Abstract
Strawberries are nutrient-rich fruits containing beneficial phytochemicals and bioactive compounds with significant health benefits. Among secondary metabolites, the polyphenolic compounds have been extensively studied. However, limited research exists on bioactive alkaloids in strawberry fruits. Additionally, the local “Akihime” strawberry variety cultivated in the high altitude of Zhaotong city is of inferior quality; in contrast, the “Red Face” variety cultivated in the lower altitude of Dandong city demonstrates superior quality attributes. This study aimed to introduce the “Red Face” strawberry variety from the lower altitude region of Dandong to the high altitudes of Zhaotong. The primary objectives were to enhance local strawberry quality and investigate the diversity of alkaloids and their biosynthesis genes in response to varying ecological conditions. In this study, a transcriptomic and LC–MS/MS approach identified several biosynthesis genes, 33 alkaloids, and 38 other bioactive compounds, reported for the first time in the strawberry fruits. Five alkaloids ergotamine, 3-indoleacrylic acid, L-pipecolic acid, 8-hydroxyquinoline, and indole, were abundantly found in both strawberry varieties. Principal component analysis and hierarchical cluster analysis revealed significant variation in the individual alkaloid compounds among the different strawberry varieties and ecological conditions. Cultivation of the “Red Face” variety at high-altitude environments modified the gene expressions and enhanced the total alkaloid contents and the antioxidant activity and capacity of strawberry fruits. Our study concluded that strawberries possess a diversity of bioactive alkaloid compounds, and introducing the “Red Face” variety at high-altitude environments produces superior quality of strawberry fruits with improved total alkaloid contents and antioxidant activities.
1. Introduction
Strawberries are globally recognized as the most significant and widely consumed soft fruit, with a production surge of 40% over the past decade [1,2]. Research highlights that strawberries are abundant in essential metabolites such as vitamin C, folate, phenolic substances, flavonoids, ellagitannins, and anthocyanins [3,4,5]. These berries play a significant role in the local economy and are consumed fresh as well as in jams, juices, and jellies [6,7]. Diets rich in fruits and vegetables like strawberries are acknowledged for their potential in preventing various health issues including cancer, cardiovascular diseases, and neurodegenerative conditions [8,9,10,11,12,13]. China is the world’s largest strawberry producer, and this fruit crop plays a significant role in the country’s economy [14,15]. Strawberry cultivation is widespread across China, with the provinces of Hebei, Shandong, and Liaoning ranking among the top producers nationally. The widespread cultivation and substantial output of strawberries underscore their crucial role in China’s agricultural sector [15]. Among the different cultivated varieties, the Dandong 99 strawberry variety (Fragaria × ananassa “Red Face”), has garnered significant attention in both domestic and international markets due to its exceptional organoleptic properties, including vibrant coloration, distinctive flavor profile, and superior taste characteristics [16,17].
Strawberry fruits are abundant in essential metabolites, making them a subject of keen interest in nutritional profile and health research [18]. Strawberries are particularly rich in vitamin C, a potent antioxidant crucial for immune function and skin health [19,20]. Additionally, they contain significant amounts of folate, a B-vitamin essential for DNA synthesis and cell division [21]. In plants, the metabolites such as lipids, flavonoids, alkaloids, ellagitannins, and anthocyanins are present in substantial quantities and have been associated with various health benefits, including cardiovascular protection, anti-inflammatory effects, anticancer activities, antimicrobial properties, reducing the risk of chronic diseases, and improving cognitive function [19,22,23,24,25,26,27,28]. In strawberry fruits, metabolic concentrations exhibit significant alterations in response to various environmental factors, including light quality and intensity, temperature fluctuations, and water availability [29,30,31,32,33,34]. Strawberries are predominantly recognized for their abundance in antioxidant polyphenols. However, recent research has shed light on their alkaloid content, a class of nitrogen-containing compounds with various biological activities [35]. Alkaloids have garnered increasing attention in fruit research due to their potential pharmacological properties [21,24]. However, the diversity and distribution of alkaloids in strawberry fruits remain largely unexplored, presenting a significant gap in our understanding of strawberry biochemistry.
In Dandong city, the Dandong 99 strawberry (Fragaria × ananassa “Red Face”) is rich in secondary metabolites and of superior quality [16]. Conversely, the Zhaotong city local variety (Fragaria × ananassa Duch. cv. “Akihime”) has inferior quality with lower sugar content and secondary metabolites [17]. Notably, Zhaotong city is known for producing high-quality apples due to significant diurnal temperature variations and abundant sunlight exposure [17,36]. Zhaotong city is located in southwestern China at an altitude of 1900 m, while Dandong city is located in northeastern China at a lower altitude of 20 m [16,36]. In this study, we aimed to introduce the Dandong 99 strawberry variety from Dandong city to Zhaotong city to enhance local strawberry fruit quality. We employed liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify the diversity of bioactive alkaloids in strawberry fruits and their corresponding biosynthesis genes under different ecological conditions. Furthermore, we evaluated the total alkaloid content and antioxidant activities in the strawberry fruits.
2. Materials and Methods
2.1. Strawberry Varieties, Environmental Data Collection, and Cultivation Conditions
The Dandong 99 “Red Face” strawberry, known as “99”, was originally cultivated in Dandong, a northeastern Chinese city located at 40°07′ N, 124°23′ E, with an elevation of 20 m above sea level. This low-altitude variety was subsequently introduced to Zhaotong city (27.34° N, 103.72° E) at an elevation of 1900 m. For simplicity, the “99” variety cultivated in Dandong (DD) was named as DD99 and the variety cultivated in Zhaotong (ZT) was named as ZT99. Concurrently, “Akihime”, a local strawberry variety cultivated in Zhaotong, was named as ZT. DD99 was cultivated in the lower elevations of Dandong (https://globalsolaratlas.info/map?s=40.123766,124382175&m=site&c=41668151,119,6 accessed on 20 January 2024) while both ZT and ZT99 were cultivated in Zhaotong’s higher altitude environment (https://globalsolaratlas.info/map?s=27.341751,103.714692&m=site&c=27.341751,103.711-4692,11 accessed on 20 January 2024), providing an opportunity for comparative analysis of strawberry cultivation across diverse geographical and elevational conditions.
Daily temperature data (average, maximum, and minimum) for Zhaotong and Dandong were obtained from [source: Global Solar Atlas, https://globalsolaratlas.info/ accessed on 11 April 2024] during the cultivation period (August–December 2023). Diurnal temperature shifts were calculated as the difference between daily maximum and minimum temperatures. Zhaotong exhibited larger diurnal shifts (>10 °C), while Dandong had more moderate fluctuations (<6 °C).
The ZT seedlings were obtained from the experimental fields of Zhaotong University, while DD99 seedlings were procured from Dongji Luyuan Agriculture and Animal Husbandry Co., Ltd. in Fengcheng, Dandong city. All seedlings were homogeneous, with an initial height of 10 cm. In August 2023, the seedlings were transplanted into greenhouses: ZT99 and ZT were cultivated in the experimental greenhouse at Zhaotong University under natural environmental conditions, while DD99 was grown in the greenhouse facilities at Dongji Luyuan Agriculture and Animal Husbandry Co., Ltd., (Dandong, China) also under natural environmental conditions. Standard fertilization, normal irrigation practices, sandy loam soil, and similar cultural practices were implemented for all seedlings, at both locations [17,37,38]. Strawberry samples were collected concurrently from DD99, ZT99, and ZT on 12 December 2023. Each treatment consisted of five biological replicates per sample group (each replicate has three individual plants) and homogenized in liquid nitrogen, followed by division into three aliquots, and preserved in cryotubes. These samples were initially stored in liquid nitrogen before being transferred to a −80 °C freezer for subsequent analysis. A comprehensive ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis was conducted to elucidate the diversity of bioactive compounds among the strawberry varieties.
2.2. Strawberry Metabolomic Extraction and Analysis
For the preparation and extraction of strawberry fruit samples, specifically for alkaloid analysis, freeze-dried strawberry fruit samples were pulverized in a Retsch MM 400 mixer mill (Fisher Scientific, Haan, Germany) using a zirconia bead for 1.5 min at 30 Hz. Subsequently, 50 mg of the powdered sample was extracted with 0.5 mL of a methanol/water/hydrochloric acid mixture (500:500:1, v/v/v). This mixture was vortexed for 5 min, sonicated for another 5 min, and then centrifuged at 12,000× g at 4 °C for 3 min. These extraction steps were repeated to process the remaining strawberry fruit residue. The resulting supernatants were then filtered through a 0.22 μm filter (Anpel) before being subjected to LC–MS/MS analysis.
The chemicals/reagents included high-purity liquid chromatography grade methanol (MeOH) sourced from Merck (Darmstadt, Germany). All experiments utilized MilliQ water from Millipore (St. Louis, MO, USA). Quality control (QC) utilized an internal standard obtained from BioBioPha Co., Ltd. (Kunming, China) and Sigma-Aldrich (St. Louis, MO, USA). Additional reagents like acetonitrile and hydrochloric acid were acquired from Merck and Xinyang Chemical Reagents (Changsha, China), respectively. Standard solutions were prepared at a concentration of 1 mg/mL in 50% MeOH and stored at −20 °C. These were then diluted to working solutions with 50% MeOH prior to analysis.
2.3. Metabolic Operating System Condition
The ultra-performance liquid chromatography (UPLC) conditions for analyzing the sample extracts were set up on a UPLC-ESI-MS/MS system (UPLC and 6500 Triple Quadrupole, SCIEX brand, Beijing, China). The UPLC system utilized a Waters Acquity BEH C18 column (1.7 μm, 2.1 mm × 100 mm) with a solvent system of water and methanol, both containing 0.1% formic acid. The gradient program started at 95:5 v/v for 0 min, changed to 50:50 v/v at 6 min, and then to 5:95 v/v at 12 min, holding for 2 min before returning to 95:5 v/v at 14 min and holding again for 2 min. The flow rate was maintained at 0.35 mL/min, the column temperature at 40 °C, and the injection volume was 2 μL. The effluent from the column was directed into an electrospray ionization (ESI) triple quadrupole-linear ion trap (QTRAP) mass spectrometer detection, as described earlier [39].
2.3.1. Electrospray Ionization-Mass Spectrometry (ESI-MS/MS) Techniques
Using a QTRAP mass spectrometer (API 6500 QTRAP UPLC/MS/MS, SCIEX, Beijing, China) equipped with an ESI Turbo Ion-Spray interface, scans were conducted in a linear ion trap (LIT) and triple quadrupole (QQQ) configurations. The system operated in positive ion mode and was managed using Analyst 1.6.3 software (AB Sciex). Operational parameters for the ESI source included a turbo spray ion source, a source temperature of 550 °C, an ion spray voltage of 5500 V in positive ion mode, and a curtain gas setting of 35 psi. Optimization of Declustering Potential (DP) and Collision Energy (CE) was performed for each Multiple Reaction Monitoring (MRM) transition based on the metabolites being analyzed. The multiple reactions monitoring was conducted by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China).
2.3.2. Multiple Reactions Monitoring System
Metabolic profiling was conducted using the MRM approach of the triple quadrupole mass spectrometry, as described before [23,39,40]. Initially, the MRM mode selectively screens the precursor ion of the target analyte, excluding ions of other molecular weights to minimize initial interference. Once the precursor ions enter the collision chamber, they are ionized, resulting in the formation of numerous fragment ions. These fragments are then passed through the triple quadrupole system, which selects specific characteristic fragment ions necessary for the analysis. This step helps in reducing interference from non-target ions, thereby enhancing the accuracy and repeatability of the quantification. Subsequently, the mass spectral data from various samples were analyzed, integrating the peak areas of all detected substances and correcting for variations in the peak areas of the same metabolite across different samples.
2.4. RNA Sequencing
Total RNA was isolated from strawberry fruits using the RNA extraction TRIzol kit (Invitrogen, Carlsbad, CA, USA). RNA assembly and sequencing libraries were created by Berry Hekang Biotech. (Beijing, China). For sequencing library preparation, the Illumina (NEB, Ipswich, MA, USA) RNA Library Prep Kit (NEBNext UltraTM) was utilized, following the company’s guidelines. Low-quality sequence reads were removed from all data sets, and paired-end reads were generated using the Illumina HiSeq 2500 platform (Illumina, Shanghai, China). The reference genome for this project is Fragariavescav6genome.fasta, available at http://eplantftp.njau.edu.cn/Fragaria/F._vesca/F._vesca_v6.0/ accessed on 4 December 2023. The genome structure annotation file is Fragariavescav6genome.gff. The database platform, assessed on 4 December 2023, showed that the gene IDs in the raw transcriptomic data corresponded to the reference genome of Fragariavescav6genome (Table S2), and clean reads mapping was performed using hisat2. For further gene annotation analysis, the following databases were used: Kyoto Encyclopedia of Genes and Genomes Ortholog database (KO); proteinfamily (Pfam); gene ontology (GO); non-redundant nucleotide sequences, NCBI (Nt); Swiss-Prot (a protein sequence database); clusters of orthologous groups of proteins (KOG/COG); and non-redundant protein sequences, NCBI (Nr). The cutadapt tool was used to filter adaptor sequences, while fastqc was employed for the quality check of RNA-seq data. Differentially expressed genes (DEGs) were identified using cuffdiff, with significant DEGs determined by |Log2FoldChange| ≥ 1 and p-value < 0.05. GO enrichment analysis of DEGs was conducted using the Wallenius non-central hypergeometric (GOseq) R package (version 2.18.0). KOBAS 2.0 software was used for statistical enrichment analysis of DEGs in KEGG pathways.
2.5. Hydroxyl Radical Scavenging Assay
To assess antioxidant activities of strawberry fruits, a modified hydroxyl radical scavenging assay was performed [41]. The assay mixture included 2.8 mL of deionized water, 0.5 mL of 9 mmol/L salicylic acid-ethanol, 0.5 mL of 9 mmol/L FeSO4, and 200 μL of extracts at a concentration of 3.2 mg/mL. The reaction was initiated by adding 0.5 mL of hydrogen peroxide (8.8 mM). Strawberry fruit samples were then incubated at 37 °C for 30 min, and the absorbance was measured at 510 nm using a Shimadzu UV-1800 spectrophotometer (SHIMADZU CORPORATION, Kyoto, Japan). Butylated hydroxytoluene (BHT) served as a positive control. The scavenging ability percentage was calculated using the formula:
Ability to Scavenging reactive oxygen species (%) = [({A_0 − A_i}{A_0}) × 100]
(A_0) is the absorbance of the control (0.5 mL deionized water replacing the sample) and (A_i) is the absorbance of the sample extract.
2.6. Alkaloid Content and Antioxidant Properties
To determine the total alkaloids, 100 mg of fresh strawberry fruit powder was utilized, employing the bromocresol green method as previously outlined [18]. Absorbance for strawberry fruit samples was measured at 470 nm using a UV-1800 spectrophotometer from Shimadzu, Kyoto, Japan. The ferric reducing antioxidant power (FRAP) assay was applied to assess antioxidant capacity [42]. In this process, Fe3+ bonded with 2,4,6-tris(2-pyridyl)-1,2,3-triazine (TPTZ) is converted to the Fe2+ -TPTZ complex, resulting in a blue color. Spectrophotometric readings of this blue color were taken at 700 nm. Additionally, the 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) method was utilized [43]. For the DPPH assay, 1 mL of an extraction mixture containing ethanol (70%), water (29%), and acetic acid (1%) was blended with 100 mg of strawberry fruit powder and centrifuged at 8000× g for 8 min. Subsequently, 30 μL of the supernatant was combined with 2.97 mL of DPPH, incubated in the dark for 30 min, and absorbance was recorded at 517 nm using the same spectrophotometer. Trolox was employed as a reference standard in both tests. The following formula was used for calculating DPPH antioxidant activity (AA).
DPPH AA (%) = 100 × [1 − {Absorbance of sample/Absorbance of control}].
2.7. Statistical Analysis
Hierarchical cluster analysis (HCA) and principal component analysis (PCA) were conducted by using R software (Rstudio version 2022.07.2 Build 576) (https://www.r-project.org/, accessed on 9 May 2025). For HCA the rows were normalized. Peak areas were utilized to construct the HCA and PCA, and raw LC-MS/MS data can be found in (Table S1). The least significant difference (LSD) test was applied to determine significant differences in alkaloid contents and their antioxidant properties at a significance level of p < 0.05, using Statistix 8.1 software (Tallahassee, FL, USA). Alkaloid content, antioxidant activity and capacity assay graphs, alkaloids and antioxidant assay correlation analysis, and standard errors were analyzed using Microsoft Excel (2010 version) (Redmond, WA, USA).
3. Results
In this study, a diverse array of 71 distinct bioactive compounds were identified and characterized in the fruits of strawberry varieties. The compounds included 33 alkaloids, categorized as follows: 7 plumerane, 5 pyridine, 5 quinoline, 3 piperidine, 2 isoquinoline, 1 pyrrole (retronecine), 1 guanidine (zarzissine), 1 quinazoline (vasicinone), 1 β-carbolines (Lycoperodine-1), 1 monoamine (phenylethanolamine), 1 phenethylamine (synephrine), and 5 other alkaloids. Additionally, 38 other compounds were detected (Table 1). The specific details of each compound include the Q1 and Q3 values, molecular weights, chemical formulas, ionization modes, Chemical Abstracts Service (CAS) registry numbers, and KEGG compound IDs are represented in Table 1.

Table 1.
Illustration of the bioactive compounds details identified in the strawberry fruits.
3.1. Clustering Analysis of Bioactive Compounds from Strawberry Fruit
The hierarchical cluster analysis (HCA) and heatmap analysis were conducted to thoroughly evaluate the variation, distribution, and abundance of bioactive compounds in the strawberry fruits (Figure 1). In the DD99 variety, a significant increment was observed in the bioactive compounds such as histamine, ergotamine, pantetheine, tatarine, zarzissine, trigonelline, 3-carbamyl-1-methylpyridinium, o-phosphocholine, phenylethanolamine, asiaticumine A, L-pipecolic acid, pipecolic acid, histidinol, and hydroxymenisdaurin D (Figure 1). Interestingly, when the 99 variety was subjected to cultivation in a high-elevation environment in Zhaotong city, subsequently named ZT99, there was an obvious decrease in the levels of these compounds (Figure 1). This suggests that varying ecological conditions play a crucial role in modulating the biosynthesis of these bioactive compounds.
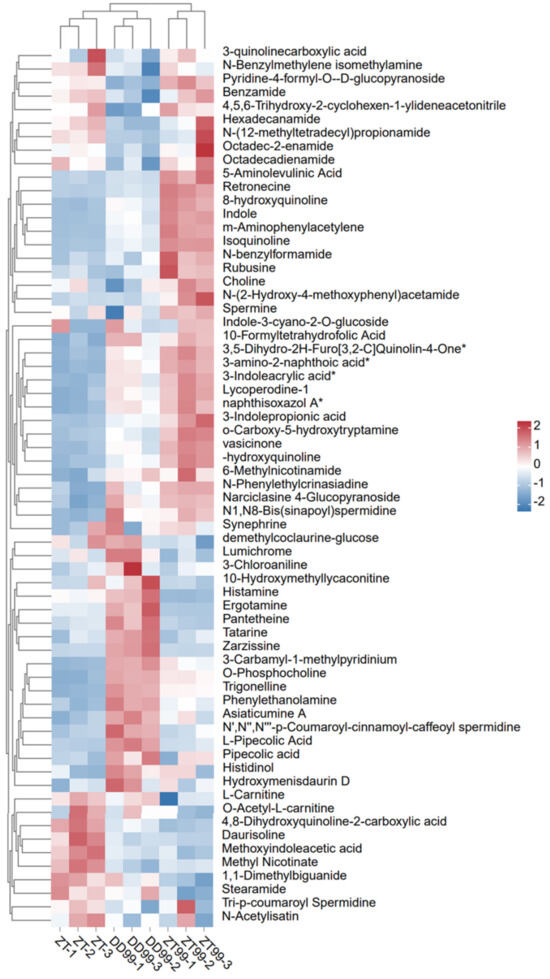
Figure 1.
Hierarchical clustering and heatmap analysis of bioactive compounds in strawberry fruits. The data rows have been normalized and * means isomers. Each row indicates the relative abundance of bioactive compounds in the strawberry fruits, while each column corresponds to different strawberry varieties.
Conversely, the ZT99 exhibited an increased level of compounds including octadec-2-enamide, octadecadienamide, 5-aminolevulinic acid, retronecine, 8-hydroxyquinoline, indole, m-aminophenylacetylene, isoquinoline, N-benzylformamide, rubusine, choline, and spermine (Figure 1). The results revealed the adaptive responses of strawberry plant to growth conditions at biochemical level that may lead toward improved nutritional and pharmaceutical potential. ZT (local strawberry variety in Zhaotong) showed lower levels of bioactive compounds mentioned above (Figure 1). Nevertheless, the amounts of daurisoline, methoxyindoleacetic acid, and methyl nicotinate were higher in the fruits of ZT variety than DD99 and ZT99. The HCA analysis suggests that ecological conditions of Dandong to Zhaotong significantly altered the biosynthesis of bioactive compounds in strawberry fruits. Moreover, the introduction of the “Red Face” strawberry variety from Dandong to Zhaotong significantly improved the abundance of several bioactive compounds than the local Zhaotong strawberry variety “Akihime”. Furthermore, the identification of several alkaloids and other bioactive compounds may have multiple therapeutic benefits.
3.2. Principal Component Analysis of Bioactive Compounds from Strawberry Fruit
The principal component analysis (PCA) was conducted to assess the variation and distribution of bioactive compounds present in the different strawberry fruits (Figure 2). In this PCA, the first principal component (PC1) accounted for 44.1 percent of the variance on x-axis, while the second principal component (PC2) accounted 32.7 percent of the variance on y-axis (Figure 2A). This substantial variance highlights the distinctive biochemical compositions of strawberry varieties.
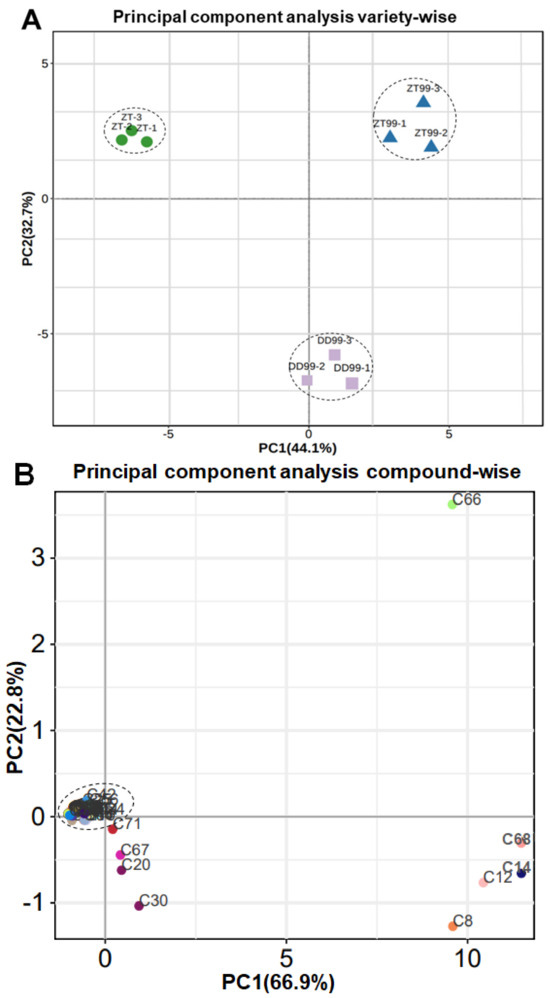
Figure 2.
Principal component analysis of compounds in strawberry fruits. (A): Strawberry variety-wise PCA, (B): Compound-wise PCA. The C1-C71 names are consistent with the serial number of bioactive compounds represented in Table 1.
The positioning of the ZT variety within the PCA plot is on the extreme left upper quadrant, which contrasts with the positioning of the ZT99, which is located on the opposite extreme right upper quadrant (Figure 2A). The DD99 variety occupies a unique position in the lower middle side of the y-axis on the PCA plot (Figure 2A). This unique placement suggests a distinctive concentration of bioactive compounds in DD99 compared to the ZT and ZT99. The PCA analysis showed that the introduction of the “Red Face” strawberry from Dandong to Zhaotong appears to have a profound impact on the biosynthesis and accumulation of both bioactive alkaloid and other compounds, particularly due to different ecological conditions.
In compound-wise PCA, a comprehensive clustering of the bioactive compounds was observed at the intersection of the x-axis and y-axis, with the exception of eight compounds (Figure 2B). These eight compounds include C67 (m-aminophenylacetylene), C20 (8-hydroxyquinoline), C30 (Indole), C8 (3,5-Dihydro-2H-Furo [3,2-C]Quinolin-4-One*), C12 (3-Indoleacrylic acid), C14 (3-amino-2-naphthoic acid), C68 (naphthisoxazol A), and C66 (demethylcoclaurine-glucose) (Figure 2B). The PCA results revealed that PC1 accounted for 66.9% of the total variance, while PC2 explained 22.8% of the variance along the y-axis (Figure 2B). Compounds such as C67, C20, and C30 were positioned very close to the central intersection point of the axis, suggesting a minimal deviation from the mean in both principal component dimensions (Figure 2B). In contrast, compounds C8, C12, C14, and C68 were located on the lower right quadrant of the PCA plot, indicating a distinct pattern or grouping away from the central cluster (Figure 2B). Notably, compound C66 exhibited a unique positioning, being isolated on the upper right quadrant of the PCA plot (Figure 2B). This distinct placement highlights its unique abundance and possibly distinct biochemical properties or roles compared to the other compounds.
3.3. Chemical Structure of Major Strawberry Alkaloids
Fifteen distinct alkaloid and other compounds were identified in significant quantities in the strawberry varieties. These compounds include3,5-dihydro-2H-Furo[3,2-C]quinolin-4-One, A-hydroxyquinoline, N-benzylmethyleneisomethylamine, naphthisoxazol A, pyridine-4-formyl-O-β-D-glucopyranoside, demethylcoclaurine-glucose, ergotamine, 3-indoleacrylic acid, L-pipecolic acid, 8-hydroxyquinoline, Indole, m-aminophenylacetylene, N-(2-Hydroxy-4-methoxyphenyl) acetamide, N-benzylformamide, and 3-chloroaniline. The available chemical structures of these aforementioned compounds are represented in Figure 3. All these compounds were reported for the first time in the strawberry fruits. The identification of these alkaloid compounds in strawberries not only expands our understanding of the plant’s metabolic pathways but also presents new opportunities for pharmacological research and applications.
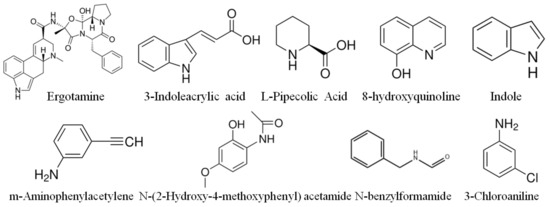
Figure 3.
Structure of major alkaloids and other compounds found abundantly in the fruit of strawberry varieties.
3.4. Clustering and PCA Analysis of Gene Expressions from Strawberry Fruits
Transcriptomic data HCA analysis was performed to assess the distribution and abundance of alkaloid biosynthesis genes in the strawberry fruits (Figure 4A). In DD99, a total of 19 genes were significantly upregulated in the alkaloid biosynthesis pathways (Figure 4A). The higher expression of genes in DD99 suggests that this variety may synthesize bioactive compounds with enhanced therapeutic potentials compared to ZT and ZT99. Interestingly, when the DD99 cultivated in a high-elevation environment, consequently named ZT99, the expression levels of these genes were decreased (Figure 4A). This decrease in the expression levels suggests an adaptive mechanism at the molecular level, enabling the plant to optimize its growth and metabolic functions under specific environmental conditions. In contrast, the ZT99 showed an elevated expression level of 15 genes (Figure 4A). The findings indicated the strawberry plant’s adaptive responses to growth conditions at the expression level, which might result in different nutritional and pharmaceutical potential compared to DD99. For the ZT, only 12 genes related to alkaloid biosynthesis exhibited higher expression than the other strawberries (Figure 4A). This unique expression profile highlights the influence of genetic diversity and environmental factors on the biosynthetic capabilities of the strawberry plant.
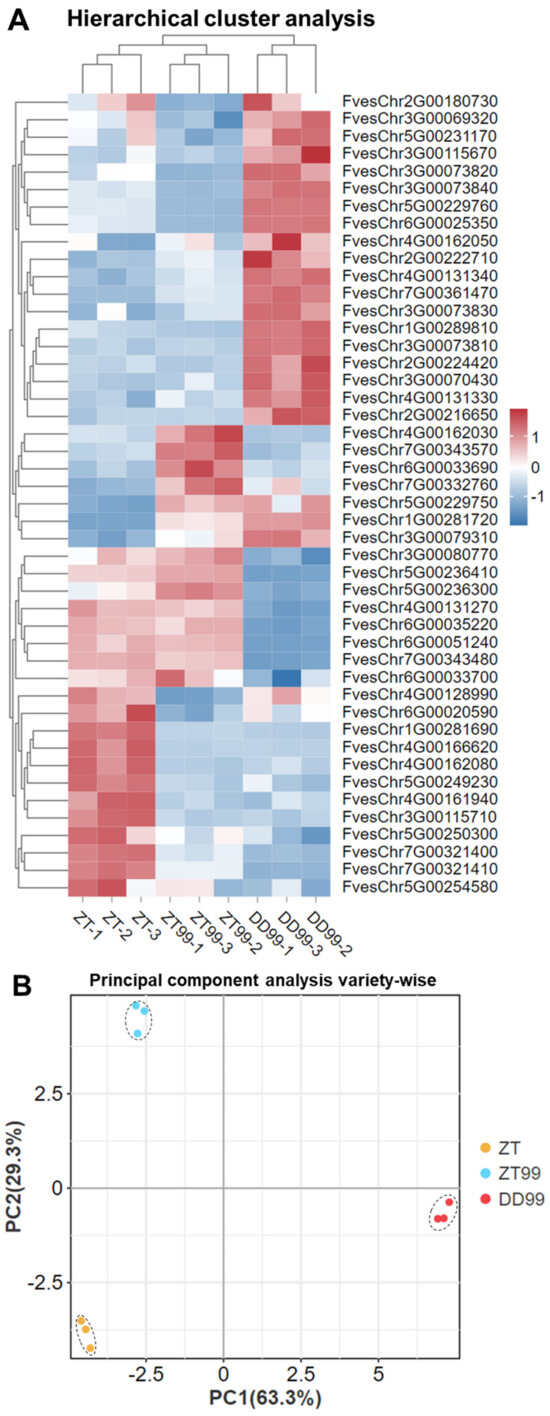
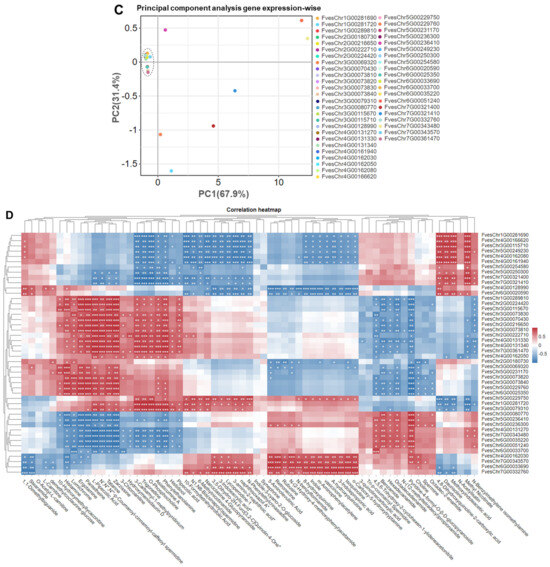
Figure 4.
Hierarchical clustering analysis and principal component analysis of genes expression in strawberry fruits. (A): HCA of genes, where the rows have been normalized. Each row indicates the expression of genes in the strawberry fruits, while each column corresponds to different strawberry varieties, (B): Strawberry variety-wise PCA, (C): Gene expression-wise PCA, (D): Correlation heatmap among genes and phytocompounds, Correlations between metabolites/genes across strawberry varieties. Asterisks denote statistical significance: * p < 0.05, ** p < 0.005, *** p < 0.0001.
The transcriptomic data PCA analysis reveals that PC1 explains 63.3% of the variance along the x-axis, while PC2 accounts for 29.3% of the variance along the y-axis (Figure 4B). This significant variance indicates distinct expression profiles among the strawberry varieties. The ZT99 is positioned in the extreme upper left quadrant of the PCA plot, contrasting with the ZT variety, which is located in the extreme lower left quadrant (Figure 4B). This spatial distinction highlights the notable differences in the distribution and abundance of genes involved in alkaloid biosynthesis between these two strawberries. Such differences may reflect the variety’ responses to their unique genetic backgrounds. The DD99 variety is uniquely positioned in the extreme right middle quadrant on the x-axis of the PCA plot (Figure 4B), suggesting a distinctive expression of genes compared to the other strawberries. Changes in environmental conditions seem to significantly affect the biosynthesis of genes associated with alkaloid bioactive compounds in ZT99 compared to DD99, as evidenced by the PCA analysis.
In the gene expression-wise PCA, a significant clustering of genes was observed mainly on the left side, near the x-axis and y-axis intersection point (Figure 4C). However, seven genes (FvesChr1G00281720, FvesChr5G00229760, FvesChr5G00236410, FvesChr6G00051240, FvesChr7G00321400, FvesChr7G00321410, and FvesChr7G00343480) exhibited distinct distribution patterns across the PCA plot, diverging from the main cluster (Figure 4C). This unique positioning suggests differential abundance and expression profiles for these seven genes relative to the other genes. The PC1 accounted for 67.9% of the total variance, while the PC2 explained 31.4% of the variance along the y-axis (Figure 4C). The spatial arrangement of genes within the PCA plot provides insights into their relative expression levels and potential interactions. The observed clustering and dispersion patterns offer a comprehensive view of expression dynamics among the biosynthesis genes, which may have implications for understanding genetic regulation mechanisms.
Correlation analysis and hierarchical clustering revealed significant associations between the expression of genes FvesChr3G00115710, FvesChr3G00073840, and FvesChr6G00025350 and various bioactive compounds, including histamine, lumichrome, ergotamine, pantetheine, N’,N″,N‴-p-coumaroyl-cinnamoyl-caffeoyl spermidine, tatarine, zarzissine, and L-pipecolic acid (Figure 4D). These findings suggest a potential regulatory role for these genes in alkaloid biosynthesis or related metabolic pathways. Additionally, the expression of FvesChr3G00080770 exhibited a significant correlation with pyridine-4-formyl-O-β-D-glucopyranoside, indicating its potential functional importance in pyridine derivative metabolism. Notably, FvesChr3G00115710 demonstrated strong correlations with several distinct compounds, including methyl nicotinate, 4,8-dihydroxyquinoline-2-carboxylic acid (a quinoline alkaloid), daurisoline (an isoquinoline alkaloid), and methoxyindoleacetic acid (a plumerane compound) (Figure 4D). These diverse associations suggest that FvesChr3G00115710 may play a critical role in the biosynthesis or regulation of various alkaloid and plumerane compounds. The observed correlations indicate a complex interaction network and regulatory mechanisms, warranting further investigation to elucidate the precise biological functions of these candidate genes.
3.5. Total Alkaloids and Bioactivity in Strawberry Fruits
Analysis of total alkaloid content revealed that ZT99 strawberry fruits contained the highest levels of alkaloids, followed by DD99 and ZT (Figure 5). This elevated alkaloid content in ZT99 strawberries correlated with enhanced bioactivity, particularly in terms of radical scavenging capabilities. The HRSA assay indicated that ZT99 strawberries possessed the greatest scavenging capabilities, while ZT strawberries exhibited the least (Figure 5). This suggests a direct correlation between alkaloid content and antioxidant efficacy, highlighting the superior potential health benefits of ZT99 strawberries. The FRAP assay on alkaloid extracts demonstrated that the ZT99 and DD99 strawberry fruits exhibited superior radical scavenging abilities compared to ZT (Figure 5). The DPPH assays showed that alkaloid extracts from ZT99 strawberries had significantly higher antioxidant activities and capacities than those from DD99, while ZT strawberries showed the lowest values (Figure 5). These results corroborate the findings from the HRSA and FRAP assays, further substantiating the superior antioxidant capabilities of ZT99 strawberries.
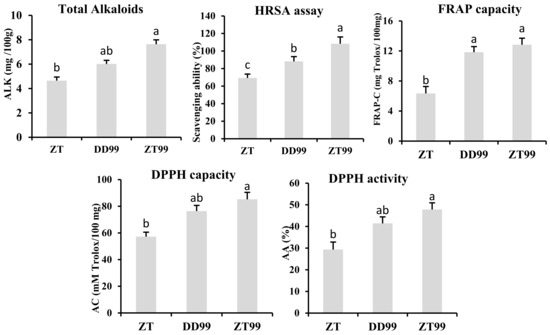
Figure 5.
Analysis of total alkaloid content along with the antioxidant properties in samples of strawberry fruit alkaloids. Abbreviations: ALK: Alkaloids; HRSA: Hydroxyl radical scavenging ability; FRAP: Ferric reducing antioxidant power capacity; AC: Antioxidant capacity; DPPH: 2,2-diphenyl-1-picrylhydrazyl; AA: Antioxidant activity. The column data consists of three biological replicates, and statistical significance was determined by comparing means with the least significant difference (LSD) at a significance level of p < 0.05 (a, b, c).
Across all conducted assays, ZT strawberries consistently demonstrated the minimum radical scavenging abilities and the lowest levels of alkaloids (Figure 5). These results indicate that the ecological conditions of Zhaotong significantly enhanced the biosynthesis of alkaloid compounds and their antioxidant activities in the “Red Face” strawberry fruits compared to those grown in Dandong. Moreover, the introduction of the “Red Face” strawberry variety in Zhaotong (ZT99) resulted in significantly higher total contents of bioactive alkaloids relative to the local Zhaotong variety “Akihime”. These findings suggest potential for improving local strawberry fruit quality through the cultivation of varieties with enhanced bioactive alkaloid profiles and antioxidant properties, which may confer increased therapeutic benefits.
Correlation analyses elucidated the relationship between alkaloid content and antioxidant assays (Table 2). The HRSA assay exhibits significant and positive correlation with alkaloid content, followed by FRAP capacity and DPPH activities (Table 2). These results indicate a significant role of alkaloids in enhancing the antioxidant capabilities of strawberry fruits. The findings suggest that strawberries with higher alkaloid content, such as ZT99, may confer greater benefits in mitigating oxidative stress. This study advances the understanding of strawberry alkaloids and their antioxidant properties, providing a foundation for further investigation into their potential health benefits.

Table 2.
Correlation analysis of alkaloids with the antioxidant activity and capacity of alkaloids.
3.6. Environmental Conditions During Strawberry Cultivation
Daily temperature data (average and maximum/minimum) revealed significant differences between the Zhaotong (high-altitude) and Dandong (low-altitude) sites (Figure 6). Zhaotong exhibited lower average temperatures specially during the strawberry growing season (August–September: 20–15 °C) with larger diurnal shifts (>10 °C) throughout the strawberry cultivation period (Figure 6). However, Dandong maintained warmer, stable conditions (August–September: 22–28 °C) with moderate diurnal shifts (<6 °C). Notably, during the strawberry maturing stage the diurnal temperature shifts showed significant difference at both sites, with Zhaotong’s showing (>15 °C) while the Dandong showed (<10 °C) diurnal temperatures shifts (Figure 6). The increased contents of alkaloids in strawberry samples harvested from Zhaotong coincides with the elevated diurnal temperatures shifts in Zhaotong (Table 1 and Figure 6). These results show that the altitudinal environmental changes (particularly pronounced diurnal temperature shifts) in Zhaotong directly influence strawberry fruit quality by enhancing bioactive alkaloid production (Table 1 and Figure 6). These results align with previous studies on fruit crops, which showed that elevated diurnal temperature shifts increase the secondary metabolite accumulation in fruits [17,36]. This aligns with the broader context of climate-driven impacts on fruit secondary metabolism, underscoring the need to adapt cultivation practices to optimize nutritional and pharmaceutical value under varying ecological conditions.
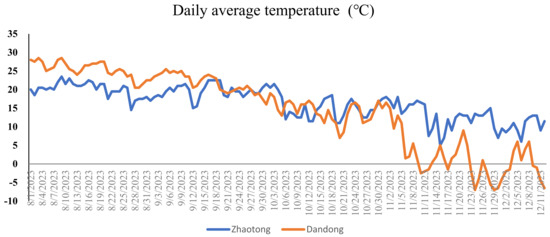
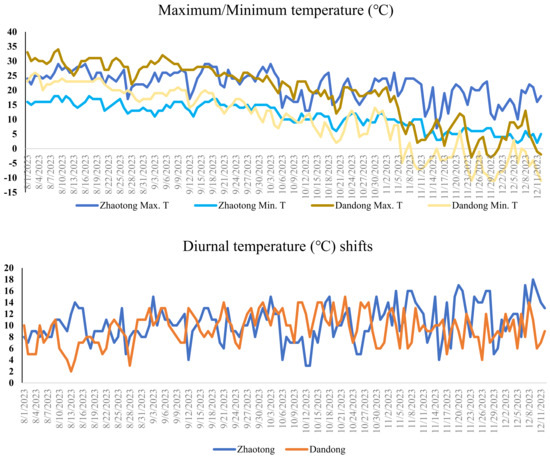
Figure 6.
Daily temperature profiles (August–December 2023) for Zhaotong and Dandong. The first graph shows average temperatures of both sites while the second graph denotes maximum/minimum temperature ranges at both sites, whereas the third graph shows diurnal temperature shifts.
4. Discussion
Plant species synthesize a variety of antioxidants and secondary metabolites that are transformed into pharmaceuticals [44]. Certain phytochemicals, such as alkaloids, exhibit potent antioxidant properties and are valuable in medicine due to their dynamic effects beneficial to the health of plants, humans, and animals [45,46]. Currently, there is no comprehensive study available on the alkaloid content in strawberry fruits. Despite the importance of alkaloids, research on strawberries has predominantly focused on phenolic compounds, taste, sweetness, and nutritional components. Only a few alkaloids have been reported in strawberries so far [47]. Our study aimed to fill this gap by providing a thorough analysis of alkaloids and other bioactive compounds in strawberry fruits. Moreover, this study aimed to introduce the “Red Face” variety into Zhaotong’s higher altitudes to improve local production and analyze variations in alkaloid profiles and their biosynthesis genes under different ecological conditions. Our study identified 71 different compounds, including 33 alkaloids, in strawberry fruits. The ecological conditions of Zhaotong city were found to be favorable for the introduction of the “Red Face” variety, resulting in increased total alkaloid content and enhanced antioxidant activities compared to the local “Akihime” variety cultivated in the same region. In strawberries, alkaloids contribute to various physiological functions and can enhance the fruit’s medicinal properties [48,49].
Previous studies reported that dragon fruit cultivated at high elevation exhibited the highest levels of alkaloid compounds, nucleotides and their derivatives, flavonoids, vitamins, and amino acids and their derivatives, indicating superior quality [50]. These findings suggest that the ecological conditions at higher altitude are optimal for producing highly nutritious and therapeutic dragon fruits. Similarly, Naryal et al. observed that the weight of apricot fruits decreased with elevation, while water content increased as altitude decreased [51]. Additionally, it has been shown that the sweetness of blueberry fruits increases with altitude, whereas fruits from lower altitudes are more acidic [52]. Our findings suggest that high-altitude regions such as Zhaotong city may be ideal for producing highly nutritious and therapeutic strawberry fruits with improved alkaloids contents. However, our study focused on the primary fruiting season (August–December), future work should evaluate seasonal stability of alkaloid profiles across multiple growth cycles. Furthermore, they provide a fundamental basis for future research on ecological-based strawberry production for specific purposes.
Our research identified different categories of alkaloid compounds that show promising antioxidant capabilities (Figure 1 and Figure 5) and potential therapeutic benefits. Recent research highlights the significant role of alkaloids in enhancing plant resilience to both abiotic and biotic stresses [53]. Dopamine not only promotes plant growth but also fortifies plants against various environmental stresses [18,54,55,56]. Similarly, cadaverine, an alkaloid precursor synthesized from pyruvate and lysine, is crucial for plant development and response to stresses like heat, drought, and salinity [57]. Besides this, alkaloids serve diverse functions in both humans and animals. Extracts from Ziziphus oxyphylla Edgw and Coptis chinensis, for example, show potential anti-diabetic properties [58,59]. In various animals, including goats, swine, and cattle, certain piperidine alkaloids can induce toxicity, yet they are also known for their neurostimulatory effects in cases involving hemlock alkaloids [60]. Our study revealed that L-pipecolic acid, A19, and pipecolic acid are present in strawberry fruits, with L-pipecolic acid being the predominant compound. Furthermore, a significant correlation was observed between L-pipecolic acid and three genes: FvesChr3G00115710, FvesChr3G00073840, and FvesChr6G00025350 (Figure 4D). These findings extend our understanding of strawberry fruit metabolism and genetic regulation.
Our results demonstrate that strawberry fruits harvested from different locations possess distinct levels of bioactive alkaloid compounds in strawberry fruits (Figure 1). The dissimilar concentrations of these compounds suggest that DD99 strawberries may possess enhanced therapeutic potential, making them a promising candidate for functional food or nutraceutical applications. Many of these compounds exhibit significant bioactivity relevant to human health [3,24,61]. For instance, histamine, a key mediator of allergic and inflammatory responses, acts through H1–H4 receptors to regulate gastric secretion, vasodilation, and neurotransmission [62]. The presence of ergotamine, a vasoconstrictive alkaloid used in migraine treatment [63], further highlights DD99 strawberries potential pharmacological value. Additionally, pantetheine, a precursor of coenzyme A, contributes to antioxidant and lipid-modulating effects [64], while trigonelline, known for its antidiabetic and neuroprotective properties [65], may enhance the fruit’s metabolic benefits. Other compounds, though less studied, also hold therapeutic promise. Asiaticumine A exhibits cytotoxic activity against cancer cells [66], and L-pipecolic acid and pipecolic acid serve as biomarkers for metabolic disorders and are important for plant immunity [67]. While tatarine and zarzissine require further characterization, their elevated levels in DD99 strawberries suggest possible unexplored bioactivities. Histidinol, an intermediate in histidine biosynthesis, has been explored for antimicrobial applications [68,69]. The higher abundance of these pharmacologically active compounds in DD99 strawberries compared to ZT99 and ZT varieties implies that DD99 may offer superior health benefits. These findings align with growing interest in strawberries as functional foods, particularly those rich in bioactive metabolites [17,70]. Further research should explore the bioavailability and in vivo effects of these compounds in DD99 strawberries to validate their therapeutic potential. Additionally, breeding or cultivation strategies could be optimized to enhance the production of these valuable metabolites.
Alkaloids from Litsea glutinosa have been found to reduce body fat in mice, and those from Murraya koenigii demonstrate cytotoxic effects against human leukemia cells [71]. Our results demonstrate that strawberry cultivation under the ecological conditions of Zhaotong yields a substantial quantity of bioactive alkaloid compounds. High levels of spermine, another alkaloid precursor, offer robust protection against oxidative damage in yeast, bacteria, and mammalian cells, and influence growth and brain receptor activities [72]. Pre-treatment with spermine in citrus plants enhances dehydration tolerance by influencing stomatal behavior and boosting antioxidant activities [73]. Our results showed that the ZT99 strawberry fruits exhibit elevated levels of spermine, which may contribute to their enhanced drought tolerance. This research provides valuable insights into the potential of strawberries as a source of alkaloid bioactive compounds. Furthermore, the unique ecological conditions of Zhaotong and Dandong appear to promote a distinct array of bioactive alkaloids in strawberries.
5. Conclusions
In conclusion, this study provides novel insights into the alkaloid diversity in strawberry fruits under different ecological conditions. Our findings reveal that strawberries contain a wide range of bioactive compounds including 33 alkaloids and 38 other compounds, many of which were reported for the first time in this fruit. The introduction of the “Red Face” variety to high-altitude environments resulted in enhanced alkaloid content, antioxidant activity, and overall fruit quality. Furthermore, the “Akihime” local variety in Zhaotong city exhibited the lowest alkaloid content and antioxidant activities. These results demonstrate the potential for improving strawberry cultivation through strategic variety selection and environmental manipulation. The identification of key biosynthesis genes (FvesChr3G00115710, FvesChr3G00073840, and FvesChr6G00025350) opens new avenues for future functional verification of these genes to enhance the nutritional properties of strawberries. In the future, research should focus on elucidating the health benefits of these identified alkaloids and optimizing cultivation practices for maximizing their production in strawberry fruits. Additionally, future studies will employ knockout or overexpression of candidate genes (identified in our study e.g., FvesChr3G00115710, etc.,) to validate their roles in alkaloid biosynthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11050533/s1, Table S1: Raw metabolic data. Table S2: Raw transcriptomic data used in this study.
Author Contributions
Conceptualization, M.J.R., M.D. and X.D.; methodology, A.R., M.I., H.W., H.L., S.J. (Shiping Jiang), M.D. and X.D.; software, M.D., M.J.R., M.I., A.R. and M.F.K.; formal analysis, M.I., H.W., M.D., A.R., X.D., M.F.K. and H.L.; investigation, K.S., M.J.R., X.D., S.J. (Shu Jiang), M.I., J.F., A.R., M.F.K. and M.D.; data curation, M.I., M.T., A.R., M.F.K., M.D., X.D. and M.J.R.; writing—original draft preparation, M.D. and M.J.R.; writing—review and editing, all authors; supervision, M.J.R. and S.Y.; project administration, M.D. and S.Y.; funding acquisition, S.Y. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly funded by Yunnan Provincial First-Class Discipline Construction Project: Cold Highland Characteristic Crops and Biological Resources Science, Grant number: 202210; National University Student Innovation Training Program, Grant number: 202310683002; Project for Reserve Talents of Young and Middle-aged Academic and Technical Leaders—Shunqiang Yang, Grant number: 202305AC160057; The project of Scientific research start-up funds for doctoral talents of Zhaotong University—Mingzheng Duan, Grant number: 202406; Young Talent Project of Talent Support Program for the Development of Yunnan. Grant number: 210604199008271015; Team Project of the “Xingzhao Talent Support Plan” in Zhaotong City—Honggao Liu. Grant No.: ZhaodangRencai[2023]No.3.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
Thank you to Pan Wenjuan, Fu Xiaoting, and Zhang Lin for their assistance during the strawberry cultivation process in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simpson, D. The Economic Importance of Strawberry Crops. In The Genomes of Rosaceous Berries and Their Wild Relatives; Springer: Cham, Switzerland, 2018; pp. 1–7. [Google Scholar]
- Arias, F.; Appelmann, T.; Giraldo, L.F.G. Import and Export of Strawberries in a Global Market. In Ciencias económicas y contables, desafíos y retos para la competitividad; Sepúlveda, J.A., Ed.; Corporación Universitaria Americana (Coruniamericana): Medellín, Colombia, 2020; pp. 280–291. [Google Scholar]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A. Phenolic Contents of Lettuce, Strawberry, Raspberry, and Blueberry Crops Cultivated under Plastic Films Varying in Ultraviolet Transparency. Food Chem. 2010, 119, 1224–1227. [Google Scholar] [CrossRef]
- Panico, A.M.; Garufi, F.; Nitto, S.; Di Mauro, R.; Longhitano, R.C.; Magrì, G.; Catalfo, A.; Serrentino, M.E.; De Guidi, G. Antioxidant Activity and Phenolic Content of Strawberry Genotypes from Fragaria x Ananassa. Pharm. Biol. 2009, 47, 203–208. [Google Scholar] [CrossRef]
- Figuerola, F.E. Berry Jams and Jellies. In Food Science and Technology; Marcel Dekker: New York, NY, USA, 2007; Volume 168, p. 367. [Google Scholar]
- Aguilera, J.M. Berries as Foods: Processing, Products, and Health Implications. Annu. Rev. Food Sci. Technol. 2024, 15, 1–26. [Google Scholar] [CrossRef]
- Hannum, S.M. Potential Impact of Strawberries on Human Health: A Review of the Science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a Health Promoter: An Evidence Based Review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Taher, M.; Shukry, N.A.S.B.; Susanti, D.; Saleh, W.M.N.H.W.; Syukri, Y. Citrus Flavonoids in Preventing Cardiovascular Diseases. In Plant-Derived Bioactives; Springer: Singapore, 2020; pp. 495–508. [Google Scholar]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A Promising Anticancer Agent from Nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Ramakrishnan, R. Anticancer Properties of Zingiber Officinale–Ginger: A Review. Int. J. Med. Pharm. Sci. 2013, 3, 11–20. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Lei, J.J.; Jiang, S.; Ma, R.Y.; Xue, L.; Zhao, J.; Dai, H.P. Current Status of Strawberry Industry in China. In Proceedings of the IX International Strawberry Symposium, Rimini, Italy, 1–5 May 2021; Volume 1309, pp. 349–352. [Google Scholar]
- Zhang, Y.; Wang, G.; Chang, L.; Dong, J.; Zhong, C.; Wang, L. Current Status of Strawberry Production and Research in China. Acta Hortic. 2014, 1049, 67–71. [Google Scholar] [CrossRef]
- Kun, Q.; Yuenan, L.; Yingxue, L. Analysis of Dandong 99 Strawberry Market Competitiveness Based on Porter’s Five Forces Model. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 253, p. 03058. [Google Scholar]
- Rao, M.J.; Wang, H.; Lei, H.; Zhang, H.; Duan, X.; Bao, L.; Yang, C.; Han, D.; Zhang, Y.; Yang, S.; et al. LC-MS/MS-Based Metabolomic Study Provides Insights into Altitude-Dependent Variations in Flavonoid Profiles of Strawberries. Front. Plant Sci. 2025, 15, 1527212. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The Strawberry: Composition, Nutritional Quality, and Impact on Human Health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Batool, A.; Yaqub, S.; Iqbal, A.; Kauser, S.; Arif, M.R.; Ali, S.; Gorsi, F.I.; Nisar, R.; Firdous, N. Effects of Spray Drying and Ultrasonic Assisted Extraction on the Phytochemicals, Antioxidant and Antimicrobial Activities of Strawberry Fruit. Food Chem. Adv. 2024, 5, 100755. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Kokko, H.; Mintz-Oron, S.; Venger, I.; Kärenlampi, S.; Aharoni, A. Non-Targeted Analysis of Spatial Metabolite Composition in Strawberry (Fragaria × Ananassa) Flowers. Phytochemistry 2008, 69, 2463–2481. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Wang, J.; Han, S.; Ma, L.; Mo, X.; Li, M.; Hu, L.; Wang, L. Transcriptomic and Widely Targeted Metabolomic Approach Identified Diverse Group of Bioactive Compounds, Antiradical Activities, and Their Associated Genes in Six Sugarcane Varieties. Antioxidants 2022, 11, 1319. [Google Scholar] [CrossRef]
- Rao, M.J.; Tahir Ul Qamar, M.; Wang, D.; Ali, Q.; Ma, L.; Han, S.; Duan, M.; Hu, L.; Wang, L. A High-Throughput Lipidomics and Transcriptomic Approach Reveals Novel Compounds from Sugarcane Linked with Promising Therapeutic Potential against COVID-19. Front. Nutr. 2022, 9, 988249. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Wei, X.; Zuo, H.; Ma, L.; Tahir Ul Qamar, M.; Li, M.; Han, S.; Hu, L.; Wang, L. LC–MS/MS-Based Metabolomics Approach Revealed Novel Phytocompounds from Sugarcane Rind with Promising Pharmacological Value. J. Sci. Food Agric. 2022, 102, 6632–6642. [Google Scholar] [CrossRef]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Tahir Ul Qamar, M.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.B.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-Based Metabolomics Approach Identified Novel Antioxidant Flavonoids Associated with Drought Tolerance in Citrus Species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Eman, M.; Yuan, H.; Sharma, A.; Zheng, B. Comparative Analysis of Citrus Species’ Flavonoid Metabolism, Gene Expression Profiling, and Their Antioxidant Capacity under Drought Stress. Antioxidants 2024, 13, 1149. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Shad, M.A.; Aslam, M.Z.; Wang, J.; Wang, L. Widely Targeted LC-MS/MS Approach Provides Insights into Variations in Bioactive Flavonoid Compounds and Their Antioxidant Activities in Green, Red, and Purple Sugarcane. LWT Food Sci. Technol. 2024, 209, 116792. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant Flavonoids in Cancer Chemoprevention: Role in Genome Stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; da Silva Messias, R.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-Dependent Salt and Drought Stress Improve Strawberry Fruit Quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Pedrozo, P.; Vicente, E.; Moltini, A.I.; Ibáñez, F.; Lado, B.; Fariña, L.; Ares, G.; Lado, J. Strawberry Fruit Quality: Impacts of the Harvest Date with a Breeding Perspective. JSFA Rep. 2023, 3, 597–608. [Google Scholar] [CrossRef]
- Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Borsetta, G.; Caprioli, G.; Mannozzi, C.; Marconi, R.; Piatti, D.; Santanatoglia, A.; Sagratini, G. Volatile Profile of Strawberry Fruits and Influence of Different Drying Methods on Their Aroma and Flavor: A Review. Molecules 2023, 28, 5810. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; do Nascimento, J.R.O.; Lajolo, F.M. Physico-Chemical Changes Related to Quality of Five Strawberry Fruit Cultivars during Cool-Storage. Food Chem. 2003, 83, 167–173. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.-Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef]
- Ullah, I.; Toor, M.D.; Yerlikaya, B.A.; Mohamed, H.I.; Yerlikaya, S.; Basit, A.; ur Rehman, A. High-Temperature Stress in Strawberry: Understanding Physiological, Biochemical and Molecular Responses. Planta 2024, 260, 118. [Google Scholar] [CrossRef]
- Karlund, A.; Hanhineva, K.; Lehtonen, M.; Karjalainen, R.O.; Sandell, M. Nontargeted Metabolite Profiles and Sensory Properties of Strawberry Cultivars Grown Both Organically and Conventionally. J. Agric. Food Chem. 2015, 63, 1010–1019. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.-C.; Ma, J.; Cheng, C.-G.; Wang, K.-Y.; Mao, L.-X.; He, Y.-B.; Qiu, M.-J.; Yang, X.-J. Evaluation of Potential Climatic Production of Apple during the Possible Growing Period at Zhaotong, Yunnan across Cool Highland of Southwest China. Chin. J. Agrometeorol. 2021, 42, 87. [Google Scholar]
- Reganold, J.P.; Andrews, P.K.; Reeve, J.R.; Carpenter-Boggs, L.; Schadt, C.W.; Alldredge, J.R.; Ross, C.F.; Davies, N.M.; Zhou, J. Fruit and Soil Quality of Organic and Conventional Strawberry Agroecosystems. PLoS ONE 2010, 5, e12346. [Google Scholar] [CrossRef]
- Bottoms, T.G.; Bolda, M.P.; Gaskell, M.L.; Hartz, T.K. Determination of Strawberry Nutrient Optimum Ranges through Diagnosis and Recommendation Integrated System Analysis. Horttechnology 2013, 23, 312–318. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Yang, M.; Fan, H.; Shen, S.; Hu, L.; Wang, L. Novel Insights into Anthocyanin Metabolism and Molecular Characterization of Associated Genes in Sugarcane Rinds Using the Metabolome and Transcriptome. Int. J. Mol. Sci. 2022, 23, 338. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, X.; Zhang, H. Correlations between Antioxidant Activity and Alkaloids and Phenols of Maca (Lepidium meyenii). J. Food Qual. 2017, 2017, 3185945. [Google Scholar] [CrossRef]
- Damiano, S.; Forino, M.; De, A.; Vitali, L.A.; Lupidi, G.; Taglialatela-Scafati, O. Antioxidant and Antibiofilm Activities of Secondary Metabolites from Ziziphus Jujuba Leaves Used for Infusion Preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Jing, H.; Liu, J.; Liu, H.; Xin, H. Histochemical Investigation and Kinds of Alkaloids in Leaves of Different Developmental Stages in Thymus Quinquecostatus. Sci. World J. 2014, 2014, 839548. [Google Scholar] [CrossRef]
- Swamy, M.K. Plant-Derived Bioactives: Chemistry and Mode of Action; Springer Nature: Singapore, 2020; ISBN 9789811523618. [Google Scholar]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, S.; Butt, Z.A.; Rehman, K.U.; Ullah, S.; Khan, S.S. Flavonoids, Alkaloids, and Saponins as Antimicrobial Agents from Fragaria vesca L. Pure Appl. Biol. (PAB) 2021, 10, 761–769. [Google Scholar] [CrossRef]
- Stynoski, J.L.; Torres-Mendoza, Y.; Sasa-Marin, M.; Saporito, R.A. Evidence of Maternal Provisioning of Alkaloid Based Chemical Defenses in the Strawberry Poison Frog Oophaga Pumilio. Ecology 2014, 95, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Jayaramaiah, R.H.; Gupta, R.; Kim, S.W.; An, J.U.; Jziyu, W.; Li, M.; Kang, N.J.; Hong, K.-P.; Kang, J.-S. Evaluation of Bioactive Compounds in Strawberry Fruits by a Targeted Metabolomic Approach. Hortic. Sci. Technol. 2017, 35, 805–819. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Chen, J.; Zhang, N.; Zhou, W.; Song, Y. Altitudinal Variation of Dragon Fruit Metabolite Profiles as Revealed by UPLC-MS/MS-Based Widely Targeted Metabolomics Analysis. BMC Plant Biol. 2024, 24, 344. [Google Scholar] [CrossRef] [PubMed]
- Naryal, A.; Dolkar, D.; Bhardwaj, A.K.; Kant, A.; Chaurasia, O.P.; Stobdan, T. Effect of Altitude on the Phenology and Fruit Quality Attributes of Apricot (Prunus armeniaca L.) Fruits. Def. Life Sci. J. 2020, 5, 18–24. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, G.; Tian, L.; Wu, H.; Ren, Y.; Tamir, G.; Huang, W.; Yu, H. High Altitude Is Beneficial for Antioxidant Components and Sweetness Accumulation of Rabbiteye Blueberry. Front. Plant Sci. 2020, 11, 573531. [Google Scholar] [CrossRef]
- Rao, M.J.; Wu, S.; Duan, M.; Wang, L. Antioxidant Metabolites in Primitive, Wild, and Cultivated Citrus and Their Role in Stress Tolerance. Molecules 2021, 26, 5801. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of Dopamine in Plants: A Review. Plant Signal Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X. Dopamine-Induced Abiotic Stress Tolerance in Horticultural Plants. Sci. Hortic. 2023, 307, 111506. [Google Scholar] [CrossRef]
- Du, J.; Xu, H.; Zhang, D.; Feng, S. Chelation and Nanoparticle Delivery of Monomeric Dopamine to Increase Plant Salt Stress Resistance. Nat. Commun. 2025, 16, 4157. [Google Scholar] [CrossRef]
- Jancewicz, A.L.; Gibbs, N.M.; Masson, P.H. Cadaverine’s Functional Role in Plant Development and Environmental Response. Front. Plant Sci. 2016, 7, 870. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Adhikari, A.; Rasheed, S.; Marasini, B.P.; Hussain, N.; Kaleem, W.A. Cyclopeptide Alkaloids of Ziziphus Oxyphylla Edgw as Novel Inhibitors of α-Glucosidase Enzyme and Protein Glycation. Phytochem. Lett. 2011, 4, 404–406. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.-H.; Ali, M.Y.; Min, B.-S.; Kim, G.-D.; Jung, H.A. Coptis Chinensis Alkaloids Exert Anti-Adipogenic Activity on 3T3-L1 Adipocytes by Downregulating C/EBP-α and PPAR-γ. Fitoterapia 2014, 98, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Bowman, W.C.; Sanghvi, I.S. Pharmacological Actions of Hemlock (Conium Maculatum) Alkaloids. J. Pharm. Pharmacol. 1963, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Thurmond, R.L.; Gelfand, E.W.; Dunford, P.J. The Role of Histamine H1 and H4 Receptors in Allergic Inflammation: The Search for New Antihistamines. Nat. Rev. Drug Discov. 2008, 7, 41–53. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.K.; Manikyam, H.K.; Krishna, A.B. Ergot Alkaloids: A Review on Therapeutic Applications. Eur. J. Med. Plants 2016, 14, 1–17. [Google Scholar] [CrossRef]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and α-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. Int. J. Mol. Sci. 2024, 25, 3385. [Google Scholar] [CrossRef]
- Kim, N.Y.; Dukanya, D.; Sethi, G.; Girimanchanaika, S.S.; Yang, J.; Nagaraja, O.; Swamynayaka, A.; Vishwanath, D.; Venkantesha, K.; Basappa, S. Oxazine Drug-Seed Induces Paraptosis and Apoptosis through Reactive Oxygen Species/JNK Pathway in Human Breast Cancer Cells. Transl. Oncol. 2024, 49, 102101. [Google Scholar] [CrossRef]
- Hartmann, M.; Kim, D.; Bernsdorff, F.; Ajami-Rashidi, Z.; Scholten, N.; Schreiber, S.; Zeier, T.; Schuck, S.; Reichel-Deland, V.; Zeier, J. Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity. Plant Physiol. 2017, 174, 124–153. [Google Scholar] [CrossRef]
- Chen, H.; Yu, C.; Wu, H.; Li, G.; Li, C.; Hong, W.; Yang, X.; Wang, H.; You, X. Recent Advances in Histidine Kinase-Targeted Antimicrobial Agents. Front. Chem. 2022, 10, 866392. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.M.; De Simone, G.; D’Ambrosio, K. L-Histidinol Dehydrogenase as a New Target for Old Diseases. Curr. Top. Med. Chem. 2016, 16, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Krakowska-Sieprawska, A.; Walczak-Skierska, J.; Pomastowski, P.; Sobolewska, R.; Głogowski, J.; Bernat, C.; Rafińska, K. Advanced Extraction Techniques for Bioactive Compounds from Berry Fruits: Enhancing Functional Food Applications. Foods 2024, 13, 4115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, Y.; Wu, Y.; Zhang, C.; Jin, D.; Zheng, Q.; Li, Y. Anti-Hyperglycemic and Anti-Hyperlipidemia Effects of the Alkaloid-Rich Extract from Barks of Litsea Glutinosa in Ob/Ob Mice. Sci. Rep. 2018, 8, 12646. [Google Scholar] [CrossRef]
- Pegg, A.E. The Function of Spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef]
- Shi, J.; Fu, X.Z.; Peng, T.; Huang, X.S.; Fan, Q.J.; Liu, J.H. Spermine Pretreatment Confers Dehydration Tolerance of Citrus In Vitro Plants via Modulation of Antioxidative Capacity and Stomatal Response. Tree Physiol. 2010, 30, 914–923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).