Growth and Floral Induction in Okra (Abelmoschus esculentus L.) Under Blue and Red LED Light and Their Alternation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Soil Properties
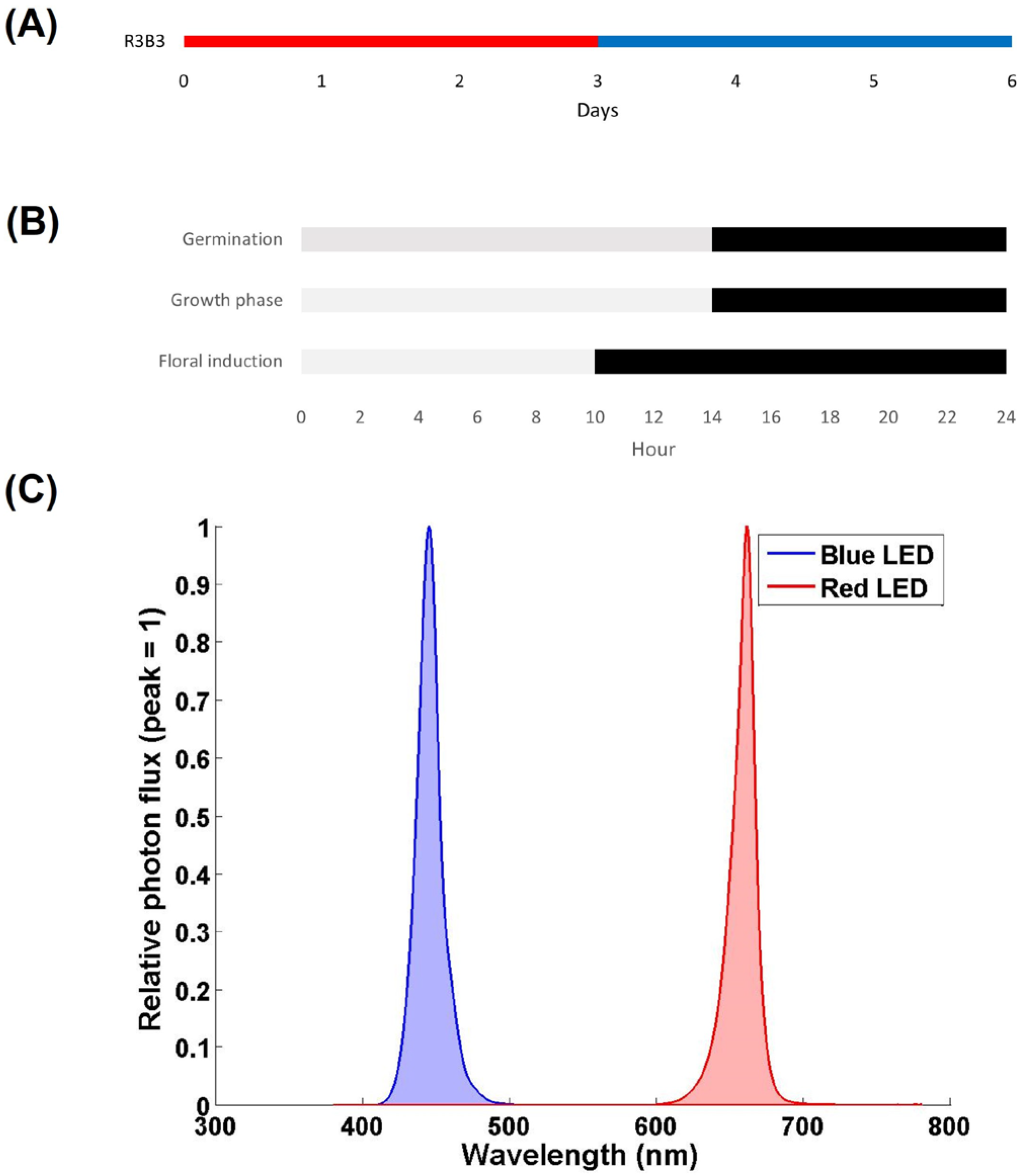
2.2. Experimental Design and Lighting Treatments
2.3. Outdoor Light Conditions
2.4. Using IoT for Plant Environmental Monitoring Using Home Automation Software
2.5. Germination Observations
2.6. Flowering Observations
2.7. Growth and Morphology Observations
2.8. Statistical Design and Analysis
3. Results
3.1. Germination Parameters
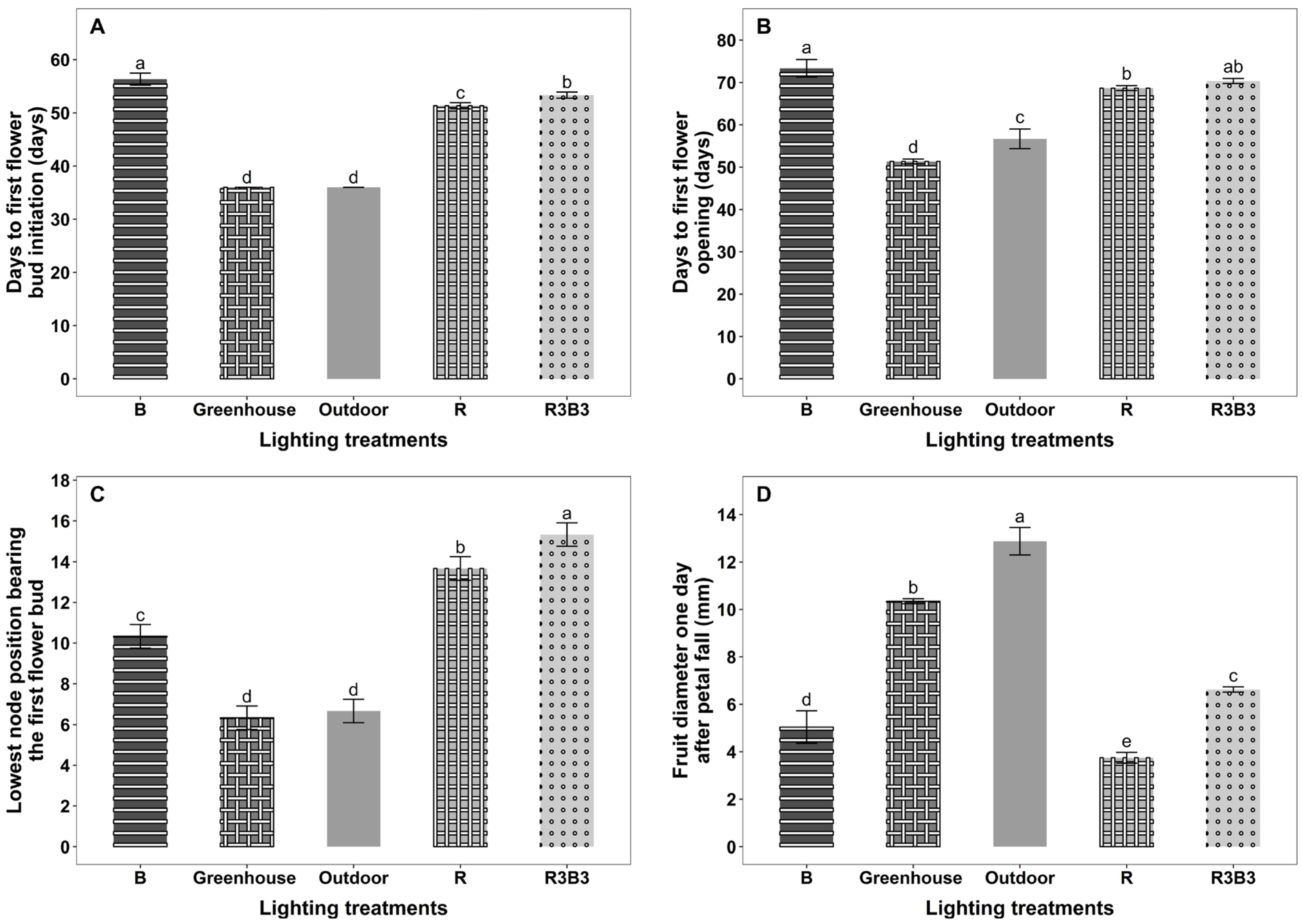
3.2. Flowering and Fruiting
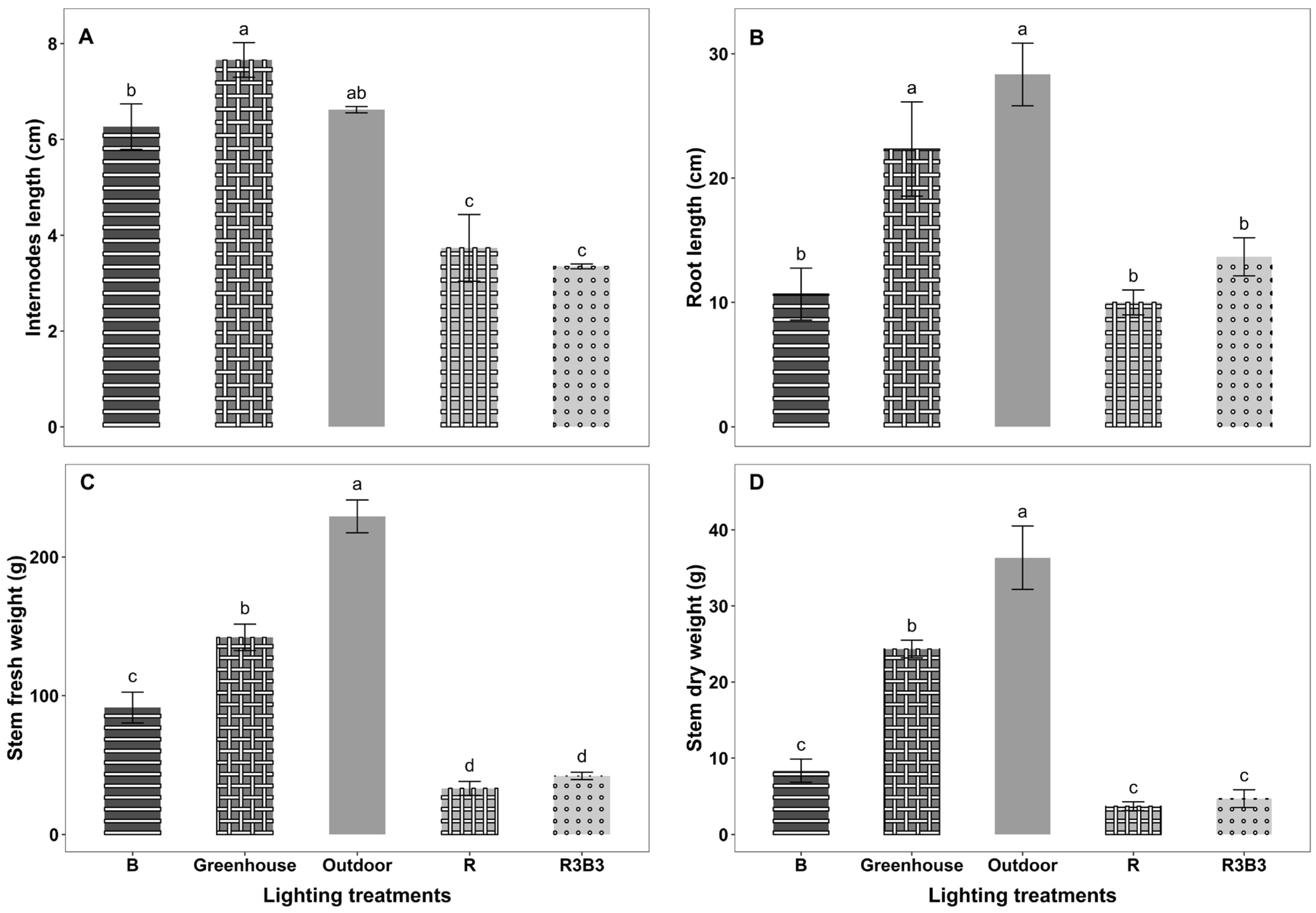
3.3. Growth Parameters
Some Temporal Growth Parameters
4. Discussion
4.1. Effects of Light Spectrum on Okra Seed Germination
4.2. Effects of Light Spectrum on Growth Parameters of Okra
4.3. Effects of Light Spectrum on Time to First Flower Bud Initiation and First Flower Open on Okra
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seydou, T.; Dapah Sara Fatim, K.; N’Doua Bertrand, G.; Souleymane, S.; Barakissa, B.; Gnénakan, Y.; Meless Patrice, N.; Omyon Jean Anselme, A.; Daouda, K. Assessment of the Agronomic Performance of Seven Okra (Abelmoschus esculentus (L.), Moench) Cultivars in Southern Côte d’Ivoire. World J. Agric. Res. 2023, 11, 87–97. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 15 January 2025).
- Khan, S.; Rafi, Z.; Baker, A.; Shoaib, A.; Alkhathami, A.G.; Asiri, M.; Alshahrani, M.Y.; Ahmad, I.; Alraey, Y.; Hakamy, A.; et al. Phytochemical Screening, Nutritional Value, Anti-Diabetic, Anti-Cancer, and Anti-Bacterial Assessment of Aqueous Extract from Abelmoschus esculentus Pods. Processes 2022, 10, 183. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, J.; Luo, J.; Qin, W.; Luo, Q.; Zhang, Q.; Wu, D.; Lin, D.; Li, S.; Dong, H.; et al. Okra in Food Field: Nutritional Value, Health Benefits and Effects of Processing Methods on Quality. Food Rev. Int. 2021, 37, 67–90. [Google Scholar] [CrossRef]
- Fabianová, J.; Šlosár, M.; Kopta, T.; Vargová, A.; Timoracká, M.; Mezeyová, I.; Andrejiová, A. Yield, Antioxidant Activity and Total Polyphenol Content of Okra Fruits Grown in Slovak Republic. Horticulturae 2022, 8, 966. [Google Scholar] [CrossRef]
- Akogo, Y.; Aidam, A.; Odah, K.; Quashie, A.M. Micropropagation in Vitro de Deux Espèces de Gombo: Abelmoshus Esculentus et Abelmoshus Cannabinus. Cah. Agric. 1996, 5, 109–111. [Google Scholar]
- Miezan N’guettia, C.A.; San-Whouly Ouali-N’goran, M.; Fatogoman, S.; Daouda, K. ARPN Journal of Agricultural and Biological Science Distribution of insects according to the phenological stages of okra (Abelmoschus esculentus) and phytosanitary practices in anna (bingerville, côte d’ivoire). ARPN J. Agric. Biol. Sci. 2017, 12, 171–181. [Google Scholar]
- Maiti, R.K.; Singh, V.P. A Review on Recent Research in Okra (Abelmoschus esculentus L.). Farming Manag. 2021, 6, 77–107. [Google Scholar] [CrossRef]
- Ekoja, E.E.; Adanu, G.; Utag, T.A. Variations in Insect-Induced Fruit Damage and Yield of Okra Abelmoschus esculentus after Insecticide Treatments at Different Phenological Growth Stages. J. Crop Prot. 2023, 12, 79–91. [Google Scholar]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light Quality in Plant Tissue Culture: Does It Matter? Vitr. Cell. Developmental. Biol.-Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop Photosynthetic Response to Light Quality and Light Intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- McCree, K.J. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Kondratev, V.M.; Osipova, G.S.; Kiselev, M.V. Growth and Development of Tomato (Lycopersicon Esculentum Mill.) under Light Culture Conditions. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Timisoara, Romania, 20–24 September 2010; IOP Publishing Ltd.: Bristol, UK, 2022; Volume 1010. [Google Scholar]
- Kong, Y.; Zheng, Y. Diverse Flowering Response to Blue Light Manipulation: Application of Electric Lighting in Controlled-Environment Plant Production. Horticulturae 2024, 10, 578. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y.; et al. Effects of Different Light Intensity on the Growth of Tomato Seedlings in a Plant Factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef]
- Bachouch, L.; Sewraj, N.; Dupuis, P.; Canale, L.; Zissis, G.; Bouslimi, L.; El Amraoui, L. An approach for designing mixed light-emitting diodes to match greenhouse plant absorption spectra. Sustainability 2021, 13, 4329. [Google Scholar] [CrossRef]
- Kohyama, F.; Whitman, C.; Runkle, E.S. Comparing Flowering Responses of Long-Day Plants under Incandescent and Two Commercial Light-Emitting Diode Lamps. HortTechnol. Hortte 2014, 24, 490–495. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M.; van Ieperen, W. Floral Induction in the Short-Day Plant Chrysanthemum Under Blue and Red Extended Long-Days. Front. Plant Sci. 2021, 11, 610041. [Google Scholar] [CrossRef]
- SharathKumar, M.; Luo, J.; Xi, Y.; van Ieperen, W.; Marcelis, L.F.M.; Heuvelink, E. Several Short-Day Species Can Flower under Blue-Extended Long Days, but This Response Is Not Universal. Sci. Hortic. 2024, 325, 112657. [Google Scholar] [CrossRef]
- Zakurin, A.O.; Shchennikova, A.V.; Kamionskaya, A.M. Artificial-Light Culture in Protected Ground Plant Growing: Photosynthesis, Photomorphogenesis, and Prospects of LED Application. Russ. J. Plant Physiol. 2020, 67, 413–424. [Google Scholar] [CrossRef]
- Li, H. Growth and Characteristics of Abelmoschus esculentus Plantelets in Vitro under Different Quality Lights. Acta Bot. Boreal. -Occident. Sin. 2016, 36, 996–1003. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The Importance of Blue Light for Leaf Area Expansion, Development of Photosynthetic Apparatus, and Chloroplast Ultrastructure of Cucumis Sativus Grown under Weak Light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on Net Photosynthetic Rate, Growth and Leaf Stomata of Chrysanthemum Plantlets in Vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Wada, M. Chloroplast Movement. Plant Sci. 2013, 210, 177–182. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of Light-Emitting Diodes on Growth and Morphogenesis of Upland Cotton (Gossypium Hirsutum L.) Plantlets in Vitro. Plant Cell Tissue Organ. Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Weng, F. Growth, physiological and stomatal characteristics of red okra (Abelmoschus esculentus L. Moench) seedlings at different light intensities. Pak. J. Bot. 2024, 56, 945–950. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue Light Alleviates ‘Red Light Syndrome’ by Regulating Chloroplast Ultrastructure, Photosynthetic Traits and Nutrient Accumulation in Cucumber Plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of Spectral Quality of Monochromatic LED Lights on the Growth of Artichoke Seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef]
- Sulistyowati, Y.; Wahyuni; Hartati, N.S.; Kim, J.; Cho, E.; Seskar, M.; Raskin, I.; Sudarmonowati, E. Effect of Various LED Light on the Growth and Reproductive of in Vitro-Derived Indonesian Chili Pepper (Capsicum annum) Kopay and Laris Varieties. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bali, Indonesia, 16–18 May 2023; Institute of Physics: London, UK, 2023; Volume 1255. [Google Scholar]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving Spinach, Radish, and Lettuce Growth under Red Light-Emitting Diodes (LEDs) with Blue Light Supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Ohtake, N.; Ju, Y.; Ishikura, M.; Suzuki, H.; Adachi, S.; Yamori, W. Alternating Red/Blue Light Increases Leaf Thickness and Mesophyll Cell Density in the Early Growth Stage, Improving Photosynthesis and Plant Growth in Lettuce. Environ. Control Biol. 2021, 59, 59–67. [Google Scholar] [CrossRef]
- Takasu, S.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Photosynthesis and Morphology of Leaf Lettuce (Lactuca sativa L. Cv. Greenwave) Grown under Alternating Irradiation of Red and Blue Light. Environ. Control Biol. 2019, 57, 93–98. [Google Scholar] [CrossRef]
- Hamamoto, H.; Yamazaki, K. Reproductive Response of Okra and Native Rosella to Long-Day Treatment with Red, Blue, and Green Light-Emitting Diode Lights. HortScience Horts 2009, 44, 1494–1497. [Google Scholar] [CrossRef]
- Amaki, W.; Kunii, M. Effects of Light Quality on the Flowering Responses in Kalanchoe Blossfeldiana. Acta Hortic. 2015, 1107, 279–283. [Google Scholar] [CrossRef]
- Maduagwuna, A.E.; Asiegbu, J.E.; Onyeonagu, C.C. Responses of Okra (Abelmoschus esculentus (L.) Moench) Varieties to Photoperiod. Direct Res. J. Agric. Food Sci. 2021, 9, 242–252. [Google Scholar]
- Rrrhichaii, P.; Fujimei’, Y.; Sukprakarn2, S.; Okudaii, N.; Date’satoshi, S. Flowering Responseto Photoperiod In okra (Abeimoschus esculentus). J. Jpn. Soc. Agric. Technol. Manag. 2004, 11, 33–41. [Google Scholar]
- Nwoke, F.I.O. Effects of Plant Age on Photoperiodic Induction and Development of Flowers and Fruits in Abelmoschus esculentus (L.) Moench. Z. Für Pflanzenphysiol. 1983, 110, 393–400. [Google Scholar] [CrossRef]
- CNRA Répertoire Des Variétés Améliorées de Cultures Vivrières (CNRA Directory of Improved Varieties of Food Crops). Available online: https://ftech.byethost7.com/wp-content/uploads/2022/08/DGN1848.pdf (accessed on 10 May 2024).
- Degni, B.F.; Haba, C.T.; Dibi, W.G.; Soro, D.; Zoueu, J.T. Effect of Light Spectrum on Growth, Development, and Mineral Contents of Okra (Abelmoschus esculentus L.). Open Agric. 2021, 6, 276–285. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter and a Proposed Modification of the Chromic Acid Titration Method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WL, USA, 1982; pp. 159–165. [Google Scholar]
- Al-Ubaydi, R.M.; Al-Shakry, E.F.; Al-Samara, M.A.; Al-Mohmadawy, S.M. Effect of irrigation intervals on growth, flowering and fruits quality of okra Abelmoschus esculentus (L.) Monech. Afr. J. Agric. Res. 2017, 12, 2036–2040. [Google Scholar] [CrossRef]
- Degni, B.F.; Haba, C.T.; Dibi, W.G.; Gbogbo, Y.A.; Niangoran, N.U. Impact of Light Spectrum and Photosynthetic Photon Flux Density on the Germination and Seedling Emergence of Okra. Light. Res. Technol. 2020, 52, 595–606. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and Accumulation of Secondary Metabolites in Perilla as Affected by Photosynthetic Photon Flux Density and Electrical Conductivity of the Nutrient Solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- POWER|DAV. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 18 May 2025).
- Velasco, M.H. Enabling Year-Round Cultivation in the Nordics-Agrivoltaics and Adaptive LED Lighting Control of Daily Light Integral. Agriculture 2021, 11, 1255. [Google Scholar] [CrossRef]
- Blonquist, J.M.; Bugbee, B. Solar, Net, and Photosynthetic Radiation. In Agroclimatology; Wiley: Hoboken, NJ, USA, 2018; pp. 1–49. ISBN 9780891183587. [Google Scholar]
- Sunrise and Sunset in Yamoussoukro, Ivory Coast. Available online: https://www.sunrise-and-sunset.com/fr/sun/cote-d'ivoire/yamoussoukro (accessed on 18 May 2025).
- Wang, X.; Yu, H. Research on Control System of Intelligent Greenhouse of IoT Based on Zigbee. J. Phys. Conf. Ser. 2019, 1345, 042036. [Google Scholar] [CrossRef]
- Danev, V.; Kirilov, L.; Nikolov, R. Creating Smart Home Environment Based on Open Source Home Automation Software. In Proceedings of the ACM International Conference Proceeding Series, Ruse, Bulgaria, 18 June 2021; Association for Computing Machinery: New York, NY, USA; pp. 81–86. [Google Scholar]
- Zhang, H.; Srinivasan, R.; Ganesan, V. Low Cost, Multi-Pollutant Sensing System Using Raspberry Pi for Indoor Air Quality Monitoring. Sustainability 2021, 13, 370. [Google Scholar] [CrossRef]
- ZigBee Communication Protocol. Available online: https://Engocontrols.Com/En/Zigbee-Communication-Protocol/ (accessed on 21 March 2024).
- CC2531USB-RD Reference Design|TI.com. Available online: https://www.ti.com/sitesearch/en-us/docs/universalsearch.tsp?langPref=en-US&nr=27&searchTerm=CC2531USB-RD#q=CC2531USB-RD (accessed on 18 May 2025).
- Koenkk Kanters. Available online: https://Github.Com/Koenkk (accessed on 21 March 2024).
- Froiz-Míguez, I.; Fernández-Caramés, T.M.; Fraga-Lamas, P.; Castedo, L. Design, Implementation and Practical Evaluation of an Iot Home Automation System for Fog Computing Applications Based on MQTT and ZigBee-WiFi Sensor Nodes. Sensors 2018, 18, 266. [Google Scholar] [CrossRef]
- Domoticz. Available online: https://www.domoticz.com/ (accessed on 22 March 2024).
- Brasil, R.; Bot, V. Calculating Germination Measurements and Organizing Spreadsheets. Braz. J. Bot. 2009, 32, 849–855. [Google Scholar]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C.C.; Baskin, J.M. Problems with Using Mean Germination Time to Calculate Rate of Seed Germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Hoyt, P.; Bradfield, R. Effect of Varying Leaf Area by Partial Defoliation and Plant Density on Dry Matter Production in Corn 1. Agron. J. 1962, 54, 523–525. [Google Scholar] [CrossRef]
- Takahata, K.; Mine, Y.; Miura, H. Optimum Emergence Temperature for Okra Seeds. Trop. Agr. Develop. 2011, 55, 93–96. [Google Scholar]
- Limprasitwong, P.; Thongchaisuratkrul, C. Plant Growth Using Automatic Control System under LED, Grow, and Natural Light. In Proceedings of the ICAICTA 2018—5th International Conference on Advanced Informatics: Concepts Theory and Applications, Krabi, Thailand, 14–17 August 2018; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018; pp. 192–195. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2010; Volume 91, pp. 29–66. [Google Scholar]
- Randall, W.C.; Lopez, R.G.; Currey, C.; Gerovac, J.; Mahan, C.; Buschkoetter, J.; Hilligoss, A.; Patz, B. Comparison of Supplemental Lighting from High-Pressure Sodium Lamps and Light-Emitting Diodes during Bedding Plant Seedling Production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, J.; Ju, J.; Hu, Y.; Liu, X.; He, R.; Song, J.; Huang, Y.; Liu, H. The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory. Agronomy 2025, 15, 70. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and Photomorphogenesis of Pepper Plants under Red Light-Emitting Diodes with Supplemental Blue or Far-Red Lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Huber, B.M.; Louws, F.J.; Hernández, R. Impact of Different Daily Light Integrals and Carbon Dioxide Concentrations on the Growth, Morphology, and Production Efficiency of Tomato Seedlings. Front. Plant Sci. 2021, 12, 615853. [Google Scholar] [CrossRef]
- Ji, F.; Wei, S.Q.; Liu, N.; Xu, L.J.; Yang, P. Growth of Cucumber Seedlings in Different Varieties as Affected by Light Environment. Int. J. Agric. Biol. Eng. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Ihlebekk, H.; Eilertsen, S.; Junttila, S.; Grindal, G.; Moe, R. Control of plant height in campanula isophylla by temperature alternations; involvement of gas. Acta Hortic. 1995, 394, 347–355. [Google Scholar] [CrossRef]
- Panţer, E.; Pele, M.; Drăghici, E.M. Influence of illumination with leds on growth and development of lettuce seedlings. Horticulture 2015, LIX, 2285–5653. [Google Scholar]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of Blue and Red Light on Stem Elongation and Flowering of Tomato Seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Kirchhoff, H. Chloroplast Ultrastructure in Plants. New Phytol. 2019, 223, 565–574. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-Regulated Transcriptional Networks in Higher Plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.Y. Vegetative Propagation of Six Pachyphytum Species as Influenced by Different LED Light Qualities. Hortic. Sci. Technol. 2023, 41, 237–249. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The Role of Light-Regulated Auxin Signaling in Root Development. Int. J. Mol. Sci. 2023, 24, 5253. [Google Scholar] [CrossRef]
- Kircher, S.; Schopfer, P. Photosynthetic Sucrose Acts as Cotyledon-Derived Long-Distance Signal to Control Root Growth during Early Seedling Development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef]
- Gil, C.S.; Kwon, S.J.; Jeong, H.Y.; Lee, C.; Lee, O.J.; Eom, S.H. Blue Light Upregulates Auxin Signaling and Stimulates Root Formation in Irregular Rooting of Rosemary Cuttings. Agronomy 2021, 11, 1725. [Google Scholar] [CrossRef]
- Warner, R.M.; Erwin, J.E. Effect of Photoperiod and Daily Light Integral on Flowering of Five Hibiscus sp. Sci. Hortic. 2003, 97, 341–351. [Google Scholar] [CrossRef]
- Usman, M.; Zeeshan, M.; Mehrab, E.; Marium, S.; Sarwar, M.K.S. The Photoperiodic Floral Transition in Cotton (Gossypium hirsutum). Trends Anim. Plant Sci. 2024, 4, 118–132. [Google Scholar] [CrossRef]
- Tenga, A.Z.; Ormrod, D.P. Responses of Okra (Hibiscus esculentus L.) Cultivars to Photoperiod and Temperature. Sci. Hortic. 1985, 27, 177–187. [Google Scholar] [CrossRef]
- DADA, V.A.; Adejumo, S.A. Growth and Yield of Okra (Abelmoschus esculentus Moench) as Influenced by Compost Application under Different Light Intensities. Not. Sci. Biol. 2015, 7, 217–226. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; An, G. The Control of Flowering Time by Environmental Factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Lee, Z.; Kim, S.; Choi, S.J.; Joung, E.; Kwon, M.; Park, H.J.; Shim, J.S. Regulation of Flowering Time by Environmental Factors in Plants. Plants 2023, 12, 3680. [Google Scholar] [CrossRef]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and Identity of Florigen: Flowering Locus T Moves Center Stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Leung, C.C.; Tarté, D.A.; Gendron, J.M. Plants Distinguish Different Photoperiods to Independently Control Seasonal Flowering and Growth. Science 2024, 383, 605. [Google Scholar] [CrossRef] [PubMed]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor Regulation of CONSTANS Protein in Photoperiodic Flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Tanigawa, Y.; Murakami, T.; Araki, T.; Nagatani, A. Phytochrome-Dependent Late-Flowering Accelerates Flowering through Physical Interactions with Phytochrome B and CONSTANS. Proc. Natl. Acad. Sci. USA 2013, 110, 18017–18022. [Google Scholar] [CrossRef]
- Cerdán, P.D.; Chory, J. Regulation of Flowering Time by Light Quality. Nature 2003, 423, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Lin, C. Update on Development Photoreceptors and Regulation of Flowering Time 1. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kadowaki, M.; Che, J.; Takahashi, S.; Horiuchi, N.; Ogiwara, I. Influence of Light Quality on Flowering Characteristics, Potential for Year-Round Fruit Production and Fruit Quality of Blueberry in a Plant Factory. Fruits 2019, 74, 3–10. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.H.; Chung, S.O. Effects of Temperature, Relative Humidity, and Carbon Dioxide Concentration on Growth and Glucosinolate Content of Kale Grown in a Plant Factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef]







| Growth Parameters Under Different Light Treatments | ||||||
|---|---|---|---|---|---|---|
| Lighting Treatments | Plant Height (cm) | Stem Diameter (mm) | Node Number | Leaf Number | LA (cm2) | LAI |
| B | 20 ± 3.2 b | 6 ± 1.20 a | 3.7 ± 0.58 b | 6.3 ± 0.58 a | 83 ± 25 c | 0.5 ± 0.18 b |
| Greenhouse | 31 ± 3 a | 8 ± 1.10 a | 3.3 ± 0.58 b | 6.0 ± 1.00 a | 199 ± 38 a | 1.4 ± 0.33 a |
| Outdoor | 21 ± 1.2 b | 7.9 ± 0.68 a | 3.7 ± 0.58 b | 6.3 ± 0.58 a | 156 ± 25 ab | 0.98 ± 0.06 ab |
| R | 16 ± 1.5 bc | 6 ± 1.50 a | 4.7 ± 0.58 ab | 7.3 ± 0.58 a | 79 ± 22 c | 0.7 ± 0.24 b |
| R3B3 | 12 ± 1.7 c | 7.4 ± 0.38 a | 5.3 ± 0.58 a | 7.7 ± 0.58 a | 102 ± 3.4 bc | 0.8 ± 0.11 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.H.; Degni, B.F.; Dupuis, P.; Canale, L.; Fanny, A.K.; Haba, C.T.; Zissis, G. Growth and Floral Induction in Okra (Abelmoschus esculentus L.) Under Blue and Red LED Light and Their Alternation. Horticulturae 2025, 11, 548. https://doi.org/10.3390/horticulturae11050548
Yao YH, Degni BF, Dupuis P, Canale L, Fanny AK, Haba CT, Zissis G. Growth and Floral Induction in Okra (Abelmoschus esculentus L.) Under Blue and Red LED Light and Their Alternation. Horticulturae. 2025; 11(5):548. https://doi.org/10.3390/horticulturae11050548
Chicago/Turabian StyleYao, Yao Hervé, Banah Florent Degni, Pascal Dupuis, Laurent Canale, Arouna Khalil Fanny, Cissé Théodore Haba, and Georges Zissis. 2025. "Growth and Floral Induction in Okra (Abelmoschus esculentus L.) Under Blue and Red LED Light and Their Alternation" Horticulturae 11, no. 5: 548. https://doi.org/10.3390/horticulturae11050548
APA StyleYao, Y. H., Degni, B. F., Dupuis, P., Canale, L., Fanny, A. K., Haba, C. T., & Zissis, G. (2025). Growth and Floral Induction in Okra (Abelmoschus esculentus L.) Under Blue and Red LED Light and Their Alternation. Horticulturae, 11(5), 548. https://doi.org/10.3390/horticulturae11050548








