Hydroponic Wastewater Treatment with Microalgae: A Sustainable Alternative for Irrigating Pelargonium × hortorum
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Production
2.2. Pelargonium Experiment Facilities
2.3. Plant Material
2.4. Treatments
2.5. Sampling and Analyses
2.5.1. Water Analysis
2.5.2. Biometric Parameters
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Irrigation Water Quality
3.1.1. Irrigation Water Analysis
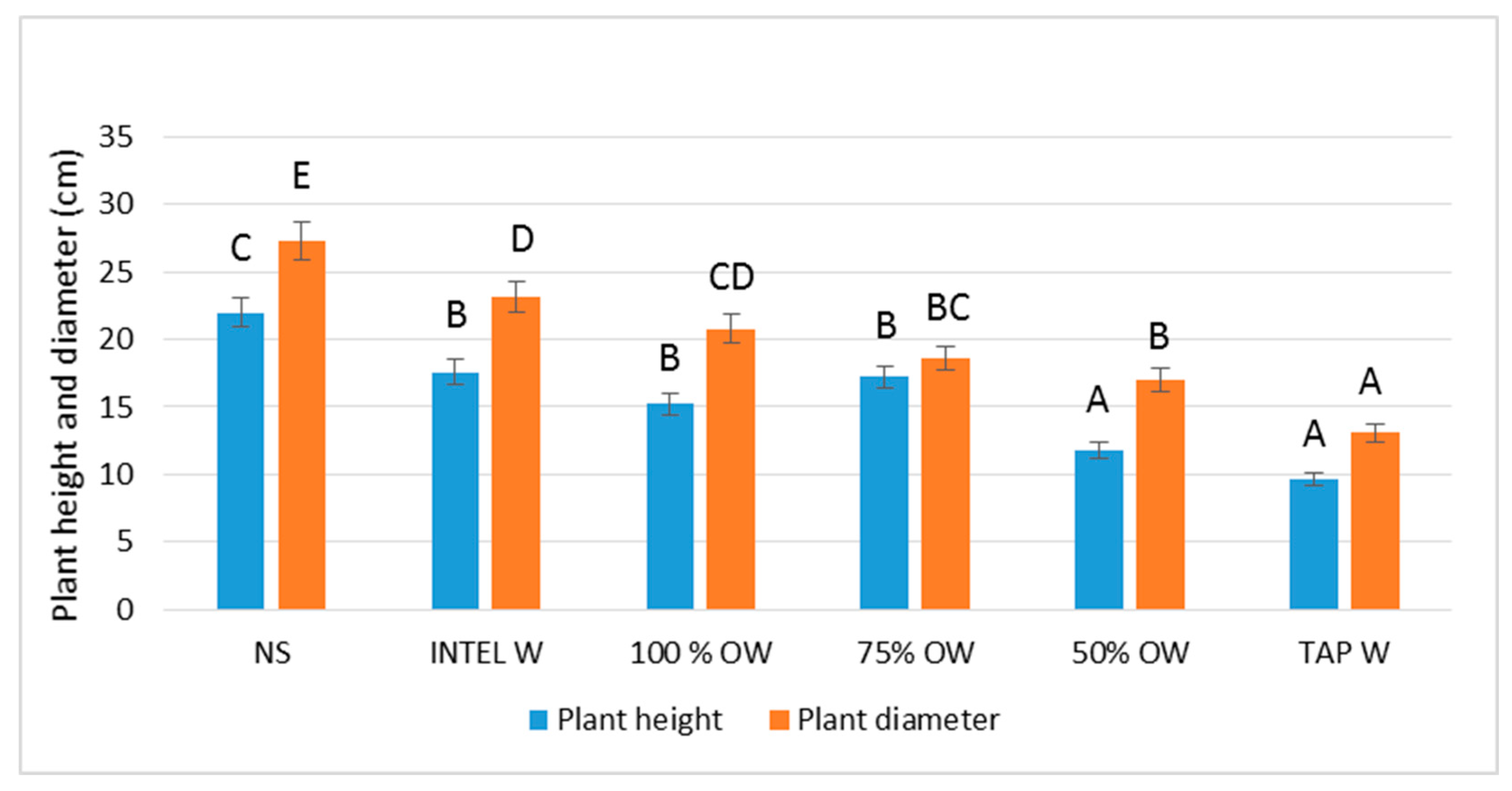
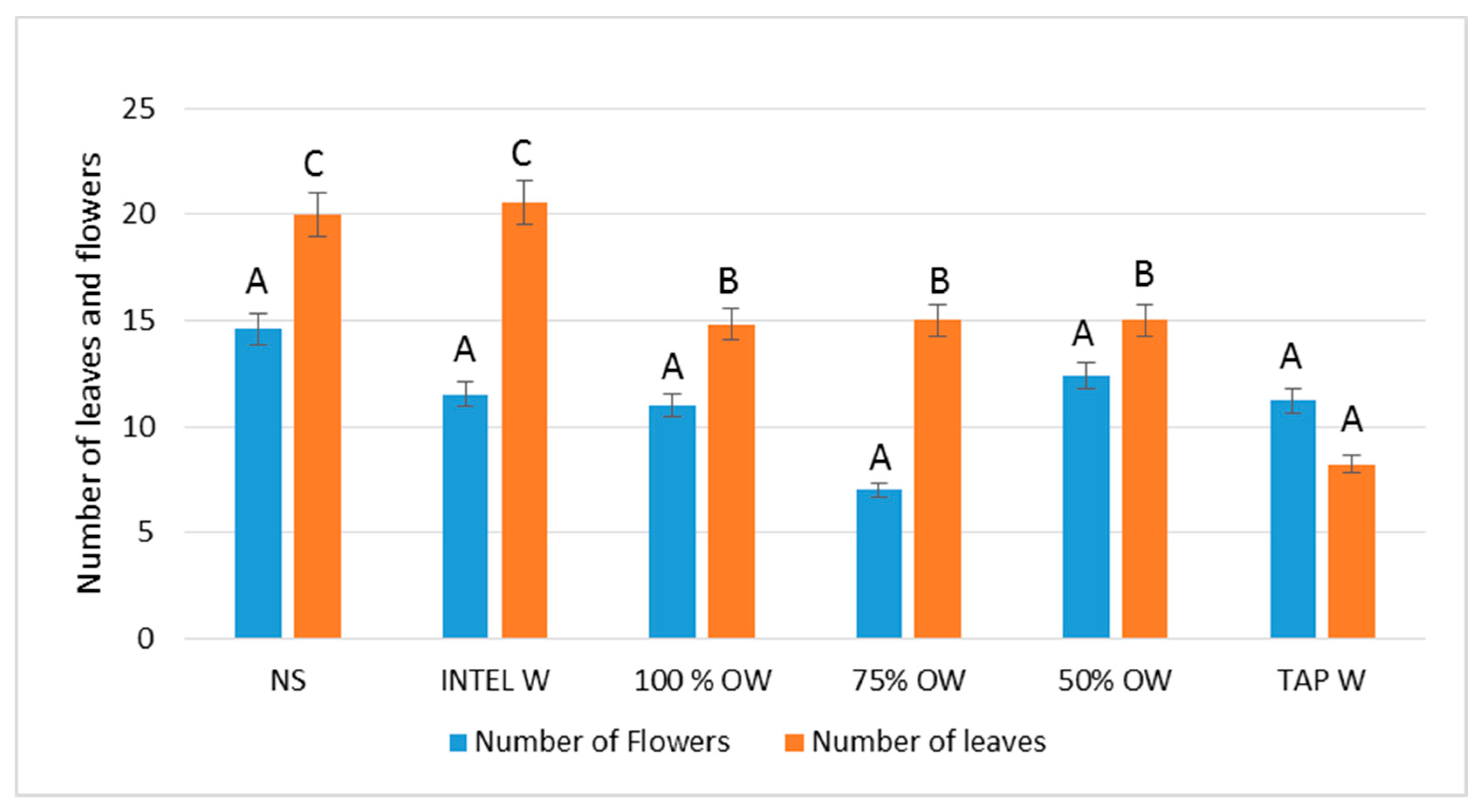
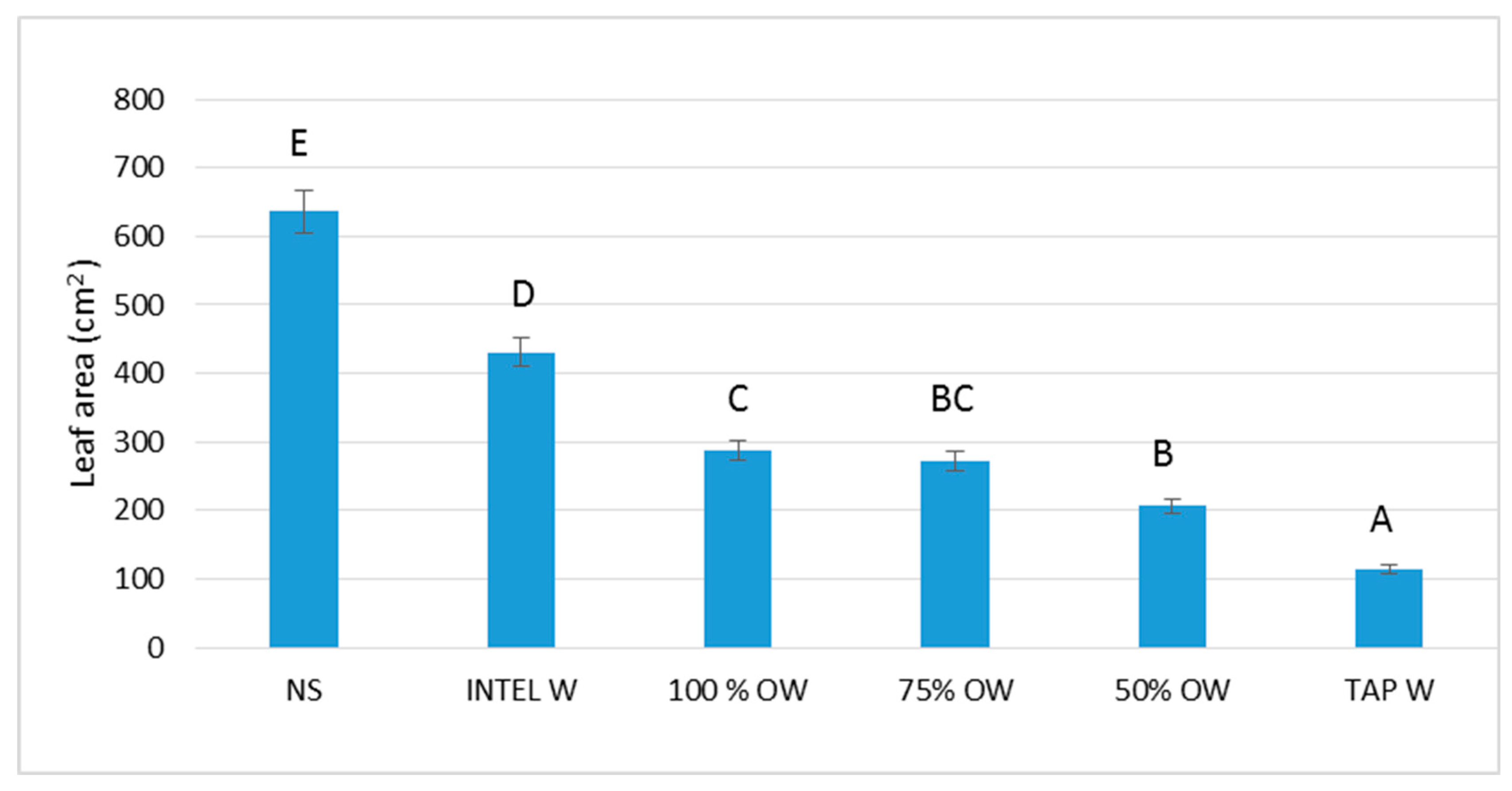
3.1.2. Plant Biometric Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Renganathan, P.; Puente, E.O.R.; Sukhanova, N.V.; Gaysina, L.A. Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture. BioTech 2024, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.; Prajapati, S.K. Use of Microalgal-Fungal Pellets for Hydroponics Effluent Recycling and High-Value Biomass Production. Heliyon 2024, 10, e37539. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Balasubramanian, P. Enhancing Nutrient Removal from Hydroponic Effluent with Simultaneous Production of Lipid-Rich Biomass through Mixotrophic Cultivation of Microalgae. J. Appl. Phycol. 2025, 37, 957–971. [Google Scholar] [CrossRef]
- Pekkoh, J.; Wichaphian, A.; Kamngoen, A.; Sriket, N.; Zin, M.T.; Lomakool, S.; Maneechote, W.; Chromkaew, Y.; Pathom-aree, W.; Cheirsilp, B.; et al. Heterotrophic Upcycling of Hydroponic Wastewater Supplemented with Glucose and Indole-3-Acetic Acid into High-Quality Chlorella Biomass for Zero-Waste Multiproduct Microalgal Biorefinery. Environ. Technol. Innov. 2024, 36, 103813. [Google Scholar] [CrossRef]
- Yolanda, Y.D.; Kim, S.; Sohn, W.; Shon, H.K.; Yang, E.; Lee, S. Simultaneous Nutrient-Abundant Hydroponic Wastewater Treatment, Direct Carbon Capture, and Bioenergy Harvesting Using Microalgae–Microbial Fuel Cells. Desalination Water Treat. 2025, 321, 100941. [Google Scholar] [CrossRef]
- Faliagka, S.; Kountrias, G.; Dimitriou, E.; Álvarez-Gil, M.; Blanco-Vieites, M.; Magrassi, F.; Notari, M.; Pechlivani, E.M.; Katsoulas, N. Development of a Greenhouse Wastewater Stream Utilization System for On-Site Microalgae-Based Biostimulant Production. AgriEngineering 2024, 6, 1898–1923. [Google Scholar] [CrossRef]
- Sriket, N.; Wichaphian, A.; Kamngoen, A.; Pekkoh, J.; Chromkaew, Y.; Pathom-aree, W.; Maneechote, W.; Cheirsilp, B.; Srinuanpan, S. Hydroponic Effluent Recycling and Resource Recovery Using Heterotrophic Chlorella Microalgae: Advancing a Circular Bioeconomy. J. Water Process Eng. 2024, 67, 106176. [Google Scholar] [CrossRef]
- Rápalo-Cruz, A.; Gómez-Serrano, C.; González-López, C.V.; Morillas-España, A.; Jiménez-Becker, S. Utilization of Treated Wastewater Derived from Microalgae Production for the Irrigation of Horticultural Crops. J. Appl. Phycol. 2024, 36, 1259–1268. [Google Scholar] [CrossRef]
- Rápalo-Cruz, A.; Gómez-Serrano, C.; González-López, C.V.; Hassanpouraghdam, M.B.; Jiménez-Becker, S. Alterations in the Growth Responses of Pelargonium × Hortorum Irrigated with Microalgae Production Wastewater. Horticulturae 2024, 10, 921. [Google Scholar] [CrossRef]
- Rivera-Sánchez, E.; Villaró-Cos, S.; Jiménez-Becker, S.; Rapalo-Cruz, A.; Lafarga, T. Biostimulant Effect of a Novel Seawater-Adapted Strain of Scenedesmus Almeriensis on Garden Geranium. Algal Res. 2025, 86, 103918. [Google Scholar] [CrossRef]
- Tejada-Ruiz, S.; Gonzalez-Lopez, C.; Rojas, E.; Jiménez-Becker, S. Effect of the Foliar Application of Microalgae Hydrolysate (Arthrospira Platensis) and Silicon on the Growth of Pelargonium Hortorum L.H. Bailey under Salinity Conditions. Agronomy 2020, 10, 1713. [Google Scholar] [CrossRef]
- Giuffrida, F.; Rouphael, Y.; Toscano, S.; Scuderi, D.; Romano, D.; Rivera, C.M.; Colla, G.; Leonardi, C. A Simple Model for Nondestructive Leaf Area Estimation in Bedding Plants. Photosynthetica 2011, 49, 380. [Google Scholar] [CrossRef]
- Wen, B.; Xiao, W.; Mu, Q.; Li, D.; Chen, X.; Wu, H.; Li, L.; Peng, F. How Does Nitrate Regulate Plant Senescence? Plant Physiol. Biochem. 2020, 157, 60–69. [Google Scholar] [CrossRef]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An Overview of Important Enzymes Involved in Nitrogen Assimilation of Plants; Humana: New York, NY, USA, 2020; pp. 1–13. [Google Scholar]
- Guan, C.; Zhang, D.; Chu, C. Interplay of Light and Nitrogen for Plant Growth and Development. Crop J. 2025. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between Nitrate and Ammonium in Their Uptake, Allocation, Assimilation, and Signaling in Plants. J. Exp. Bot. 2016, 68, 2501–2512. [Google Scholar] [CrossRef]
- Fan, H.; Quan, S.; Qi, S.; Xu, N.; Wang, Y. Novel Aspects of Nitrate Regulation in Arabidopsis. Front. Plant Sci. 2020, 11, 574246. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Dar, T.A.; Uddin, M.; Ali, A.; Khan, M.M.A.; ul Hassan Dar, T. Understanding the Dynamics of Phosphorus Starvation and Plant Growth. In Essential Plant Nutrients; Springer International Publishing: Cham, Switzerland, 2017; pp. 147–154. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Ródenas, R.; Lara, A.; Martínez, V.; Rubio, F. The Combination of K + Deficiency with Other Environmental Stresses: What Is the Outcome? Physiol. Plant 2019, 165, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent Advances and Perspectives in the Treatment of Hydroponic Wastewater: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Saxena, P.; Bassi, A. Removal of Nutrients from Hydroponic Greenhouse Effluent by Alkali Precipitation and Algae Cultivation Method. J. Chem. Technol. Biotechnol. 2013, 88, 858–863. [Google Scholar] [CrossRef]
- Žitnik, M.; Šunta, U.; Godič Torkar, K.; Krivograd Klemenčič, A.; Atanasova, N.; Griessler Bulc, T. The Study of Interactions and Removal Efficiency of Escherichia Coli in Raw Blackwater Treated by Microalgae Chlorella Vulgaris. J. Clean. Prod. 2019, 238, 117865. [Google Scholar] [CrossRef]
- Kumar, R.R.; Cho, J.Y. Reuse of Hydroponic Waste Solution. Environ. Sci. Pollut. Res. 2014, 21, 9569–9577. [Google Scholar] [CrossRef] [PubMed]
- Fascella, G.; Mammano, M.M.; Rouphael, Y.; Cirillo, C. Agronomical and Physiological Responses of Containerized Ornamentals to Salinity Induced by Major Nutrients. Acta Hortic. 2017, 1170, 635–642. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Ibrahimi, M.-K.; Feki, N.; Lhomme, J.-P.; Bouri, S. Soil Salinisation and Irrigation Management of Date Palms in a Saharan Environment. Environ. Monit. Assess. 2016, 188, 497. [Google Scholar] [CrossRef] [PubMed]
- LeBude, A.V.; Owen, J.S.; Holmes, C. High pH, Low Alkalinity Pond Water Used for Overhead Irrigation Does Not Affect Plant Growth of Select Flowering Shrubs1. J. Environ. Hortic. 2021, 39, 22–32. [Google Scholar] [CrossRef]
- Kingsta, R.M.; Saumi, A.S.; Saranya, P. Design and Construction of Arduino Based PH Control System for Household Waste Water Reuse. In Proceedings of the 2019 3rd International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 23–25 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1037–1041. [Google Scholar]
- Sousa, J.F.; Amaro, H.M.; Ribeirinho-Soares, S.; Esteves, A.F.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M. Native Microalgae-Bacteria Consortia: A Sustainable Approach for Effective Urban Wastewater Bioremediation and Disinfection. Microorganisms 2024, 12, 1421. [Google Scholar] [CrossRef]
- Ljumović, K.; Betterle, N.; Baietta, A.; Ballottari, M. Valorization of Wastewater from Industrial Hydroponic Cultivations Using the Microalgal Species Chlorella Vulgaris. Algal Res. 2024, 81, 103570. [Google Scholar] [CrossRef]
- Rápalo-Cruz, A.; Gómez-Serrano, C.; González-López, C.V.; Morillas-España, A.; Jiménez-Becker, S. Driving Sustainability: Reusing Microalgae Wastewater to Grow Pelargonium × hortorum. Commun. Soil Sci. Plant Anal. 2025, 56, 1586–1598. [Google Scholar] [CrossRef]
- Bonomelli, C.; de Freitas, S.T.; Aguilera, C.; Palma, C.; Garay, R.; Dides, M.; Brossard, N.; O’Brien, J.A. Ammonium Excess Leads to Ca Restrictions, Morphological Changes, and Nutritional Imbalances in Tomato Plants, Which Can Be Monitored by the N/Ca Ratio. Agronomy 2021, 11, 1437. [Google Scholar] [CrossRef]
- Utkin, A.; Ermolova, L.; Utkina, I.; Dulepova, N.; Rosbakh, S. Utkin et al.’s Dataset on Specific Leaf Area. Ecology 2022, 103, e3714. [Google Scholar] [CrossRef]
- van Iersel, M.W.; Weaver, G.; Legendre, R.; Kim, C. Converting Light into Biomass: Quantifying the Conversion Efficiency. Acta Hortic. 2022, 1337, 65–72. [Google Scholar] [CrossRef]
- Nakata, M.T.; Nakao, M.; Denda, A.; Onoda, Y.; Ueda, H.; Demura, T. Estimating the Flexural Rigidity of Arabidopsis Inflorescence Stems: Free-Vibration Test vs. Three-Point Bending Test. Plant Biotechnol. 2020, 37, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ratkowsky, D.A.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf Fresh Weight Versus Dry Weight: Which Is Better for Describing the Scaling Relationship between Leaf Biomass and Leaf Area for Broad-Leaved Plants? Forests 2019, 10, 256. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, A.K.; Jiang, Z.; Chen, G.; Feng, B.; Wang, Z.; Li, H.; Si, J.; Zhang, B.; Bi, X.; et al. Nitrogen Fertilizer Reduction and Postponing for Improving Plant Photosynthetic Physiological Characteristics to Increase Wheat-Maize and Annual Yield and Economic Return. Sci. Agric. Sin. 2024, 57, 868–884. [Google Scholar]
- Aanderud, Z.T.; Bledsoe, C.S.; Richards, J.H. Contribution of Relative Growth Rate to Root Foraging by Annual and Perennial Grasses from California Oak Woodlands. Oecologia 2003, 136, 424–430. [Google Scholar] [CrossRef]



| NS | INTEL W | 100%OW | 75%OW | 50%OW | TAP W | |
|---|---|---|---|---|---|---|
| pH | 5.30 | 6.40 | 8.50 | 8.70 | 9.00 | 9.40 |
| EC dSm−1 | 2.30 | 3.40 | 3.75 | 2.86 | 2.20 | 0.40 |
| NO3− ppm | 634.86 | 418.24 | 151.27 | 114.42 | 77.67 | 2.24 |
| NH4+ ppm | 34.05 | 16.10 | 0.83 | 0.62 | 0.35 | 0.02 |
| PO43− ppm | 188.70 | 154.75 | 22.29 | 17.28 | 11.46 | 0.02 |
| K+ ppm | 304.61 | 146.92 | 139.34 | 105.52 | 71.58 | 2.48 |
| Ca2+ ppm | 97.02 | 106.28 | 73.18 | 63.04 | 52.54 | 29.11 |
| Mg2+ ppm | 10.49 | 75.34 | 79.35 | 62.92 | 46.14 | 12.04 |
| SO42− ppm | 89.60 | 258.61 | 304.82 | 230.67 | 158.24 | 12.66 |
| Cl− ppm | 146.99 | 326.57 | 510.29 | 423.25 | 228.09 | 158.90 |
| Na+ ppm | 84.91 | 189.14 | 296.31 | 250.00 | 191.00 | 85.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rápalo-Cruz, A.; Gómez-Serrano, C.; González-López, C.V.; Urrestarazu-Gavilán, M.; Jiménez-Becker, S. Hydroponic Wastewater Treatment with Microalgae: A Sustainable Alternative for Irrigating Pelargonium × hortorum. Horticulturae 2025, 11, 547. https://doi.org/10.3390/horticulturae11050547
Rápalo-Cruz A, Gómez-Serrano C, González-López CV, Urrestarazu-Gavilán M, Jiménez-Becker S. Hydroponic Wastewater Treatment with Microalgae: A Sustainable Alternative for Irrigating Pelargonium × hortorum. Horticulturae. 2025; 11(5):547. https://doi.org/10.3390/horticulturae11050547
Chicago/Turabian StyleRápalo-Cruz, Alejandro, Cintia Gómez-Serrano, Cynthia Victoria González-López, Miguel Urrestarazu-Gavilán, and Silvia Jiménez-Becker. 2025. "Hydroponic Wastewater Treatment with Microalgae: A Sustainable Alternative for Irrigating Pelargonium × hortorum" Horticulturae 11, no. 5: 547. https://doi.org/10.3390/horticulturae11050547
APA StyleRápalo-Cruz, A., Gómez-Serrano, C., González-López, C. V., Urrestarazu-Gavilán, M., & Jiménez-Becker, S. (2025). Hydroponic Wastewater Treatment with Microalgae: A Sustainable Alternative for Irrigating Pelargonium × hortorum. Horticulturae, 11(5), 547. https://doi.org/10.3390/horticulturae11050547









