Onion Male Sterility: Genetics, Genomics and Breeding
Abstract
1. Introduction
2. Male Sterility in Onion
2.1. Types of Male Sterility Systems in Onion
2.2. Utility of Male Sterile Lines in Onion Hybrid Breeding
3. Genetic and Molecular Basis of Male Sterility in Onion
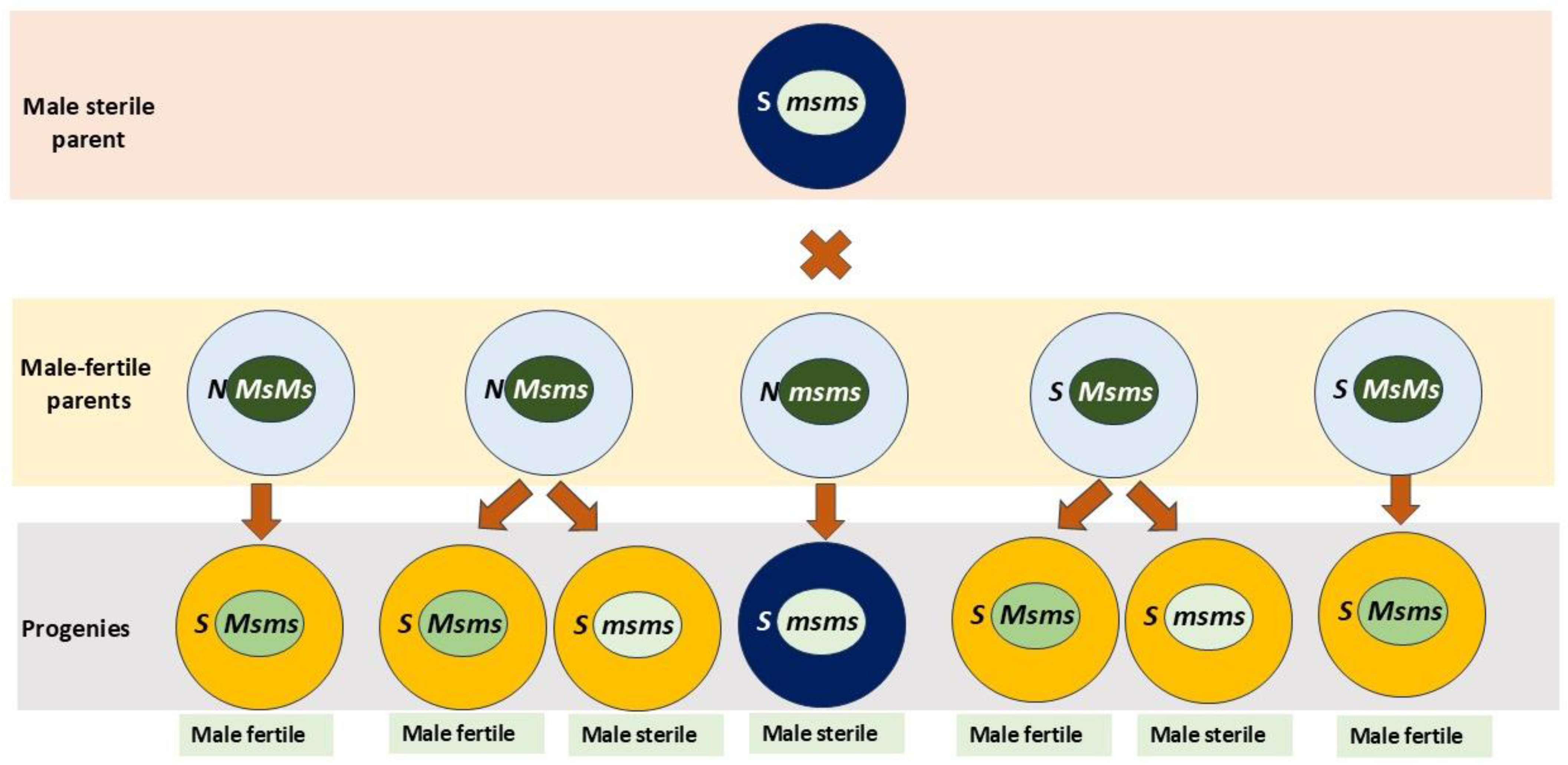
3.1. Genetics of Male Fertility and Sterility in Onion
3.2. Utility of Molecular Markers in Onion Male Sterility
4. Phenotypic Features
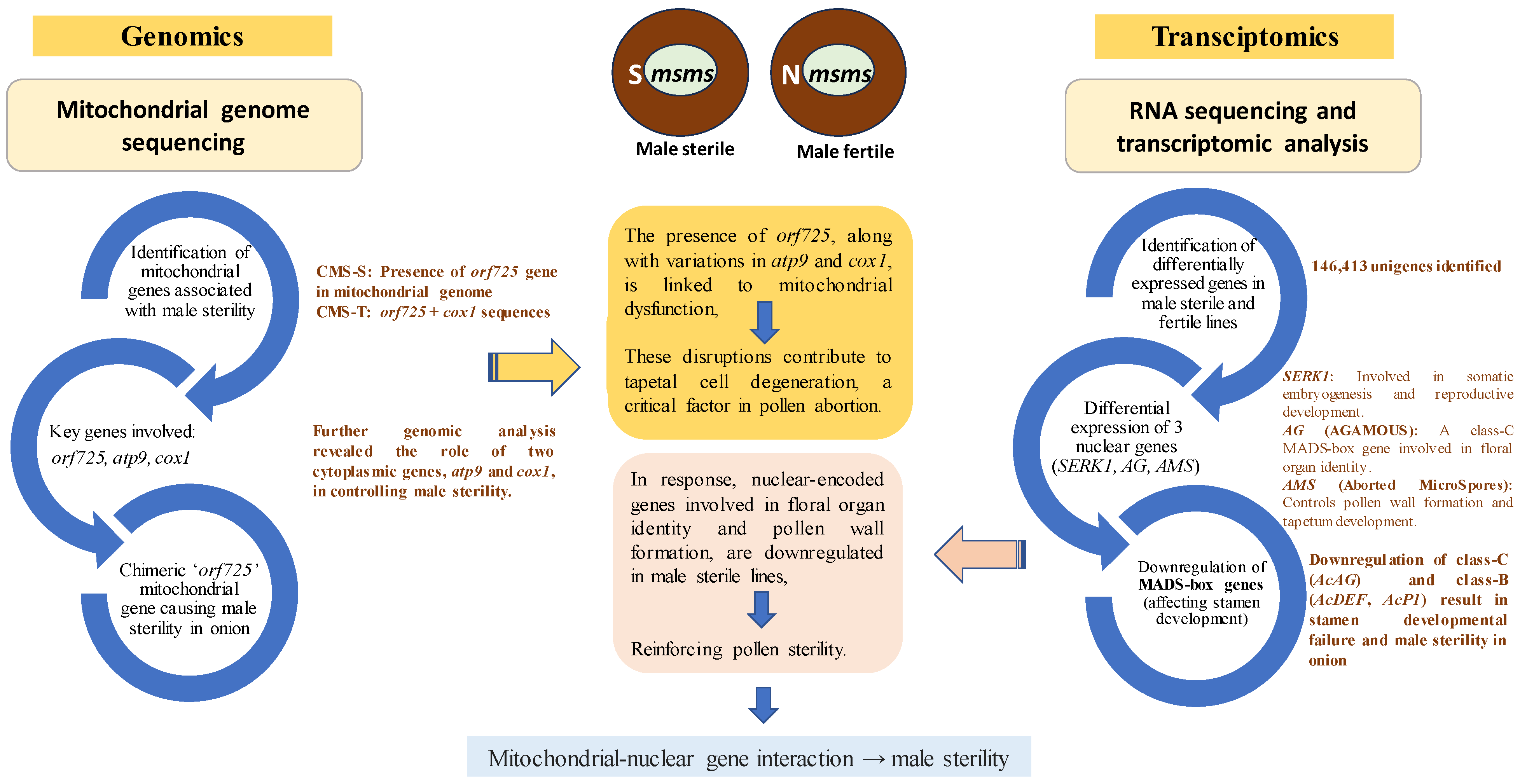
5. Genomic and Transcriptomic Insights into Onion Male Sterility
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teotia, D.; Agrawal, A.; Goyal, H.; Jain, P.; Singh, V.; Verma, Y.; Perveen, K.; Bukhari, N.A.; Chandra, A.; Malik, V. Pharmacophylogeny of genus Allium L. J. King Saud Univ. Sci. 2024, 36, 103330. [Google Scholar] [CrossRef]
- Ricroch, A.; Yockteng, R.; Brown, S.C.; Nadot, S. Evolution of genome size across some cultivated Allium species. Genome 2005, 48, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Marcussen, T.; Sandve, S.R.; Heier, L.; Spannagl, M.; Pfeifer, M.; Jakobsen, K.S.; Wulff, B.B.H.; Steuernagel, B.; Mayer, K.F.X.; Olsen, O.-A. Ancient hybridizations among the ancestral genomes of bread wheat. Science 2014, 345, 1250092. [Google Scholar] [CrossRef]
- Rashid, H.A.; Cheng, W.; Thomas, B. Temporal and spatial expression of arabidopsis gene homologs control daylength adaptation and bulb formation in onion (Allium cepa L.). Sci. Rep. 2019, 9, 14629. [Google Scholar] [CrossRef]
- Atif, M.J.; Ahanger, M.A.; Amin, B.; Ghani, M.I.; Ali, M.; Cheng, Z. Mechanism of allium crops bulb enlargement in response to photoperiod: A review. Int. J. Mol. Sci. 2020, 21, 1325. [Google Scholar] [CrossRef] [PubMed]
- Currah, L.; Ockendon, D.J. Protandry and the sequence of flower opening in the onion (Allium cepa L.). New Phytol. 1978, 81, 419–429. [Google Scholar] [CrossRef]
- Ricciardi, L.; Mazzeo, R.; Marcotrigiano, A.R.; Rainaldi, G.; Iovieno, P.; Zonno, V.; Pavan, S.; Lotti, C. Assessment of genetic diversity of the “Acquaviva Red Onion” (Allium cepa L.) apulian landrace. Plants 2020, 9, 260. [Google Scholar] [CrossRef]
- Chalbi, A.; Chikh-Rouhou, H.; Tlahig, S.; Mallor, C.; Garcés-Claver, A.; Haddad, M.; Sta-Baba, R.; Bel-Kadhi, M.S. Biochemical characterization of local onion genotypes (Allium cepa L.) in the arid regions of Tunisia. Pol. J. Environ. Stud. 2022, 32, 15–26. [Google Scholar] [CrossRef]
- Jimenez, L.; Alarcón, E.; Trevithick-Sutton, C.; Gandhi, N.; Scaiano, J.C. Effect of γ-radiation on green onion DNA integrity: Role of ascorbic acid and polyphenols against nucleic acid damage. Food Chem. 2011, 128, 735–741. [Google Scholar] [CrossRef]
- Chalbi, A.; Chikh-Rouhou, H.; Mezghani, N.; Slim, A.; Fayos, O.; Bel-Kadhi, M.S.; Garcés-Claver, A. Genetic diversity analysis of Onion (Allium cepa L.) from the arid region of Tunisia using phenotypic traits and SSR markers. Horticulturae 2023, 9, 1098. [Google Scholar] [CrossRef]
- Sharma, P.; Nair, S.A.; Sharma, P. Male sterility and its commercial exploitation in hybrid seed production of vegetable crops: A review. Agric. Rev. 2019, 40, 261–270. [Google Scholar] [CrossRef]
- Jo, J.; Purushotham, P.M.; Han, K.; Lee, H.-R.; Nah, G.; Kang, B.-C. Development of a genetic map for onion (Allium cepa L.) using reference-free genotyping-by-sequencing and SNP assays. Front. Plant Sci. 2017, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Singh, S.; Nandi, A. Male sterility systems in the genomics era for expediting vegetable breeding. Sci. Hortic. 2024, 338, 113774. [Google Scholar] [CrossRef]
- Mallor, C.; Arnedo, M.S.; Garcés, A. Assessing the genetic diversity of Spanish Allium cepa landraces for onion breeding using microsatellite markers. Sci. Hortic. 2014, 170, 24–31. [Google Scholar] [CrossRef]
- Manjunathagowda, D.C. Perspective and application of molecular markers linked to the cytoplasm types and male-fertility restorer locus in onion (Allium cepa). Plant Breed. 2021, 140, 732–744. [Google Scholar] [CrossRef]
- Serra, A.D.B.; Currah, L. Agronomy of Onions. Allium Crop Science: Recent Advances. CABI. 2002, pp. 187–232. Available online: https://www.cabi.org/VetMedResource/ebook/20023117383 (accessed on 1 December 2024).
- Jones, H.A.; Emsweller, S.L. Development of the flower and macrogametophyte of Allium cepa. Hilgardia 1936, 10, 415–428. [Google Scholar] [CrossRef]
- McCallum, J. Onion. In Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2008; Volume 5, p. 331. [Google Scholar]
- Singh, S.; Bhatia, R.; Kumar, R.; Sharma, K.; Dash, S.; Dey, S.S. Cytoplasmic male sterile and doubled haploid lines with desirable combining ability enhances the concentration of important antioxidant attributes in Brassica oleracea. Euphytica 2018, 214, 207. [Google Scholar] [CrossRef]
- Singh, S.; Dey, S.S.; Bhatia, R.; Kumar, R.; Behera, T.K. Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding. Plant Reprod. 2019, 32, 231–256. [Google Scholar] [CrossRef]
- Havey, M.J. Diversity among male-sterility-inducing and male-fertile cytoplasms of onion. Theor. Appl. Genet. 2000, 101, 778–782. [Google Scholar] [CrossRef]
- Soto, V.C.; Caselles, A.; Silva, M.F.; Galmarini, C.R. Onion hybrid seed production: Relation with nectar composition and flower traits. J. Econ. Entomol. 2018, 111, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dey, S.S.; Bhatia, R.; Kumar, R.; Sharma, K.; Behera, T.K. Heterosis and combining ability in cytoplasmic male sterile and doubled haploid based Brassica oleracea progenies and prediction of heterosis using microsatellites. PLoS ONE 2019, 14, e0210772. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatia, R.; Kumar, R.; Behera, T.K.; Kumari, K.; Pramanik, A.; Ghemeray, H.; Sharma, K.; Bhattacharya, R.C.; Dey, S.S. Elucidating mitochondrial DNA markers of ogura-based cms lines in indian cauliflowers (Brassica oleracea var. botrytis L.) and their floral abnormalities due to diversity in cytonuclear interactions. Front. Plant Sci. 2021, 12, 631489. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Kumar, R.; Das, A.; Ghemeray, H.; Behera, T.K.; Dey, S.S. Characterization and genetic analysis of OguCMS and doubled haploid based large genetic arsenal of Indian cauliflowers (Brassica oleracea var. botrytis L.) for morphological, reproductive and seed yield traits revealed their breeding potential. Genet. Resour. Crop. Evol. 2021, 68, 1603–1623. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry: Cambridge, UK, 2010; 480p. [Google Scholar]
- Chen, L.; Liu, Y.-G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 2013, 65, 579–606. [Google Scholar] [CrossRef]
- Hanson, M.R.; Bentolila, S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 2004, 16, S154–S169. [Google Scholar] [CrossRef]
- Bohra, A.; Jha, U.C.; Adhimoolam, P.; Bisht, D.; Singh, N.P. Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep. 2016, 35, 967–993. [Google Scholar] [CrossRef]
- Chase, C.D. Cytoplasmic male sterility: A window to the world of plant mitochondrial–nuclear interactions. Trends Genet. 2007, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.A.; Clarke, A.E. Inheritance of male sterility in the onion and the production of hybrid seed. Proc. Am. Soc. Hortic. Sci. 1943, 43, 189–194. [Google Scholar]
- Jones, H.A.; Davis, G. Inbreeding and Heterosis and Their Relation to the Development of New Varieties of Onions; Technical Bulletin 874; USDA: Washington, DC, USA, 1944.
- Berninger, E. Contribution à l’étude de la stérilité male de l’oignon (Allium cepa L.). Ann. Amelior. Plant 1965, 15, 183–199. [Google Scholar]
- Brewster, J.L. Onions and Other Vegetable Alliums, 2nd ed.; Crop Production Science in Horticulture, 15; CABI Publishing: Wallingford, UK, 2008; 432p, ISBN 13:978-1845933999. [Google Scholar]
- Schweisguth, B. Etude d’un nouveau type de stérilité male chez l’oignon, Allium cepa. Annu. Amélior Plant 1973, 23, 221–233. [Google Scholar]
- Manjunathagowda, D.C.; Muthukumar, P.; Gopal, J.; Prakash, M.; Bommesh, J.C.; Nagesh, G.C.; Megharaj, K.C.; Manjesh, G.N.; Anjanappa, M. Male sterility in onion (Allium cepa L.): Origin, evolutionary status, and their prospectus. Genet. Resour. Crop. Evol. 2021, 68, 421–439. [Google Scholar] [CrossRef]
- Havey, M.J. A putative donor of S-cytoplasm and its distribution among open-pollinated populations of onion. Theor. Appl. Genet. 1993, 86, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gokçe, A.F.; Havey, M.J. Linkage equilibrium among tightly linked RFLPs and the Ms locus in open-pollinated onion populations. J. Am. Soc. Hortic. Sci. 2002, 127, 944–946. [Google Scholar] [CrossRef]
- Havey, M.J.; Kim, S. Molecular marker characterization of commercially used cytoplasmic male sterilities in onion. J. Am. Soc. Hortic. Sci. 2021, 146, 351–355. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S. Identification of a novel haplotype of the Ms locus controlling restoration of male-fertility and its implication in origination of cytoplasmic male-sterility in onion (Allium cepa L.). J. Hortic. Sci. Biotechnol. 2021, 96, 750–758. [Google Scholar] [CrossRef]
- Yu, N.; Kim, S. Identification of Ms2, a novel locus controlling male-fertility restoration of cytoplasmic male-sterility in onion (Allium cepa L), and development of tightly linked molecular markers. Euphytica 2021, 217, 191. [Google Scholar] [CrossRef]
- Khar, A.; Galván, G.A.; Singh, H. Allium Breeding Against Biotic Stresses. In Genomic Designing for Biotic Stress Resistant Vegetable Crops; Kole, C., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Kim, B.; Kim, C.-W.; Kim, S. Inheritance of fertility restoration of male-sterility conferred by cytotype Y and identification of instability of male fertility phenotypes in onion (Allium cepa L.). J. Hortic. Sci. Biotechnol. 2019, 94, 341–348. [Google Scholar] [CrossRef]
- Jones, H.A.; Perry, B.A. Vegetative propagation of short-day varieties of onions as an aid in a breeding program. Proc. Am. Soc. Hortic. Sci. 1949, 53, 367–370. [Google Scholar]
- Fujieda, K.; Matsuoka, N.; Fujita, Y. Vegetative multiplication of onion, Allium cepa L., through tissue culture. J. Jpn. Soc. Hortic. Sci. 1979, 48, 186–194. [Google Scholar] [CrossRef]
- Pike, L.M.; Yoo, K.S. A tissue culture technique for the clonal propagation of onion using immature flower buds. Sci. Hortic. 1990, 45, 31–36. [Google Scholar] [CrossRef]
- Manjunathagowda, D.C.; Anjanappa, M. Identification and development of male sterile and their maintainer lines in short-day onion (Allium cepa L.) genotypes. Genet. Resour. Crop. Evol. 2020, 67, 357–365. [Google Scholar] [CrossRef]
- Ahmad, R.; Hassan, M.; Akhtar, G.B.; Saeed, S.; Khan, S.A.; Shah, M.K.N.; Khan, N. Identification and characterization of important sterile and maintainer lines from various genotypes for advanced breeding programmes of onion (Allium cepa). Plant Breed. 2020, 139, 988–995. [Google Scholar] [CrossRef]
- Kazakova, A.A.; Yakovlev, G.V. Expression of heterosis in onion hybrid produced on male sterility basis. Proc. Bot. Genet. 1973, 49, 268–280. [Google Scholar]
- Sharma, P.K. Studies on general and specific combining ability effects in onion using male sterile, maintainer and restorer lines and hybrids. Ekin J. Crop Breed. Genet. 2022, 8, 77–85. [Google Scholar]
- Pathak, C.S.; Singh, D.P.; Deshpande, A.A. Annual Report; Indian Institute Horticultral Research (IIHR): Bangalore, India, 1980; pp. 34–36. [Google Scholar]
- Pathak, C. A possible new source of male sterility in onion. Acta Hortic. 1997, 4, 313–316. [Google Scholar] [CrossRef]
- Havey, M.J. Seed yield, floral morphology, and lack of male-fertility restoration of male-sterile onion (Allium cepa L.) populations possessing the cytoplasm of Allium galanthum Kir. et Kar. J. Am. Soc. Hortic. Sci. 1999, 124, 626–629. [Google Scholar] [CrossRef]
- Pathak, C.; Gowda, R.V. Breeding for the development of onion hybrids in India: Problems and prospects. Acta Hortic. 1993, 358, 239–242. [Google Scholar] [CrossRef]
- Gökçe, A.F.; McCallum, J.; Sato, Y.; Havey, M.J. Molecular tagging of the Ms locus in onion. J. Am. Soc. Hortic. Sci. 2002, 127, 576–582. [Google Scholar] [CrossRef]
- Bang, H.; Cho, D.Y.; Yoo, K.S.; Yoon, M.K.; Patil, B.S.; Kim, S. Development of simple PCR–based markers linked to the Ms locus, a restorer–of– fertility gene in onion (Allium cepa L.). Euphytica 2011, 179, 439–449. [Google Scholar] [CrossRef]
- Huo, Y.; Miao, J.; Liu, B.; Yang, Y.; Zhang, Y.; Tahara, Y.; Meng, Q.; He, Q.; Kitano, H.; Wu, X. The expression of pectin methylesterase in onion flower buds is associated with the dominant male-fertility restoration allele. Plant Breed. 2012, 131, 211–216. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Huo, Y.M.; Miao, J.; Liu, B.J.; Kong, S.P.; Gao, L.M.; Liu, C.; Wang, Z.B.; Tahara, Y.; Kitano, H.; et al. Identification of two SCAR markers co-segregated with the dominant Ms and recessive ms alleles in onion (Allium cepa L.). Euphytica 2013, 190, 267–277. [Google Scholar] [CrossRef]
- Lee, H.-I.; Havey, M.J. Variable penetrance among different sources of the male fertility restoration allele of onion. HortScience 2020, 55, 543–546. [Google Scholar] [CrossRef]
- Kim, S. A codominant molecular marker in linkage disequilibrium with a restorer–of–Fertility gene (Ms) and its applica-tion in reevaluation of inheritance of fertility restoration in onions. Mol. Breed. 2014, 34, 769–778. [Google Scholar] [CrossRef]
- Kim, B.; Yang, T.-J.; Kim, S. Identification of a gene responsible for cytoplasmic male-sterility in onions (Allium cepa L.) using comparative analysis of mitochondrial genome sequences of two recently diverged cytoplasms. Theor. Appl. Genet. 2019, 132, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bellaoui, M.; Martin-Canadell, A.; Pelletier, G.; Budar, F. Low-copy-number molecules are produced by recombination, actively maintained and can be amplified in the mitochondrial genome of Brassicaceae: Relationship to reversion of the male sterile phenotype in some cybrids. Mol. Genet. Genom. 1998, 257, 177–185. [Google Scholar] [CrossRef]
- Janska, H.; Sarria, R.; Woloszynska, M.; Arrieta-Montiel, M.; Mackenzie, S.A. Stoichiometric shifts in the common bean mitochondrial genome leading to male sterility and spontaneous reversion to fertility. Plant Cell 1998, 10, 1163–1180. [Google Scholar] [CrossRef]
- Sandhu, A.P.; Abdelnoor, R.V.; Mackenzie, S.A. Transgenic induction of mitochondrial rearrangements for cytoplasmic male sterility in crop plants. Proc. Natl. Acad. Sci. USA 2007, 104, 1766–1770. [Google Scholar] [CrossRef]
- Schnable, P. The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci. 1998, 3, 175–180. [Google Scholar] [CrossRef]
- Budar, F.; Touzet, P.; De Paepe, R. The nucleo-mitochondrial conflict in cytoplasmic male sterilities revised. Genetica 2003, 117, 3–16. [Google Scholar] [CrossRef]
- Martin, W.J.; McCallum, J.; Shigyo, M.; Jakse, J.; Kuhl, J.C.; Yamane, N.; Pither-Joyce, M.; Gokce, A.F.; Sink, K.C.; Town, C.D.; et al. Genetic mapping of expressed sequences in onion and in silico comparisons with rice show scant colinearity. Mol. Genet. Genom. 2005, 274, 197–204. [Google Scholar] [CrossRef]
- Kim, Y.; Zhang, D. Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci. 2018, 23, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Small, I.; Suffolk, R.; Leaver, C.J. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 1989, 58, 69–76. [Google Scholar] [CrossRef]
- Albert, B.; Godelle, B.; Gouyon, P.H. Evolution of the plant mitochondrial genome: Dynamics of duplication and deletion of sequences. J. Mol. Evol. 1998, 46, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, B.; Woloszynska, M.; Janska, H. Heteroplasmy as a common state of mitochondrial genetic information in plants and animals. Curr. Genet. 2006, 50, 149–159. [Google Scholar] [CrossRef]
- Woloszynska, M.; Trojanowski, D. Counting mtDNA molecules in Phaseolus vulgaris: Sublimons are constantly produced by recombination via short repeats and undergo rigorous selection during substoichiometric shifting. Plant Mol. Biol. 2009, 70, 511–521. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Singh, S.; Nandi, A. Molecular advances in research and applications of male sterility systems in tomato. Plant Physiol. Biochem. 2025, 220, 109503. [Google Scholar] [CrossRef]
- Gaborieau, L.; Brown, G.G.; Mireau, H. The propensity of pentatricopeptide repeat genes to evolve into restorers of cytoplasmic male sterility. Front. Plant Sci. 2016, 7, 1816. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wise, R.P.; Schnable, P.S. The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 1996, 272, 1334–1336. [Google Scholar] [CrossRef]
- Fujii, S.; Toriyama, K. Suppressed expression of retrograde-regulated male sterility restores pollen fertility in cytoplasmic male sterile rice plants. Proc. Natl. Acad. Sci. USA 2009, 106, 9513–9518. [Google Scholar] [CrossRef]
- Hu, J.; Wang, K.; Huang, W.; Liu, G.; Gao, Y.; Wang, J.; Huang, Q.; Ji, Y.; Qin, X.; Wan, L.; et al. The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant Cell 2012, 24, 109–122. [Google Scholar] [CrossRef]
- Kitazaki, K.; Arakawa, T.; Matsunaga, M.; Yui-Kurino, R.; Matsuhira, H.; Mikami, T.; Kubo, T. Post-translational mechanisms are associated with fertility restoration of cytoplasmic male sterility in sugar beet (Beta vulgaris). Plant J. 2015, 83, 290–299. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.T.; Cho, D.Y.; Han, T.; Bang, H.; Patil, B.S.; Ahn, Y.K.; Yoon, M.-K. Identification of a novel chimeric gene, orf725, and itsuse in development of a molecular marker for distinguishing among three cytoplasm typesinonion (Allium cepa L.). Theor. Appl. Genet. 2009, 118, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, K.; Yang, T.; Kim, S. Completion of the mitochondrial genome sequence of onion (Allium cepa L.) containing the CMS-S male-sterile cytoplasm and identification of an independent event of the ccm FN gene split. Curr. Genet. 2016, 62, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, M.; Kaneko, T.; Sakamoto, T.; Kimura, S.; Shigyo, M.; Yamagishi, H.; Terachi, T. Multichromosomal structure of the onion mitochondrial genome and a transcript analysis. Mitochondrion 2019, 46, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.; Park, M.; Choi, D. Identification of candidate genes associated with fertility restoration of cytoplasmic male sterility in onion (Allium cepa L.) using a combination of bulked segregant analysis and RNA-seq. Theor. Appl. Genet. 2015, 128, 2289–2299. [Google Scholar] [CrossRef]
- Ahn, W.; Kim, S. Identification of a candidate gene responsible for male sterility conferred by CMS-T cytoplasm in onion (Allium cepa L.) and development of molecular markers for detection of CMS-T cytoplasm. Euphytica 2023, 219, 28. [Google Scholar] [CrossRef]
- Engelke, T.; Tatlioglu, T. APCR-marker for the CMS1 inducing cytoplasm in chives derived from recombination events affecting the mitochondrial gene atp9. Theor. Appl. Genet. 2002, 104, 698–702. [Google Scholar] [CrossRef]
- Khar, A.; Zimik, M.; Verma, P.; Singh, H.; Mangal, M.; Singh, M.C.; Gupta, A.J. Molecular marker-based characterization of cytoplasm and restorer of male sterility (Ms) locus in commercially grown onions in India. Mol. Biol. Rep. 2022, 49, 5535–5545. [Google Scholar] [CrossRef]
- Park, J.; Bang, H.; Cho, D.Y.; Yoon, M.-K.; Patil, B.S.; Kim, S. Construction of high-resolution linkage map of the Ms locus, a restorer-of-fertility gene in onion (Allium cepa L.). Euphytica 2013, 192, 267–278. [Google Scholar] [CrossRef]
- Sato, Y. PCR amplification of CMS-specific mitochondrial nucleotide sequences to identify cytoplasmic genotypes of onion (Allium cepa L.). Theor. Appl. Genet. 1998, 96, 367–370. [Google Scholar] [CrossRef]
- Engelke, T.; Terefe, D.; Tatlioglu, T. APCR-based marker system monitoring CMS-(S), CMS-(T) and (N)-cytoplasm in the onion (Allium cepa L.). Theor. Appl. Genet. 2003, 107, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, S. Identification of a variant of CMS-T cytoplasm and development of high resolution melting markers for distinguishing cytoplasm types and genotypinga restorer-of-fertility locus in onion (Allium cepa L.). Euphytica 2019, 215, 164. [Google Scholar] [CrossRef]
- Khrustaleva, L.; Nzeha, M.; Ermolaev, A.; Nikitina, E.; Romanov, V. Two-step identification of N-, S-, R- and T-cytoplasm types in onion breeding lines using high-resolution melting (HRM)-based markers. Int. J. Mol. Sci. 2023, 24, 1605. [Google Scholar] [CrossRef] [PubMed]
- Holford, P.; Croft, J.H.; Newbury, H.J. Differences between, and possible origins of the cytoplasms found in fertile and male-sterile onions (Allium cepa L.). Theor. Appl. Genet. 1991, 82, 737–744. [Google Scholar] [CrossRef]
- Khar, A.; Saini, N. Limitations of PCR based molecular markers to identify male sterile and maintainer plants from Indian onion (Allium cepa L.) populations. Plant Breed. 2016, 135, 519–524. [Google Scholar] [CrossRef]
- Saini, N.; Hedau, N.K.; Khar, A.; Yadav, S.; Bhatt, J.C.; Agrawal, P.K. Successful deployment of marker assisted selection (MAS) for inbred and hybrid development in long-day onion (Allium cepa L.). Indian J. Genet. Plant Breed. 2015, 75, 93. [Google Scholar] [CrossRef][Green Version]
- Singh, H.; Khar, A. Perspectives of onion hybrid breeding in India: An overview. Indian J. Agric. Sci. 2021, 91, 1426–1432. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.; Priyadarsini, S.; Ola, A.L. Genomics empowering conservation action and improvement of celery in the face of climate change. Planta 2024, 259, 42. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Abdedayem, W.; Solmaz, I.; Sari, N.; Garcés-Claver, A. Melon (Cucumis melo L.): Genomics and Breeding. In Smart Plant Breeding for Vegetable Crops in Post-Genomics Era; Singh, S., Sharma, D., Sharma, S.K., Singh, R., Eds.; Springer: Singapore, 2023; pp. 25–52. [Google Scholar] [CrossRef]
- Finkers, R.; van Kaauwen, M.; Ament, K.; Burger-Meijer, K.; Egging, R.; Huits, H.; Kodde, L.; Kroon, L.; Shigyo, M.; Sato, S.; et al. Insights from the first genome assembly of Onion (Allium cepa). G3 Genes|Genomes|Genet. 2021, 11, jkab243. [Google Scholar] [CrossRef]
- Bishnoi, R.; Solanki, R.; Singla, D.; Mittal, A.; Chhuneja, P.; Meena, O.P.; Dhatt, A.S. Comparative mitochondrial genome analysis reveals a candidate ORF for cytoplasmic male sterility in tropical onion. 3 Biotech 2024, 14, 6. [Google Scholar] [CrossRef]
- Yuan, Q.; Song, C.; Gao, L.; Zhang, H.; Yang, C.; Sheng, J.; Ren, J.; Chen, D.; Wang, Y. Transcriptome de novo assembly and analysis of differentially expressed genes related to cytoplasmic male sterility in onion. Plant Physiol. Biochem. 2018, 125, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lan, Y.; Wen, C.; Zhao, H.; Wang, J.; Wang, Y. Transcriptome sequencing analyses between the cytoplasmic male sterile line and its maintainer line in welsh onion (Allium fistulosum L.). Int. J. Mol. Sci. 2016, 17, 1058. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Huang, Y.; Zhu, Z.; Xu, Q. Cloning and expression analysis of onion (Allium cepa L.) Mads-Box genes and regulation mechanism of cytoplasmic male sterility. Biochem. Genet. 2023, 61, 2116–2134. [Google Scholar] [CrossRef] [PubMed]



| Features | CMS-S | CMS-T | CMS-R | CMS-Y |
|---|---|---|---|---|
| Description | First identified in the material of Italian-Red in 1925 | Discovered in the French variety “Jaune Paille Des Vertus” in 1965 | Originated from the onion cultivar “Rijnsburge” | Reported in two onion accessions, PI273626 and PI236025 |
| Male sterility mechanism | Increased genomic shift of the mitochondrial gene orf725 along with a reduction in the copy number of coxI gene | Enhanced genomic shift of orf725 gene along with an increase in the copy number of coxI gene | Male sterility is linked to a specific mitochondrial gene, orf725 | Male sterility is linked to the mitochondrial gene, orf725 with the cox1 gene |
| Fertility restoration (Nuclear Rf gene) | Fertility is restored by a single nuclear dominant locus Ms | Three independent nuclear loci are involved in fertility restoration (A, C, D) | Restored by a nuclear dominant Ms locus | Potentially restored by an Rf gene (not fully characterized) |
| Utility | Stable and widely exploited | Not used commercially | Less common as compared to CMS-S cytoplasm | Unstable and rarerly used |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikh-Rouhou, H.; Singh, S.; Priyadarsini, S.; Mallor, C. Onion Male Sterility: Genetics, Genomics and Breeding. Horticulturae 2025, 11, 539. https://doi.org/10.3390/horticulturae11050539
Chikh-Rouhou H, Singh S, Priyadarsini S, Mallor C. Onion Male Sterility: Genetics, Genomics and Breeding. Horticulturae. 2025; 11(5):539. https://doi.org/10.3390/horticulturae11050539
Chicago/Turabian StyleChikh-Rouhou, Hela, Saurabh Singh, Srija Priyadarsini, and Cristina Mallor. 2025. "Onion Male Sterility: Genetics, Genomics and Breeding" Horticulturae 11, no. 5: 539. https://doi.org/10.3390/horticulturae11050539
APA StyleChikh-Rouhou, H., Singh, S., Priyadarsini, S., & Mallor, C. (2025). Onion Male Sterility: Genetics, Genomics and Breeding. Horticulturae, 11(5), 539. https://doi.org/10.3390/horticulturae11050539









