Variations in Physical and Chemical Characteristics of Terminalia catappa Nuts
Abstract
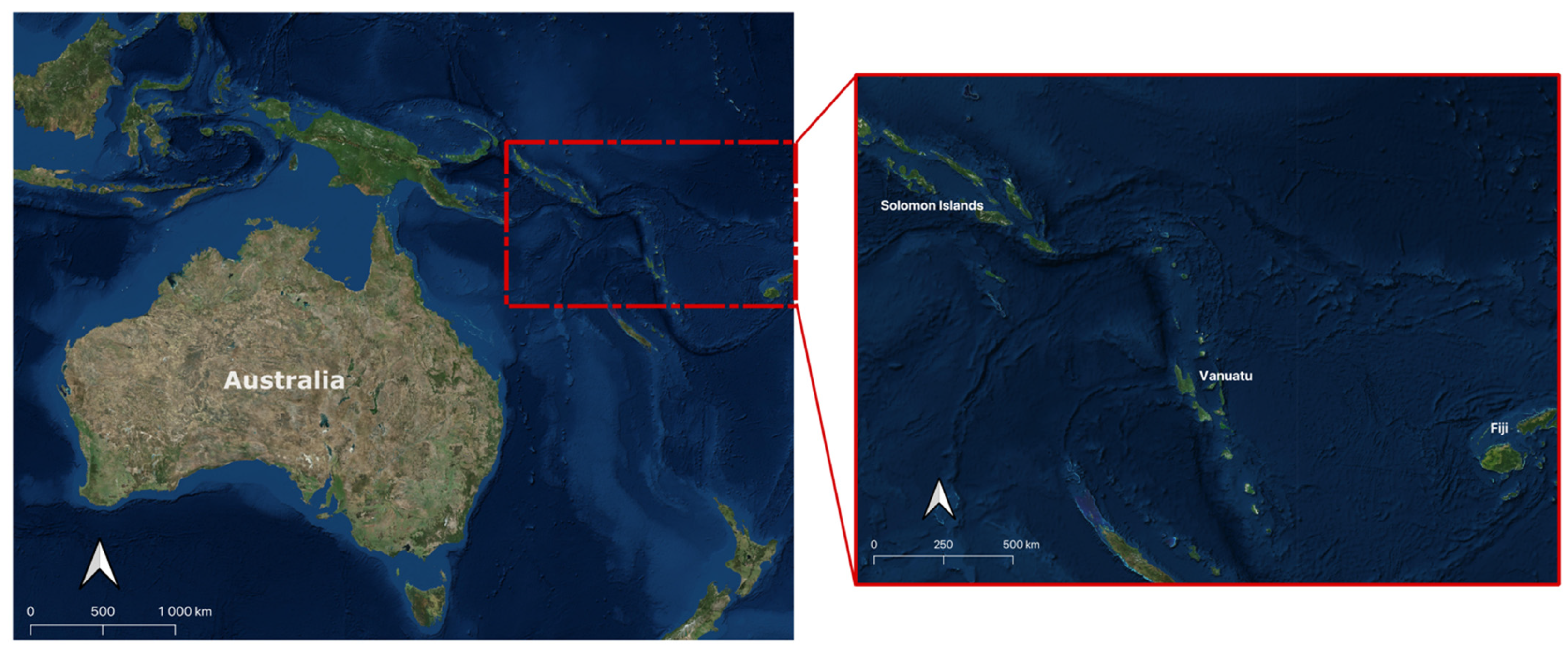
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Oil Extraction and Chemical Analyses
2.3. Oil Stability Using Accelerated Ageing
2.4. Calculations
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dao, T.H.H.; Hölscher, D. Impact of Non-Timber Forest Product Use on the Tree Community in North-Western Vietnam. Forests 2018, 9, 431. [Google Scholar] [CrossRef]
- Epanda, M.A.; Tsafack Donkeng, R.; Ngo Nonga, F.; Frynta, D.; Adi, N.N.; Willie, J.; Speelman, S. Contribution of Non-Timber Forest Product Valorisation to the Livelihood Assets of Local People in the Northern Periphery of the Dja Faunal Reserve, East Cameroon. Forests 2020, 11, 1019. [Google Scholar] [CrossRef]
- Sardeshpande, M.; Shackleton, C. Wild Edible Fruits: A Systematic Review of an Under-Researched Multifunctional NTFP (Non-Timber Forest Product). Forests 2019, 10, 467. [Google Scholar] [CrossRef]
- Jansen, M.; Guariguata, M.R.; Raneri, J.E.; Ickowitz, A.; Chiriboga-Arroyo, F.; Quaedvlieg, J.; Kettle, C.J. Food for thought: The underutilized potential of tropical tree-sourced foods for 21st century sustainable food systems. People Nat. 2020, 2, 1006–1020. [Google Scholar] [CrossRef]
- Bai, S.H.; Brooks, P.; Gama, R.; Nevenimo, T.; Hannet, G.; Hannet, D.; Randall, B.; Walton, D.; Grant, E.; Wallace, H.M. Nutritional quality of almond, canarium, cashew and pistachio and their oil photooxidative stability. J. Food Sci. Technol. 2019, 56, 792–798. [Google Scholar] [CrossRef]
- International Nut & Dried Fruit Council. World Nut and Dried Fruit Trade Map. Available online: https://inc.nutfruit.org/inc-publishes-trade-maps-series/ (accessed on 21 October 2024).
- Wallace, H.; Randall, B.; Grant, E.; Jones, K.; Walton, D.; Poienou, M.; Nevenimo, T.; Moxon, J.; Pauku, R.L. Processing methods for Canarium nuts in the Pacific. Acta Hortic. 2016, 1128, 145–149. [Google Scholar] [CrossRef]
- Wallace, H.; Komolong, B.; Nevenimo, T.; Waaii, C.; Hannett, D.; Hannett, G.; Kapi Ling, S.; Grant, E.; Hodges, B.; Kill, E.; et al. Enhancing Private Sector-Led Development of the Canarium Industry in PNG: ACIAR Project FST/2014/099 Final Report; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2019; pp. 1–135. Available online: https://www.aciar.gov.au/publication/technical-publications/enhancing-private-sector-led-development-canarium-nut-industry-papua-new-guinea-final (accessed on 21 October 2024).
- Hosseini Bai, S.; Darby, I.; Nevenimo, T.; Hannet, G.; Hannet, D.; Poienou, M.; Grant, E.; Brooks, P.; Walton, D.; Randall, B.; et al. Effects of roasting on kernel peroxide value, free fatty acid, fatty acid composition and crude protein content. PLoS ONE 2017, 12, e0184279. [Google Scholar] [CrossRef]
- Wallace, H.M.; Hannet, D.; Hannet, G.; Hosseini-Bai, S.; Jones, K.; Komolong, B. Commercialising an indigenous agroforestry tree: Overview of commercial processing methods for Canarium indicum (galip) nuts in Papua New Guinea. Acta Hortic. 2022, 1355, 345–350. [Google Scholar] [CrossRef]
- Soriano, M.; Mohren, F.; Ascarrunz, N.; Dressler, W.; Peña-Claros, M. Socio-ecological costs of Amazon nut and timber production at community household forests in the Bolivian Amazon. PLoS ONE 2017, 12, e0170594. [Google Scholar] [CrossRef]
- Leakey, R.R. Converting ‘trade-offs’ to ‘trade-ons’ for greatly enhanced food security in Africa: Multiple environmental, economic and social benefits from ‘socially modified crops’. Food Secur. 2018, 10, 505–524. [Google Scholar] [CrossRef]
- Randall, B.W.; Walton, D.A.; Grant, E.L.; Zekele, P.; Gua, B.; Pauku, R.; Wallace, H.M. Selection of the tropical nut Canarium indicum for early fruiting, nut-in-shell size and kernel size. Acta Hortic. 2016, 1109, 169–174. [Google Scholar] [CrossRef]
- Bai, S.H.; Randall, B.; Grant, E.; Gama, R.; Gua, B.; Keli, D.; Negalevu, P.; Oakeshott, J.; Wallace, H.M. Tree-to-tree variation of kernel size in two underutilized tree nuts in Pacific. Acta Hortic. 2022, 1340, 141–144. [Google Scholar] [CrossRef]
- Leakey, R.; Fuller, S.; Treloar, T.; Stevenson, L.; Hunter, D.; Nevenimo, T.; Binifa, J.; Moxon, J. Characterization of tree-to-tree variation in morphological, nutritional and medicinal properties of Canarium indicum nuts. Agrofor. Syst. 2008, 73, 77–87. [Google Scholar] [CrossRef]
- Pauku, R.; Lowe, A.; Rrb, L. Domestication of indigenous fruit and nut trees for agroforestry in the Solomon Islands. For. Trees Livelihoods 2010, 19, 269–287. [Google Scholar] [CrossRef]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Hosseini Bai, S. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef]
- Leakey, R.; Shackleton, S.; Plessis, P.D. Domestication potential of Marula (Sclerocarya birrea subsp caffra) in South Africa and Namibia: 1. Phenotypic variation in fruit traits. Agrofor. Syst. 2005, 64, 25–35. [Google Scholar] [CrossRef]
- Levis, C.; Flores, B.M.; Moreira, P.A.; Luize, B.G.; Alves, R.P.; Franco-Moraes, J.; Lins, J.; Konings, E.; Peña-Claros, M.; Bongers, F.; et al. How People Domesticated Amazonian Forests. Front. Ecol. Evol. 2018, 5, 171. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Page, T. The ‘ideotype concept’ and its application to the selection of cultivars of trees providing agroforestry tree products. For. Trees Livelihoods 2006, 16, 5–16. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.; Trueman, S.; Hosseini Bai, S. Quality and shelf life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Oxidative stability of tree nut oils. J. Agric. Food Chem. 2008, 56, 4751–4759. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of Nuts and Their Potential Health Benefits-An Overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef]
- Kodad, O.; Socias i Company, R.; Prats, M.S.; LÓpez Ortiz, M.C. Variability in tocopherol concentrations in almond oil and its use as a selection criterion in almond breeding. J. Hortic. Sci. Biotech. 2006, 81, 501–507. [Google Scholar] [CrossRef]
- Pedley, L.; Kodela, P.G. Terminalia catappa. Available online: https://profiles.ala.org.au/opus/foa/profile/Terminalia%20catappa (accessed on 9 April 2025).
- Thomson, L.A.; Evans, B. Terminalia catappa (tropical almond). Species Profiles Pac. Isl. Agrofor. 2006, 2, 1–20. [Google Scholar]
- Smith, A.C. Studies of Pacific Island Plants, XXIV. The genus Terminalia (Combretaceae) in Fiji, Samoa, and Tonga. Brittonia 1971, 23, 394–412. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Trueman, S.J.; Gama, T.; Jones, K.; Walton, D.; Randall, B.; Wallace, H.M. Shelf life of macadamia kernels of different origin. Acta Hortic. 2019, 1256, 375–378. [Google Scholar] [CrossRef]
- Blaikie, S.J.; O’Farrell, P.J.; Chacko, E.K.; Müller, W.J.; Wei, X.; Steele Scott, N.; Sykes, S.R. Assessment and selection of new hybrids from the Australian cashew breeding program. Aust. J. Exp. Agric. 2002, 42, 615–623. [Google Scholar] [CrossRef]
- Walton, D.A.; Wallace, H. Genetic and postharvest factors affecting macadamia kernel quality. Afri J. Agric. Res. 2012, 7, 2490–2495. [Google Scholar] [CrossRef]
- Ogunsina, B.S.; Bamgboye, A.I. Pre-shelling parameters and conditions that influence the whole kernel out-turn of steam-boiled cashew nuts. J. Saudi Soc. Agric. Sci. 2014, 13, 29–34. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Gama, R.; Jones, K.; Hannet, D.; Hannet, G.; Komolong, B.; Brooks, P.; Grant, E.; Elliott, B.; Wallace, H.M. Presence of Testa and Shell Maintains Oil Stability in Almond and Canarium Nuts. Horticulturae 2023, 9, 1003. [Google Scholar] [CrossRef]
- O’Connor, K.; Hayes, B.; Topp, B. Prospects for increasing yield in macadamia using component traits and genomics. Tree Genet. Genomes 2018, 14, 7. [Google Scholar] [CrossRef]
- Stephenson, R. Macadamia: Domestication and commercialization. Chr. Hortic. 2005, 45, 11–15. [Google Scholar]
- Amaral, J.S.; Casal, S.; Seabra, R.M.; Oliveira, B.P. Effects of roasting on hazelnut lipids. J. Agric. Food Chem. 2006, 54, 1315–1321. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Nevenimo, T.; Hannet, G.; Hannet, D.; Jones, K.; Trueman, S.; Grant, E.; Walton, D.A.; Randall, B.; Wallace, H.M. Freezing, roasting and salt dipping impacts on peroxide value, free fatty acid and fatty acid concentrations of nut kernels. Acta Hortic. 2019, 1256, 71–76. [Google Scholar] [CrossRef]
- Griffin, L.; Dean, L. Nutrient Composition of Raw, Dry-Roasted, and Skin-On Cashew Nuts. J. Food Res. 2024, 6, 13. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Winter-Smith, J.; Selak, V.; Harwood, M.; Ameratunga, S.; Grey, C. Cardiovascular disease and its management among Pacific people: A systematic review by ethnicity and place of birth. BMC Cardiov. Disord. 2021, 21, 515. [Google Scholar] [CrossRef]
- Glenn, A.J.; Aune, D.; Freisling, H.; Mohammadifard, N.; Kendall, C.W.C.; Salas-Salvadó, J.; Jenkins, D.J.A.; Hu, F.B.; Sievenpiper, J.L. Nuts and Cardiovascular Disease Outcomes: A Review of the Evidence and Future Directions. Nutrients 2023, 15, 911. [Google Scholar] [CrossRef]
- Vidaković, A.; Radunić, M.; Poljak, I. Variation in chemical composition and fruit morphometric traits of almond-leaved pear (Pyrus spinosa Forssk.) natural populations. Genet. Resour. Crop Evol. 2025, 72, 1495–1510. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.M.; Trueman, S.; Hosseini Bai, S. Variability in crude protein and mineral nutrient concentrations of almonds. Acta Hortic. 2018, 1219, 213–218. [Google Scholar] [CrossRef]
- Kucukyumuk, Z.; Erdal, I. Rootstock and cultivar effect on mineral nutrition, seasonal nutrient variation and correlations among leaf, flower and fruit nutrient concentrations in apple trees. Bulg. J. Agric. Sci. 2011, 17, 633–641. [Google Scholar]
- Sathe, S.K.; Seeram, N.P.; Kshirsagar, H.H.; Heber, D.; Lapsley, K.A. Fatty Acid Composition of California Grown Almonds. J. Food Sci. 2008, 73, C607–C614. [Google Scholar] [CrossRef] [PubMed]
- Trueman, S.J.; Penter, M.G.; Malagodi-Braga, K.S.; Nichols, J.; De Silva, A.L.; Ramos, A.T.M.; Moriya, L.M.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; et al. High Outcrossing Levels among Global Macadamia Cultivars: Implications for Nut Quality, Orchard Designs and Pollinator Management. Horticulturae 2024, 10, 203. [Google Scholar] [CrossRef]


| Solomon Islands | Vanuatu | Fiji | |
|---|---|---|---|
| Fruit mass (g) | |||
| Mean ± SE | 29.21 ± 0.52 b | 33.72 ± 0.90 a | 7.97 ± 0.17 c |
| N | 418 | 486 | 746 |
| Minimum | 10.17 | 5.90 | 2.60 |
| Maximum | 67.49 | 142.19 | 35.00 |
| Kernel mass (g) | |||
| Mean ± SE | 1.66 ± 0.04 a | 1.58 ± 0.03 b | 0.33 ± 0.01 c |
| N | 416 | 486 | 746 |
| Minimum | 0.10 | 0.05 | 0.01 |
| Maximum | 3.93 | 3.94 | 0.82 |
| Kernel-to-fruit weight (%) | |||
| Mean ± SE | 5.64 ± 0.10 a | 5.61 ± 0.13 a | 4.75 ± 0.09 b |
| N | 416 | 486 | 746 |
| Minimum | 0.28 | 0.35 | 0.12 |
| Maximum | 15.53 | 15.28 | 11.13 |
| Total oil (%) | |||
| Mean ± SE | 59.37 ± 1.08 a | 55.34 ± 1.21 b | 56.86 ± 0.30 ab |
| N | 85 | 105 | 150 |
| Minimum | 46.27 | 28.21 | 47.32 |
| Maximum | 77.55 | 84.19 | 65.56 |
| TS (%) | |||
| Mean ± SE | 46.04 ± 0.55 a | 44.30 ± 0.37 b | 38.60 ± 0.28 c |
| N | 68.00 | 90.00 | 150.00 |
| Minimum | 36.92 | 32.86 | 31.96 |
| Maximum | 64.65 | 52.11 | 45.99 |
| TUS (%) | |||
| Mean ± SE | 54.44 ± 0.52 c | 55.70 ± 0.37 b | 61.40 ± 0.27 a |
| N | 68.00 | 90.00 | 150.00 |
| Minimum | 49.33 | 47.89 | 54.01 |
| Maximum | 68.55 | 67.14 | 68.04 |
| Solomon Islands | Vanuatu | Fiji | |
|---|---|---|---|
| C14:0 (myristic) | 0.04 ± 0.001 | 0.03 ± 0.002 | 0.02 ± 0.001 |
| C16:0 (palmitic) | 40.37 ± 0.570 | 39.86 ± 0.354 | 34.16 ± 0.255 |
| C18:0 (stearic) | 5.22 ± 0.068 | 4.28 ± 0.079 | 4.29 ± 0.061 |
| C20:0 (arachidic) | 0.35 ± 0.014 | 0.11 ± 0.003 | 0.11 ± 0.002 |
| C22:0 (behenic) | 0.06 ± 0.004 | 0.03 ± 0.002 | 0.03 ± 0.001 |
| C16:1 (palmitoleic) | 0.26 ± 0.012 | 0.08 ± 0.003 | 0.06 ± 0.001 |
| C18:2 (linoleic) | 23.81 ± 0.411 | 20.31 ± 0.388 | 20.83 ± 0.337 |
| C18:1 cis (oleic) | 29.73 ± 0.485 | 34.9 ± 0.343 | 40.15 ± 0.326 |
| C18:1 trans (elaidic) | 0.61 ± 0.015 | 0.37 ± 0.034 | 0.33 ± 0.006 |
| C20:1 (eicosenoic) | 0.04 ± 0.003 | 0.03 ± 0.002 | 0.03 ± 0.001 |
| (a) Fatty Acid Compositions | (b) Kernel Characteristics * | |||||
|---|---|---|---|---|---|---|
| PCA1 | PCA2 | PCA3 | PCA1 | PCA2 | PCA3 | |
| Eigenvalues | 4.85 | 1.52 | 1.18 | 2.85 | 1.16 | 1.07 |
| Percentages of variance | 35.44 | 22.32 | 17.87 | 45.49 | 21.49 | 18.10 |
| Cumulative percentages of variance | 35.44 | 57.77 | 75.64 | 45.49 | 66.98 | 85.09 |
| C16:1 (palmitoleic) | 0.90 | 0.03 | 0.01 | |||
| C20:0 (arachidic) | 0.90 | 0.11 | −0.04 | |||
| C14:0 (myristic) | 0.81 | −0.11 | 0.18 | |||
| C22:0 (behenic) | 0.81 | 0.34 | 0.15 | |||
| C18:1 cis (oleic) | −0.67 | 0.63 | 0.27 | |||
| C18:1 trans (elaidic) | 0.65 | 0.15 | 0.41 | |||
| C20:1 (eicosenoic) | 0.60 | 0.50 | 0.21 | |||
| C18:0 (stearic) | 0.58 | −0.14 | −0.34 | |||
| C16:0 (palmitic) | 0.31 | −0.83 | 0.40 | |||
| C18:2 (linoleic) | 0.51 | 0.11 | −0.75 | |||
| Fruit mass (g) | 0.72 | −0.19 | 0.39 | |||
| Kernel mass (g) | 0.82 | 0.395 | 0.19 | |||
| Kernel-to-fruit weight (%) | 0.26 | 0.91 | −0.27 | |||
| Total oil (%) | −0.02 | 0.18 | 0.86 | |||
| TS (%) | 0.89 | −0.24 | −0.20 | |||
| TUS (%) | −0.89 | 0.23 | 0.20 | |||
| Incubation Period (Days) | ||||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | |
| Hexanal Concentration (ppm) | 30.20 ± 6.67 c | 97.59 ± 5.67 b | 132.27 ± 9.17 ab | 142.99 ± 12.51 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini Bai, S.; Randall, B.; Gama, R.; Gua, B.; Keli, D.; Jones, K.; Elliott, B.; Wallace, H.M. Variations in Physical and Chemical Characteristics of Terminalia catappa Nuts. Horticulturae 2025, 11, 540. https://doi.org/10.3390/horticulturae11050540
Hosseini Bai S, Randall B, Gama R, Gua B, Keli D, Jones K, Elliott B, Wallace HM. Variations in Physical and Chemical Characteristics of Terminalia catappa Nuts. Horticulturae. 2025; 11(5):540. https://doi.org/10.3390/horticulturae11050540
Chicago/Turabian StyleHosseini Bai, Shahla, Bruce Randall, Repson Gama, Basil Gua, Doni Keli, Kim Jones, Brittany Elliott, and Helen M. Wallace. 2025. "Variations in Physical and Chemical Characteristics of Terminalia catappa Nuts" Horticulturae 11, no. 5: 540. https://doi.org/10.3390/horticulturae11050540
APA StyleHosseini Bai, S., Randall, B., Gama, R., Gua, B., Keli, D., Jones, K., Elliott, B., & Wallace, H. M. (2025). Variations in Physical and Chemical Characteristics of Terminalia catappa Nuts. Horticulturae, 11(5), 540. https://doi.org/10.3390/horticulturae11050540







