A Contemporary Review of Preharvest Mineral Nutrient Management and Defense Elicitor Treatments for Robust Fresh Produce
Abstract
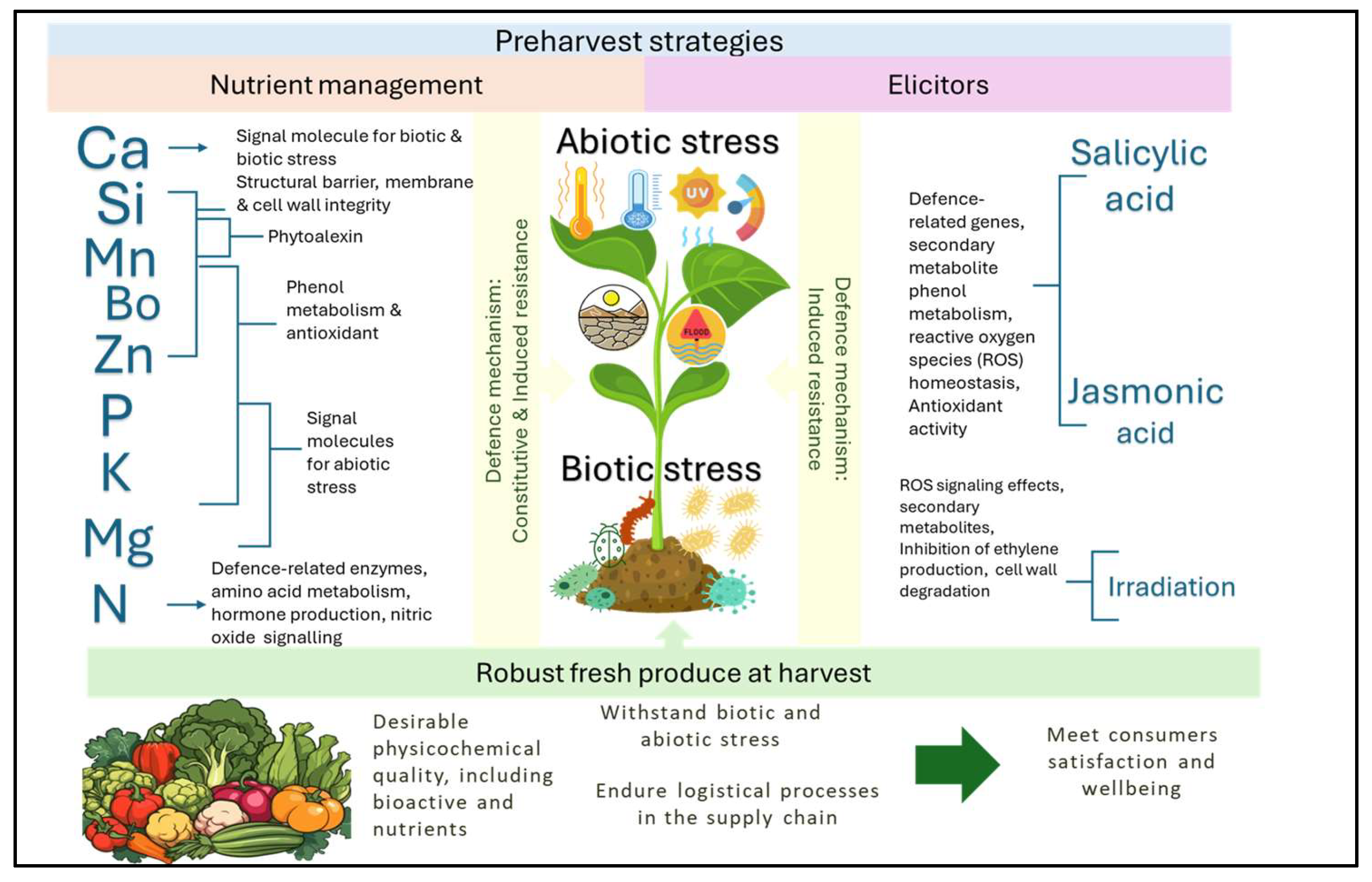
1. Introduction
2. Optimizing Preharvest Strategies to Produce Robust Fresh Produce
2.1. Plant Nutrient Management
2.1.1. Nitrogen
2.1.2. Calcium
2.1.3. Silicon
2.1.4. Other Macronutrients and Micronutrients
2.1.5. Interaction of Mineral Nutrients
2.2. Role of Elicitors
2.2.1. Elicitors
2.2.2. Crosstalk Interaction of Elicitors
2.2.3. Factors Affecting Elicitor Efficacy
3. Measuring and Monitoring Fresh Produce Robustness
4. Challenges and Recommendations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parajuli, R.; Thoma, G.; Matlock, M.D. Environmental sustainability of fruit and vegetable production supply chains in the face of climate change: A review. Sci. Total Environ. 2019, 650, 2863–2879. [Google Scholar] [CrossRef] [PubMed]
- de Castro Moura Duarte, A.L.; Picanço Rodrigues, V.; Bonome Message Costa, L. The sustainability challenges of fresh food supply chains: An integrative framework. Environ. Dev. Sustain. 2024, 1–25. [Google Scholar] [CrossRef]
- Kabir, A.N.M.F.; Alam, M.J.; Begum, I.A.; McKenzie, A.M. Consumers’ preference for purchasing vegetables in bangladesh: What matters? Soc. Sci. Humanit. Open 2023, 8, 100685. [Google Scholar] [CrossRef]
- Petrescu, D.C.; Vermeir, I.; Petrescu-Mag, R.M. Consumer understanding of food quality, healthiness, and environmental impact: A cross-national perspective. Int. J. Environ. Res. Public Health 2019, 17, 169. [Google Scholar] [CrossRef]
- Das, K.; Chatterjee, R.; Sinha, T. Efficacy of iron fortification to augment the nutritional quality of some winter season leafy vegetables. Curr. J. Appl. Sci. Technol. 2020, 39, 75–83. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]
- Topalcengiz, Z.; Chandran, S.; Gibson, K.E. A comprehensive examination of microbial hazards and risks during indoor soilless leafy green production. Int. J. Food Microbiol. 2024, 411, 110546. [Google Scholar] [CrossRef]
- Xavier, I.B.; de Lima Tavares, J.; Pontes, E.D.S.; Magnani, M.; Alvarenga, V.O. Understanding food safety on sprouts and microgreens: Contamination routes, outbreaks and challenges. Food Res. Int. 2025, 214, 116589. [Google Scholar] [CrossRef]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Lindén, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The hurdle approach–a holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy green vegetables. A review. Front. Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef]
- Brandl, M.T. Plant lesions promote the rapid multiplication of Escherichia coli o157:H7 on postharvest lettuce. Appl. Environ. Microbiol. 2008, 74, 5285–5289. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An alarming decline in the nutritional quality of foods: The biggest challenge for future generations’ health. Foods 2024, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D. Improved Fruit Quality and Robustness in Avocado Supply Chains (Mineral Nutrition); Horticulture Innovation: Sydney, Australia, 2021; pp. 1–82. [Google Scholar]
- Ullah, M.A.; Joyce, D.C. Avocado (Persea americana cv. ‘Hass’) fruit mineral composition at canopy level towards sustainable quality. Sustainability 2024, 16, 750. [Google Scholar] [CrossRef]
- Ullah, M.A.; Khanal, A.; Joyce, P.; White, N.; Macnish, A.; Joyce, D. Internal disorders of mango fruit and their management—Physiology, biochemistry, and role of mineral nutrients. Plants 2024, 13, 2596. [Google Scholar] [CrossRef]
- Péneau, S.; Linke, A.; Escher, F.; Nuessli, J. Freshness of fruits and vegetables: Consumer language and perception. Br. Food J. 2009, 111, 243–256. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Gil, M.I.; Izumi, H.; Colelli, G.; Watkins, C.B.; Zude, M. Quality and safety of fresh horticultural commodities: Recent advances and future perspectives. Food Packag. Shelf Life 2017, 14, 2–11. [Google Scholar] [CrossRef]
- Joyce, D.C. The quality cycle. In Crop Management and Postharvest Handling of Horticultural Products; Dris, R., Niskanen, R., Jain, S.M., Eds.; Science Publishers, Inc.: Plymouth, UK, 2001; Volume 1, pp. 1–11. [Google Scholar]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Dietrich, R.; Ploß, K.; Heil, M. Constitutive and induced resistance to pathogens in arabidopsis thaliana depends on nitrogen supply. Plant Cell Environ. 2004, 27, 896–906. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (isr) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef]
- Huber, D.; Römheld, V.; Weinmann, M. Chapter 10-relationship between nutrition, plant diseases and pests. In Marschner’s Mineral Nutrition of Higher Plants (Third Edition); Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 283–298. [Google Scholar]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Gupta, R.; Leibman-Markus, M.; Anand, G.; Rav-David, D.; Yermiyahu, U.; Elad, Y.; Bar, M. Nutrient elements promote disease resistance in tomato by differentially activating immune pathways. Phytopathology 2022, 112, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, M.; Agapov, V.; Reuveni, R. Foliar spray of micronutrient solutions induces local and systemic protection against powdery mildew (Sphaerotheca fuliginia) in cucumber plants. Eur. J. Plant Pathol. 1997, 103, 581–588. [Google Scholar] [CrossRef]
- Salman, M.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; Akhtar, G.; Faried, H.N.; Hussain, A.; Amin, M.; Khalid, S. Combined foliar application of calcium, zinc, boron and time influence leaf nutrient status, vegetative growth, fruit yield, fruit biochemical and anti-oxidative attributes of “chandler” strawberry. J. Plant Nutr. 2022, 45, 1837–1848. [Google Scholar] [CrossRef]
- Haleema, B.; Shah, S.T.; Basit, A.; Hikal, W.M.; Arif, M.; Khan, W.; Said-Al Ahl, H.A.H.; Fhatuwani, M. Comparative effects of calcium, boron, and zinc inhibiting physiological disorders, improving yield and quality of Solanum lycopersicum. Biology 2024, 13, 766. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Martínez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the roles of nitrogen nutrition in plant disease defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Zhang, F.; Li, C. Effect of nitrogen application on enhancing high-temperature stress tolerance of tomato plants during the flowering and fruiting stage. Front. Plant Sci. 2023, 14, 1172078. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Barto, E.K.; Menexes, G.; Rillig, M.C. Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol. 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Shi, H.; Hou, L.; Xu, X.; Zhu, Y.; Zhai, B.; Liu, Z. Effects of different rates of nitrogen fertilizer on apple yield, fruit quality, and dynamics of soil moisture and nitrate in soil of rainfed apple orchards on the loess plateau, china. Eur. J. Agron. 2023, 150, 126950. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Wu, Y.; Wu, W.; Lyu, L.; Li, W. Effects of nitrogen application level on the physiological characteristics, yield and fruit quality of blackberry. Sci. Hortic. 2023, 313, 111915. [Google Scholar] [CrossRef]
- Gülüt, K.Y.; Şentürk, G.G. Impact of nitrogen fertilizer type and application rate on growth, nitrate accumulation, and postharvest quality of spinach. PeerJ 2024, 12, e17726. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Porat, R.; Yermiyahu, U.; Heler, Y.; Holland, D.; Dag, A. Effects of nitrogen fertilization on pomegranate fruit, aril and juice quality. J. Sci. Food Agric. 2020, 100, 1678–1686. [Google Scholar] [CrossRef]
- Krężel, J.; Kołota, E. Source of nitrogen affects the yield and quality of spinach cultivars grown for autumn harvest. Acta Agric. Scand Sect. B—Soil Plant Sci. 2014, 64, 583–589. [Google Scholar] [CrossRef]
- Sinha, A.; Jawandha, S.K.; Gill, P.P.S.; Singh, H. Influence of pre-harvest sprays of calcium nitrate on storability and quality attributes of plum fruits. J. Food Sci. Technol. 2019, 56, 1427–1437. [Google Scholar] [CrossRef]
- Lobos, T.E.; Retamales, J.B.; Hanson, E.J. Early preharvest calcium sprays improve postharvest fruit quality in ‘liberty’ highbush blueberries. Sci. Hortic. 2021, 277, 109790. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Q.; Zheng, S.; Liu, X.; Wu, D.; Gu, Q.; Wei, Q. Effect of pre-harvest calcium treatment on post-harvest fruit quality of nanfeng tangerine. Horticulturae 2024, 10, 381. [Google Scholar] [CrossRef]
- Goutam, M.; Dhaliwal, H.S.; Mahajan, B.V. Effect of pre-harvest calcium sprays on post-harvest life of winter guava (Psidium guajava L.). J. Food Sci. Technol. 2010, 47, 501–506. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Mignani, I.; Diamantidis, G.; Tzavella-Klonari, K. The effect of preharvest calcium sprays on quality attributes, physicochemical aspects of cell wall components and susceptibility to brown rot of peach fruits (Prunus persica L. Cv. Andross). Sci. Hortic. 2005, 107, 43–50. [Google Scholar] [CrossRef]
- Madani, B.; Muda Mohamed, M.T.; Biggs, A.R.; Kadir, J.; Awang, Y.; Tayebimeigooni, A.; Shojaei, T.R. Effect of pre-harvest calcium chloride applications on fruit calcium level and post-harvest anthracnose disease of papaya. Crop Prot. 2014, 55, 55–60. [Google Scholar] [CrossRef]
- Ma, X.; Liu, B.; Zhang, Y.; Su, M.; Zheng, B.; Wang, S.; Wu, H. Unraveling correlations between calcium deficiency and spongy tissue in mango fruit flesh. Sci. Hortic. 2023, 309, 111694. [Google Scholar] [CrossRef]
- Huai, B.; Wu, Y.; Liang, C.; Tu, P.; Mei, T.; Guan, A.; Yao, Q.; Li, J.; Chen, J. Effects of calcium on cell wall metabolism enzymes and expression of related genes associated with peel creasing in citrus fruits. PeerJ 2022, 10, e14574. [Google Scholar] [CrossRef] [PubMed]
- Percival, G.C.; Haynes, I. The influence of calcium sprays to reduce fungicide inputs against apple scab [Venturia inaequalis (cooke) g. Wint.]. Arboric. Urban For. (AUF) 2009, 35, 263–270. [Google Scholar] [CrossRef]
- Peryea, F.J.; Neilsen, G.H.; Faubion, D. Start-timing for calcium chloride spray programs influences fruit calcium and bitter pit in ‘Braeburn’ and ‘Honeycrisp’ apples. J. Plant Nutr. 2007, 30, 1213–1227. [Google Scholar] [CrossRef]
- Mazumder, M.N.N.; Misran, A.; Ding, P.; Wahab, P.E.M.; Mohamad, A. Preharvest foliar spray of calcium chloride on growth, yield, quality, and shelf life extension of different lowland tomato varieties in malaysia. Horticulturae 2021, 7, 466. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Y.; Sang, Z.; Ge, Y.; Bai, C.; Fu, A.; Wang, Q.; Watkins, C.B.; Zuo, J. Multi-omics analysis reveals the mechanism of calcium-reduced quality deterioration in mechanically injured green pepper fruit. Postharvest Biol. Technol. 2023, 204, 112437. [Google Scholar] [CrossRef]
- Torres, E.; Carrasco-Cuello, F. Foliar- and root-applied silicon-based biostimulant increases the nutritional efficiency, fruit firmness and storage potential of nectarines. Sci. Hortic. 2022, 1333, 269–274. [Google Scholar] [CrossRef]
- da Silva, A.D.; Pio, R.; Reis, L.A.C.; Afridi, M.S.; Suárez, N.F.; Peche, P.M.; Ribeiro, C.H.M. Silicon application for the production and quality of raspberry fruit in a subtropical region. Pesqui. Agropecuária Bras. 2023, 58, e03371. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; da Cunha-Chiamolera, T.P.L.; Peña, A.A.; Urrestarazu, M. Silicon enhances production and quality of blueberry fruits (Vaccinium corymbosum L.). J. Plant Nutr. 2022, 45, 1563–1571. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Skodra, C.; Molassiotis, A.; Tanou, G. Silicon influenced ripening metabolism and improved fruit quality traits in apples. Plant Physiol. Biochem. 2021, 166, 270–277. [Google Scholar] [CrossRef]
- Gulzar, N.; Ali, S.; Shah, M.A.; Kamili, A.N. Silicon supplementation improves early blight resistance in lycopersicon esculentum mill. By modulating the expression of defense-related genes and antioxidant enzymes. 3 Biotech 2021, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, N.; Xie, Y.; Zhu, W.; Yang, Y.; Wang, J.; Lei, Y.; Liu, W.; Wang, S.; Jin, L.; et al. Improvements in the appearance and nutritional quality of tomato fruits resulting from foliar spraying with silicon. Foods 2024, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Téllez, L.I.; García-Jiménez, A.; Escobar-Sepúlveda, H.F.; Ramírez-Olvera, S.M.; Bello-Bello, J.J.; Gómez-Merino, F.C. Silicon induces hormetic dose-response effects on growth and concentrations of chlorophylls, amino acids and sugars in pepper plants during the early developmental stage. PeerJ 2020, 8, e9224. [Google Scholar] [CrossRef]
- Almeselmani, M.; Pant, R.C.; Singh, B. Potassium level and physiological response and fruit quality in hydroponically grown tomato. Int. J. Veg. Sci. 2009, 16, 85–99. [Google Scholar] [CrossRef]
- Zikalala, B.O.; Nkomo, M.; Araya, H.; Ngezimana, W.; Mudau, F.N. Nutritional quality of baby spinach (Spinacia oleracea L.) as affected by nitrogen, phosphorus and potassium fertilisation. S. Afr. J. Plant Soil 2017, 34, 79–86. [Google Scholar] [CrossRef]
- Tian, G.; Qin, H.; Liu, C.; Xing, Y.; Feng, Z.; Xu, X.; Liu, J.; Lyu, M.; Jiang, H.; Zhu, Z.; et al. Magnesium improved fruit quality by regulating photosynthetic nitrogen use efficiency, carbon-nitrogen metabolism, and anthocyanin biosynthesis in ‘red fuji’ apple. Front. Plant Sci. 2023, 14, 1136179. [Google Scholar] [CrossRef]
- Quddus, M.A.; Siddiky, M.A.; Hussain, M.J.; Rahman, M.A.; Ali, M.R.; Masud, M.A.T. Magnesium influences growth, yield, nutrient uptake, and fruit quality of tomato. Int. J. Veg. Sci. 2022, 28, 441–464. [Google Scholar] [CrossRef]
- Turhan, A.; Ozmen, N.; Turhan, A.; Ozmen, N. Effects of iron fertilization on plant growth, yield components and quality traits of industrial tomatoes. Mediterr. Agric. Sci. 2022, 35, 1–6. [Google Scholar] [CrossRef]
- Sabatino, L.; D’Anna, F.; Iapichino, G.; Moncada, A.; D’Anna, E.; De Pasquale, C. Interactive effects of genotype and molybdenum supply on yield and overall fruit quality of tomato. Front. Plant Sci. 2019, 9, 1922. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of molybdenum rate on yield and quality of lettuce, escarole, and curly endive grown in a floating system. Agronomy 2018, 8, 171. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Basumatary, A.; Kumar, A.; Dutta, S.; Kalita, P.; Singh, L.K.; Bora, S.S.; Devi, S.H.; Gudade, B.A.; Bhagowati, S.; et al. Physiological performance, yield and quality of crops as influenced by boron. Indian J. Agric. Sci. 2022, 91, 568–572. [Google Scholar] [CrossRef]
- Davis, J.M.; Sanders, D.C.; Nelson, P.V.; Lengnick, L.; Sperry, W.J. Boron improves growth, yield, quality, and nutrient content of tomato. J. Am. Soc. Hortic. Sci. 2003, 128, 441–446. [Google Scholar] [CrossRef]
- Silber, A.; Bar-Tal, A.; Levkovitch, I.; Bruner, M.; Yehezkel, H.; Shmuel, D.; Cohen, S.; Matan, E.; Karni, L.; Aktas, H.; et al. Manganese nutrition of pepper (Capsicum annuum L.): Growth, mn uptake and fruit disorder incidence. Sci. Hortic. 2009, 123, 197–203. [Google Scholar] [CrossRef]
- Sajid, M.; Haq, S.U.; Jan, A.; Noor, F.; Ali, Q.S.; Alam, M.; Zaman, A.; Shah, F.A.; Mosa, W.F.A.; Abada, H.S. Effect of foliar application with potassium nitrate and copper sulfate on fruit yield and quality of pear (Pyrus communis L.) trees. Int. J. Fruit. Sci. 2022, 22, 759–768. [Google Scholar] [CrossRef]
- Rasouli, M.; Koushesh Saba, M. Pre-harvest zinc spray impact on enzymatic browning and fruit flesh color changes in two apple cultivars. Sci. Hortic. 2018, 240, 318–325. [Google Scholar] [CrossRef]
- Aminzade, R.; Ramezanian, A.; Eshghi, S.; Hosseini, S.M.H. The potential of postharvest zinc treatment for preservation of pomegranate aril quality. Sci. Rep. 2024, 14, 1067. [Google Scholar] [CrossRef] [PubMed]
- Grasso, R.; Peña-Fleitas, M.T.; de Souza, R.; Rodríguez, A.; Thompson, R.B.; Gallardo, M.; Padilla, F.M. Nitrogen effect on fruit quality and yield of muskmelon and sweet pepper cultivars. Agronomy 2022, 12, 2230. [Google Scholar] [CrossRef]
- Swarts, N.D.; Mertes, E.; Close, D.C. Role of nitrogen fertigation in sweet cherry fruit quality and consumer perception of quality: At- and postharvest. Acta Hortic. 2017, 1161, 503–510. [Google Scholar] [CrossRef]
- Mukkun, L.; Singh, Z.; Phillips, D. Nitrogen nutrition affects fruit firmness, quality and shelf life of strawberry. Acta Hortic. 2017, 553, 69–71. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Espino-Díaz, M.; Dávila-Aviña, J.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F.; Molina-Corral, F.J.; Parra-Quezada, R.A.; Orozco, G.I.O. Preharvest nitrogen application affects quality and antioxidant status of two tomato cultivars. Bragantia 2020, 79, 134–144. [Google Scholar] [CrossRef]
- Kreidl, S.; Holmes, R.; Partington, D.; Hanger, B.; Karavarsamis, N. The effect of calcium and nitrogen nutrition on the development of postharvest rots in pak choi. Acta Hortic. 2012, 944, 73–82. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Wang, Z.-H.; Stewart, B.A. Chapter five-responses of crop plants to ammonium and nitrate n. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 118, pp. 205–397. [Google Scholar]
- Liu, G.; Du, Q.; Li, J. Interactive effects of nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci. Hortic. 2017, 214, 41–50. [Google Scholar] [CrossRef]
- Doyle, J.W.; Nambeesan, S.U.; Malladi, A. Physiology of nitrogen and calcium nutrition in blueberry (Vaccinium sp.). Agronomy 2021, 11, 765. [Google Scholar] [CrossRef]
- Park, C.-J.; Shin, R. Calcium channels and transporters: Roles in response to biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 964059. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Perring, M.A.; Jackson, C.H. The mineral composition of apples. Calcium concentration and bitter pit in relation to mean mass per apple. J. Sci. Food Agric. 1975, 26, 1493–1502. [Google Scholar] [CrossRef]
- de Freitas, S.T.; Mitcham, E.J. Factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar]
- Dhatt, A.S.; Mahajan, B.V.C.; Bhatt, A.R. Effect of pre and post-harvest calcium treatments on the storage life of Asian pear. Acta Hortic. 2005, 696, 497–501. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Wang, X.; Yan, L.; Hu, X.; Lian, M. Effect of foliar calcium fertilization on fruit quality, cell wall enzyme activity and expression of key genes in chinese cherry. Int. J. Fruit. Sci. 2023, 23, 200–216. [Google Scholar] [CrossRef]
- Najafi, R.; Barzegar, T. The effect of foliar spray of different calcium sources on antioxidant properties and quality of cauliflower (Brassica oleracea cv. Botrytis ‘Romanesco’). J. Hortic. Sci. 2022, 36, 577–589. [Google Scholar] [CrossRef]
- Santos, E.; Montanha, G.S.; Agostinho, L.F.; Polezi, S.; Marques, J.P.R.; de Carvalho, H.W.P. Foliar calcium absorption by tomato plants: Comparing the effects of calcium sources and adjuvant usage. Plants 2023, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Recasens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘golden delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of spraying calcium fertilizer on photosynthesis, mineral content, sugar–acid metabolism and fruit quality of fuji apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]
- Manivannan, A.; Ahn, Y.-K. Silicon regulates potential genes involved in major physiological processes in plants to combat stress. Front. Plant Sci. 2017, 8, 1346. [Google Scholar] [CrossRef]
- Osei, A.F.; Jin, X.L.; Abdullah, W.Z.B.W.; Sidique, S.N.M. Silicon improves strawberry plants nutrient uptake and epicuticular wax formation in a rhizosphere cooling system. Asian J. Agric. Biol. 2023, 2, 2. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Sheng, H.; Chen, S. Plant silicon-cell wall complexes: Identification, model of covalent bond formation and biofunction. Plant Physiol. Biochem. 2020, 155, 13–19. [Google Scholar] [CrossRef]
- Chan-in, P.; Wangkaew, B.; Anankul, N.; Teerawatsakul, Y.; Thiyagaraja, V.; Tamakaew, N.; Withee, P.; Haituk, S.; Cheewangkoon, R.; Pusadee, T. Effect of silicon on the fruit quality and disease response to gummy stem blight in cucumber. Chiang Mai J. Sci. 2023, 50, e2023073. [Google Scholar] [CrossRef]
- Liu, B.; Davies, K.; Hall, A.; Sarma, B. Silicon builds resilience in strawberry plants against both strawberry powdery mildew podosphaera aphanis and two-spotted spider mites tetranychus urticae. PLoS ONE 2020, 15, e0241151. [Google Scholar] [CrossRef]
- Ferreira, H.A.; Nascimento, C.W.A.d.; Datnoff, L.E.; Nunes, G.H.d.S.; Preston, W.; Souza, E.B.d.; Mariano, R.d.L.R. Effects of silicon on resistance to bacterial fruit blotch and growth of melon. Crop Prot. 2015, 78, 277–283. [Google Scholar] [CrossRef]
- Abd-El-Kareem, F.; Elshahawy, I.E.; Abd-Elgawad, M.M.M. Effectiveness of silicon and silicate salts for controlling black root rot and induced pathogenesis-related protein of strawberry plants. Bull. Natl. Res. Cent. 2019, 43, 91. [Google Scholar] [CrossRef]
- Jayawardana, H.A.R.K.; Weerahewa, H.L.D.; Saparamadu, M.D.J.S. Effect of root or foliar application of soluble silicon on plant growth, fruit quality and anthracnose development of capsicum. Trop. Agric. Res. 2015, 26, 74. [Google Scholar] [CrossRef]
- Mburu, K.; Oduor, R.; Mgutu, A.; Tripathi, L. Silicon application enhances resistance to xanthomonas wilt disease in banana. Plant Pathol. 2016, 65, 807–818. [Google Scholar] [CrossRef]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Xu, J.; Hassan, M.U.; Yuan, H.; Liu, L.; He, B.; Ejaz, I.; White, P.J.; Cakmak, I.; et al. Improvement of nutritional quality of food crops with fertilizer: A global meta-analysis. Agron. Sustain. Dev. 2023, 43, 74. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, T.Q.; Tan, C.S.; Astatkie, T. Responses of fruit yield and quality of processing tomato to drip-irrigation and fertilizers phosphorus and potassium. Agron. J. 2011, 103, 1339–1345. [Google Scholar] [CrossRef]
- Lu, M.; Liu, D.; Shi, Z.; Gao, X.; Liang, Y.; Yao, Z.; Zhang, W.; Wang, X.; Chen, X. Nutritional quality and health risk of pepper fruit as affected by magnesium fertilization. J. Sci. Food Agric. 2021, 101, 582–592. [Google Scholar] [CrossRef]
- Tavanti, T.R.; Melo, A.A.R.d.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.d.S.; Silva, R.M.d.; Reis, A.R.d. Micronutrient fertilization enhances ros scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Steiner, F.; Zoz, T.; Zuffo, A.M.; Pereira-Machado, P.; Zoz, J.; Zoz, A. Foliar application of molybdenum enhanced quality and yield of crispleaf lettuce (Lactuca sativa L., cv. Grand rapids). Acta Agron. 2018, 67, 73–78. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Niu, J.; Guo, X.; Wu, H.; Han, D.; Shuai, L.; Wu, Z. Preharvest zinc sulfate spray improves the storability of longan (Dimocarpus longan Lour.) fruits by protecting the cell wall components and antioxidants of pericarp. J. Sci. Food Agric. 2019, 99, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Bonomelli, C.; de Freitas, S.T.; Aguilera, C.; Palma, C.; Garay, R.; Dides, M.; Brossard, N.; O’Brien, J.A. Ammonium excess leads to ca restrictions, morphological changes, and nutritional imbalances in tomato plants, which can be monitored by the n/ca ratio. Agronomy 2021, 11, 1437. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Zhou, Y.; Ren, C.; Zhang, S.; Liu, L. Relative availability of nitrogen and calcium regulates the growth of poplar seedlings due to transcriptome changes. Forests 2023, 14, 1899. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N.; Abdel-Sattar, M.; Aboukarima, A.M. Effect of ammonium sulphate incorporated with calcium nitrate fertilizers on nutritional status, fruit set and yield of pomegranate trees cv. Wonderful. Agronomy 2022, 12, 971. [Google Scholar] [CrossRef]
- Naseri, L.; Arzani, K.; Babalar, M. Foliar boron, copper and manganese uptakes and concentrations of apple leaves cv. Golden delicious on m9 and b9 rootstocks. Acta Hortic. 2002, 594, 229–235. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; González-Lázaro, M.; Alonso-Ortiz de Urbina, D.; Sáenz de Urturi, I.; Marín-San Román, S.; Murillo-Peña, R.; Torres-Díaz, L.L.; Pérez-Álvarez, E.P.; Fernández, V. Foliar applications of calcium, silicon and their combination: A tool to improve grape composition and quality. Appl. Sci. 2023, 13, 7217. [Google Scholar] [CrossRef]
- Cano-Reinoso, D.M.; Soesanto, L.; Kharisun, K.; Wibowo, C. Effect of pre-harvest foliar calcium and silicon fertilization on pineapple quality and fruit collapse incidence. Agrivita 2022, 44, 405–418. [Google Scholar] [CrossRef]
- da Silva, D.L.; de Mello Prado, R.; Tenesaca, L.F.L.; da Silva, J.L.F.; Mattiuz, B.-H. Silicon attenuates calcium deficiency by increasing ascorbic acid content, growth and quality of cabbage leaves. Sci. Rep. 2021, 11, 1770. [Google Scholar] [CrossRef]
- Tripathi, P.; Subedi, S.; Khan, A.L.; Chung, Y.S.; Kim, Y. Silicon effects on the root system of diverse crop species using root phenotyping technology. Plants 2021, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Takahashi, E. Interaction between calcium and silicon in water-cultured rice plants. Plant Soil 1993, 148, 107–113. [Google Scholar] [CrossRef]
- Mao, Y.; Chai, X.; Zhong, M.; Zhang, L.; Zhao, P.; Kang, Y.; Guo, J.; Yang, X. Effects of nitrogen and magnesium nutrient on the plant growth, quality, photosynthetic characteristics, antioxidant metabolism, and endogenous hormone of chinese kale (brassica albograbra bailey). Sci. Hortic. 2022, 303, 111243. [Google Scholar] [CrossRef]
- Asgarian, Z.S.; Karimi, R.; Palou, L. Pre-harvest foliar spraying of calcium and zinc preserves berries quality and mitigates chilling injury of grape during cold storage. Sci. Hortic. 2024, 338, 113557. [Google Scholar] [CrossRef]
- Tavallali, V.; Esmaili, S.; Karimi, S. Nitrogen and potassium requirements of tomato plants for the optimization of fruit quality and antioxidative capacity during storage. J. Food Meas. Charact. 2018, 12, 755–762. [Google Scholar] [CrossRef]
- Vilhena, N.Q.; Quiñones, A.; Rodríguez, I.; Gil, R.; Fernández-Serrano, P.; Salvador, A. Leaf and fruit nutrient concentration in rojo brillante persimmon grown under conventional and organic management, and its correlation with fruit quality parameters. Agronomy 2022, 12, 237. [Google Scholar] [CrossRef]
- Joyce, D.C.; Kiloes, A.; Ullah, M.; Aziz, A. Growing robust avocado fruit—A systems thinking approach to inform decision making. In Proceedings of the 10th World Avocado Congress, Auckland, New Zealand, 2–5 April 2023. [Google Scholar]
- Müller, B.; Hartung, J.; von Cossel, M.; Lewandowski, I.; Müller, T.; Bauerle, A. On-farm use of recycled liquid ammonium sulphate in southwest germany using a participatory approach. Nutr. Cycl. Agroecosyst. 2024, 129, 459–474. [Google Scholar] [CrossRef]
- Reddy, K.S.; Mohanty, M.; Rao, D.L.N.; Rao, A.S.; Pandey, M.; Singh, M.; Dixit, S.K.; Dalal, R.C.; Blamey, F.P.C.; Menzies, N.W. Farmer involvement in the development and adoption of improved nutrient management technologies using the mother–baby trial approach in vertisols. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 51–62. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Reglinski, T.; Havis, N.; Rees, H.J.; de Jong, H. The practical role of induced resistance for crop protection. Phytopathology 2023, 113, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Mulugeta, T.; Lankinen, Å.; Liljeroth, E.; Andreasson, E. Plant resistance inducers against pathogens in solanaceae species-from molecular mechanisms to field application. Int. J. Mol. Sci. 2016, 17, 1673. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Panahirad, S.; Morshedloo, M.R.; Ali, S.; Hano, C.; Kulak, M. Secondary metabolites and their potential roles in plant tolerance against abiotic and biotic stress. Plant Stress 2023, 10, 100292. [Google Scholar] [CrossRef]
- Gong, D.; Bi, Y.; Li, Y.; Wang, Y.; Prusky, D.; Alkan, N. Preharvest elicitors spray improves antioxidant activity, alleviates chilling injury, and maintains quality in harvested fruit. Horticulturae 2022, 8, 1208. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Corio-Costet, M.-F.; Burdziej, A.; Laurens, M.; Bodin, E.; Bellée, A.; Da Costa, G.; Le Mao, I.; Cluzet, S. Effects of three elicitors on primary metabolism six days after treatment in healthy vitis vinifera leaves and eight days after treatment in healthy and downy mildew-inoculated leaves. OENO One 2024, 58, 1–17. [Google Scholar] [CrossRef]
- Ahmad, B.; Zaid, A.; Sadiq, Y.; Bashir, S.; Wani, S.H. Role of selective exogenous elicitors in plant responses to abiotic stress tolerance. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 273–290. [Google Scholar]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Wang, Y.H.; Irving, H.R. Developing a model of plant hormone interactions. Plant Signal Behav. 2011, 6, 494–500. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Lozano-Pastor, P.; Artés-Hernández, F.; Artés, F.; Aguayo, E. Preharvest uv-c treatment improves the quality of spinach primary production and postharvest storage. Postharvest Biol. Technol. 2019, 155, 130–139. [Google Scholar] [CrossRef]
- Huang, J.Y.; Xu, F.; Zhou, W. Effect of led irradiation on the ripening and nutritional quality of postharvest banana fruit. J. Sci. Food Agric. 2018, 98, 5486–5493. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, Z.; Iqbal, Z. Effect of preharvest sprays of salicylic acid on the shelf life and quality of ‘lane late’ sweet orange (Citrus sinensis L.) cold storage. In Proceedings of the VII International Postharvest Symposium, Kuala Lumpur, Malaysia, 25–29 June 2012; pp. 103–112. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef] [PubMed]
- Emilda, D.; Sutanto, A.; Sukartini; Jumjunidang. Application of salicylic acid to induce disease resistance against fusarium wilt on banana. IOP Conf. Ser. Earth Environ. Sci. 2020, 468, 12026. [Google Scholar] [CrossRef]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Erogul, D.; Özsoydan, İ. Effect of pre-harvest salicylic acid treatments on the quality and shelf life of the ‘cresthaven’ peach cultivar. Folia Hortic. 2020, 32, 221–227. [Google Scholar] [CrossRef]
- Supapvanich, S.; Mitsang, P.; Youryon, P. Preharvest salicylic acid application maintains physicochemical quality of ‘taaptimjaan’ wax apple fruit (Syzygium samarangenese) during short-term storage. Sci. Hortic. 2017, 215, 178–183. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic acid preharvest treatment improves green pepper fruit quality and antioxidant capacity during postharvest storage. Acta Hortic. 2024, 1396, 471–476. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Salicylic acid-induced differential resistance to the tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Akter, M.A.; Miyaji, N.; Shimizu, M.; Takasaki-Yasuda, T.; Dennis, E.S.; Fujimoto, R. The role of salicylic acid responsive genes in disease resistance in brassica rapa vegetable. Acta Hortic. 2023, 1362, 305–312. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.; Xu, W.; Fan, S.; Wan, T.; Zhang, J.; Cai, Y. Methyl jasmonate induces postharvest disease resistance to decay caused by alternaria alternata in sweet cherry fruit. Sci. Hortic. 2022, 292, 110624. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Li, Y.; Li, M.; Jin, P.; Zheng, Y. Involvement of ppwrky70 in the methyl jasmonate primed disease resistance against rhizopus stolonifer of peaches via activating phenylpropanoid pathway. Postharvest Biol. Technol. 2021, 174, 111466. [Google Scholar] [CrossRef]
- Li, M.; Qu, X.; Gong, D.; Huang, T.; Wang, Y.; Yang, Y.; Gao, Z.; Zhang, Z.; Sun, J.; Hu, M. Induced resistance to control postharvest stem-end rot by methyl jasmonate in mango fruit. Physiol. Mol. Plant Pathol. 2024, 134, 102426. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Methyl jasmonate-induced suppression of anthracnose rot in tomato fruit. Crop Prot. 2007, 26, 1507–1513. [Google Scholar] [CrossRef]
- Huang, T.; Liu, G.; Zhu, L.; Liu, J.; Xiang, Y.; Xu, X.; Zhang, Z. Mitigation of chilling injury in mango fruit by methyl jasmonate is associated with regulation of antioxidant capacity and energy homeostasis. Postharvest Biol. Technol. 2024, 211, 112801. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Zhang, X.; Zhu, X.; Hou, Y.; Chen, J.; Cui, K.; Li, X.; Wu, W. Methyl jasmonate attenuates chilling injury of prune fruit by maintaining ros homeostasis and regulating gaba metabolism and energy status. Postharvest Biol. Technol. 2025, 220, 113303. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Han, C.; Duan, R.; Yang, J.; Xue, H. Effects of methyl jasmonate on fruit coloration and quality improvement in pears (Pyrus bretschneideri). Agronomy 2023, 13, 2409. [Google Scholar] [CrossRef]
- Shuai, L.; Xue, P.; Liao, L.; Liu, Y.; Song, M.; Shang, F.; Cai, W.; Yin, F.; Cai, J. Methyl jasmonate suppressed the pericarp browning in postharvest longan fruit by modulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2024, 209, 112681. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Feng, L.; Yang, E.N.; Su, X.; Jiang, Y. Effect of methyl jasmonate on pericarp browning of postharvest lychees: Effect of methyl jasmonate on lychee browning. J. Food Process Preserv. 2011, 35, 417–422. [Google Scholar] [CrossRef]
- Baek, M.W.; Choi, H.R.; Lee, H.C.; Lee, J.H.; Lee, O.-H.; Hong, J.S.; Jeong, C.S.; Tilahun, S. Preharvest methyl jasmonate and salicylic acid treatments improve the nutritional qualities and postharvest storability of tomato. Sci. Hortic. 2023, 321, 112332. [Google Scholar] [CrossRef]
- Fu, A.; Zheng, Y.; Lv, Y.; Watkins, C.B.; Bai, C.; Ma, L.; Yuan, S.; Zheng, S.; Jia, L.e.; Gao, L.; et al. Multi-omics analysis reveals specific modifications associated with reduced chilling injury in bell pepper fruit by methyl jamonate. Postharvest Biol. Technol. 2022, 185, 111799. [Google Scholar] [CrossRef]
- Ku, K.M.; Juvik, J.A. Environmental stress and methyl jasmonate-mediated changes in flavonoid concentrations and antioxidant activity in broccoli florets and kale leaf tissues. HortScience 2013, 48, 996–1002. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2007, 55, 10366–10372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Trouth, F.; Yang, T. Preharvest uv-b treatment improves strawberry quality and extends shelf life. Horticulturae 2023, 9, 211. [Google Scholar] [CrossRef]
- de Oliveira, I.R.; Crizel, G.R.; Severo, J.; Renard, C.M.G.C.; Chaves, F.C.; Rombaldi, C.V. Preharvest uv-c radiation influences physiological, biochemical, and transcriptional changes in strawberry cv. Camarosa. Plant Physiol. Biochem. 2016, 108, 391–399. [Google Scholar] [CrossRef]
- Anja Dieleman, J.; Marjolein Kruidhof, H.; Weerheim, K.; Leiss, K. Led lighting strategies affect physiology and resilience to pathogens and pests in eggplant (Solanum melongena L.). Front. Plant Sci. 2021, 11, 610046. [Google Scholar] [CrossRef]
- Obande, M.A.; Tucker, G.A.; Shama, G. Effect of preharvest uv-c treatment of tomatoes (Solanum Lycopersicon Mill.) on ripening and pathogen resistance. Postharvest Biol. Technol. 2011, 62, 188–192. [Google Scholar] [CrossRef]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. Uv-c treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- Sidibé, A.; Charles, M.T.; Lucier, J.-F.; Xu, Y.; Beaulieu, C. Preharvest uv-c hormesis induces key genes associated with homeostasis, growth and defense in lettuce inoculated with Xanthomonas campestris pv. Vitians. Front. Plant Sci. 2022, 12, 793989. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of pre-harvest supplemental uv-a/blue and red/blue led lighting on lettuce growth and nutritional quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Skowron, E.; Trojak, M.; Pacak, I. Effects of uv-b and uv-c spectrum supplementation on the antioxidant properties and photosynthetic activity of lettuce cultivars. Int. J. Mol. Sci. 2024, 25, 9298. [Google Scholar] [CrossRef]
- Lee, J.-H.; Oh, M.-M.; Son, K.-H. Short-term ultraviolet (uv)-a light-emitting diode (led) radiation improves biomass and bioactive compounds of kale. Front. Plant Sci. 2019, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, W.; Yang, T.; Luo, Y.; Chen, P. Preharvest uvb application increases glucosinolate contents and enhances postharvest quality of broccoli microgreens. Molecules 2021, 26, 3247. [Google Scholar] [CrossRef] [PubMed]
- Huyskens-Keil, S.; Schreiner, M.; Krumbein, A.; Reichmuth, C.H.; Janata, E.; Ulrichs, C.H. UV-B and gamma irradiation as physical elicitors to promote phytochemicals in brassica sprouts. Acta Hortic. 2010, 858, 37–41. [Google Scholar] [CrossRef]
- Xie, Z.; Charles, M.T.; Charlebois, D.; Rolland, D.; Roussel, D.; Deschênes, M.; Dubé, C.; Khanizadeh, S.; Fan, J. Preharvest exposure to uv-c radiation: Impact on strawberry fruit quality. Acta Hortic. 2015, 1079, 589–592. [Google Scholar] [CrossRef]
- Mishra, S.; Roychowdhury, R.; Ray, S.; Hada, A.; Kumar, A.; Sarker, U.; Aftab, T.; Das, R. Salicylic acid (sa)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress 2024, 11, 100427. [Google Scholar] [CrossRef]
- Li, S.; Xiao, L.; Chen, M.; Cao, Q.; Luo, Z.; Kang, N.; Jia, M.; Chen, J.; Xiang, M. The involvement of the phenylpropanoid and jasmonate pathways in methyl jasmonate-induced soft rot resistance in kiwifruit (Actinidia chinensis). Front. Plant Sci. 2022, 13, 1097733. [Google Scholar] [CrossRef]
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Urban, L.; Chabane Sari, D.; Orsal, B.; Lopes, M.; Miranda, R.; Aarrouf, J. UV-C light and pulsed light as alternatives to chemical and biological elicitors for stimulating plant natural defenses against fungal diseases. Sci. Hortic. 2018, 235, 452–459. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhang, W.; Yang, W.; An, S.; Guo, M.; Chen, G. Salicylic acid treatment ameliorates postharvest quality deterioration in ‘france’ prune (Prunus domestica L. ‘Ximei’) fruit by modulating the antioxidant system. Foods 2024, 13, 2871. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Xu, Y.; An, Y.; Hu, Z.; Xiong, A.; Wang, G. Effects of jasmonic acid on stress response and quality formation in vegetable crops and their underlying molecular mechanisms. Plants 2024, 13, 1557. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Li, Y.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Supplemental uv-a affects growth and antioxidants of chinese kale baby-leaves in artificial light plant factory. Horticulturae 2021, 7, 294. [Google Scholar] [CrossRef]
- Sidibé, A.; Charles, M.T.; Nicolas, O.; Beaulieu, C. Preharvest uv-c affects lettuce resistance to xanthomonas campestris pv. Vitians and quality. Sci. Hortic. 2021, 285, 110094. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J.; Aghdam, M.S. Salicylic acid treatment of peach trees maintains nutritional quality of fruits during cold storage. Adv. Hortic. Sci. 2017, 32, 33–40. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Coutand, C. Mechanosensing and thigmomorphogenesis, a physiological and biomechanical point of view. Plant Sci. 2010, 179, 168–182. [Google Scholar] [CrossRef]
- Börnke, F.; Rocksch, T. Thigmomorphogenesis–control of plant growth by mechanical stimulation. Sci. Hortic. 2018, 234, 344–353. [Google Scholar] [CrossRef]
- Telewski, F.W. Mechanosensing and plant growth regulators elicited during the thigmomorphogenetic response. Front. For. Glob. Change 2021, 3, 574096. [Google Scholar] [CrossRef]
- Saidi, I.; Ammar, S.; Demont-Caulet, N.; Thévenin, J.; Lapierre, C.; Bouzid, S.; Jouanin, L. Thigmomorphogenesis in solanum lycopersicum: Morphological and biochemical responses in stem after mechanical stimulation. Plant Signal Behav. 2010, 5, 122–125. [Google Scholar] [CrossRef]
- Porter, B.W.; Zhu, Y.J.; Webb, D.T.; Christopher, D.A. Novel thigmomorphogenetic responses in carica papaya: Touch decreases anthocyanin levels and stimulates petiole cork outgrowths. Ann. Bot. 2009, 103, 847–858. [Google Scholar] [CrossRef]
- Hunt, E.R.; Jaffe, M.J. Thigmomorphogenesis: The interaction of wind and temperature in the field on the growth of Phaseolus vulgaris L. Ann. Bot. 1980, 45, 665–672. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Moritz, L.; Pieterse, C.M.; Van Wees, S.C. Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front. Plant Sci. 2015, 6, 639. [Google Scholar] [CrossRef]
- El-kenawy, M.A. Effect of chitosan, salicylic acid and fulvic acid on vegetative growth, yield and fruit quality of thompson seedless grapevines. Egypt. J. Hortic. 2017, 44, 45–59. [Google Scholar] [CrossRef]
- Contreras-Medina, L.M.; Feregrino-Perez, A.A.; Guevara-González, R.G.; Jimenez-Garcia, S.N.; Garcia-Mier, L.; Torres-Pacheco, I. Elicitor mixtures significantly increase bioactive compounds, antioxidant activity, and quality parameters in sweet bell pepper. J. Chem. 2015, 2015, 269296. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Guillén, F.; Habibi, F.; Golding, J.B. Editorial: Pre-and postharvest treatments with elicitors on the development of bioactive compounds and nutritional quality of fruit and vegetables. Front. Nutr. 2022, 9, 1070945. [Google Scholar] [CrossRef]
- Vyas, P.B.; Rao Tadapaneni, V.R.; Thakkar, V.R. Chemical elicitors improve the shelf life of phalsa (Grewia asiatica L.) by inducing antioxidants and controlling microbes. Fruits 2016, 71, 307–317. [Google Scholar] [CrossRef]
- Martínez-Camacho, J.E.; Guevara-González, R.G.; Rico-García, E.; Tovar-Pérez, E.G.; Torres-Pacheco, I. Delayed senescence and marketability index preservation of blackberry fruit by preharvest application of chitosan and salicylic acid. Front. Plant Sci. 2022, 13, 796393. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Deng, M.; Gui, R.; Liu, Y.; Chen, X.; Lin, Y.; Li, M.; Wang, Y.; He, W.; et al. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chem. X 2022, 15, 100384. [Google Scholar] [CrossRef]
- Heil, M.; Hilpert, A.; Kaiser, W.; Linsenmair, K.E. Reduced growth and seed set following chemical induction of pathogen defence: Does systemic acquired resistance (sar) incur allocation costs? J. Ecol. 2000, 88, 645–654. [Google Scholar] [CrossRef]
- Leibman-Markus, M.; Schneider, A.; Gupta, R.; Marash, I.; Rav-David, D.; Carmeli-Weissberg, M.; Elad, Y.; Bar, M. Immunity priming uncouples the growth-defense trade-off in tomato. Development 2023, 150, dev201158. [Google Scholar] [CrossRef]
- Kiremit, M.S. Optimization of salicylic acid dose to improve lettuce growth, physiology and yield under salt stress conditions. J. Crop Health 2024, 76, 269–283. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Xie, J.; Yu, J.; Li, J.; Lv, J.; Gao, Y.; Niu, T.; Patience, B.E. Effects of preharvest methyl jasmonate and salicylic acid treatments on growth, quality, volatile components, and antioxidant systems of chinese chives. Front. Plant Sci. 2021, 12, 767335. [Google Scholar] [CrossRef]
- Kim, Y.L.; Yeom, M.-S.; Sim, H.-S.; Lee, G.O.; Kang, I.-J.; Yang, G.-S.; Yun, J.G.; Son, K.-H. Effect of pre-harvest intermittent uv-b exposure on growth and secondary metabolites in achyranthes japonica nakai microgreens in a vertical farm. Horticulturae 2024, 10, 1040. [Google Scholar] [CrossRef]
- Seki, H.; Murakami, H.; Ma, T.; Tsuchikawa, S.; Inagaki, T. Evaluating soluble solids in white strawberries: A comparative analysis of vis-nir and nir spectroscopy. Foods 2024, 13, 2274. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, L.; Wang, W.; Zhang, X.; Gu, Q.; Zhu, Y.; Chen, R.; Zhang, C. Visible/near-infrared spectroscopy and hyperspectral imaging facilitate the rapid determination of soluble solids content in fruits. Food Eng. Rev. 2024, 16, 470–496. [Google Scholar] [CrossRef]
- Jayas, D.S.; Singh, C.B.; Paliwal, J. Chapter 15-classification of wheat kernels using near-infrared reflectance hyperspectral imaging. In Hyperspectral Imaging for Food Quality Analysis and Control; Sun, D.-W., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 449–470. [Google Scholar]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of near-infrared (nir) spectroscopy and hyperspectral imaging for quality and safety assessment of fruits: An overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Rocha, W.F.d.C.; Prado, C.B.d.; Blonder, N. Comparison of chemometric problems in food analysis using non-linear methods. Molecules 2020, 25, 3025. [Google Scholar] [CrossRef]
- Mishra, P.; Passos, D.; Marini, F.; Xu, J.; Amigo, J.M.; Gowen, A.A.; Jansen, J.J.; Biancolillo, A.; Roger, J.M.; Rutledge, D.N.; et al. Deep learning for near-infrared spectral data modelling: Hypes and benefits. TrAC Trends Anal. Chem. 2022, 157, 116804. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Ueno, N.; Huck, C.W. Analyzing the quality parameters of apples by spectroscopy from vis/nir to nir region: A comprehensive review. Foods 2023, 12, 1946. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G. Application of visible/nir spectroscopy for the estimation of soluble solids, dry matter and flesh firmness in stone fruits. J. Sci. Food Agric. 2021, 101, 2100–2107. [Google Scholar] [CrossRef]
- Beghi, R.; Giovenzana, V.; Tugnolo, A.; Guidetti, R. Application of visible/near infrared spectroscopy to quality control of fresh fruits and vegetables in large-scale mass distribution channels: A preliminary test on carrots and tomatoes. J. Sci. Food Agric. 2018, 98, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Devianti; Sufardi; Hafsah, S.; Sariadi; Ahmad, F.; Arianti, N.D.; Saputra, E.; Hartuti, S. Rapid and non-destructive determination of vitamin c and antioxidant activity of intact red chilies using visible near-infrared spectroscopy and machine learning tools. Case Stud. Chem. Environ. Eng. 2023, 8, 100435. [Google Scholar] [CrossRef]
- Adesokan, M.; Alamu, E.O.; Otegbayo, B.; Maziya-Dixon, B. A review of the use of near-infrared hyperspectral imaging (nir-hsi) techniques for the non-destructive quality assessment of root and tuber crops. Appl. Sci. 2023, 13, 5226. [Google Scholar] [CrossRef]
- Tanzilli, D.; Cocchi, M.; Amigo, J.M.; D’Alessandro, A.; Strani, L. Does hyperspectral always matter? A critical assessment of near infrared versus hyperspectral near infrared in the study of heterogeneous samples. Curr. Res. Food Sci. 2024, 9, 100813. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Xu, W.; Yan, T.; Yan, J.; Gao, P.; Zhang, C. Application of near-infrared spectroscopy and hyperspectral imaging combined with machine learning algorithms for quality inspection of grape: A review. Foods 2022, 12, 132. [Google Scholar] [CrossRef]
- Okere, E.E.; Ambaw, A.; Perold, W.J.; Opara, U.L. Vis-nir and swir hyperspectral imaging method to detect bruises in pomegranate fruit. Front. Plant Sci. 2023, 14, 1151697. [Google Scholar] [CrossRef]
- Yu, X.; Lu, H.; Wu, D. Development of deep learning method for predicting firmness and soluble solid content of postharvest korla fragrant pear using vis/nir hyperspectral reflectance imaging. Postharvest Biol. Technol. 2018, 141, 39–49. [Google Scholar] [CrossRef]
- Weng, S.; Yu, S.; Dong, R.; Pan, F.; Liang, D. Nondestructive detection of storage time of strawberries using visible/near-infrared hyperspectral imaging. Int. J. Food Prop. 2020, 23, 269–281. [Google Scholar] [CrossRef]
- He, M.; Li, C.; Cai, Z.; Qi, H.; Zhou, L.; Zhang, C. Leafy vegetable freshness identification using hyperspectral imaging with deep learning approaches. Infrared Phys. Technol. 2024, 138, 105216. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, C.; Zeng, S.; Yang, W.; Chen, Y. Hyperspectral imaging coupled with deep learning model for visualization and detection of early bruises on apples. J. Food Compos. Analy 2024, 134, 106489. [Google Scholar] [CrossRef]
- Song, H.; Yoon, S.-R.; Dang, Y.-M.; Yang, J.-S.; Hwang, I.M.; Ha, J.-H. Nondestructive classification of soft rot disease in napa cabbage using hyperspectral imaging analysis. Sci. Rep. 2022, 12, 14707. [Google Scholar] [CrossRef] [PubMed]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 82–8214. [Google Scholar] [CrossRef]
- Clément, A.; Dorais, M.; Vernon, M. Nondestructive measurement of fresh tomato lycopene content and other physicochemical characteristics using visible−nir spectroscopy. J. Agric. Food Chem. 2008, 56, 9813–9818. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Ouyang, A. Nondestructive measurement of soluble solid content of navel orange fruit by visible–nir spectrometric technique with plsr and pca-bpnn. LWT-Food Sci. Technol. 2010, 43, 602–607. [Google Scholar] [CrossRef]
- Gómez, A.H.; He, Y.; Pereira, A.G. Non-destructive measurement of acidity, soluble solids and firmness of satsuma mandarin using vis/nir-spectroscopy techniques. J. Food Eng. 2006, 77, 313–319. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of nir spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Determination of phenolic compounds of grape skins during ripening by nir spectroscopy. LWT-Food Sci. Technol. 2011, 44, 847–853. [Google Scholar] [CrossRef]
- Li, J.; Huang, B.; Wu, C.; Sun, Z.; Xue, L.; Liu, M.; Chen, J. Nondestructive detection of kiwifruit textural characteristic based on near infrared hyperspectral imaging technology. Int. J. Food Prop. 2022, 25, 1697–1713. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, H.; Feng, Q.; Xu, L.; Lin, Q.; Cai, K. Rapid detection of pomelo fruit quality using near-infrared hyperspectral imaging combined with chemometric methods. Front. Bioeng. Biotechnol. 2021, 8, 616943. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Yu, W.-H.; Song, D.-J.; Chun, S.-W.; Kim, M.S.; Lee, A.; Kim, G.; Shin, B.-S.; Mo, C. Prediction of soluble-solid content in citrus fruit using visible–near-infrared hyperspectral imaging based on effective-wavelength selection algorithm. Sensors 2024, 24, 1512. [Google Scholar] [CrossRef] [PubMed]
- Teo, V.X.; Dhandapani, S.; Ang Jie, R.; Philip, V.S.; Teo Ju Teng, M.; Zhang, S.; Park, B.S.; Olivo, M.; Dinish, U.S. Early detection of n, p, k deficiency in choy sum using hyperspectral imaging-based spatial spectral feature mining. Front. Photon. 2024, 5, 1418246. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; He, H.-J.; Kamruzzaman, M. Advancements, limitations and challenges in hyperspectral imaging for comprehensive assessment of wheat quality: An up-to-date review. Food Chem. X 2024, 21, 101235. [Google Scholar] [CrossRef]
- Mishra, P. Developing multifruit global near-infrared model to predict dry matter based on just-in-time modeling. J. Chemom. 2024, 38, e3540. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhan, B.; Wang, Z.; Jiang, Y. Determination of ssc in pears by establishing the multi-cultivar models based on visible-nir spectroscopy. Infrared Phys. Technol. 2019, 102, 103066. [Google Scholar] [CrossRef]
- Pissard, A.; Marques, E.J.N.; Dardenne, P.; Lateur, M.; Pasquini, C.; Pimentel, M.F.; Fernández Pierna, J.A.; Baeten, V. Evaluation of a handheld ultra-compact nir spectrometer for rapid and non-destructive determination of apple fruit quality. Postharvest Biol. Technol. 2021, 172, 111375. [Google Scholar] [CrossRef]
- Du, X.-l.; Li, X.-y.; Liu, Y.; Zhou, W.-h.; Li, J.-l. Genetic algorithm optimized non-destructive prediction on property of mechanically injured peaches during postharvest storage by portable visible/shortwave near-infrared spectroscopy. Sci. Hortic. 2019, 249, 240–249. [Google Scholar] [CrossRef]
- Blakey, R.J. Evaluation of avocado fruit maturity with a portable near-infrared spectrometer. Postharvest Biol. Technol. 2016, 121, 101–105. [Google Scholar] [CrossRef]
- Posom, J.; Klaprachan, J.; Rattanasopa, K.; Sirisomboon, P.; Saengprachatanarug, K.; Wongpichet, S. Predicting marian plum fruit quality without environmental condition impact by handheld visible–near-infrared spectroscopy. ACS Omega 2020, 5, 27909–27921. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Oh, E.U.; Lim, D.C. Performance evaluation of a portable near-infrared spectrometer for assessment of main quality parameters in yellow-fleshed kiwifruit. Acta Hortic. 2022, 1332, 393–398. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Nondestructive detection of postharvest quality of cherry tomatoes using a portable nir spectrometer and chemometric algorithms. Food Anal. Methods 2019, 12, 914–925. [Google Scholar] [CrossRef]
- dos Santos Neto, J.P.; de Assis, M.W.D.; Casagrande, I.P.; Cunha Júnior, L.C.; de Almeida Teixeira, G.H. Determination of ‘palmer’ mango maturity indices using portable near infrared (vis-nir) spectrometer. Postharvest Biol. Technol. 2017, 130, 75–80. [Google Scholar] [CrossRef]
- Lamptey, F.P.; Teye, E.; Abano, E.E.; Amuah, C.L.Y. Application of handheld nir spectrometer for simultaneous identification and quantification of quality parameters in intact mango fruits. Smart Agric. Technol. 2023, 6, 100357. [Google Scholar] [CrossRef]
- Das, A.J.; Wahi, A.; Kothari, I.; Raskar, R. Ultra-portable, wireless smartphone spectrometer for rapid, non-destructive testing of fruit ripeness. Sci. Rep. 2016, 6, 32504. [Google Scholar] [CrossRef]
- Goisser, S.; Wittmann, S.; Fernandes, M.; Mempel, H.; Ulrichs, C. Comparison of colorimeter and different portable food-scanners for non-destructive prediction of lycopene content in tomato fruit. Postharvest Biol. Technol. 2020, 167, 111232. [Google Scholar] [CrossRef]
- Wang, T.; Chen, J.; Fan, Y.; Qiu, Z.; He, Y. Seefruits: Design and evaluation of a cloud-based ultra-portable nirs system for sweet cherry quality detection. Comput. Electron. Agric. 2018, 152, 302–313. [Google Scholar] [CrossRef]
- Martins, V.; Unlubayir, M.; Teixeira, A.; Gerós, H.; Lanoue, A. Calcium and methyl jasmonate cross-talk in the secondary metabolism of grape cells. Plant Physiol. Biochem. 2021, 165, 228–238. [Google Scholar] [CrossRef]
- Yeganehpoor, F.; Zehtab-Salmasi, S.; Shafagh-Kolvanagh, J.; Ghassemi-Golezani, K.; Dastborhan, S. Can application of nitrogen fertilizers and salicylic acid improve growth and fruit yield of coriander under water deficit? Acta Sci. Pol. Hortorum Cultus 2019, 18, 87–97. [Google Scholar] [CrossRef]
- Rawat, S.; Ravita; Tyagi, K.; Kumar, K.; Kumari, G.; Kaintura, S.S.; Pandey, A.; Ginwal, H.S.; Barthwal, S. Silicon and salicylic acid: Individual and combined impact on plant growth and stress tolerance. Plant Biosyst. 2023, 157, 1003–1013. [Google Scholar] [CrossRef]
| Nutrient | Effect | Produce | References |
|---|---|---|---|
| Nitrogen | Increase total soluble solids, leaf chlorophyll, photosynthesis efficiency, antioxidant enzyme activity, anthocyanin, phenolics, flavonoids, lycopene, antioxidant capacity fresh weight and vitamin C | Apple, blackberry, tomato, spinach | [33,34,35,37] |
| Calcium | Improve color, enhance shelf life, reduce weight loss, maintain anthocyanin, retain sensory quality, firmness, increase total phenolic content, TSS, vitamin C, reduce deterioration, pathological (e.g., brown rot, anthracnose) and physiological diseases (e.g., spongy tissue, peel creasing scab, bitter pit, blossom end rot) | Plum, blueberry, tangerine, guava, peach, papaya, mango, orange, tomato, green pepper, broccoli microgreens | [6,38,39,40,41,42,43,44,45,46,47,48,49] |
| Silicon | Increase dry matter content, fruit weight, firmness, TSS, decrease shriveling, respiration rate, improves color, increase total phenolic, total anthocyanin compounds, resistance to disease (e.g., early blight), increase chlorophyll | Nectarine, raspberry, blueberry, apples, tomato, and pepper. | [50,51,52,53,54,55,56] |
| Potassium | Improves protein, ascorbic acid, lycopene, TSS, reducing sugar levels, titratable acidity, increases total phenols, total antioxidants activity, total flavonoids, vitamin C | Tomato, spinach | [57,58] |
| Phosphorus | Increase total phenols, total antioxidants activity, total flavonoids, vitamin C, dry biomass of stems and leaves | Spinach, tomato | [57,58] |
| Magnesium | Increase anthocyanin, vitamin C, carotene, protein content, firmness | Apple, tomato | [59,60] |
| Iron | Increase dry matter, TSS, total acidity, ascorbic acid content, vitamin A, chlorophyll content | Tomato, leafy vegetables (e.g., mustard, onion) | [5,61] |
| Molybdenum | Increase fresh weight, polyphenol content ascorbic acid | Tomato, lettuce, escarole, curly endive | [62,63] |
| Boron | Inhibit powdery mildew, improve shelf life, fruit firmness, increase ascorbic acid, protein and starch | Cucumber, tomato, cauliflower, cowpea, okra | [25,64,65] |
| Manganese | Inhibit powdery mild, reduce heat-damaged fruit | Cucumber, bell pepper | [25,66] |
| Copper | Inhibit powdery mildew, increase firmness, fruit juice, TSS, ascorbic acid | Pear, cucumber, | [25,67] |
| Zinc | Reduce rotting rate, browning, maintain colour, cellulose, pectin, flavonoid, and phenolics, increase ascorbic acid, total antioxidant activity, TSS, titratable acidity, anthocyanin | Longan, apple, strawberry | [67,68,69] |
| Elicitor | Disease and Physicochemical Quality | Bioactive and Nutritional Quality | Produce | References |
|---|---|---|---|---|
| Salicylic acid/methyl salicylate (MeSa) | Disease resistance against black mold disease, yellow leaf curl virus, Fusarium wilt, Delayed ripening and senescence, prolonged shelf life, reduced fresh weight loss, improved firmness, colour, and acidity, maintained chlorophyll levels, titratable acidity | Increased ascorbic acid, antioxidant capacity, glutathione, total phenolic, total flavonoid, anthocyanins, total chlorophyll content | Orange, grape, banana cherry, peach, wax apple, grape, peach, pepper, tomato, pointed gourd, Chinese chives, Brassica rapa | [138,139,140,141,142,143,144,145,146] |
| Jasmonic acid or methyl jasmonate (MeJa) | Disease resistance against Alternaria alternata, Rhizopus stolonifera, stem end rot anthracnose rot Improved colour, total soluble and sugar content alleviating chilling injury reducing pericarp browning | Increased phenolics, flavonoids, total chlorophyll, phenols and flavonoids, vitamin C, volatile components | Sweet cherry, peach, mango, pear, prune, longan, lychee, tomato, bell pepper broccoli florets, kale leaf, lettuce | [147,148,149,150,151,152,153,154,155,156,157,158,159] |
| Irradiation UV-B, UV-C, UV-A, LEDs, gamma, far-red, red and blue light | Disease resistance against powdery mildew, Penicillium digitatum, Xanthomonas campestris Retained firmness, delayed ripening, increased growth, reduced chilling injury, electrolyte leakage, respiration, improved biomass, thicker and more compact leaves | Increased carotenoids, anthocyanins, flavonoids, glucosinolate, phenolic compounds, vitamin C content, antioxidant capacity | Strawberry, blueberry, eggplant, tomato, lettuce, kale, broccoli microgreens, brassica sprouts | [160,161,162,163,164,165,166,167,168,169,170,171] |
| Feature | Visible/Near-Infrared (vis/NIR) | Near-Infrared Hyperspectral Imaging | Hyperspectral Imaging |
|---|---|---|---|
| Spectral acquisition | Spectral data invisible region (380–780 nm) and near-infrared region (780–2500 nm), spot-scan measurement | Spectral data with imaging, both spectral and spatial information across the NIR region, 1100 to 2500 nm | Spatial and spectral data across a broader wavelength range 1000 nm to 2500 nm, in line and area scan techniques |
| Data information | One-dimensional spectral data for each point/sample, chemical quality such as sugar, moisture, and acidity | Hyperspectral data cube, two-dimensional geometric space and one-dimensional spectral information, distribution of physicochemical properties | Hyperspectral data cube, two-dimensional geometric space and one-dimensional spectral information (hundreds of bands of continuous wavelengths) for extensive data on physicochemical qualities |
| Fresh produce | Orange, mandarin, grape, | Strawberry, kiwi, citrus, pomelo | Apple, spinach, and Chinese cabbage, leafy vegetables |
| Quality | SSC, color, content, color, firmness, pH, flavanols, TPC, vitamin C, and antioxidant activity | Prediction of storage time, textural profile analysis, SSC, vitamin C and organic acid contents | Early bruise detection, diseases N, P, K deficiency, freshness quality |
| Cost | Less expensive, less complex setup, less computationally demanding. | Moderate cost, but becomes more expensive with higher wavelengths up to 2500 nm | Expensive setup, including advanced computing to process large datasets |
| References | [205,207,212,213,216,223,224,225,226,227,228] | [205,214,217,219,223,229,230,231] | [207,216,217,220,221,222,232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secretaria, L.B.; Hoffman, E.; Bekker, M.; Joyce, D. A Contemporary Review of Preharvest Mineral Nutrient Management and Defense Elicitor Treatments for Robust Fresh Produce. Horticulturae 2025, 11, 596. https://doi.org/10.3390/horticulturae11060596
Secretaria LB, Hoffman E, Bekker M, Joyce D. A Contemporary Review of Preharvest Mineral Nutrient Management and Defense Elicitor Treatments for Robust Fresh Produce. Horticulturae. 2025; 11(6):596. https://doi.org/10.3390/horticulturae11060596
Chicago/Turabian StyleSecretaria, Leizel B., Eleanor Hoffman, Marlize Bekker, and Daryl Joyce. 2025. "A Contemporary Review of Preharvest Mineral Nutrient Management and Defense Elicitor Treatments for Robust Fresh Produce" Horticulturae 11, no. 6: 596. https://doi.org/10.3390/horticulturae11060596
APA StyleSecretaria, L. B., Hoffman, E., Bekker, M., & Joyce, D. (2025). A Contemporary Review of Preharvest Mineral Nutrient Management and Defense Elicitor Treatments for Robust Fresh Produce. Horticulturae, 11(6), 596. https://doi.org/10.3390/horticulturae11060596





