Genome-Wide Association Studies Provide Molecular Insights into the Genetic Determination of the Fruit Shape-Related Traits of Actinidia eriantha
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Collection
2.2. Determination of the Fruit Shape-Related Traits and Weight
2.3. DNA Extraction and Whole-Genome Resequencing
2.4. SNP Calling
2.5. Genome-Wide Association Studies
2.6. Statistical Analysis
3. Results
3.1. Descriptive Statistics of Fruit Shape-Related Traits
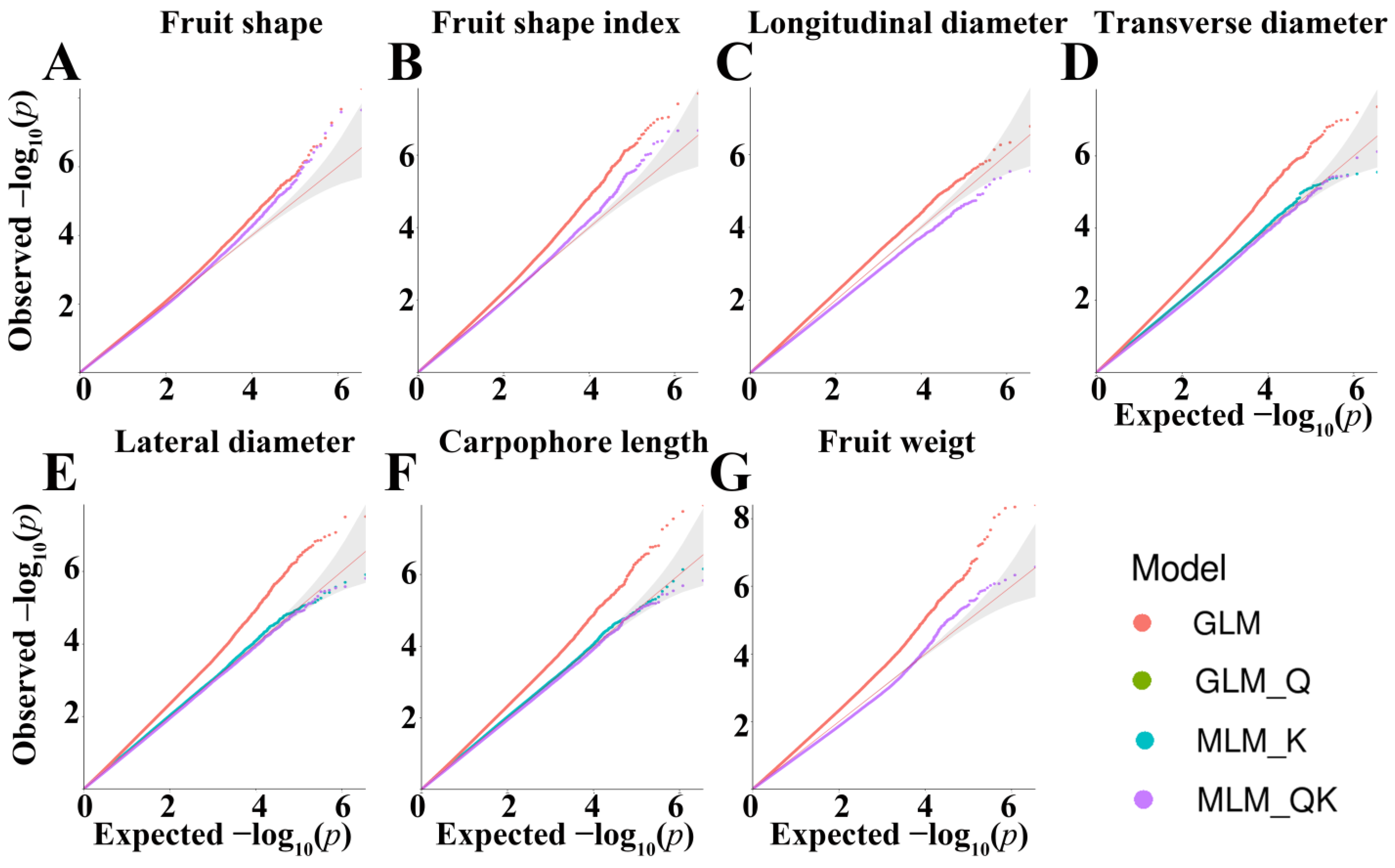
3.2. Comparison of Different Model for GWAS
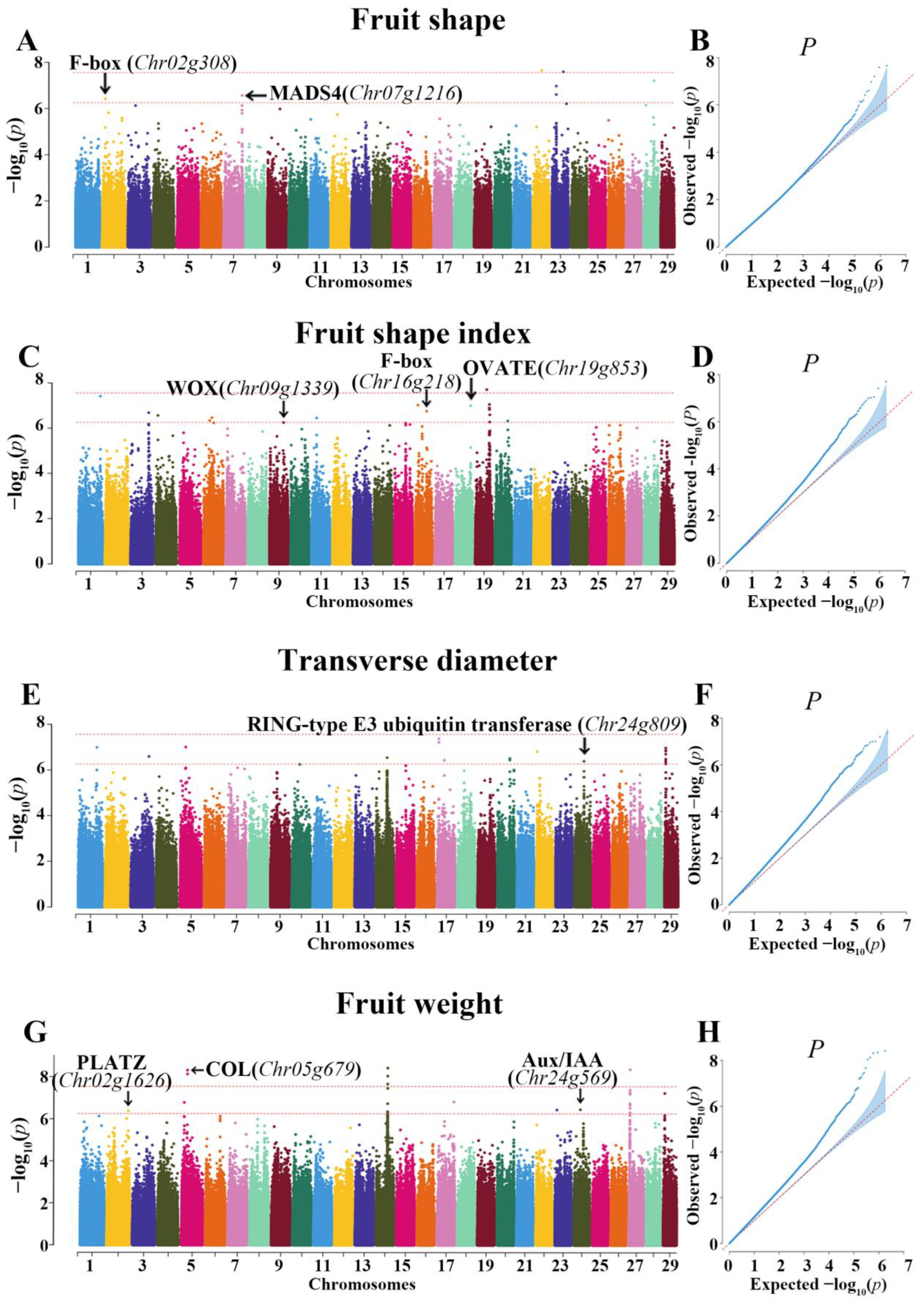
3.3. GWAS Results of Fruit Shape
3.4. GWAS Results of Fruit Shape Index
3.5. GWAS Results of Transverse Diameter
3.6. GWAS Results of Fruit Weight
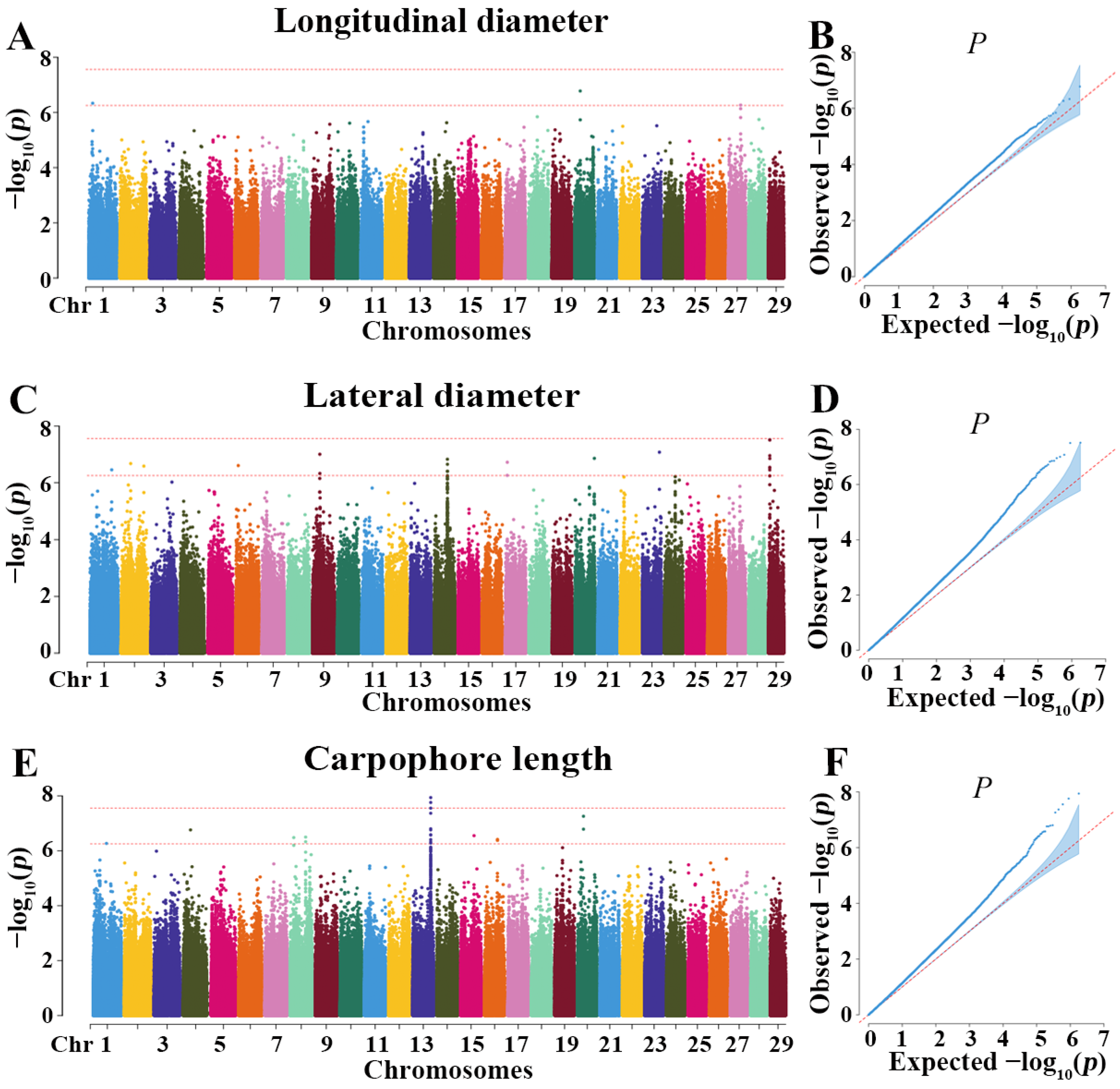
3.7. GWAS Results of Longitudinal Diameter, Lateral Diameter, and Carpophore Length
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SNP | single nucleotide polymorphisms |
| GWAS | genome-wide association analysis |
| CV | coefficient of variation |
| QTLs | quantitative trait loci |
References
- Huang, H.W.; Liu, Y.F. Natural hybridization, introgression breeding, and cultivar improvement in the genus Actinidia. Tree Genet. Genomes 2014, 10, 1113–1122. [Google Scholar] [CrossRef]
- GB/T 19557.11-2022; Guidelines for the Conduct of Test for Distinctness, Uniformity and Stability-Actinidia (Actinidia L.). Chinese National Standard: Beijing, China, 2022.
- Liao, G.L.; Xu, X.B.; Huang, C.H.; Zhong, M.; Jia, D.F. Resource evaluation and novel germplasm mining of Actinidia eriantha. Sci. Hortic. 2021, 282, 110037. [Google Scholar] [CrossRef]
- Xu, X.B.; Liao, G.L.; Huang, C.H.; Zhong, M. Germplasm Resources of Actinidia eriantha; Science Press: Beijing, China, 2021. [Google Scholar]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; Pascale, S.D.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, F.Z.; Chen, Y.H.; Lian, Y. Research progress on inheritance of fruit shape in horticultural crops. Acta Hortic. Sin. 2011, 38, 1385–1396. [Google Scholar]
- Pan, Y.P.; Wang, Y.H.; McGregor, C.; Liu, S.; Luan, F.S.; Gao, M.L.; Weng, Y.Q. Genetic architecture of fruit size and shape variation in cucurbits: A comparative perspective. Theor. Appl. Genet. 2020, 133, 1–21. [Google Scholar] [CrossRef]
- Chunthawodtiporn, J.; Hill, T.; Stoffel, K.; Van, D.A. Quantitative trait loci controlling fruit size and other horticultural traits in bell pepper (Capsicum annuum). Plant Genome 2018, 11, 160125. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.Y.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; et al. A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nat. Commun. 2018, 9, 4734. [Google Scholar] [CrossRef]
- Shan, W.; Han, X.; Antonio, C.; Tea, M.; Esther, V.D.K. SUN regulates vegetative and reproductive organ shape by changing cell division patterns. Plant Physiol. 2011, 157, 1175–1186. [Google Scholar]
- Knaap, E.; Chakrabarti, M.; Chu, Y.H.; Clevenger, J.P.; Illa-Berenguer, E.; Huang, Z.; Keyhaninejad, N.; Mu, Q.; Sun, L.; Wang, Y.; et al. What lies beyond the eye: The molecular mechanisms regulating tomato fruit weight and shape. Front. Plant Sci. 2014, 5, 227. [Google Scholar]
- Wei, Q.Z.; Fu, W.Y.; Wang, Y.Z.; Qin, X.D.; Wang, J.; Li, J.; Lou, Q.F.; Chen, J.F. Rapid identification of fruit length loci in cucumber (Cucumis sativus L.) using next-generation sequencing (NGS)-based QTL analysis. Sci. Rep. 2016, 6, 27496. [Google Scholar] [CrossRef]
- Weng, Y.Q.; Colle, M.; Wang, Y.H.; Yang, L.; Rubinstein, M.; Sherman, A.; Ophir, R.; Grumet, R. QTL mapping in multiple populations and development stages reveals dynamic quantitative trait loci for fruit size in cucumbers of different market classes. Theor. Appl. Genet. 2015, 128, 1747–1763. [Google Scholar] [CrossRef]
- Pan, Y.P.; Liang, X.J.; Gao, M.L.; Liu, H.Q.; Meng, H.W.; Weng, Y.Q.; Cheng, Z.H. Round fruit shape in WI7239 cucumber is controlled by two interacting quantitative trait loci with one putatively encoding a tomato SUN homolog. Theor. Appl. Genet. 2017, 130, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Wu, Y.; Wang, X.L.; Ren, W.M.; Chen, Q.Y.; Zhang, S.J.; Zhang, F.; Lin, Y.Z.; Yue, J.Y.; Liu, Y.S. Genome wide association analysis identifies candidate genes for fruit quality and yield in Actinidia eriantha. J. Integr. Agric. 2024, 23, 1929–1939. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xue, C.; Hu, H.J.; Li, J.M.; Xue, Y.S.; Wang, R.Z.; Fan, J.; Zou, C.; Tao, S.T.; Qin, M.F.; et al. Genome-wide association studies provide insights into the genetic determination of fruit traits of pear. Nat. Commun. 2021, 12, 1144. [Google Scholar] [CrossRef]
- Zhang, S.W.; Wu, S.Q.; Jia, Z.Q.; Zhang, J.H.; Li, Y.; Ma, X.Y.; Fan, B.L.; Wang, P.Q.; Gao, Y.N.; Ye, Z.B.; et al. Exploring the influence of a single-nucleotide mutation in EIN4 on tomato fruit firmness diversity through fruit pericarp microstructure. Plant Biotechnol. J. 2024, 22, 2379–2394. [Google Scholar] [CrossRef]
- Hu, L.; Xu, T.; Cai, Y.; Qin, Y.; Zheng, Q.; Chen, T.; Gong, L.; Yang, J.; Zhao, Y.; Chen, J.; et al. Identifying candidate genes for grape (Vitis vinifera L.) fruit firmness through genome-wide association studies. J. Agric. Food Chem. 2025, 73, 8413–8425. [Google Scholar] [CrossRef]
- Zaidan, I.R.; Ferreira, M.F.; Pereira do Couto, D.; Santos, J.G.; Silva, M.A.; Canal, G.B.; Bernardes, C.; Azevedo, C.F.; Ferreira, A. Genome-wide association analysis of traits related to development, abiotic and biotic stress resistance in Coffea canephora. Sci. Hortic. 2025, 341, 114004. [Google Scholar] [CrossRef]
- Whitt, L.; Bennett, J.S.; Collum, T.D.; Evans, B.; Raines, D.; Gutierrez, B.; Janisiewicz, W.J.; Jurick, W.M.; Gottschalk, C. Genome-wide associations within diverse wild apple germplasm for postharvest blue mold resistance to Penicillium expansum. Postharvest Biol. Technol. 2025, 225, 113513. [Google Scholar] [CrossRef]
- Liao, G.L.; Zhong, M.; Jiang, Z.Q.; Tao, J.J.; Jia, D.F.; Qu, X.Y.; Huang, C.H.; Liu, Q.; Xu, X.B. Genome-wide association studies provide insights into the genetic determination of flower and leaf traits of Actinidia eriantha. Front. Plant Sci. 2021, 12, 730890. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Qu, D.; Sun, W.; Yu, Y.; Zhang, Y. Study on population structure of kiwifruit and GWAS for hairiness character. Gene 2022, 821, 146276. [Google Scholar] [CrossRef]
- Lu, X.M.; Yu, X.F.; Li, G.Q.; Qu, M.H.; Wang, H.; Liu, C.; Man, Y.P.; Jiang, X.H.; Li, M.Z.; Wang, J.; et al. Genome assembly of autotetraploid Actinidia arguta highlights adaptive evolution and enables dissection of important economic traits. Plant Commun. 2024, 5, 100856. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Wang, R.; Lin, M.M.; Gu, H.; Li, Y.K.; Zhang, M.; Feng, X.; Qi, X.J. Construction of a high-density genetic map and QTL mapping of growth traits in kiwifruit. Sci. Hortic. 2025, 339, 113816. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.S.; Gao, H.; Cao, J.L.; Qian, J.Q.; Zheng, K.X.; Jia, D.F.; Gao, Z.; Xu, X.B. A model for selecting kiwifruit (Actinidia eriantha) germplasm resources with excellent fruit quality. Foods 2024, 13, 4014. [Google Scholar] [CrossRef]
- Kartavya, V.; Caroline, G.; Simon, A.; Christel, K. Librarian: A quality control tool to analyse sequencing library compositions. F1000Res 2022, 11, 1122. [Google Scholar]
- Chen, S.F. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Yang, Y.D.; Huang, L.Y.; Xu, C.Y.; Qi, L.; Wu, Z.Y.; Li, J.; Chen, H.X.; Wu, Y.; Fu, T.; Zhu, H.; et al. Chromosome-scale genome assembly of areca palm (Areca catechu). Mol. Ecol. Resour. 2021, 21, 2504–2519. [Google Scholar] [CrossRef]
- Warden, C.D.; Adamson, A.; Neuhausen, S.L.; Wu, X.W. Detailed comparison of two popular variant calling packages for exome and targeted exon studies. Peerj 2014, 2, e600. [Google Scholar] [CrossRef]
- Zheng, X.W.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef]
- Long, P.; Tan, H.; Chen, B.; Wang, L.; Quan, R.; Hu, Z.; Zeng, M.; Greenbaum, J.; Shen, H.; Deng, H.; et al. Dissecting the shared genetic architecture between anti-Müllerian hormone and age at menopause based on genome-wide association study. Am. J. Obstet. Gynecol. 2024, 231, 634.e1–634.e11. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, L.W.; Fang, J.G. Genome-wide association study of the candidate genes for grape berry shape-related traits. BMC Plant Biol. 2022, 22, 42. [Google Scholar] [CrossRef]
- Zhou, Y.; Ge, L.; Hu, L.; Yang, Y.; Liu, S. A cucumber AGAMOUS-LIKE 15 (AGL15) MADS-Box gene mediates abnormal leaf morphology in Arabidopsis. Agronomy 2018, 8, 265. [Google Scholar] [CrossRef]
- Tsaballa, A.; Pasentsis, K.; Darzenta, N.; Tsaftaris, A. Multiple evidence for the role of an Ovate-like gene in determining fruit shape in pepper. BMC Plant Biol. 2011, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Cui, C. Analysis of the Mechanism of Wheat RING typeE3 Ubiquitin Ligase TaBB Regulating Grain Size. Master’s Thesis, Northwest A&F University, Yangling, China, 2023. [Google Scholar]
- Fu, Y.X.; Cheng, M.P.; Li, M.L.; Guo, X.J.; Wu, Y.R.; Wang, J.R. Identification and characterization of PLATZ transcription factors in wheat. Int. J. Mol. Sci. 2020, 21, 8934. [Google Scholar] [CrossRef]
- Borovsky, Y.; Raz, A.; Doron-Faigenboim, A.; Zemach, H.; Karavani, E.; Paran, I. Pepper fruit elongation is controlled by capsicum annuum ovate family protein 20. Front. Plant Sci. 2022, 12, 815589. [Google Scholar] [CrossRef] [PubMed]
- Dujak, C.; Alcudia, V.C.; Aranzana, M.J. Genomic analysis of fruit size and shape traits in apple: Unveiling candidate genes through GWAS analysis. Hortic. Res. 2024, 11, uhad270. [Google Scholar] [CrossRef]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef]
- Rodríguez, G.; Muños, S.; Anderson, C.; Sim, S.C.; Michel, A.; Causse, M.; Gardener, B.M.; Francis, D.; Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011, 156, 275–285. [Google Scholar] [CrossRef]
- Tan, Q.P.; Liu, X.; Gao, H.R.; Xiao, W.; Chen, X.D.; Fu, X.L.; Li, L.; Li, D.M.; Gao, D.S. Comparison between flat and round peaches, genomic evidences of heterozygosity events. Front. Plant Sci. 2019, 10, 592. [Google Scholar]
- Zhou, H.; Ma, R.; Gao, L.; Zhang, J.; Zhang, A.; Zhang, X.; Ren, F.; Zhang, W.; Liao, L.; Yang, Q.; et al. A 1.7-Mb chromosomal inversion downstream of a PpOFP1 gene is responsible for flat fruit shape in peach. Plant Biotechnol. J. 2020, 19, 192–205. [Google Scholar] [CrossRef]
- Hickey, J.M.; Crossa, J.; Babu, R.; Campos, G.D.L. Factors affecting the accuracy of genotype imputation in populations from several maize breeding programs. Crop Sci. 2012, 52, 654–663. [Google Scholar] [CrossRef]
- Macnee, N.; Hilario, E.; Tahir, J.; Currie, A.; Warren, B.; Rebstock, R.; Hallett, I.C.; Chagné, D.; Schaffer, R.J.; Bulley, S.M. Peridermal fruit skin formation in Actinidia spp. (kiwifruit) is associated with genetic loci controlling russeting and cuticle formation. BMC Plant Biol. 2021, 21, 334. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.K.; Zhang, Y.; Tu, Y.; Wang, Y.Z.; Cheng, W.J.; Yang, Y.W. Overexpression of an EIN3-binding F-box protein2-like gene caused elongated fruit shape and delayed fruit development and ripening in tomato. Plant Sci. 2018, 272, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xu, Y.; Chang, F.Q.; Li, X.D.; Ma, R.C. Cloning and characterization of two MADS box genes from peach (Prunus persica). J. Genet. Genom. 2004, 30, 908–918. [Google Scholar]
- Lenhard, M.; Bohnert, A.; Jürgens, G.; Laux, T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 2001, 105, 805–814. [Google Scholar] [CrossRef]
- Rodríguez, G.R.; Kim, H.J.; Knaap, V.D.E. Mapping of two suppressors of OVATE (sov) loci in tomato. Heredity 2013, 111, 256–264. [Google Scholar] [CrossRef]
- Guo, X.J.; Fu, Y.X.; Lee, Y.R.J.; Chern, M.; Li, M.L.; Cheng, M.; Dong, H.X.; Yuan, Z.W.; Gui, L.X.; Yin, J.J.; et al. The PGS1 basic helix-loop-helix (bHLH) protein regulates Fl3 to impact seed growth and grain yield in cereals. Plant Biotechnol. J. 2022, 20, 1311–1326. [Google Scholar] [CrossRef]
- Zhao, F.F.; Zhang, J.J.; Weng, L.; Li, M.; Wang, Q.H.; Xiao, H. Fruit size control by a zinc finger protein regulating pericarp cell size in tomato. Mol. Hortic. 2021, 1, 6. [Google Scholar] [CrossRef]
- Han, X.; Rong, H.; Tian, Y.T.; Qu, Y.S.; Xu, M.; Xu, L. Genome-wide Identification of PLATZ transcription factors in ginkgo biloba L. and their expression characteristics during seed development. Front. Plant Sci. 2022, 13, 946194. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.Y.; Xiao, Q.L.; Luo, L.; Zhang, C.X.; Mao, C.Q.; Du, J.; Long, T.D.; Cao, Y.; Yi, Q.; et al. Transcription factor ZmPLATZ2 positively regulate the starch synthesis in maize. Plant Growth Regul. 2021, 93, 291–302. [Google Scholar] [CrossRef]
- Su, L.Y.; Audran, C.; Bouzayen, M.; Roustan, J.P.; Chervin, C. The Aux/IAA, Sl-IAA17 regulates quality parameters over tomato fruit development. Plant Signal. Behav. 2015, 10, e1071001. [Google Scholar] [CrossRef]
- Bu, H.D.; Sun, X.H.; Yue, P.T.; Qiao, J.L.; Sun, J.M.; Wang, A.D.; Yuan, H.; Yu, W.Q. The MdAux/IAA2 transcription repressor regulates cell and fruit size in apple fruit. Int. J. Mol. Sci. 2022, 23, 9454. [Google Scholar] [CrossRef]
- Liu, F.F.; Jiang, Y.; Cao, Y.Y.; Jing, J.Z.; Wang, L.F.; Li, J.J.; Wang, H.; Li, H.Y. Expression pattern assay of ZmGW2,a RING-domain E3 ubiquitin ligase gene in maize. J. Maize Sci. 2013, 21, 47–51. [Google Scholar]


| Traits | Range | Med | Mean | SD | CV | H’ | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| Fruit shape index | 1.10–2.82 | 1.60 | 1.64 | 0.27 | 16.62 | 5.32 | 0.98 | 1.93 |
| Longitudinal diameter (mm) | 20.21–53.78 | 37.38 | 37.32 | 6.03 | 16.15 | 5.36 | 0.28 | 0.11 |
| Transverse diameter (mm) | 14.52–32.48 | 23.21 | 23.14 | 3.12 | 13.49 | 5.37 | 0.10 | 0.07 |
| Lateral diameter (mm) | 13.41–30.70 | 21.21 | 21.09 | 2.84 | 13.47 | 5.37 | 0.27 | 0.26 |
| Carpophore length (mm) | 7.49–24.40 | 13.05 | 13.19 | 2.73 | 20.72 | 5.35 | 0.34 | 0.38 |
| Fruit weight (g) | 3.39–25.41 | 10.60 | 11.40 | 3.89 | 34.09 | 5.36 | 0.63 | 0.19 |
| GLM | GLM-Q | MLM-K | MLM-QK | |
|---|---|---|---|---|
| Fruit shape | 7 | 7 | 9 | 7 |
| Fruit shape index | 17 | 5 | 17 | 5 |
| Longitudinal diameter | 3 | 0 | 3 | 0 |
| Transverse diameter | 20 | 0 | 0 | 0 |
| Lateral diameter | 21 | 0 | 0 | 0 |
| Carpophore length | 20 | 0 | 0 | 0 |
| Fruit weight | 27 | 2 | 22 | 2 |
| Total | 115 | 14 | 51 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Jia, D.; Liu, Y.; Gao, H.; Mao, J.; Xu, X. Genome-Wide Association Studies Provide Molecular Insights into the Genetic Determination of the Fruit Shape-Related Traits of Actinidia eriantha. Horticulturae 2025, 11, 538. https://doi.org/10.3390/horticulturae11050538
Chen L, Jia D, Liu Y, Gao H, Mao J, Xu X. Genome-Wide Association Studies Provide Molecular Insights into the Genetic Determination of the Fruit Shape-Related Traits of Actinidia eriantha. Horticulturae. 2025; 11(5):538. https://doi.org/10.3390/horticulturae11050538
Chicago/Turabian StyleChen, Lu, Dongfeng Jia, Yansong Liu, Huan Gao, Jipeng Mao, and Xiaobiao Xu. 2025. "Genome-Wide Association Studies Provide Molecular Insights into the Genetic Determination of the Fruit Shape-Related Traits of Actinidia eriantha" Horticulturae 11, no. 5: 538. https://doi.org/10.3390/horticulturae11050538
APA StyleChen, L., Jia, D., Liu, Y., Gao, H., Mao, J., & Xu, X. (2025). Genome-Wide Association Studies Provide Molecular Insights into the Genetic Determination of the Fruit Shape-Related Traits of Actinidia eriantha. Horticulturae, 11(5), 538. https://doi.org/10.3390/horticulturae11050538







