Effect of Plant Growth-Promoting Rhizobacteria Synthetic Consortium on Growth, Yield, and Metabolic Profile of Lettuce (Lactuca sativa L.) Grown Under Suboptimal Nutrient Regime
Abstract
1. Introduction
2. Materials and Methods
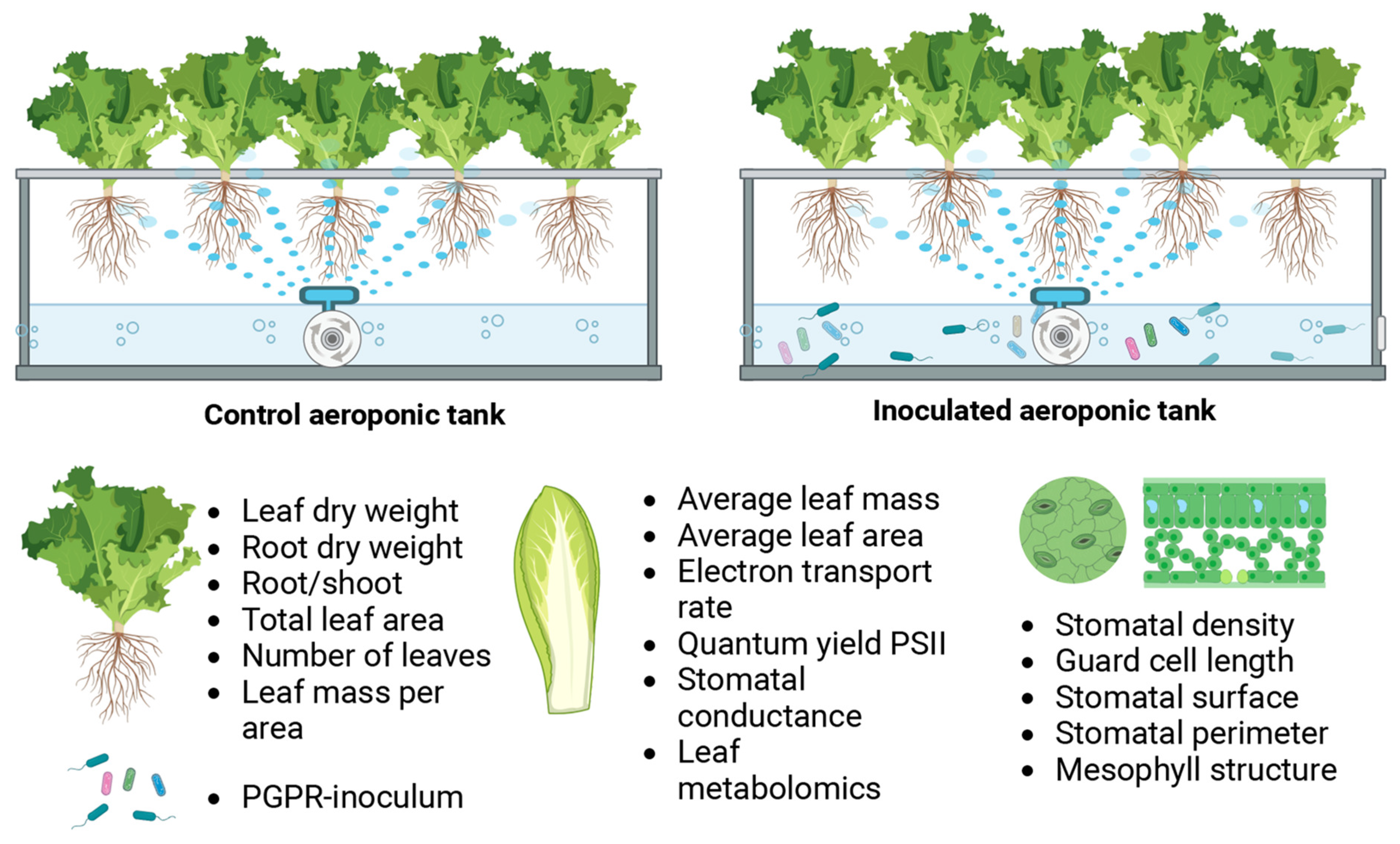
2.1. Experimental Design
2.2. Treatment with the PGPR Synthetic Consortium
2.3. Plant Material Collection
2.4. Plant Biomass Determination and Plant Structure Analysis
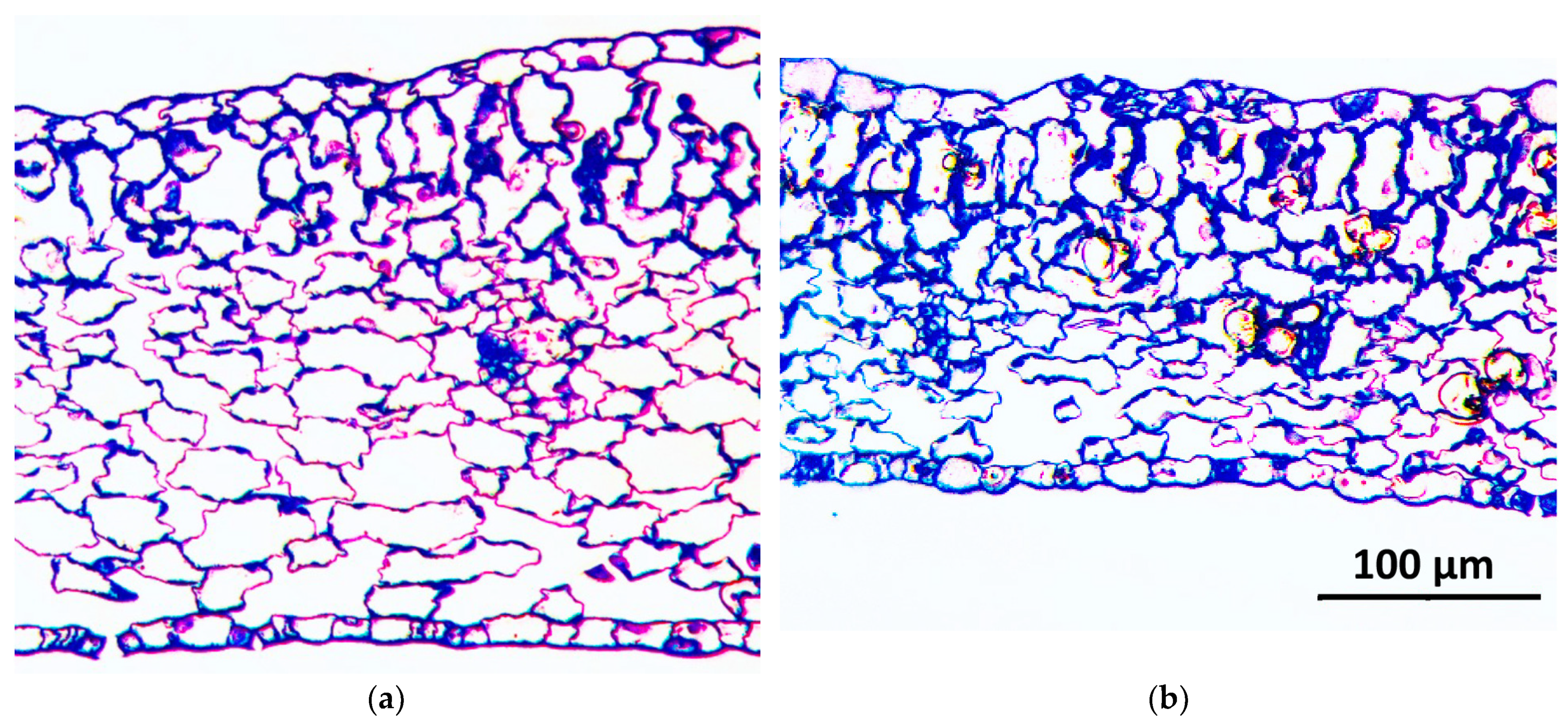
2.5. Leaf Anatomical Analysis
2.6. Leaf Physiological Measurements and Net Assimilation Rate
2.7. Leaf C and N Content Determination
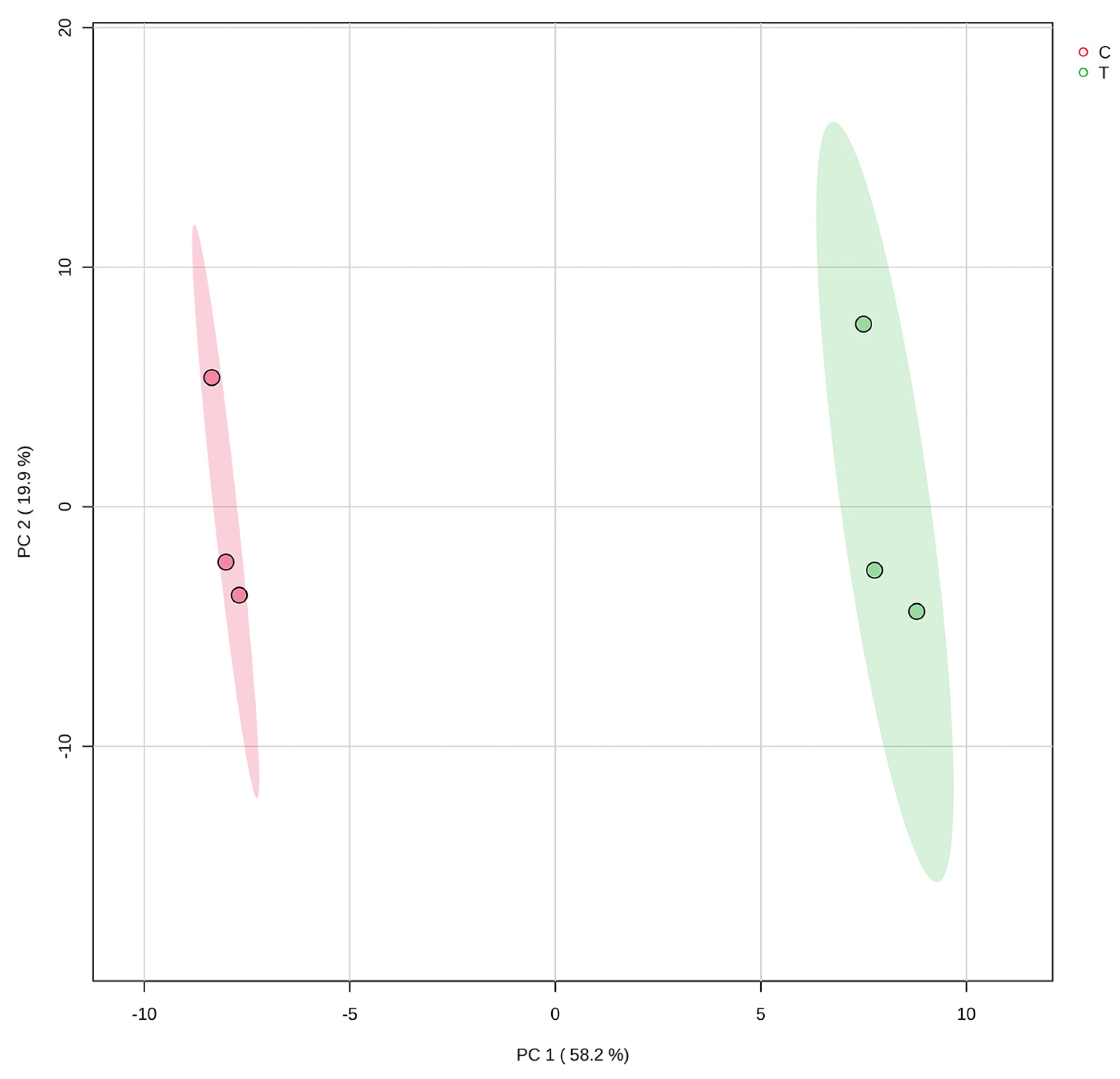
2.8. Untargeted Metabolomics
2.9. Statistical Analysis
3. Results
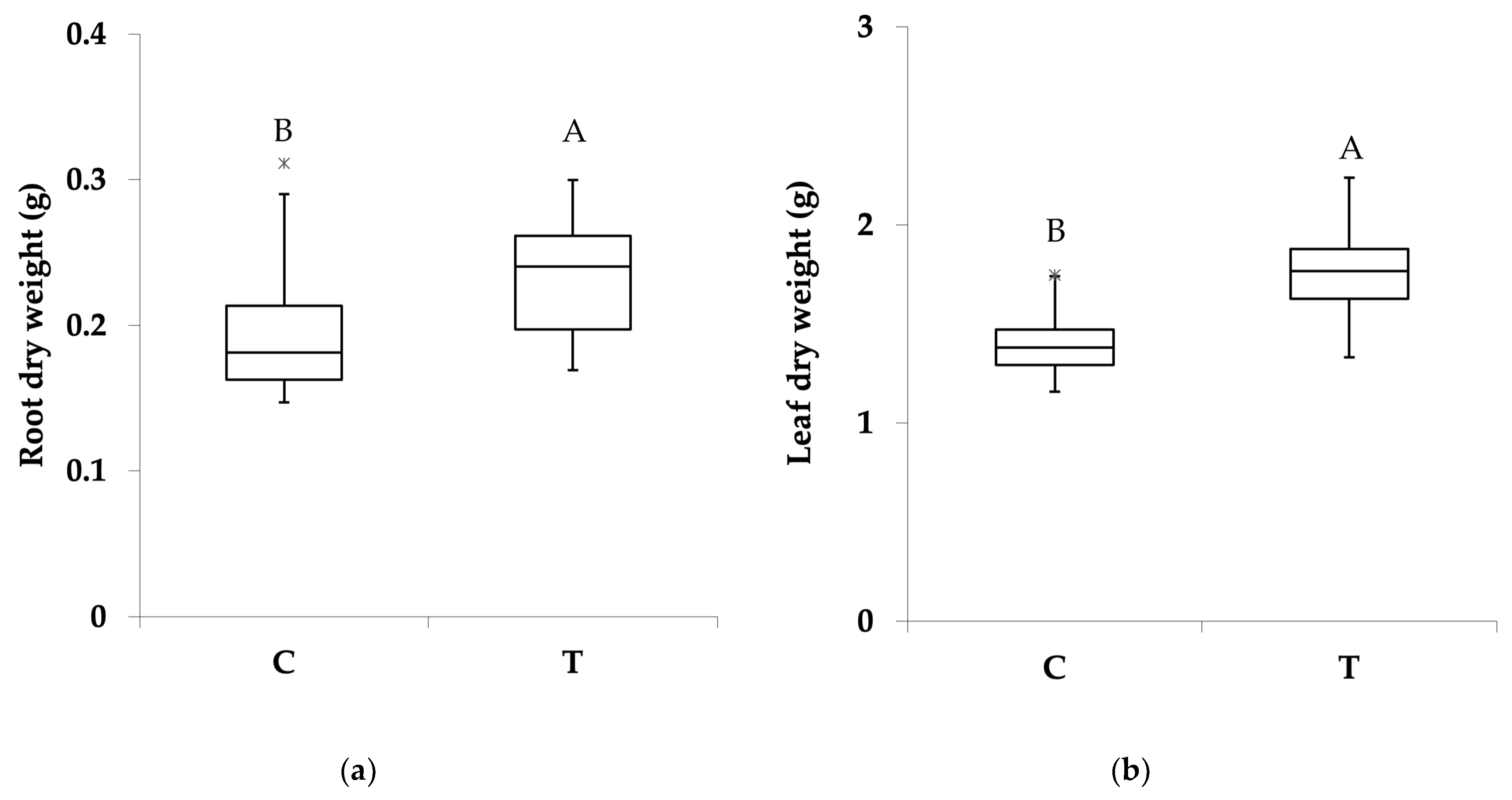
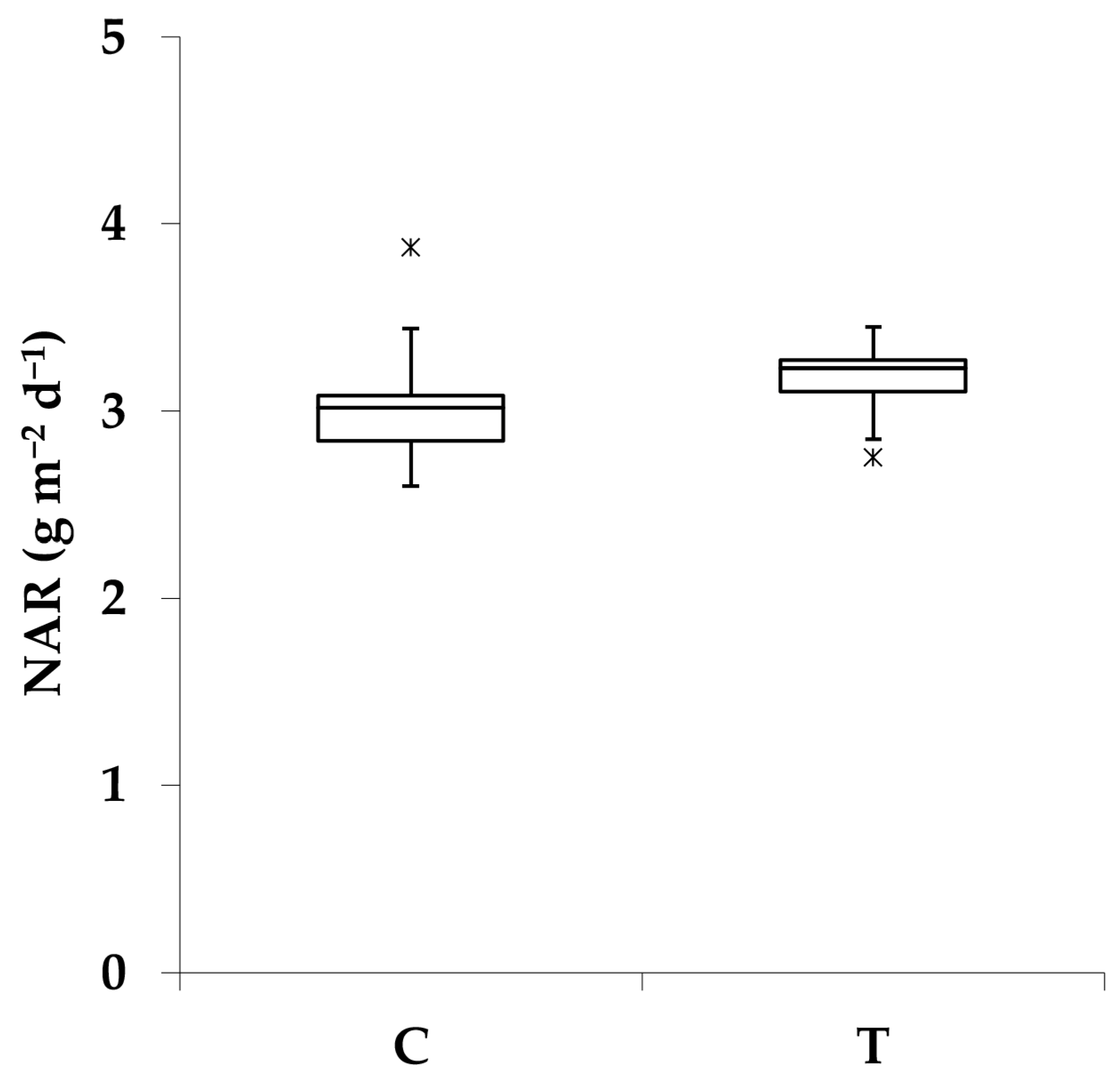
3.1. Effect of PGPR Consortium on Biomass Production and Plant Structure
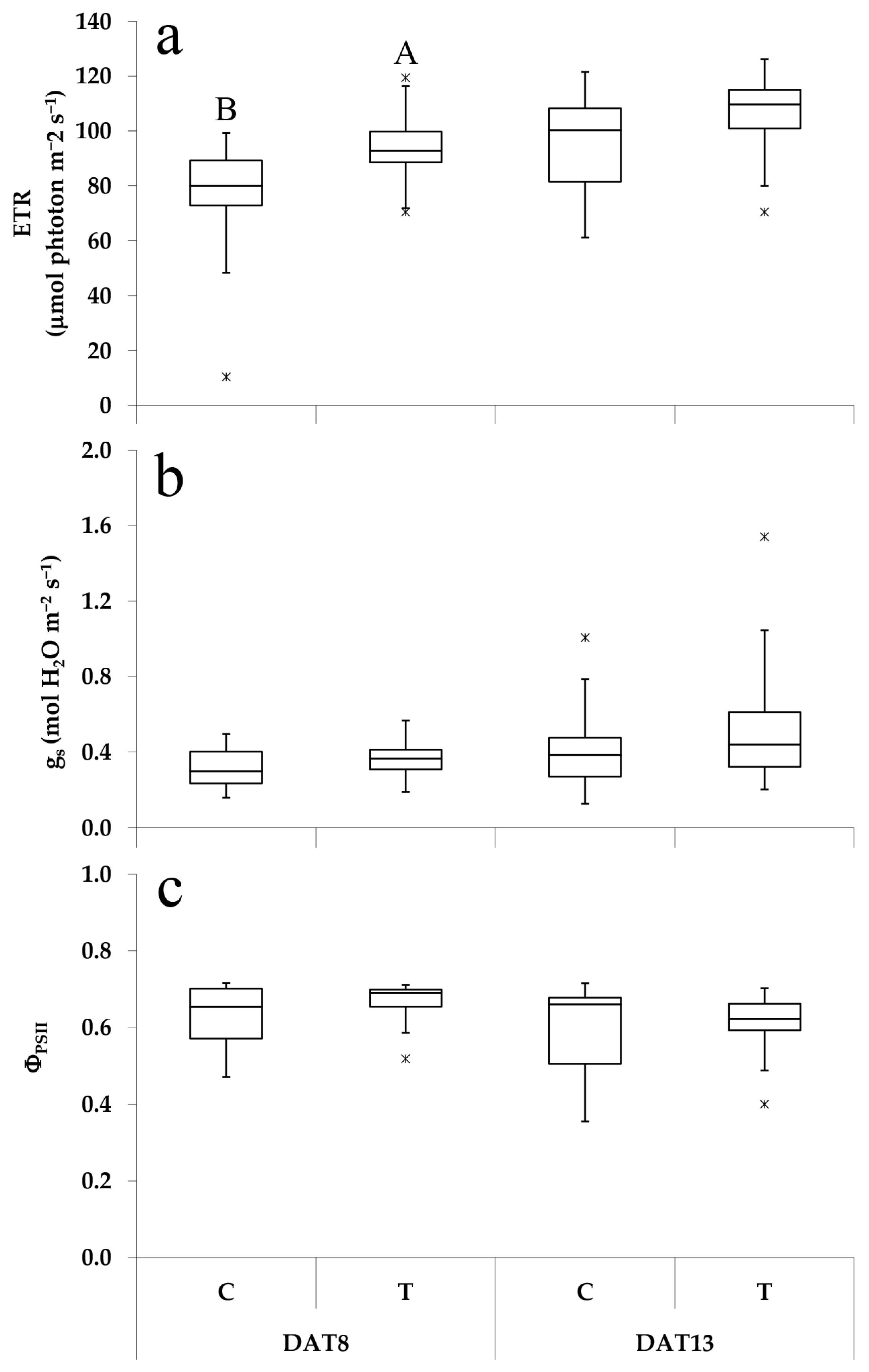
3.2. Effect of PGPR Consortium on Leaf Physiological Responses
3.3. Effect of PGPR on Leaf Carbon (C) and Nitrogen (N) Contents
3.4. Effect of PGPR Consortium on Leaf Anatomy
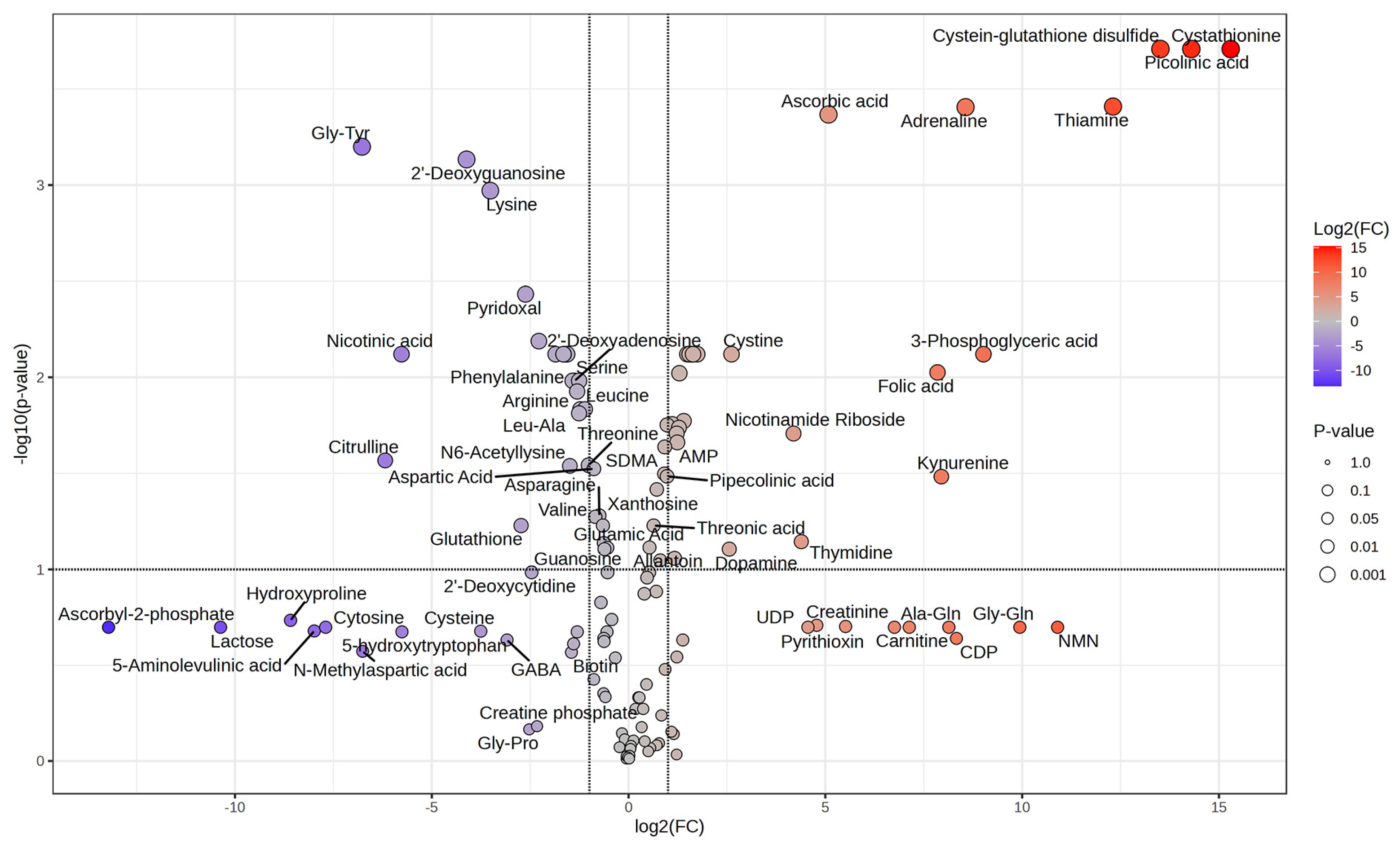
3.5. Effect of PGPR Consortium on Leaf Metabolome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point; Synthesis Report 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- OECD; FAO. Food Security and Trade 2023; OECD: Paris, France; FAO: Rome, Italy, 2023. [Google Scholar]
- Ortiz, A.; Sansinenea, E. Recent advancements for microorganisms and their natural compounds useful in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 891–897. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Abraham, P.E.; Michener, J.K.; Muchero, W.; Cregger, M.A. Ecosystem consequences of introducing plant growth promoting rhizobacteria to managed systems and potential legacy effects. New Phytol. 2022, 234, 1914–1918. [Google Scholar] [CrossRef]
- Ikiz, B.; Dasgan, H.Y.; Gruda, N.S. Utilizing the power of plant growth promoting rhizobacteria on reducing mineral fertilizer, improved yield, and nutritional quality of Batavia lettuce in a floating culture. Sci. Rep. 2024, 14, 1616. [Google Scholar] [CrossRef] [PubMed]
- Fürnkranz, M.; Wanek, W.; Richter, A.; Abell, G.; Rasche, F.; Sessitsch, A. Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J. 2008, 2, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, A.S.; Bonatelli, M.L.; Chia, M.A.; Peressim, L.; Quecine, M.C. Opposite sides of Pantoea agglomerans and its associated commercial outlook. Microorganisms 2022, 10, 2072. [Google Scholar] [CrossRef] [PubMed]
- Raymond, N.S.; Gómez-Muñoz, B.; van der Bom, F.J.T.; Nybroe, O.; Jensen, L.S.; Müller-Stöver, D.S.; Oberson, A.; Richardson, A.E. Phosphate-solubilising microorganisms for improved crop productivity: A critical assessment. New Phytol. 2021, 229, 1268–1277. [Google Scholar] [CrossRef]
- Wang, Y.P.; Huang, Y.; Augusto, L.; Goll, D.S.; Helfenstein, J.; Hou, E. Toward a global model for soil inorganic phosphorus dynamics: Dependence of exchange kinetics and soil bioavailability on soil physicochemical properties. Glob. Biogeochem. Cycles 2022, 36, e2021GB007061. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, P.; Sirari, A.; Singh, I.; Gill, B.S.; Singh, U.; Saharan, K. Synergism of Pseudomonas aeruginosa (LSE-2) nodule endophyte with Bradyrhizobium sp. (LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World J. Microbiol. Biotechnol. 2019, 35, 47. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar] [CrossRef]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Wang, B.; Mei, C.; Seiler, J.R. Early growth promotion and leaf level physiology changes in Burkholderia phytofirmans strain PsJN inoculated switchgrass. Plant Physiol. Biochem. 2015, 86, 16–23. [Google Scholar] [CrossRef]
- Bisht, S.; Singh, S.; Singh, M.; Sharma, J.G. Augmentative role of Piriformospora indica fungus and plant growth promoting bacteria in mitigating salinity stress in Trigonella foenum-graecum. J. Appl. Biol. Biotechnol. 2022, 10, 85–94. [Google Scholar]
- Mantelin, S.; Desbrosses, G.; Larcher, M.; Tranbarger, T.J.; Cleyet-Marel, J.C.; Touraine, B. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta 2006, 223, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Bellini, C.; Kowalczyk, M.; Touraine, B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 2010, 232, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Ait Barka, E.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In Plant Hormones; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. [Google Scholar]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Gallei, M.; Luschnig, C.; Friml, J. Auxin signalling in growth: Schrödinger’s cat out of the bag. Curr. Opin. Plant Biol. 2020, 53, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Blázquez, M.A.; Sansinenea, E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef] [PubMed]
- Shahid, R.; Kumar, R.; Altaf, M.M.; Kumar, A.; Khan, L.U.; Saqib, M.; Nawaz, M.A.; Saddiq, B.; Bahadur, S.; Tiwari, R.K.; et al. Phytohormones mediated modulation of abiotic stress tolerance and potential crosstalk in horticultural crops. J. Plant Growth Regul. 2023, 42, 4724–4750. [Google Scholar]
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Batlang, U.; Emongor, V.E.; Pule-Meulenburg, F. Effect of benzyladenine plus gibberellins and gibberellic acid on yield and yield components of cucumber (Cucumis sativus L. cv. ‘tempo’). J. Agron. 2006, 5, 418–423. [Google Scholar]
- Li, Y.; Li, H.; Han, X.; Han, G.; Xi, J.; Liu, Y.; Zhang, Y.; Xue, Q.; Guo, Q.; Lai, H. Actinobacteria biofertilizer improves the yields of different plants and alters the assembly processes of rhizosphere microbial communities. Appl. Soil. Ecol. 2022, 171, 104345. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated microbial consortia perform better than single strains in living soil: A meta-analysis. Appl. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Tholen, D.; Zhu, X.-G. The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol. 2011, 156, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Weraduwage, S.M.; Sharkey, T.D. Prospects for enhancing leaf photosynthetic capacity by manipulating mesophyll cell morphology. J. Exp. Bot. 2019, 70, 1153–1165. [Google Scholar] [CrossRef]
- Clarke, V.C.; Danila, F.R.; von Caemmerer, S. CO2 diffusion in tobacco: A link between mesophyll conductance and leaf anatomy. Interface Focus 2021, 11, 20200040. [Google Scholar] [CrossRef] [PubMed]
- Abou Jaoudé, R.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. A Plant’s perception of growth-promoting bacteria and their metabolites. Front. Plant Sci. 2024, 14, 1332864. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci. 2017, 8, 674. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Shi, Q.; Liu, X.; Yang, Z.; Han, P.; Li, J.; Wei, Z.; Hu, T.; Liu, F. The interactive effects of deficit irrigation and Bacillus pumilus inoculation on growth and physiology of tomato plant. Plants 2023, 12, 670. [Google Scholar] [CrossRef]
- Larcher, M.; Muller, B.; Mantelin, S.; Rapior, S.; Cleyet-Marel, J.-C. Early modifications of Brassica napus root system architecture induced by a plant growth-promoting Phyllobacterium strain. New Phytol. 2003, 160, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Knoth, J.L.; Kim, S.H.; Ettl, G.J.; Doty, S.L. Effects of cross host species inoculation of nitrogen-fixing endophytes on growth and leaf physiology of maize. GCB Bioenergy 2013, 5, 408–418. [Google Scholar] [CrossRef]
- Lamont, B.B.; Pérez-Fernández, M.; Rodríguez-Sánchez, J. Soil bacteria hold the key to root cluster formation. New Phytol. 2015, 206, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- del Rosario Cappellari, L.; Santoro, M.V.; Reinoso, H.; Travaglia, C.; Giordano, W.; Banchio, E. Anatomical, morphological, and phytochemical effects of inoculation with plant growth- promoting rhizobacteria on peppermint (Mentha piperita). J. Chem. Ecol. 2015, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Saryanah, N.A.; Sulastri; Himawati, S.; Bidara, I.S.; Roswanjaya, Y.P.; Asiani, N.; Sukmadi, R.B.; Irawati, A.F.C. Salinity stress mitigation on Zea mays L. seedling by halotolerant bacteria. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1160, p. 012004. [Google Scholar]
- Yankey, R.K.; Karanja, J.K.; Okal, E.J.; Omoor, I.; Lin, H.; Bodjremou, D.M.; Li, J.; Lin, D.; Cao, X.M.; Lin, Z.X. A consortium of plant growth-promoting rhizobacteria strains synergistically assists jujuncao (Pennisetum giganteum) to remediate cadmium contaminated soils. Appl. Ecol. Environ. Res. 2021, 19, 2425–2442. [Google Scholar] [CrossRef]
- Patel, M.; Vurukonda, S.S.K.P.; Patel, A. Multi-trait halotolerant plant growth-promoting bacteria mitigate induced salt stress and enhance growth of Amaranthus viridis. J. Soil Sci. Plant Nutr. 2023, 23, 1860–1883. [Google Scholar] [CrossRef]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.M.L.M.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Topalcengiz, Z.; Chandran, S.; Gibson, K.E. A comprehensive examination of microbial hazards and risks during indoor soilless leafy green production. Int. J. Food Microbiol. 2024, 411, 110546. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Ikiz, B.; Dasgan, H.Y.; Balik, S.; Kusvuran, S.; Gruda, N.S. The use of biostimulants as a key to sustainable hydroponic lettuce farming under saline water stress. BMC Plant Biol. 2024, 24, 808. [Google Scholar] [CrossRef] [PubMed]
- Baset, M.M.; Shamsuddin, Z.H.; Wahab, Z.; Marziah, M. Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured musa plantlets under nitrogen-free hydroponics condition. Aust. J. Crop Sci. 2010, 4, 85–90. [Google Scholar]
- Tkachenko, O.V.; Evseeva, N.V.; Terentyeva, E.V.; Burygin, G.L.; Shirokov, A.; Burov, A.; Matora, L.Y.; Shchyogolev, S.Y. Improved production of high-quality potato seeds in aeroponics with plant-growth-promoting rhizobacteria. Potato Res. 2021, 64, 55–66. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2020, 275, 109733. [Google Scholar] [CrossRef]
- Gravel, V.; Martinez, C.; Antoun, H.; Tweddell, R.J. Control of greenhouse tomato root rot [Pythium ultimum] in hydroponic systems, using plant-growth-promoting microorganisms. Can. J. Plant Pathol. 2006, 28, 475–483. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, K.; He, X.; Li, S.-Q.; Zhang, Z.-H.; Shen, B.; Yang, X.-M.; Zhang, R.-F.; Huang, Q.-W.; Shen, Q.-R. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil 2011, 344, 87–97. [Google Scholar] [CrossRef]
- Jousset, A.; Lee, S.-W. Coming of age for the rhizosphere microbiome transplantation. Soil Ecol. Lett. 2023, 5, 4–5. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Samarakoon, U.; Palmer, J.; Ling, P.; Altland, J. Effects of electrical conductivity, pH, and foliar application of calcium chloride on yield and tip burn of Lactuca sativa grown using the nutrient–film technique. HortScience 2020, 55, 1265–1271. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Baldassarre Švecová, E.; Ruzzi, M. Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Front. Plant Sci. 2019, 10, 60. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Melini, F.; Ruzzi, M. Genome sequence of the plant growth-promoting rhizobacterium Pantoea agglomerans C1. Microbiol. Resour. Announc. 2019, 8, e00828-19. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Svecova, E.; Ruzzi, M. Effects of a protein hydrolysate-based biostimulant and two micronutrient-based fertilizers on plant growth and epiphytic bacterial population of lettuce. Acta Hortic. 2016, 1148, 43–48. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Syeed, R.; Mohsin, M.; Nafees, A.; Dewir, Y.H.; Mendler-Drienyovszki, N. Integrated GC-MS and UPLC-ESI-QTOF-MS based untargeted metabolomics analysis of in vitro raised tissues of Digitalis purpurea L. Front. Plant Sci. 2024, 15, 1433634. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, J.R. Yield and N assimilation of winter wheat (Triticum aestivum L., var. Norstar) inoculated with rhizobacteria. Pedobiol. 2000, 44, 97–104. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Urquiaga, S.; Döbereiner, J.; Baldani, J.I. The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil. 2002, 242, 205–215. [Google Scholar] [CrossRef]
- Felici, C.; Vettori, L.; Giraldi, E.; Forino, L.M.C.; Toffanin, A.; Tagliasacchi, A.M.; Nuti, M. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicum esculentum: Effects on plant growth and rhizosphere microbial community. Appl. Soil. Ecol. 2008, 40, 260–270. [Google Scholar] [CrossRef]
- Vanegas, J.; Uribe-Vélez, D. Selection of mixed inoculants exhibiting growth-promoting activity in rice plants from undefined consortia obtained by continuous enrichment. Plant Soil 2014, 375, 215–227. [Google Scholar] [CrossRef]
- Azizi, S.; Tabari, M.; Abad, A.R.F.N.; Ammer, C.; Guidi, L.; Bader, M.K.-F. Soil inoculation with beneficial microbes buffers negative drought effects on biomass, nutrients, and water relations of common myrtle. Front. Plant Sci. 2022, 13, 892826. [Google Scholar] [CrossRef] [PubMed]
- Mehnaz, S.; Baig, D.N.; Lazarovits, G. Genetic and phenotypic diversity of plant growth promoting rhizobacteria isolated from sugarcane plants growing in Pakistan. J. Microbiol. Biotechnol. 2010, 20, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, Y.; Gan, G.; Li, W.; Wan, W.; Jiang, Y.; Yang, T.; Zhang, Y.; Xu, Y.; Wang, Y.; et al. Exploring rhizo-microbiome transplants as a tool for protective plant-microbiome manipulation. ISME Commun. 2022, 2, 10. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, C.Y.; Teng, W.L.; Ouwehand, A.C.; Tuomola, E.M.; Salminen, S. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl. Environ. Microbiol. 2000, 66, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Zhou, D.; Chretien, R.L.; Turner, A.; Hou, G.; Evans, M.R.; Lowman, S. A Potential Application of Pseudomonas psychrotolerans IALR632 for Lettuce Growth Promotion in Hydroponics. Microorganisms 2023, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Dasgan, H.Y.; Aksu, K.S.; Zikaria, K.; Gruda, N.S. Biostimulants Enhance the Nutritional Quality of Soilless Greenhouse Tomatoes. Plants 2024, 13, 2587. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Yilmaz, D.; Zikaria, K.; Ikiz, B.; Gruda, N.S. Enhancing the Yield, Quality andAntioxidant Content of Lettucethrough Innovative and Eco-FriendlyBiofertilizer Practices in Hydroponics. Horticulturae 2023, 9, 1274. [Google Scholar] [CrossRef]
- Kean-Galeno, T.; Lopez-Arredondo, D.; Herrera-Estrella, L. The shoot apical meristem: An evolutionary molding of higher plants. Int. J. Mol. Sci. 2024, 25, 1519. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aldiyab, A.; Elgudayem, F.; Ikiz, B.; Gruda, N.S. Effect of biofertilizers on leaf yield, nitrate amount, mineral content and antioxidants of basil (Ocimum basilicum L.) in a floating culture. Sci. Rep. 2022, 12, 20917. [Google Scholar] [CrossRef]
- Villar, R.; Ruiz-Robleto, J.; Ubera, J.L.; Poorter, H. Exploring variation in leaf mass per area (LMA) from leaf to cell: An anatomical analysis of 26 woody species. Am. J. Bot. 2013, 100, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Jordan, G.J.; Carpenter, R.J.; Koutoulis, A.; Price, A.; Brodribb, T.J. Environmental adaptation in stomatal size independent of the effects of genome size. New Phytol. 2015, 205, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Jordan, G.J.; Carpenter, R.J. Unified changes in cell size permit coordinated leaf evolution. New Phytol. 2013, 199, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. Disruption of stomatal lineage signaling or transcriptional regulators has differential effects on mesophylldevelopment, but maintains coordination of gas exchange. New Phytol. 2017, 216, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef]
- Gago, J.; Daloso, D.M.; Carriquí, M.; Nadal, M.; Morales, M.; Araújo, W.L.; Nunes-Nesi, A.; Flexas, J. Mesophyll conductance: The leaf corridors for photosynthesis. Biochem. Soc. Trans. 2020, 48, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kiddle, G.; Pastori, G.M.; Bernard, S.; Pignocchi, C.; Antoniw, J.; Verrier, P.J.; Foyer, C.H. Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antioxid. Redox Signal. 2003, 5, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, M.R.; Mathers, A.; Baillie, A.L.; Dunn, J.; Wilson, M.J.; Hunt, L.; Pajor, R.; Fradera-Soler, M.; Rolfe, S.; Osborne, C.P.; et al. Mesophyll porosity is modulated by the presence of functional stomata. Nat. Commun. 2019, 10, 2825. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; John, G.P.; Pan, R.; Bartlett, M.K.; Fletcher, L.R.; Scoffoni, C.; Sack, L. A stomatal safety-efficiency trade-off constrains responses to leaf dehydration. Nat. Commun. 2019, 10, 3398. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.-M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef]
- Putra, A.M.; Anastasya, N.A.; Rachmawati, S.W.; Yusnawan, E.; Syibli, M.A.; Trianti, I.; Setiawan, A.; Aini, L.Q. Growth performance and metabolic changes in lettuce inoculated with plant growth promoting bacteria in a hydroponic system. Sci. Hortic. 2024, 327, 112868. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Bonini, P.; Muleo, R.; Gatti, L.; Meneghini, M.; Tronati, M.; Melini, F.; Ruzzi, M. A genetic and metabolomic perspective on the production of indole-3-acetic acid by Pantoea agglomerans and use of their metabolites as biostimulants in plant nurseries. Front. Microbiol. 2020, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Luziatelli, F.; Gatti, L.; Ficca, A.G.; Medori, G.; Silvestri, C.; Melini, F.; Muleo, R.; Ruzzi, M. Metabolites secreted by a plant-growth-promoting Pantoea agglomerans strain improved rooting of Pyrus communis L. cv Dar Gazi cuttings. Front. Microbiol. 2020, 11, 539359. [Google Scholar] [CrossRef]
- Comadira, G.; Rasool, B.; Karpinska, B.; Morris, J.; Verrall, S.R.; Hedley, P.E.; Foyer, C.H.; Hancock, R.D. Nitrogen deficiency in barley (Hordeum vulgare) seedlings induces molecular and metabolic adjustments that trigger aphid resistance. J. Exp. Bot. 2015, 66, 3639–3655. [Google Scholar] [CrossRef]
- Diaz-Mendoza, M.; Velasco-Arroyo, B.; Santamaria, M.E.; González-Melendi, P.; Martinez, M.; Diaz, I. Plant senescence and proteolysis: Two processes with one destiny. Genet. Mol. Biol. 2016, 39, 329–338. [Google Scholar] [CrossRef]
- Cipriano, M.A.P.; Freitas-Iório, R.P.; Dimitrov, M.R.; de Andrade, S.A.L.; Kuramae, E.E.; Silveira, A.P.D.D. Plant-growth endophytic bacteria improve nutrient use efficiency and modulate foliar N-metabolites in sugarcane seedling. Microorganisms 2021, 25, 479. [Google Scholar] [CrossRef]
- Akter, S.; Khan, M.S.; Smith, E.N.; Flashman, E. Measuring ROS and redox markers in plant cells. RSC Chem. Biol. 2021, 2, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Deepak, S.; Shailasree, S.; Kini, R.K.; Muck, A.; Mithöfer, A.; Shetty, S.H. Hydroxyproline-rich glycoproteins and plant defence. J. Phytopathol. 2010, 158, 585–593. [Google Scholar] [CrossRef]
- Viviani, A.; Verma, B.C.; Giordani, T.; Fambrini, M. L-ascorbic acid in plants: From biosynthesis to its role in plant development and stress response. Agrochimica 2021, 65, 151–171. [Google Scholar] [CrossRef]
- Smirnoff, N. Botanical briefing: The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Umapathi, C.N.; Chandrasekhar, A.; Senthil, T.; Kalaiselvi, M.K.; Kalarani, R.; Sivakumar, R.; Karthikeyan, R.; Kuttimani, S.; Anandakumar, S. Bacillus sp. and Pseudacidovorax intermedius colonization effect on biochemical and metabolites expression in drought-stressed Sorghum bicolor (L.) Moench. Plant Stress. 2024, 11, 100424. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Arrigoni, O. The ascorbic acid system in seeds: To protect and to serve. Seed Sci. Res. 2003, 13, 249–260. [Google Scholar] [CrossRef]
- Kka, N.; Rookes, J.; Cahill, D. The influence of ascorbic acid on root growth and the root apical meristem in Arabidopsis thaliana. Plant Physiol. Biochem. 2018, 129, 323–330. [Google Scholar] [CrossRef]
- Page, M.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2012, 35, 388–404. [Google Scholar] [CrossRef]
- Senn, M.E.; Grozeff, G.G.; Alegre, M.L.; Barrile, F.; De Tullio, M.C.; Bartoli, C.G. Effect of mitochondrial ascorbic acid synthesis on photosynthesis. Plant Physiol. Biochem. 2016, 104, 29–35. [Google Scholar] [CrossRef]
- Akula, R.; Mukherjee, S. New insights on neurotransmitters signaling mechanisms in plants. Plant Signal. Behav. 2020, 15, 1737450. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, R.; Akashi, M.; Taniguchi, T.; Kinose, Y.; Yaprak, A.E.; Turgay, O.C. Metabolomics analyses reveal metabolites affected by plant growth-promoting endophytic bacteria in roots of the halophyte Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2021, 22, 11813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, T.; Niu, K.; Ma, H. Integrated proteomics, transcriptomics, and metabolomics offer novel insights into Cd resistance and accumulation in Poa pratensis. J. Hazard. Mater. 2024, 474, 134727. [Google Scholar] [CrossRef] [PubMed]

| C | T | |||||
|---|---|---|---|---|---|---|
| Mean | s.e. | Mean | s.e. | |||
| Root/shoot ratio (R/S) | 0.14 | a | 0.008 | 0.13 | a | 0.006 |
| Total leaf area (cm2) | 376.0 | b | 10.3 | 450.2 | a | 12.3 |
| Number of leaves per plant | 14.0 | b | 0.4 | 15.6 | a | 0.5 |
| Average leaf mass (g) | 0.10 | b | 0.003 | 0.11 | a | 0.003 |
| Average leaf area (cm2) | 27.3 | a | 1.0 | 29.1 | a | 0.7 |
| Specific leaf area (cm2/g) | 271.3 | a | 7.0 | 256.5 | b | 4.7 |
| Root length (cm) | 36.9 | a | 1.7 | 32.8 | b | 1.4 |
| Specific root length (cm/g) | 199.1 | a | 17.2 | 143.3 | b | 9.3 |
| C | T | ||||||
|---|---|---|---|---|---|---|---|
| Mean | s.e. | n | Mean | s.e. | n | ||
| Stomatal density (number of stomata, mm−2) | 297.1 | 12.6 | 4 | 239.1 | 9.4 | 4 | ** |
| Guard cell length (µm) | 17.0 | 0.3 | 82 | 19.7 | 0.2 | 66 | *** |
| Average stomatal pore surface (µm2) | 25.1 | 2.6 | 46 | 57.4 | 5.8 | 37 | *** |
| Total stomatal pore surface (µm2 mm−2) | 7593 | 486 | 4 | 13,622 | 899 | 4 | n.s. |
| Leaf thickness (µm) | 276.1 | 8.9 | 9 | 195.6 | 5.4 | 9 | *** |
| Airspace surface to total leaf tissue (%) | 40.1 | 4.0 | 3 | 28.1 | 1.9 | 3 | * |
| Spongy parenchyma surface to total leaf tissue (%) | 45.6 | 3.3 | 3 | 39.2 | 3.1 | 3 | n.s. |
| Palisade parenchyma surface to total leaf tissue (%) | 14.4 | 0.9 | 3 | 32.6 | 2.4 | 3 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Jaoudé, R.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Effect of Plant Growth-Promoting Rhizobacteria Synthetic Consortium on Growth, Yield, and Metabolic Profile of Lettuce (Lactuca sativa L.) Grown Under Suboptimal Nutrient Regime. Horticulturae 2025, 11, 64. https://doi.org/10.3390/horticulturae11010064
Abou Jaoudé R, Luziatelli F, Ficca AG, Ruzzi M. Effect of Plant Growth-Promoting Rhizobacteria Synthetic Consortium on Growth, Yield, and Metabolic Profile of Lettuce (Lactuca sativa L.) Grown Under Suboptimal Nutrient Regime. Horticulturae. 2025; 11(1):64. https://doi.org/10.3390/horticulturae11010064
Chicago/Turabian StyleAbou Jaoudé, Renée, Francesca Luziatelli, Anna Grazia Ficca, and Maurizio Ruzzi. 2025. "Effect of Plant Growth-Promoting Rhizobacteria Synthetic Consortium on Growth, Yield, and Metabolic Profile of Lettuce (Lactuca sativa L.) Grown Under Suboptimal Nutrient Regime" Horticulturae 11, no. 1: 64. https://doi.org/10.3390/horticulturae11010064
APA StyleAbou Jaoudé, R., Luziatelli, F., Ficca, A. G., & Ruzzi, M. (2025). Effect of Plant Growth-Promoting Rhizobacteria Synthetic Consortium on Growth, Yield, and Metabolic Profile of Lettuce (Lactuca sativa L.) Grown Under Suboptimal Nutrient Regime. Horticulturae, 11(1), 64. https://doi.org/10.3390/horticulturae11010064






