5 | N/A | [160] |
| Flowering time | SHB diversity population | 95 | SNP markers from ddRADseq | 65,145 | N/A | N/A | 67 SNPs on 12 chr. | [157] |
| Fruit ripening date | SHB diversity population | 95 | SNP markers from ddRADseq | 65,145 | N/A | N/A | 18 SNPs on 9 chr. | [157] |
| Off-season flowering | SHB diversity population | 536 | SNP markers from Capture Seq | 59,910 | N/A | N/A | 17 SNPs | [156] |
| Fruiting period | SHB diversity population | 95 | SNP markers from ddRADseq | 65,145 | N/A | N/A | 3 SNPs on chr. 9 and 10 | [157] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Blueberry Orgaization (IBO). Berries’ Versatility and ‘Superfruit’ Status Fuel Decade of Strong Growth. 2023. Available online: https://www.internationalblueberry.org/2023/05/08/berries-versatility-and-superfruit-status-fuel-decade-of-strong-growth/ (accessed on 23 May 2025).
- Ballington, J.R. Collection, utilization, and preservation of genetic resources in Vaccinium. Hort. Sci. 2001, 36, 213–220. [Google Scholar]
- Bell, D.J.; Rowland, L.J.; Smagula, J.; Drummond, F.A. Recent advances in the biology and genetics of lowbush blueberry. Tech. Bul. 2009, 203, 1–28. [Google Scholar]
- Lyrene, P. Breeding rabbiteye blueberries. In Plant Breeding Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 1987. [Google Scholar]
- Ballington, J.R. The role of interspecific hybridization in blueberry improvement. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 4 March 2009; Volume 810. [Google Scholar] [CrossRef]
- Lyrene, P. Breeding southern highbush blueberries in Florida. In Proceedings of the VII International Symposium on Vaccinium Culture, Chillan, Chile, 4–9 December 2000; Volume 574. [Google Scholar] [CrossRef]
- Bassil, N.; Bidani, A.; Hummer, K.; Rowland, L.J.; Olmstead, J.; Lyrene, P.; Richards, C. Assessing genetic diversity of wild southeastern North American Vaccinium species using microsatellite markers. Genet. Resour. Crop Evol. 2018, 65, 939–950. [Google Scholar] [CrossRef]
- Draper, A.; Hancock, J. Florida 4B: Native blueberry with exceptional breeding value. J. Am. Pom. Soc. 2003, 57, 138. [Google Scholar]
- Hummer, K.; Zee, F.; Strauss, A.; Keith, L.; Nishijima, W. Evergreen production of southern highbush blueberries in Hawai’i. J. Am. Pomol. Soc. 2007, 61, 188–195. [Google Scholar]
- Phillips, D.; Williamson, J.G.; Munoz, P.R. Evergreen production system for southern highbush blueberries in Florida. EDIS 2020, 2020, HS1362. [Google Scholar] [CrossRef]
- Fang, Y.; Nunez, G.H.; Silva, M.N.D.; Phillips, D.A.; Munoz, P.R. A Review for southern highbush blueberry alternative production systems. Agronomy 2020, 10, 1531. [Google Scholar] [CrossRef]
- Scalzo, J.; Wright, G.; Boettiger, S. Adaptability of blueberries to lower chill growing regions in Australia. Acta Hort. 2016, 1117, 45–48. [Google Scholar] [CrossRef]
- Reeder, R.; Obreza, T.; Darnell, R. Establishment of a non-dormant blueberry (Vaccinium corymbosum hybrid) production system in a warm winter climate. J. Hortic. Sci. Biotechnol. 1998, 73, 655–663. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Rowland, L.J. Cold-hardiness of Vaccinium ashei and V. constablaei germplasm and the potential for northern-adapted rabbiteye cultivars. Acta Hort. 2006, 715, 77–80. [Google Scholar] [CrossRef]
- Doughty, C.; Hemerick, G. Impedance as a measurement of blueberry bud hardiness. J. Am. Soc. Hort. Sci. 1975, 100, 115–118. [Google Scholar] [CrossRef]
- Bittenbender, B.; Howell, G. Cold hardiness of flower buds from selected highbush blueberry cultivars. J. Am. Soc. Hortic. Sci. 1976, 101, 135–139. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Ogden, E.L.; Rowland, L.J.; Vinyard, B. Evaluation of midwinter cold hardiness among 25 rabbiteye blueberry cultivars. HortScience 2006, 41, 579–581. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Rowland, L.J.; Ogden, E.L.; Vinyard, B.T. Cold-hardiness, acclimation, and deacclimation among diverse blueberry genotypes. J. Amer. Soc. Hort. Sci. 2012, 137, 31–37. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Ehlenfeldt, M.K.; Arora, R. Cold tolerance of blueberry genotypes throughout the dormant period from acclimation to deacclimation. HortScience 2008, 43, 1970–1974. [Google Scholar] [CrossRef]
- Rowland, D.L.; Hancock, J.F.; Bassil, N. Bluberry, in Genetics, Genomics and Breeding of Berries; Folta, K.M., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hall, I.; Craig, D.; Aalders, L. The effect of photoperiod on the growth and flowering of the highbush blueberry (Vaccinium corymbosum L.). Proc. Am. Soc. Hort. Sci. 1963, 82, 260–263. [Google Scholar]
- Gough, R.; Shutak, V.; Hauke, R. Growth and development of highbush blueberry. II. Reproductive growth, histological studies. J. Am. Soc. Hort. Sci. 1978, 103, 476–479. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Takeda, F.; Glenn, D.M.; Ehlenfeldt, M.K.; Vinyard, B.T. Variation among highbush blueberry cultivars for frost tolerance of open flowers. HortScience 2013, 48, 692–695. [Google Scholar] [CrossRef]
- Spiers, J.M.; Marshall, D.A.; Braswell, J.H. Chilling requirement studies in blueberries. Small Fruits Rev. 2004, 3, 325–330. [Google Scholar] [CrossRef]
- Darnell, R.L.; Davies, F.S. Chilling accumulation, budbreak, and fruit set of young rabbiteye blueberry plants. HortScience 1990, 25, 635–638. [Google Scholar] [CrossRef]
- Spiers, J.M.; Draper, A.D. Effect of chilling on bud break in rabbiteye blueberry. J. Am. Soc. Hort. Sci. 1974, 99, 398–399. [Google Scholar] [CrossRef]
- Parmentier, C.C.M.; Rowland, L.J.; Line, M. Water status in relation to maintenance and release from dormancy in blueberry flower buds. J. Am. Soc. Hort. Sci. 1998, 123, 762–769. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Rowland LJOgden, E.L.; Ehlenfeldt, M.K.; Vinyard, B. Cold hardiness, deacclimation kinetics, and bud development among 12 diverse blueberry genotypes under field conditions. J. Am. Soc. Hortic. Sci. Jashs 2005, 130, 508–514. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.U.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Norvell, D.J.; Moore, J.N. An Evaluation of chilling models for estimating rest requirements of highbush blueberries (Vaccinium corymbosum L.). J. Am. Soc. Hort. Sci. 1982, 107, 54–56. [Google Scholar] [CrossRef]
- Darrow, G.M. Rest period requirements for blueberries. Proc. Amer. Soc. Hort. Sci. 1942, 41, 189–194. [Google Scholar]
- Austin, M.E.; Bondari, K. Chilling hour requirement for flower bud expansion of two rabbiteye and one highbush blueberry shoots. HortScience 1987, 22, 1247–1248. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries. In Crop Production Science in Horticulture; Atherton, J., Ed.; Cabi: Wallingford, UK, 2018; Volume 27. [Google Scholar]
- Song, G.-Q.; Walworth, A.; Zhao, D.; Jiang, N.; Hancock, J.F. The Vaccinium corymbosum FLOWERING LOCUS T-like gene (VcFT): A flowering activator reverses photoperiodic and chilling requirements in blueberry. Plant Cell Rep. 2013, 32, 1759–1769. [Google Scholar] [CrossRef]
- Lyrene, P. Components of ripening in rabbiteye blueberry. HortScience 1983, 18, 221–223. [Google Scholar] [CrossRef]
- Kovaleski, A.P.; Williamson, J.G.; Olmstead, J.W.; Darnell, R.L. Inflorescence bud initiation, development, and bloom in two southern highbush blueberry cultivars. J. Am. Soc. Hort. Sci. 2015, 140, 38–44. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef]
- Inouye, D.W. The ecological and evolutionary significance of frost in the context of climate change. Ecol. Lett. 2000, 3, 457–463. [Google Scholar] [CrossRef]
- Allstadt, A.J.; Vavrus, S.J.; Heglund, P.J.; Pidgeon, A.M.; Thogmartin, W.E.; Radeloff, V.C. Spring plant phenology and false springs in the conterminous US during the 21st century. Environ. Res. Lett. 2015, 10, 104008. [Google Scholar] [CrossRef]
- Kaharabata, S.; Desjardins, R. An indicator of freeze-kill damages to fruit trees during flowering. Int. J. Biometeorol. 2021, 65, 813–825. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef]
- Gupton, C.; Spiers, J. Variability among rabbiteye blueberry cultivars for tolerance of flowers to frost. HortScience 1983, 18, 713–714. [Google Scholar] [CrossRef]
- Olson, A.R.; Eaton, L.J. Spring frost damage to placental tissues in lowbush blueberry flower buds. Can. J. Plant Sci. 2001, 81, 779–781. [Google Scholar] [CrossRef]
- Patten, K.; Neuendorff, E.; Nimr, G.; Clark, J.R.; Fernandez, G. Cold Injury of Southern Blueberries as a Function of Germplasm and Season of Flower Bud Development. HortScience 1991, 26, 18–20. [Google Scholar] [CrossRef]
- Hancock, J.F.; Nelson, J.W.; Bittenbender, H.C.; Callow, P.W.; Cameron, J.S.; Krebs, S.L.; Pritts, M.P.; Schumann, C.M. Variation among highbush blueberry cultivars in susceptibility to spring frost. J. Am. Soc. Hort. Sci. 1987, 112, 702–706. [Google Scholar] [CrossRef]
- Spiers, J. Effect of stage of bud development on cold injury in rabbiteye blueberry. J. Am. Soc. Hort. Sci. 1978, 103, 452–455. [Google Scholar] [CrossRef]
- Flinn, C.L.; Ashworth, E.N. Seasonal changes in ice distribution and xylem development in blueberry flower buds. J. Am. Soc. Hort. Sci. 1994, 119, 1176–1184. [Google Scholar] [CrossRef]
- Marshall, D.A.; Spiers, J.; Smith, B. Spring freeze damage to rabbiteye blueberry buds and berries. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain, 31 August 2006; Volume 715. [Google Scholar] [CrossRef]
- Longstroth, M. Damage to developing blueberry buds in a spring freeze. In Proceedings of the IX International Vaccinium Symposium, Corvallis, OR, USA, 14–18 July 2008; Volume 810. [Google Scholar] [CrossRef]
- Hicklenton, P.R.; Reekie, J.Y.C.; MacKenzie, K.; Eaton, L.J.; Havard, P. Freeze damage and frost tolerance thresholds for flowers of the lowbush blueberry (Vaccinium angustifolium Ait). In Proceedings of the VII International Symposium on Vaccinium Culture, Chillan, Chile, 4–9 December 2000; Volume 574. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Krewer, G.; Lindstrom, O.M. Fruit set of rabbiteye blueberry (Vaccinium ashei) after subfreezing temperatures. J. Am. Soc. Hort. Sci. 1999, 124, 337–340. [Google Scholar] [CrossRef]
- Lin, W.; Pliszka, K. Comparison of spring frost tolerance among different highbush blueberry (Vaccinium corymbosum L.) cultivars. In Proceedings of the XXVI International Horticultural Congress: Berry Crop Breeding, Production and Utilization for a New Century, Toronto, ON, Canada, 11–17 August 2002; Volume 626. [Google Scholar]
- England, G.K. Observations of the February 2012 Freeze and Its Effect on Commercial Blueberry Plantings. In Proceedings of the Florida State Horticultural Society; CABI: Wallingford, UK, 2012. [Google Scholar]
- Bunger, M. 2022 Freeze Damage to Blueberries in Alabama, Georgia, and North Carolina; USDA, Ed.; USDA/RMA Office: Kansas City, MO, USA, 2022. [Google Scholar]
- Coneva, E. Extension Education on Newly Released Blueberry Cultivars with Improved Fruit Quality Characteristics; South Regional Small Fruit Consortium: Savannah, GA, USA, 2022. [Google Scholar]
- Smith, E.D. Cold hardiness and options for the freeze protection of southern highbush blueberry. Agriculture 2019, 9, 9. [Google Scholar] [CrossRef]
- Marino, G.P.; Kaiser, D.P.; Gu, L.; Ricciuto, D.M. Reconstruction of false spring occurrences over the southeastern United States, 1901–2007: An increasing risk of spring freeze damage? Environ. Res. Lett. 2011, 6, 024015. [Google Scholar] [CrossRef]
- Warmund, M.R.; Guinan, P.; Fernandez, G. Temperatures and cold damage to small fruit crops across the eastern United States associated with the April 2007 freeze. HortScience 2008, 43, 1643–1647. [Google Scholar] [CrossRef]
- Nesmith, D.S. Fruit set and berry weight of four rabbiteye blueberry cultivars following exposure to sub-freezing temperatures during flowering. Int. J. Fruit Sci. 2012, 12, 256–260. [Google Scholar] [CrossRef]
- Lin, W.; Pliszka, K. Comparison of spring frost tolerance among different highbush blueberry (Vaccinium corymbosum L.) cultivars. Acta Hort. 2003, 626, 337–341. [Google Scholar] [CrossRef]
- Sutton, S.; Sterns, J. Blueberry Economics: The Costs of Establishing and Producing Conventional Blueberries in the Willamette Valley; Oregon State University: Corvallis, OR, USA, 2020; Volume 61. [Google Scholar]
- Liu, J.; Sherif, S.M. Combating spring frost with ethylene. Front. Plant Sci. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Conlan, E.; Borisova, T.; Smith, E.; Williamson, J.; Olmstead, M. The use of irrigation for frost protection for blueberry in the southeastern United States. HortTech. 2018, 28, 660–667. [Google Scholar] [CrossRef]
- Krewer, G.; Nesmith, D.S.; Williamson, J.; Maus, B.; Mullinix, B. Ethephon for bloom delay of rabbiteye and southern highbush blueberries. Small Fruits Rev. 2005, 4, 43–57. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Krewer, G.; Rieger, M.; Mullinix, B. Gibberellic acid-induced fruit set of rabbiteye blueberry following freeze and physical injury. HortScience 1995, 30, 1241–1243. [Google Scholar] [CrossRef]
- Williamson, J.G.; Lyrene, P.M. Reproductive growth and development of blueberry. EDIS 2004, 2004, HS976. [Google Scholar] [CrossRef]
- Zang, Y.X.; Chun, I.J.; Zhang, L.L.; Hong, S.B.; Zheng, W.W.; Xu, K. Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbiteye blueberry. Sci. Hortic. 2016, 200, 13–18. [Google Scholar] [CrossRef]
- Moore, J.N. The blueberry industry of North America. HortTechnology 1994, 4, 96–102. [Google Scholar] [CrossRef]
- Biermann, J.; Stushnoff, C.; Burke, M. Differential thermal analysis and freezing injury in cold hardy blueberry flower buds. J. Am. Soc. Hort. Sci. 1979, 104, 444–449. [Google Scholar] [CrossRef]
- Cappiello, P.E.; Dunham, S.W. Seasonal variation in low-temperature tolerance of Vaccinium angustifolium Ait. HortScience 1994, 29, 302–304. [Google Scholar] [CrossRef]
- Cline, W.O. Infection of cold-injured blueberry stems by Botryosphaeria dothidea. J. Small Fruit Vitic. 1996, 3, 95–98. [Google Scholar] [CrossRef]
- Bryla, D.R.; Linderman, R.G. Implications of irrigation method and amount of water application on phytophthora and pythium Infection and severity of root rot in highbush blueberry. HortScience 2007, 42, 1463–1467. [Google Scholar] [CrossRef]
- Quamme, H.; Stushnoff, C.; Weiser, C. Winter hardiness of several blueberry species and cultivars in Minnesota. HortScience 1972, 7, 500–502. [Google Scholar] [CrossRef]
- Brierley, W.G.; Hildreth, A. Some studies on the hardiness of certain species of Vaccinium. Plant Physiol. 1928, 3, 303. [Google Scholar] [CrossRef]
- Strik, B.C.; Finn, C.E.; Moore, P.P. Blueberry Cultivars for the Pacific Northwest; Oregon State University: Corvallis, OR, USA, 2014. [Google Scholar]
- Rousi, A. Hybridization between Vaccinium uliginosum and cultivated blueberry. Annales Agric. Fenniae 1963, 2, 12–18. [Google Scholar]
- Moore, J.N. Improving highbush blueberries by breeding and selection. Euphytica 1965, 14, 39–48. [Google Scholar] [CrossRef]
- Hanson, E.J.; Berkheimer, S.F.; Hancock, J.F. Seasonal changes in the cold hardiness of the flower buds of highbush blueberry with varying species ancestry. J. Am. Pomol. Soc. 2007, 61, 14–18. [Google Scholar]
- Redpath, L.E.; Chavez, D.J.; Malladi, A.; Smith, E.D. Characterization of southern highbush blueberry floral bud cold hardiness through dormancy in a sub-tropical climate. J. Am Pomol. Soc. 2018, 72, 166–172. [Google Scholar]
- Arora, R.; Rowland, L.J.; Ogden, E.L.; Dhanaraj, A.L.; Marian, C.O.; Ehlenfeldt, M.K.; Vinyard, B. Dehardening kinetics, bud development, and dehydrin metabolism in blueberry cultivars during deacclimation at constant, warm temperatures. J. Am. Soc. Hort. Sci. 2004, 129, 667–674. [Google Scholar] [CrossRef]
- Finn, C.E.; Luby, J. Inheritance of fruit development interval and fruit size in blueberry progenies. J. Am. Soc. Hort. Sci. 1986, 111, 784–788. [Google Scholar] [CrossRef]
- Lyrene, P. Effects of year and genotype on flowering and ripening dates in rabbiteye blueberry. HortScience 1985, 20, 407–409. [Google Scholar] [CrossRef]
- NeSmith, D.S. ‘Suziblue’southern highbush blueberry. HortScience 2010, 45, 142–143. [Google Scholar] [CrossRef]
- Vasconez, J.P.; Delpiano, J.; Vougioukas, S.; Cheein, F.A. Comparison of convolutional neural networks in fruit detection and counting: A comprehensive evaluation. Comput. Electron. Agric. 2020, 173, 105348. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Chee, P.W.; Paterson, A.H.; Jiang, Y.; Xu, R.; Robertson, J.S.; Adhikari, J.; Shehzad, T. Three-dimensional photogrammetric mapping of cotton bolls in situ based on point cloud segmentation and clustering. ISPRS J. Photogramm. Remote Sens. 2020, 160, 195–207. [Google Scholar] [CrossRef]
- Williams, H.; Ting, C.; Nejati, M.; Jones, M.H.; Penhall, N.; Lim, J.; Seabright, M.; Bell, J.; Ahn, H.S.; Scarfe, A.; et al. Improvements to and large-scale evaluation of a robotic kiwifruit harvester. J. Field Robot. 2020, 37, 187–201. [Google Scholar] [CrossRef]
- Rahman, M.H.; Busby, S.; Hanif, S.; Maruf, M.M.; Ahmad, F.; Ru, S.; Sanz-Saez, A.; Zheng, J.; Rehman, T.U. A graph convolutional network approach for hyperspectral image analysis of blueberries physiological traits under drought stress. Smart Agric. Technol. 2025, 10, 100743. [Google Scholar] [CrossRef]
- Li, Z.; Xu, R.; Li, C.; Munoz, P.; Takeda, F.; Leme, B. In-field blueberry fruit phenotyping with a MARS-PhenoBot and customized BerryNet. Comput. Electron. Agric. 2025, 232, 110057. [Google Scholar] [CrossRef]
- Chen, S.W.; Shivakumar, S.S.; Dcunha, S.; Das, J.; Okon, E.; Qu, C.; Taylor, C.J.; Kumar, V. Counting apples and oranges with deep learning: A data-driven approach. IEEE Robot. Autom. Lett. 2017, 2, 781–788. [Google Scholar] [CrossRef]
- Zheng, Z.; Xiong, J.; Wang, X.; Li, Z.; Huang, Q.; Chen, H.; Han, Y. An efficient online citrus counting system for large-scale unstructured orchards based on the unmanned aerial vehicle. J. Field Robot. 2023, 40, 552–573. [Google Scholar] [CrossRef]
- Patrick, A.; Pelham, S.; Culbreath, A.; Holbrook, C.C.; De Godoy, I.J.; Li, C. High throughput phenotyping of tomato spot wilt disease in peanuts using unmanned aerial systems and multispectral imaging. IEEE Instrum. Meas. Mag. 2017, 20, 4–12. [Google Scholar] [CrossRef]
- Swain, K.C.; Zaman, Q.U.; Schumann, A.W.; Percival, D.C.; Bochtis, D.D. Computer vision system for wild blueberry fruit yield mapping. Biosyst. Eng. 2010, 106, 389–394. [Google Scholar] [CrossRef]
- Li, H.; Lee, W.S.; Wang, K. Identifying blueberry fruit of different growth stages using natural outdoor color images. Comput. Electron. Agric. 2014, 106, 91–101. [Google Scholar] [CrossRef]
- Tan, K.; Lee, W.S.; Gan, H.; Wang, S. Recognising blueberry fruit of different maturity using histogram oriented gradients and colour features in outdoor scenes. Biosyst. Eng. 2018, 176, 59–72. [Google Scholar] [CrossRef]
- Han, X.; Chang, J.; Wang, K. You only look once: Unified, real-time object detection. Procedia Comput. Sci. 2021, 183, 61–72. [Google Scholar] [CrossRef]
- Terven, J.; Córdova-Esparza, D.-M.; Romero-González, J.-A. A comprehensive review of yolo architectures in computer vision: From yolov1 to yolov8 and yolo-nas. Mach. Learn. Knowl. Extr. 2023, 5, 1680–1716. [Google Scholar] [CrossRef]
- Zhang, J.; Maleski, J.; Ashrafi, H.; Spencer, J.A.; Chu, Y. Open-source high-throughput phenotyping for blueberry yield and maturity prediction across environments: Neural network model and labeled dataset for breeders. Horticulturae 2024, 10, 1332. [Google Scholar] [CrossRef]
- Schumann, A.W.; Mood, N.S.; Mungofa, P.D.; MacEachern, C.; Zaman, Q.; Esau, T. Detection of three fruit maturity stages in wild blueberry fields using deep learning artificial neural networks. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019. [Google Scholar] [CrossRef]
- MacEachern, C.B.; Esau, T.J.; Schumann, A.W.; Hennessy, P.J.; Zaman, Q.U. Detection of fruit maturity stage and yield estimation in wild blueberry using deep learning convolutional neural networks. Smart Agric. Technol. 2023, 3, 100099. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, Y.; Khot, L.R.; Hoheisel, G.A.; Zhang, Q. Optical sensing for early spring freeze related blueberry bud damage detection: Hyperspectral imaging for salient spectral wavelengths identification. Comput. Electron. Agric. 2019, 167, 105025. [Google Scholar] [CrossRef]
- Gonzalez, S.; Arellano, C.; Tapia, J.E. Deepblueberry: Quantification of blueberries in the wild using instance segmentation. IEEE Access 2019, 7, 105776–105788. [Google Scholar] [CrossRef]
- Ni, X.; Li, C.; Jiang, H.; Takeda, F. Deep learning image segmentation and extraction of blueberry fruit traits associated with harvestability and yield. Hortic. Res. 2020, 7, 110. [Google Scholar] [CrossRef]
- Ni, X.; Li, C.; Jiang, H.; Takeda, F. Three-dimensional photogrammetry with deep learning instance segmentation to extract berry fruit harvestability traits. ISPRS J. Photogramm. Remote Sens. 2021, 171, 297–309. [Google Scholar] [CrossRef]
- Fear, C.D.; Lauer, F.I.; Luby, J.J.; Stucker, R.L.; Stushnoff, C. Genetic components of variance for winter injury, fall growth cessation, and off-season flowering in blueberry progenies. J. Am. Soc. Hort. Sci. 1985, 110, 262–266. [Google Scholar] [CrossRef]
- Rowland, L.; Mehra, S.; Arora, R. Identification of molecular markers associated with cold tolerance in blueberry. In Proceedings of the XXVI International Horticultural Congress: Biotechnology in Horticultural Crop Improvement: Achievements, Opportunities and Limitations, Toronto, ON, Canada, 11–17 August 2002; Volume 625. [Google Scholar] [CrossRef]
- Arora, R.; Rowland, L.J.; Lehmann, J.S.; Lim, C.C.; Panta, G.R.; Vorsa, N. Genetic analysis of freezing tolerance in blueberry (Vaccinium section Cyanococcus). Theor. Appl. Genet. 2000, 100, 690–696. [Google Scholar] [CrossRef]
- Yu, D.J.; Lee, H.J. Evaluation of freezing injury in temperate fruit trees. Hort. Environ. Biotech. 2020, 61, 787–794. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Bassil, N.; Buck, E.J.; McCallum, S.; Graham, J.; Brown, A.; Wiedow, C.; Campbell, A.M.; Haynes, K.G.; et al. Construction of a genetic linkage map of an interspecific diploid blueberry population and identification of QTL for chilling requirement and cold hardiness. Mol. Breeding 2014, 34, 2033–2048. [Google Scholar] [CrossRef]
- Lee, J.H.; Yu, D.J.; Kim, S.J.; Choi, D.; Lee, H.J. Intraspecies differences in cold hardiness, carbohydrate content and β-amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree Physiol. 2012, 32, 1533–1540. [Google Scholar] [CrossRef]
- Lim, C.C.; Arora, R.A.R.; Townsend, E.C. Comparing Gompertz and Richards functions to estimate freezing injury in Rhododendron using electrolyte leakage. J. Am. Soc. Hort. Sci. 1998, 123, 246–252. [Google Scholar] [CrossRef]
- Quamme, H.A. Application of thermal analysis to breeding fruit crops for increased cold hardiness. HortScience 1991, 26, 513–517. [Google Scholar] [CrossRef]
- Kishimoto, T.; Yamazaki, H.; Saruwatari, A.; Murakawa, H.; Sekozawa, Y.; Kuchitsu, K.; Price, W.S.; Ishikawa, M. High ice nucleation activity located in blueberry stem bark is linked to primary freeze initiation and adaptive freezing behaviour of the bark. AoB Plants 2014, 6, plu044. [Google Scholar] [CrossRef]
- Burke, M.J.; Bryant, R.G.; Weiser, C.J. Nuclear magnetic resonance of water in cold acclimating red osier dogwood stem. Plant Physiol. 1974, 54, 392–398. [Google Scholar] [CrossRef]
- Gamble, G.R. Non-invasive determination of freezing effects in blueberry fruit tissue by magnetic resonance imaging. J. Food Sci. 1994, 59, 571–573. [Google Scholar] [CrossRef]
- Ceccardi, T.L.; Heath, R.L.; Ting, I.P. Low-temperature exotherm measurement using infrared thermography. HortScience 1995, 30, 140–142. [Google Scholar] [CrossRef]
- Kerr, W.L.; Clark, C.J.; McCarthy, M.J.; De Ropp, J.S. Freezing effects in fruit tissue of kiwifruit observed by magnetic resonance imaging. Sci. Hortic. 1997, 69, 169–179. [Google Scholar] [CrossRef]
- Neuner, G.; Monitzer, K.; Kaplenig, D.; Ingruber, J. Frost survival mechanism of vegetative buds in temperate trees: Deep supercooling and extraorgan freezing vs. ice tolerance. Front. Plant Sci. 2019, 10, 537. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Wu, X.; Moriguchi, T.; Bai, S.; Teng, Y. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hort. Res. 2021, 8, 139. [Google Scholar] [CrossRef]
- Song, G.-Q.; Liu, Z.; Zhong, G.-Y. Regulatory frameworks involved in the floral induction, formation and developmental programming of woody horticultural plants: A case study on blueberries. Front. Plant Sci. 2024, 15, 1336892. [Google Scholar] [CrossRef]
- Gao, X.; Walworth, A.E.; Mackie, C.; Song, G.Q. Overexpression of blueberry FLOWERING LOCUS T is associated with changes in the expression of phytohormone-related genes in blueberry plants. Hort. Res. 2016, 3, 16053. [Google Scholar] [CrossRef]
- Walworth, A.E.; Chai, B.; Song, G.-Q. Transcript profile of flowering regulatory genes in VcFT-overexpressing blueberry plants. PLoS ONE 2016, 11, e0156993. [Google Scholar] [CrossRef]
- Song, G.Q.; Walworth, A.; Zhao, D.; Hildebrandt, B.; Leasia, M. Constitutive expression of the K-domain of a Vaccinium corymbosum SOC1-like (VcSOC1-K) MADS-box gene is sufficient to promote flowering in tobacco. Plant Cell Rep. 2013, 32, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-Q.; Chen, Q. Overexpression of the MADS-box gene K-domain increases the yield potential of blueberry. Plant Sci. 2018, 276, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Zhang, Y.; Walworth, A.; Tomaszewski, E.M.; Callow, P.; Zhong, G.Y.; Song, G.Q. Constitutive expression of an apple FLC3-like gene promotes flowering in transgenic blueberry under nonchilling conditions. Intl. J. Mol. Sci. 2019, 20, 2775. [Google Scholar] [CrossRef]
- Song, G.; Walworth, A. An invaluable transgenic blueberry for studying chilling-induced flowering in woody plants. BMC Plant Biol. 2018, 18, 265. [Google Scholar] [CrossRef]
- Song, G.-Q.; Chen, Q. Comparative transcriptome analysis of nonchilled, chilled, and late-pink bud reveals flowering pathway genes involved in chilling-mediated flowering in blueberry. BMC Plant Biol. 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhang, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and bud dormancy in perennials: Current knowledge and future perspective. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- Muthalif, M.M.; Rowland, L.J. Identification of dehydrin-like proteins responsive to chilling in floral buds of blueberry (Vaccinium, section Cyanococcus). Plant Physiol. 1994, 104, 1439–1447. [Google Scholar] [CrossRef]
- Arora, R.; Rowland, L.J.; Panta, G.R. Chill-responsive dehydrins in blueberry: Are they associated with cold hardiness or dormancy transitions? Physiol. Plant. 1997, 101, 8–16. [Google Scholar] [CrossRef]
- Levi, A.; Panta, G.R.; Parmentier, C.M.; Muthalif, M.M.; Arora, R.; Shanker, S.; Rowland, L.J. Complementary DNA cloning, sequencing and expression of an unusual dehydrin from blueberry floral buds. Physiol. Plant. 1999, 107, 98–109. [Google Scholar] [CrossRef]
- Rowland, L.J.; Panta, G.R.; Mehra, S.; Parmentier-Line, C. Molecular genetic and physiological analysis of the cold-responsive dehydrins of blueberry. J. Crop Improv. 2004, 10, 53–76. [Google Scholar] [CrossRef]
- Panta, G.; Rieger, M.; Rowland, L. Effect of cold and drought stress on blueberry dehydrin accumulation. J. Hortic. Sci. Biotechnol. 2001, 76, 549–556. [Google Scholar]
- Dhanaraj, A.L.; Slovin, J.P.; Rowland, L.J. Isolation of a cDNA clone and characterization of expression of the highly abundant, cold acclimation-associated 14 kDa dehydrin of blueberry. Plant Sci. 2005, 168, 949–957. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Arora, R.; Lim, C.C.; Lehman, J.S.; Levi, A.; Panta, G.R. Use of blueberry to study genetic control of chilling requirement and cold hardiness in woody perennials. HortScience 1999, 34, 1185–1191. [Google Scholar] [CrossRef]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal. Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef]
- Polashock, J.J.; Arora, R.; Peng, Y.; Dhananjay, N.; Rowland, L.J. Functional identification of a blueberry CBF/DREB-like element associated with cold acclimation and freezing tolerance. J. Am. Soc. Hortic. Sci 2010, 35, 40–48. [Google Scholar] [CrossRef]
- Walworth, A.E.; Rowland, L.J.; Polashock, J.J.; Hancock, J.F.; Song, G.Q. Overexpression of a blueberry-derived CBF gene enhances cold tolerance in a southern highbush blueberry cultivar. Mol. Breed. 2012, 30, 1313–1323. [Google Scholar] [CrossRef]
- Song, G.-Q.; Gao, X. Transcriptomic changes reveal gene networks responding to the overexpression of a blueberry DWARF AND DELAYED FLOWERING 1 gene in transgenic blueberry plants. BMC Plant Biol. 2017, 17, 106. [Google Scholar] [CrossRef]
- Walworth, A.; Song, G.-Q. The cold-regulated genes of blueberry and their response to overexpression of VcDDF1 in several tissues. Int. J. Mol. Sci. 2018, 19, 1553. [Google Scholar] [CrossRef]
- Dhanaraj, A.L.; Slovin, J.P.; Rowland, L.J. Analysis of gene expression associated with cold acclimation in blueberry floral buds using expressed sequence tags. Plant Sci. 2004, 166, 863–872. [Google Scholar] [CrossRef]
- Naik, D.; Dhanaraj, A.L.; Arora, R.; Rowland, L.J. Identification of genes associated with cold acclimation in blueberry (Vaccinium corymbosum L.) using a subtractive hybridization approach. Plant Sci. 2007, 173, 213–222. [Google Scholar] [CrossRef]
- Dhanaraj, A.L.; Alkharouf, N.W.; Beard, H.S.; Chouikha, I.B.; Matthews, B.F.; Wei, H.; Arora, R.; Rowland, L.J. Major differences observed in transcript profiles of blueberry during cold acclimation under field and cold room conditions. Planta 2007, 225, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Die, J.V.; Arora, R.; Rowland, L.J. Global patterns of protein abundance during the development of cold hardiness in blueberry. Environ. Exp. Bot. 2016, 124, 11–21. [Google Scholar] [CrossRef]
- Rowland, L.J.; Alkharouf, N.; Darwish, O.; Ogden, E.L.; Polashock, J.J.; Bassil, N.V.; Main, D. Generation and analysis of blueberry transcriptome sequences from leaves, developing fruit, and flower buds from cold acclimation through deacclimation. BMC Plant Biol. 2012, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Mengist, M.F.; Bostan, H.; De Paola, D.; Teresi, S.J.; Platts, A.E.; Cremona, G.; Qi, X.; Mackey, T.; Bassil, N.V.; Ashrafi, H.; et al. Autopolyploid inheritance and a heterozygous reciprocal translocation shape chromosome genetic behavior in tetraploid blueberry (Vaccinium corymbosum). New Phytol. 2023, 237, 1024–1039. [Google Scholar] [CrossRef]
- Krebs, S.L.; Hancock, J.F. The consequences of inbreeding on fertility in Vaccinium corymbosum L. J. Am. Soc. Hortic. Sci. 1988, 113, 914–918. [Google Scholar] [CrossRef]
- Rowland, L.J.; Mehra, S.; Dhanaraj, A.L.; Ogden, E.L.; Slovin, J.P.; Ehlenfeldt, M.K. Development of EST-PCR markers for DNA fingerprinting and genetic relationship studies in blueberry (Vaccinium, section Cyanococcus). J. Am. Soc. Hort. Sci. 2003, 128, 682–690. [Google Scholar] [CrossRef]
- Aruna, M.; Austin, M.E.; Ozias-Akins, P. Randomly amplified polymorphic DNA fingerprinting for identifying rabbiteye blueberry (Vaccinium ashei Reade) cultivars. J. Am. Soc. Hort. Sci. 1995, 120, 710–713. [Google Scholar] [CrossRef]
- Burgher, K.L.; Jamieson, A.R.; Lu, X. Genetic relationships among lowbush blueberry genotypes as determined by randomly amplified polymorphic DNA analysis. J. Am. Soc. Hort. Sci. 2002, 127, 98–103. [Google Scholar] [CrossRef]
- da Silva, M.N.; Benevenuto, J.; Ferrão, L.F.V.; Munoz, P.R. Genome-wide association study and transcriptome analysis reveal candidate genes for off-season flowering in blueberry. Sci. Hortic. 2024, 325, 112643. [Google Scholar] [CrossRef]
- Nagasaka, K.; Nishiyama, S.; Fujikawa, M.; Yamane, H.; Shirasawa, K.; Babiker, E.; Tao, R. Genome-wide identification of loci associated with phenology-related traits and their adaptive variations in a highbush blueberry collection. Front. Plant Sci. 2022, 12, 3147. [Google Scholar] [CrossRef]
- Cantin, C.M.; Wang, X.W.; Almira, M.; Arús, P.; Eduardo, I. Inheritance and QTL analysis of chilling and heat requirements for flowering in an interspecific almond × peach (Texas × Earlygold) F2 population. Euphytica 2020, 216, 51. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Dicenta, F.; Martínez-Gómez, P. Inheritance of chilling and heat requirements for flowering in almond and QTL analysis. Tree Genet. Genomes 2012, 8, 379–389. [Google Scholar] [CrossRef]
- Qi, X.; Ogden, E.L.; Bostan, H.; Sargent, D.J.; Ward, J.; Gilbert, J.; Iorizzo, M.; Rowland, L.J. High-density linkage map construction and QTL identification in a diploid blueberry mapping population. Front. Plant Sci. 2021, 12, 692628. [Google Scholar] [CrossRef] [PubMed]
- Colle, M.; Leisner, C.P.; Wai, C.M.; Ou, S.; Bird, K.A.; Wang, J.; Wisecaver, J.H.; Yocca, A.E.; Alger, E.I.; Tang, H.; et al. Haplotype-phased genome and evolution of phytonutrient pathways of tetraploid blueberry. GigaScience 2019, 8, giz012. [Google Scholar] [CrossRef]
- Zhao, D.; Sapkota, M.; Glaubitz, J.; Bassil, N.; Mengist, M.; Iorizzo, M.; Heller-Uszynska, K.; Mollinari, M.; Beil, C.T.; Sheehan, M. A Public Mid-Density Genotyping Platform for Cultivated Blueberry (Vaccinium spp.); Genetic Resources: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Clare, S.J.; Driskill, M.; Millar, T.R.; Chagné, D.; Montanari, S.; Thomson, S.; Espley, R.V.; Muñoz, P.; Benevenuto, J.; Zhao, D.; et al. Development of a targeted genotyping platform for reproducible results within tetraploid and hexaploid blueberry. Front. Hortic. 2024, 2, 1339310. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, X.; Guo, Y.; Wang, R.; Yao, X.; Chen, X.; Yan, T.; Wu, D.; Lu, Y.; Dong, J.; et al. Structural variations and environmental specificities of flowering time-related genes in Brassica napus. Theor. Appl. Genet. 2023, 136, 42. [Google Scholar] [CrossRef]
- Li, J.; Yuan, D.; Wang, P.; Wang, Q.; Sun, M.; Liu, Z.; Si, H.; Xu, Z.; Ma, Y.; Zhang, B.; et al. Cotton pan-genome retrieves the lost sequences and genes during domestication and selection. Genome Biol. 2021, 22, 1–26. [Google Scholar] [CrossRef]
- Yocca, A.E.; Platts, A.; Alger, E.; Teresi, S.; Mengist, M.F.; Benevenuto, J.; Ferrão, L.F.V.; Jacobs, M.; Babinski, M.; Magallanes-Lundback, M.; et al. Blueberry and cranberry pangenomes as a resource for future genetic studies and breeding efforts. Hort. Res. 2023, 10, uhad202. [Google Scholar] [CrossRef]
- Omori, M.; Yamane, H.; Osakabe, K.; Osakabe, Y.; Tao, R. Targeted mutagenesis of CENTRORADIALIS using CRISPR/Cas9 system through the improvement of genetic transformation efficiency of tetraploid highbush blueberry. J. Hort. Sci. Biotech. 2021, 96, 153–161. [Google Scholar] [CrossRef]
- Vaia, G.; Pavese, V.; Moglia, A.; Cristofori, V.; Silvestri, C. Knockout of phytoene desaturase gene using CRISPR/Cas9 in highbush blueberry. Front. Plant Sci. 2022, 13, 1074541. [Google Scholar] [CrossRef]
- Omori, M.; Yamane, H.; Osakabe, K.; Osakabe, Y.; Tao, R. The evaluation of CRISPR-Cas9-mediated editing efficiency using endogenous promoters in tetraploid blueberry. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Breeding and Effective Use of Biotechnology and Molecular Tools in Horticultural Crops, Angers, France, 14 August 2022; Volume 1362. [Google Scholar] [CrossRef]
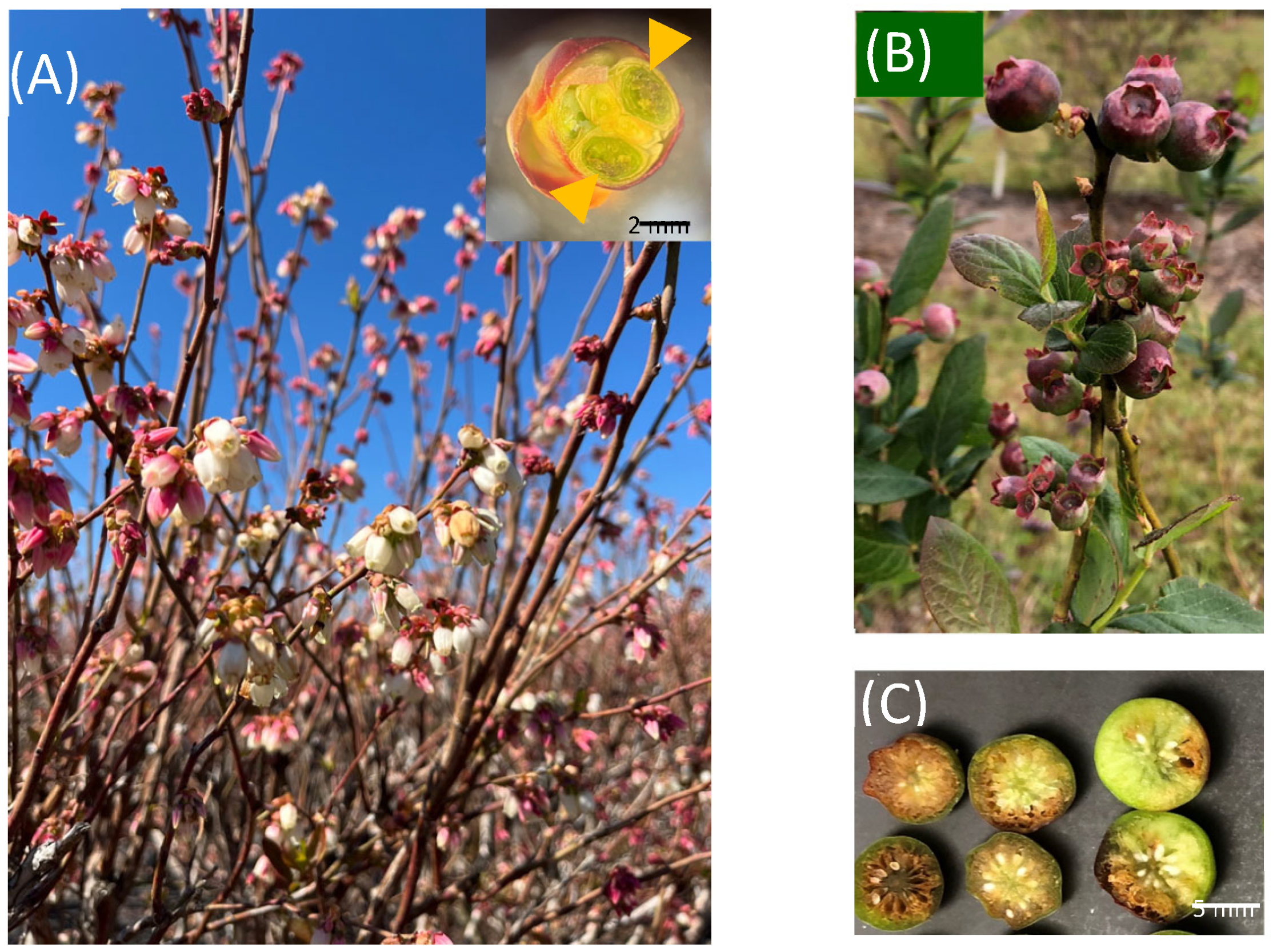

| Starting Date | Duration | Temperature | Type of Weather | Location | Blueberry Genotypes | Damages | Ref. |
|---|---|---|---|---|---|---|---|
| Spring freeze | |||||||
| 11 March 2022 | 3 days | −5 °C | Advective with high wind | Alabama, Georgia, and North Carolina | SHB and RE cultivars | 50% yield reduction | [57,58] |
| 15 March 2017 | 3 days | −6 °C | Deep freeze event | Georgia | SHB cultivars | over 60% yield reduction | [59] |
| 11 February 2012 | 2 days | −2 to 4 °C | Advective with high wind | North and central FL | SHB cultivars | 10% to 50% loss of green fruit | [56] |
| 8 March 2008 | −3.5 to 4.2 °C | Griffin, GA | 4 RE cultivars | 50% to 90% reduction in fruit set | [63] | ||
| 7 April 2007 | 3 days | −2 to 10 °C | Freezing temperature with desiccating wind | Widespread including central, mideast, and southeast US | NHB and SHB cultivars | 90% to 100% loss of NHB and 40 to 90% loss of SHB in affected production regions; post freeze fungal infection | [52] |
| 31 March 2003 | 3 days | −1.9 °C | N/A | Stone County, MS | 11 RE cultivars | Both blooms and green fruits were damaged | [51] |
| 3 May 2000 | 3 h | −6 °C | N/A | Central Poland | 9 NHB cultivars | Flower damage and fruit set reduction | [55] |
| 6 May 1996 | 3 days | −6 to 7 °C | N/A | Nova Scotia | V. angustifolium | Oxidative browning of ovaries, yield reduction | [46] |
| 4 February 1989 | 5 days | −6 to 13 °C | N/A | Overton, TX, and Clarksville, AR | 4 SHB, 4 RE, and 1 NHB cultivars | Oxidative browning of ovaries, yield reduction | [47] |
| 9 March 1982 | 3 h | −2 °C | N/A | USDA Small Fruit Research Station, Poplarville, MS | 5 RE cultivars | Browning of flower petal, yield reduction | [45] |
| 15 April 1972 | N/A | −23.4 °C | N/A | Central Poland | 9 NHB cultivars | Flower bud damage and fruit set duction | [62] |
| Winter freeze | |||||||
| January 1972 | one month | −7.6 °C | N/A | Central Poland | 9 NHB cutlivars | Shoots were killed | [62] |
| 19 February 1927 | 3 h | −32 °C | N/A | Minnesota Agricultural Experiment Station, Colquet, MN | V. pennsylvanicum, V. canadense, and V. corymbosum | Most of the shoots were killed | [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Clevenger, J.; Lee, K.; Zhang, J.; Li, C. Genetic Breeding to Improve Freeze Tolerance in Blueberries, a Review. Horticulturae 2025, 11, 614. https://doi.org/10.3390/horticulturae11060614
Chu Y, Clevenger J, Lee K, Zhang J, Li C. Genetic Breeding to Improve Freeze Tolerance in Blueberries, a Review. Horticulturae. 2025; 11(6):614. https://doi.org/10.3390/horticulturae11060614
Chicago/Turabian StyleChu, Ye, Josh Clevenger, Kendall Lee, Jing Zhang, and Changying Li. 2025. "Genetic Breeding to Improve Freeze Tolerance in Blueberries, a Review" Horticulturae 11, no. 6: 614. https://doi.org/10.3390/horticulturae11060614
APA StyleChu, Y., Clevenger, J., Lee, K., Zhang, J., & Li, C. (2025). Genetic Breeding to Improve Freeze Tolerance in Blueberries, a Review. Horticulturae, 11(6), 614. https://doi.org/10.3390/horticulturae11060614







