The Effects of Low-Temperature Stress on the Physiological Characteristics and Active Components of Ginseng Under Different Soil Moisture Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Experimental Materials and Treatments
2.3. Determination of Physiological Indices
2.4. Determination of the Ginsenoside Content
2.4.1. Preparation of the Sample Solution
2.4.2. Chromatographic Conditions
2.4.3. Mass Spectrometry Conditions
2.5. Comprehensive Evaluation of Cold Resistance
2.5.1. Evaluation Method of Cold Resistance
2.5.2. Classification Standards for Cold Resistance Evaluation
2.6. Data Processing
3. Results
3.1. Response of the Malondialdehyde (MDA) and Superoxide Dismutase (SOD) Contents in Ginseng Roots of Different Ages to Low Temperature
3.2. Response of Osmotic Adjustment Substance Content in Ginseng Roots of Different Years to Low Temperature
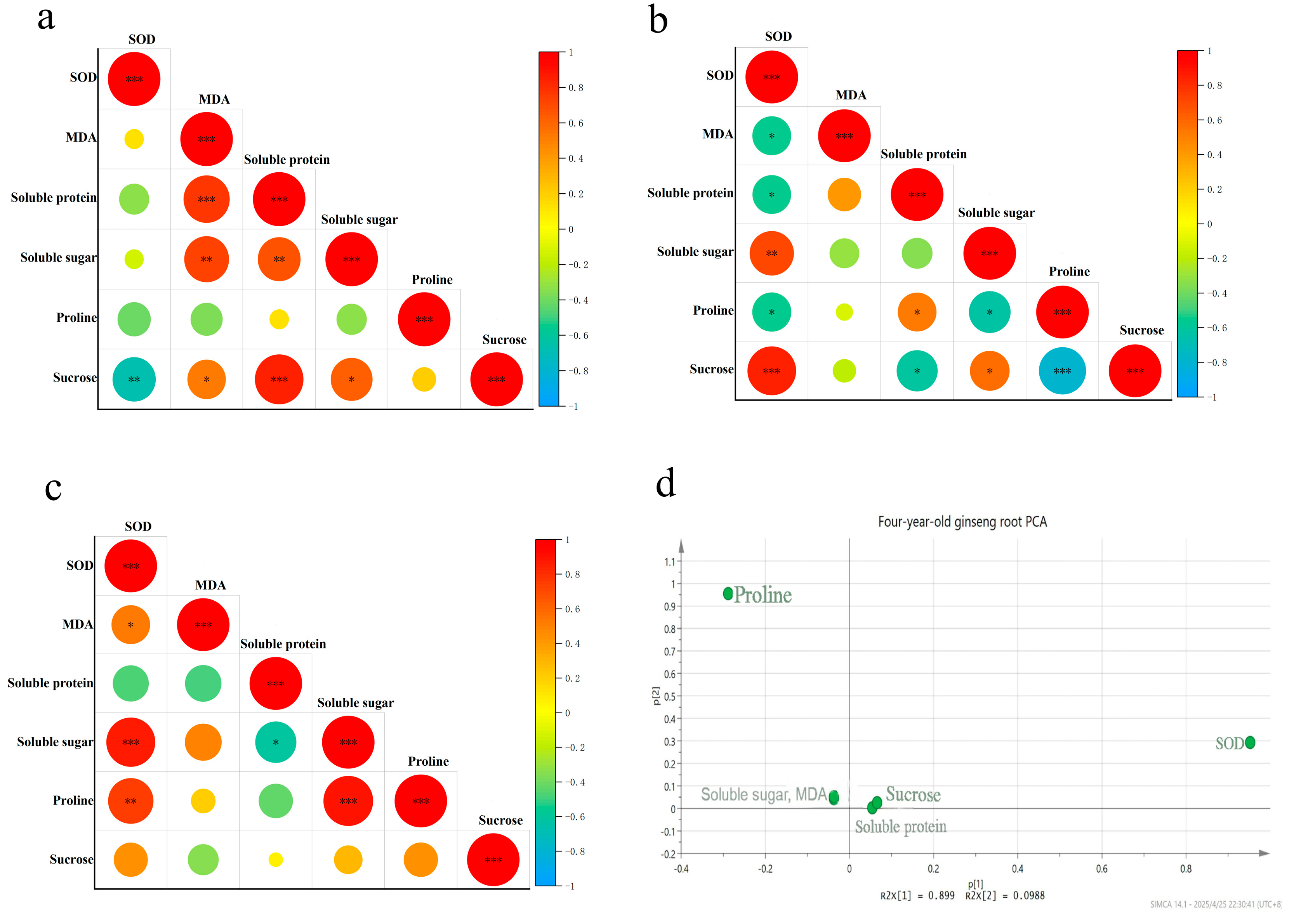
3.3. Correlation Analysis of Cold Resistance Physiological Indices in Ginseng of Different Ages Under Low-Temperature Stress
3.4. Comprehensive Evaluation of Cold Resistance in Ginseng of Two Different Ages
3.5. Analysis of the Ginsenoside Content in Ginseng Roots of Different Ages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, M.; Mok, H.; Yeo, W.S.; Ahn, J.H.; Choi, Y.K. Role of ginseng in the neurovascular unit of neuroinflammatory diseases focused on the blood-brain barrier. J. Ginseng Res. 2021, 45, 599–609. [Google Scholar] [CrossRef]
- Song, X.; Gao, W.; Shi, Y.; Li, J.; Zheng, Z. Panax ginseng and its derivatives: Promoting angiogenesis in ischemic diseases—A mechanistic overview. J. Funct. Foods 2023, 109, 105762. [Google Scholar] [CrossRef]
- An, X.; Zhang, A.L.; Yang, A.W.; Lin, L.; Wu, D.; Guo, X.; Shergis, J.L.; Thien, F.C.K.; Worsnop, C.J.; Xue, C.C. Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Respir. Med. 2010, 105, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, W.-Y.; Zhang, J.; Zuo, B.-M.; Zhang, L.-M.; Huang, L.-Q. Advances in study of ginsenoside biosynthesis pathway in Panax ginseng C. A. Meyer. Acta Physiol. Plant. 2012, 34, 397–403. [Google Scholar] [CrossRef]
- Ru, W.; Wang, D.; Xu, Y.; He, X.; Sun, Y.-E.; Qian, L.; Zhou, X.; Qin, Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.). Drug Discov. Ther. 2015, 9, 23–32. [Google Scholar] [CrossRef]
- Tian, T.; ChungNga, K.; Luo, W.; Li, D.; Yang, C. The anti-aging mechanism of ginsenosides with medicine and food homology. Food Funct. 2023, 14, 9123–9136. [Google Scholar] [CrossRef]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A Potential Neuroprotective Agent. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Liu, T.; Wang, J. Neuroprotective Effects of Ginsenosides against Cerebral Ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef]
- Ding, L.; Qi, H.; Wang, Y.; Zhang, Z.; Qing, L.; Chen, G.; Liu, J.; Chen, Z.; Jing, L.; Chen, J.; et al. Recent advances in ginsenosides against respiratory diseases: Therapeutic targets and potential mechanisms. Biomed. Pharmacother. 2023, 158, 114096. [Google Scholar] [CrossRef]
- Mohamed, B.; Gehana, S.; Tomlinson, G.E.S.; Subrata, D. Pharmacokinetics and pharmacodynamics of Rh2 and aPPD ginsenosides in prostate cancer: A drug interaction perspective. Cancer Chemother. Pharmacol. 2023, 92, 419–437. [Google Scholar]
- Li, N.; Liu, Y.; Li, W.; Zhou, L.; Li, Q.; Wang, X.; He, P. A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer’s disease. J. Ginseng Res. 2016, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, T.; Kang, X.; Han, M.; Yang, L. Preliminary study on response and its mechanism of ginsenosidebiosynthesis in Panax ginseng to water regulation. China J. Chin. Mater. Medica 2019, 44, 2768–2776. [Google Scholar]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Cameron, W.; Sheldon, H.; Yulia, K.; Sheng, L. Timing of short period water stress determines potato plant growth, yield and tuber quality. Agric. Water Manag. 2021, 247, 106731. [Google Scholar]
- Zhang, L. Effects of Low Temperature Stress on Root Physiology and Pathogen Content of Stem Base Rot of Wheat Agricultural. Master’s Thesis, University of Hebei, Baoding, China, 2021. [Google Scholar]
- Zhang, W.; Huang, S.; Wu, J.; Xie, J. Effects of low temperature stress on resistance physiological indices of leaf and root of Dendrocalamus latiflorus seedlings. Chin. J. Ecol. 2012, 31, 513–519. [Google Scholar]
- Chu, P.; Wang, H.; Jia, J.; Liang, C. Effects of Root Application of Melatonin on Root Morphology and physiology of Soybean Seedlings under Low Temperature Stress. Mol. Plant Breed. 2023, 1–12. Available online: https://link.cnki.net/urlid/46.1068.S.20231220.1404.006 (accessed on 7 May 2025).
- Li, J.; Bai, X.; Fu, R.; Zhang, C.; Yan, Y. Effects of combined extreme cold and drought stress on growth, photosynthesis, and physiological characteristics of cool-season grasses. Sci. Rep. 2024, 14, 116. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Zhang, Z.; Chen, Z. Experimental Techniques in Plant Physiology; Jilin University Press: Changchun, China, 2008. [Google Scholar]
- Skhawat, A.; Gill, R.A.; Zaid, U.; Na, Z.; Saddam, H.; Kangni, Z.; Qian, H.; Muhammad, S.; Bilal, T.M.; B Gill, M.; et al. Exogenously applied melatonin enhanced the tolerance of Brassica napus against cobalt toxicity by modulating antioxidant defense, osmotic adjustment, and expression of stress response genes. Ecotoxicol. Environ. Saf. 2023, 252, 114624. [Google Scholar]
- Xu, P.-L.; Guo, Y.-K.; Bai, J.-G.; Shang, L.; Wang, X.-J. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol. Plant. 2008, 132, 467–478. [Google Scholar] [CrossRef]
- Zhang, S.; Nimapingcuo; Xu, Y.; Miao, Y. Physiological responses to low temperature stress and cold tolerance evaluation in three Elymus species. Pratacult. Sci. 2016, 33, 1154–1163. [Google Scholar]
- Zhang, Y.; Cong, B.; Wang, X.; Zhang, Q. Correlation Analysis of Cold Resistance and Antioxidant Enzyme Activities in Alfalfa Roots. Acta Agrestia Sin. 2021, 29, 244–249. [Google Scholar]
- Li, J.; Zuo, Y.; Zhang, J.; Yang, M. Study on physiological mechanism of the seeds of Paris polyphylla var. yunnanensis under temperature and moisture of double factor stress. Plant Physiol. J. 2020, 56, 2439–2447. [Google Scholar]
- Lin, Y.; Guo, W.; Xu, Z.; Jia, Z. Cold Resistance and Changes on MDA and Soluble Sugar of Leaves of Ligustrunlucidum Ait in Winter. Chin. Agric. Sci. Bull. 2012, 28, 68–72. [Google Scholar]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, J.; Singh, B.V. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2019, 25, 667–681. [Google Scholar]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef]
- Schreiber, L. Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci. 2010, 15, 546–553. [Google Scholar] [CrossRef]
- Ole, P.; Margret, S.; David, C.T.; Mikio, N. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2020, 229, 42–49. [Google Scholar]
- Xie, C.; Suo, F.; Jia, G.; Song, J. Correlation between ecological factors and ginsenosides. Acta Ecol. Sin. 2011, 31, 7551–7563. [Google Scholar]
- Yang, L.; Zhang, T.; Yang, L.; Han, M. Effects of ecological factors on ginsenosides synthesis and its key enzyme genes expression. Chin. Tradit. Herb. Drugs 2017, 48, 4296–4305. [Google Scholar]
- Zhang, W.; Liu, W.; Wang, L.; Yu, P.; Song, X.; Yao, Y.; Liu, X.; Meng, X. Effects of water stress on secondary metabolism of Panax ginseng fresh roots. PLoS ONE 2024, 19, e0312023. [Google Scholar] [CrossRef] [PubMed]
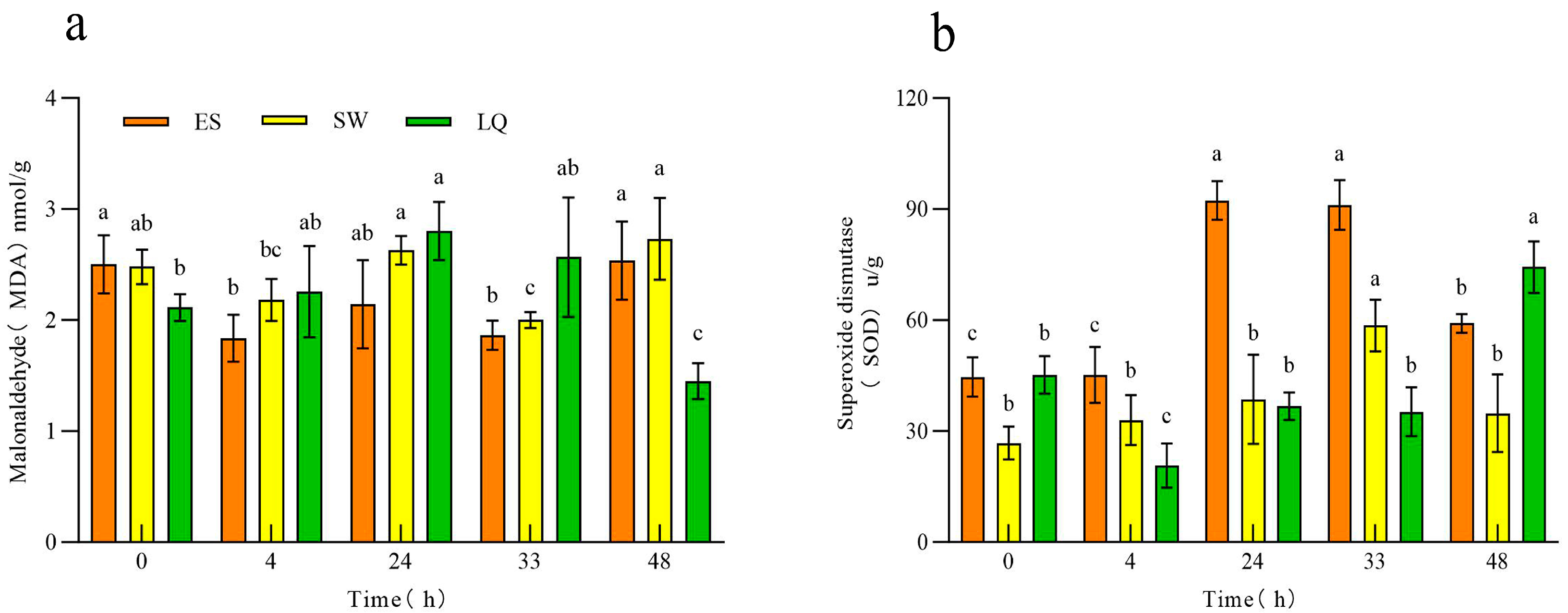
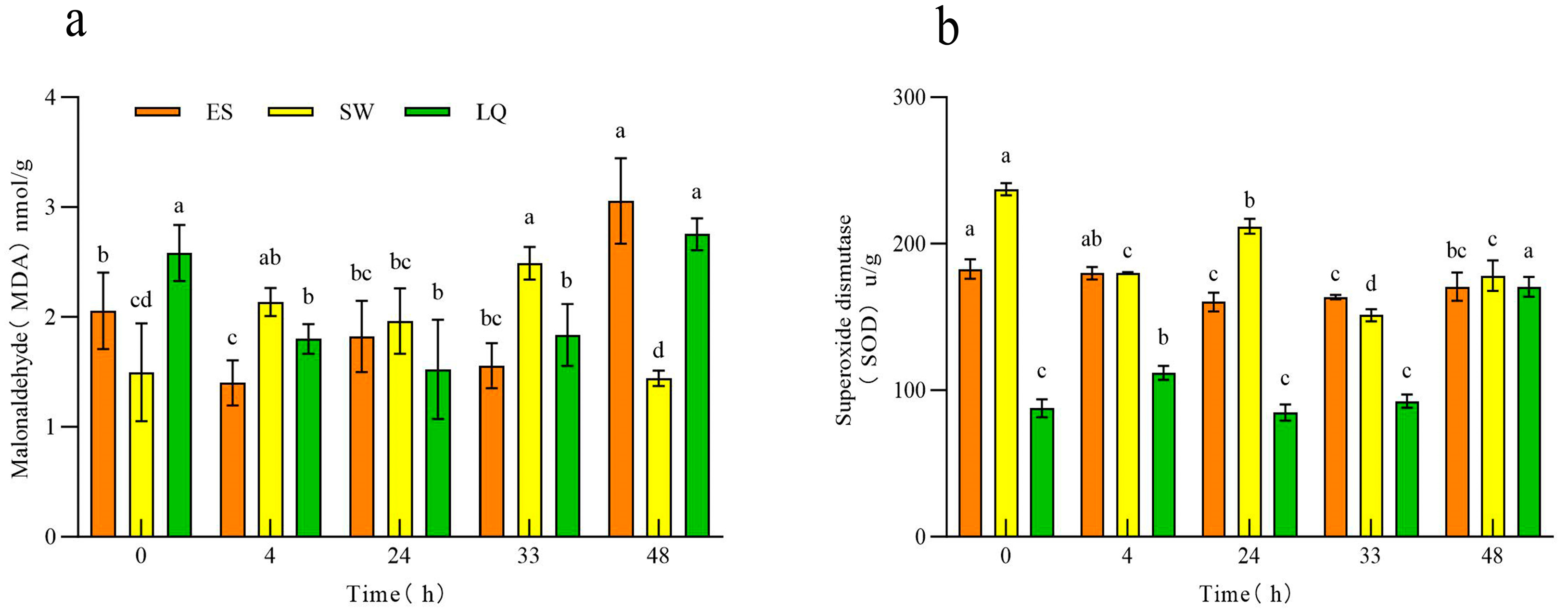
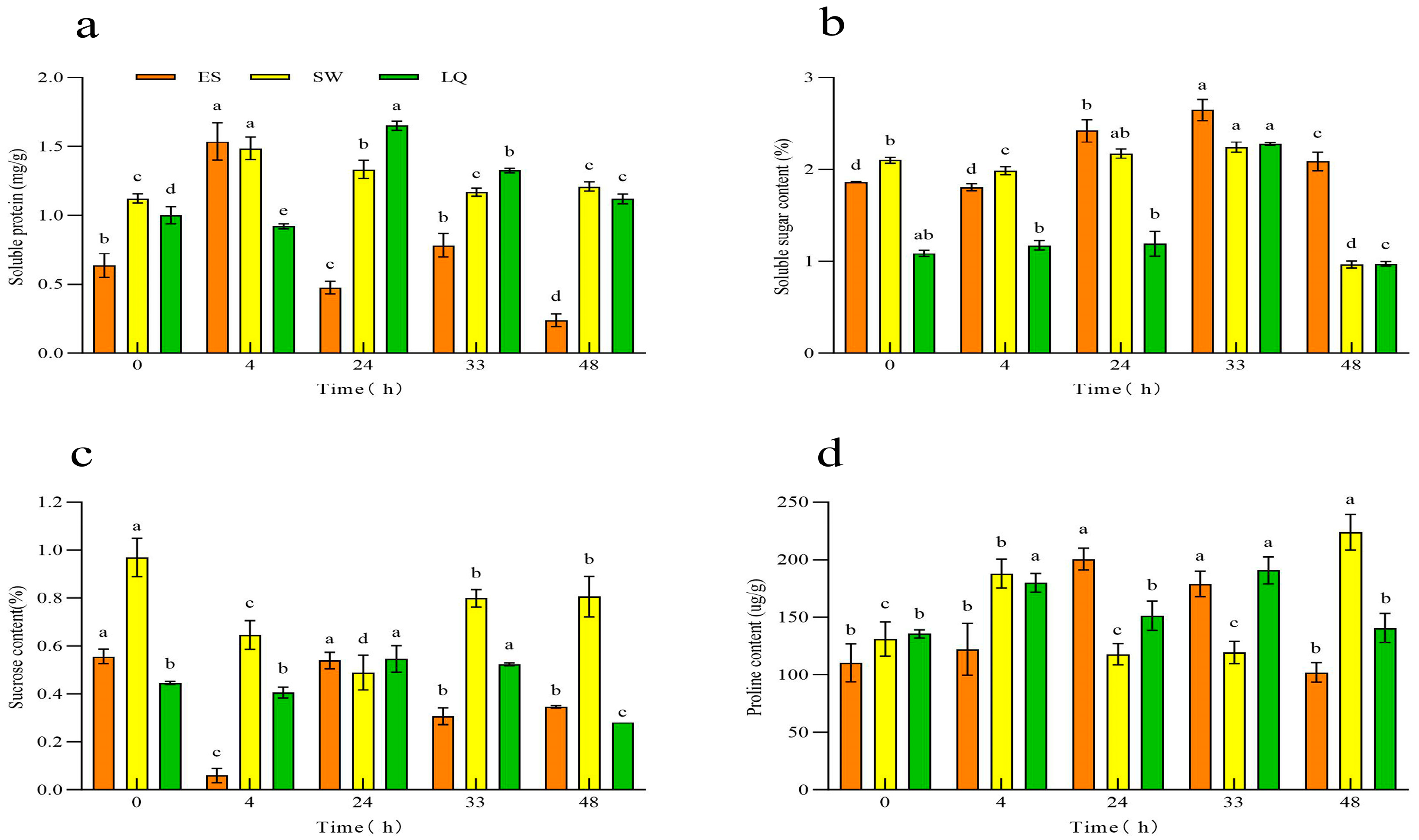
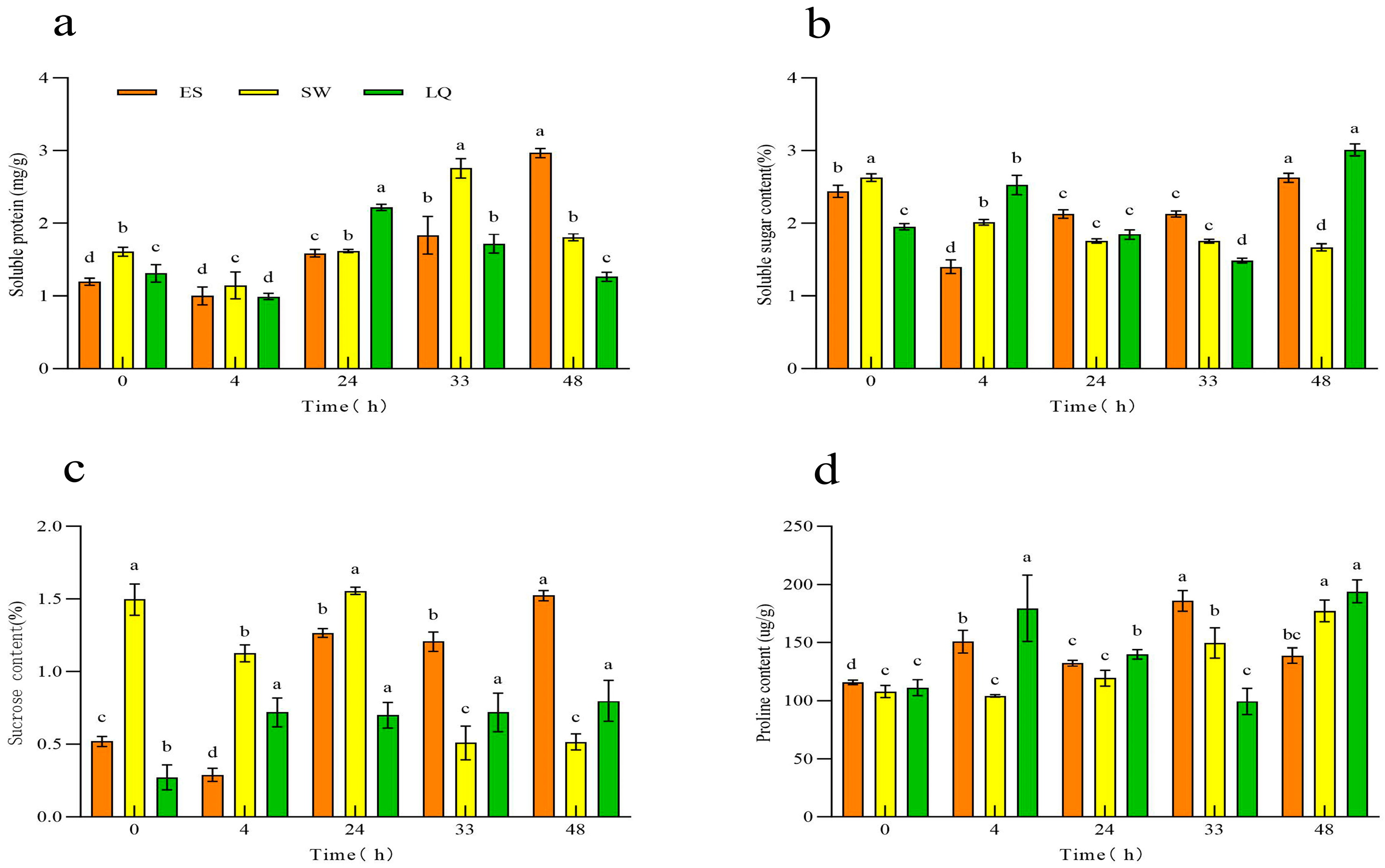


| Test Material | Subordinative Function Value | Cold Resistance Comprehensive Evaluation Value | Order | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Soluble Protein | Soluble Sugar | Sucrose | Proline | MDA | SOD | ||
| Y2ES | 0.22 | 0 | 0.25 | 0.41 | 0.74 | 0 | 0.17 | 6 |
| Y2SW | 0.14 | 0.76 | 1 | 0.16 | 0.56 | 0 | 0.27 | 4 |
| Y2LQ | 0 | 1 | 0 | 0.68 | 0.57 | 0 | 0.22 | 5 |
| Y4ES | 0.82 | 0.7 | 0.59 | 1 | 1 | 0 | 0.80 | 2 |
| Y4SW | 1 | 0.72 | 0.19 | 0 | 0.43 | 0 | 0.94 | 1 |
| Y4LQ | 0.54 | 0.46 | 0.55 | 0.99 | 0 | 0 | 0.52 | 3 |
| Ginsenoside | Ginsenoside Retention Time, min | Two-Year-Old Ginseng Roots | Four-Year-Old Ginseng Roots | ||||
|---|---|---|---|---|---|---|---|
| Group | ES/CK | SW/CK | LQ/CK | ES/CK | SW/CK | LQ/CK | |
| 08.11@PPT-G-G-G | 8.11 | 0.08 | 0.09 | 0.12 | 0.81 | 0.37 | 0.37 |
| PPT-G-G-X_2 | 8.59 | 0.41 | 0.37 | 0.54 | 0.41 | 0.44 | 0.34 |
| PPT-G-G-X@Noto_R1 | 8.70 | 0.03 | 0.12 | 0.03 | 9.89 | 1.55 | 4.50 |
| PPT-G-G-R-X | 8.88 | 0.33 | 0.25 | 0.57 | 0.42 | 0.63 | 0.37 |
| PPT-G-G-But_1 | 9.66 | 0.13 | 0.12 | 0.14 | 0.39 | 0.24 | 0.18 |
| PPT-G-G-R-Ace_1 | 9.67 | 2 | 1.58 | 2.77 | 0.44 | 0.47 | 0.53 |
| PPT-G-G-R-36_2 | 9.82 | 0.70 | 0.63 | 0.90 | 0.67 | 0.59 | 0.52 |
| PPT-G-G-R@GinsenoNJ_1 | 9.83 | 0.67 | 0.60 | 0.88 | 0.63 | 0.53 | 0.45 |
| PPT-G-G-R_1 | 9.84 | 0.53 | 0.48 | 0.91 | 0.42 | 0.48 | 0.41 |
| PPT-G-G-R-Ace_2 | 9.84 | 0.61 | 0.42 | 0.71 | 0.53 | 0.37 | 0.30 |
| 841@PPT-G-G-Ace_2 | 11.67 | 0.07 | 0.07 | 0.12 | 0.36 | 0.24 | 0.27 |
| 887@PPT-G-G-Ace_2 | 11.67 | 0.12 | 0.12 | 0.13 | 0.79 | 0.51 | 0.43 |
| PPT-G-G-Mal_2 | 11.70 | 0.14 | 0.14 | 0.14 | 0.83 | 0.56 | 0.48 |
| PPT-G-G-But-Mal_2 | 11.70 | 0.13 | 0.13 | 0.14 | 0.78 | 0.53 | 0.45 |
| PPT-G-G-diMal_3 | 11.70 | 0.10 | 0.10 | 0.12 | 0.82 | 0.55 | 0.49 |
| PPT-G-R-X | 12.54 | 0.75 | 0.76 | 1.09 | 0.63 | 0.83 | 0.55 |
| 13.35@PPT-G-G-G | 13.35 | 2.01 | 1.55 | 1.72 | 0.42 | 0.50 | 0.49 |
| PPT-G-G-R-A | 13.88 | 2.00 | 1.58 | 2.77 | 0.44 | 0.47 | 0.53 |
| PPT-G-G@GinsenosiNJ_3 | 14.70 | 0.23 | 0.17 | 0.36 | 0.46 | 0.34 | 0.21 |
| PPT-G-X_1 | 15.98 | 3.85 | 1.24 | 2.02 | 0.45 | 0.30 | 0.47 |
| PPT-G-R-36_1 | 16.62 | 0.59 | 0.52 | 1.14 | 0.46 | 0.60 | 0.42 |
| PPT-G-G-G-G-A | 19.50 | 0.18 | 0.20 | 0.42 | 1.70 | 1.78 | 1.58 |
| PPT-G-R-B | 16.62 | 0.59 | 0.52 | 1.13 | 0.43 | 0.55 | 0.37 |
| PPT-G-R | 16.63 | 0.75 | 0.76 | 1.09 | 0.63 | 0.83 | 0.55 |
| PPT-G-G-Ace_6 | 16.63 | 0.59 | 0.52 | 1.15 | 0.44 | 0.58 | 0.40 |
| PPT-G-G-R-36_5 | 16.63 | 0.38 | 0.37 | 1.34 | 0.36 | 0.29 | 0.58 |
| PPT-G-G-G-X_4 | 20.11 | 0.49 | 0.35 | 0.62 | 0.11 | 0.15 | 0.20 |
| PPT-G-G-R-36_6 | 20.11 | 0.47 | 0.35 | 0.63 | 0.11 | 0.15 | 0.17 |
| PPD-20S-Rg2 | 16.62 | 0.58 | 0.51 | 1.12 | 0.53 | 0.55 | 0.38 |
| PPD-20R-Rg2 | 17.00 | 3.08 | 1.61 | 1.37 | 1.12 | 1.13 | 1.39 |
| 1033@PPD-G-G-G-Ace_1 | 20.40 | 0.29 | 0.30 | 0.50 | 0.17 | 0.21 | 0.23 |
| PPD-G-G-G-X-Mal_3 | 19.19 | 0.13 | 0.19 | 0.32 | 0.36 | 1.08 | 0.42 |
| PPD-G-G-G-X-But-NJ_17 | 19.19 | 0.12 | 0.18 | 0.32 | 0.35 | 1.06 | 0.42 |
| PPD-G-G-G-X-But-NJ_18 | 19.19 | 0.14 | 0.19 | 0.33 | 0.50 | 1.19 | 0.43 |
| 987@PPD-G-G-G-Ace_1 | 20.40 | 0.22 | 0.21 | 0.57 | 0.11 | 0.15 | 0.19 |
| PPD-G-G-G@GinsenoNJ_23 | 20.09 | 0.46 | 0.35 | 0.61 | 0.10 | 0.14 | 0.15 |
| PPD-G-G-G-B-B | 20.10 | 0.47 | 0.34 | 0.63 | 0.10 | 0.13 | 0.15 |
| PPD-G-G-G-But_1 | 20.11 | 0.48 | 0.35 | 0.61 | 0.10 | 0.14 | 0.15 |
| PPD-M | 20.41 | 0.31 | 0.32 | 0.52 | 0.20 | 0.23 | 0.27 |
| PPD-G-G-G-B-B-B-M | 20.41 | 0.31 | 0.31 | 0.50 | 0.20 | 0.21 | 0.28 |
| PPD-G-G-G-Mal_1 | 20.40 | 0.29 | 0.30 | 0.49 | 0.17 | 0.21 | 0.23 |
| PPD-G-G-G-Mal_4 | 21.69 | 0.36 | 0.34 | 0.47 | 0.39 | 0.37 | 0.47 |
| PPD-G-G-X | 22.66 | 7.80 | 0.92 | 3.50 | 0.57 | 0.81 | 1.11 |
| PPD-G-G-X-Mal_2 | 22.77 | 0.39 | 0.29 | 0.51 | 0.45 | 0.56 | 0.40 |
| OT-G-R | 9.06 | 1.59 | 1.62 | 2.33 | 0.82 | 1.16 | 0.68 |
| OA-Ga-G-G@GinsenoNJ_6 | 18.15 | 0.05 | 0.07 | 0.13 | 0.90 | 0.46 | 0.60 |
| OA-GlcA-G-G-But-NJ_25 | 20.40 | 0.29 | 0.31 | 0.50 | 0.19 | 0.22 | 0.26 |
| OA-GlcA-G-G-But-NJ_26 | 20.40 | 0.28 | 0.28 | 0.47 | 0.19 | 0.20 | 0.28 |
| Total saponins | 0.39 | 0.37 | 0.59 | 0.46 | 0.40 | 0.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jin, H.; Wang, Y.; Liu, X.; Xu, Y.; Yang, H. The Effects of Low-Temperature Stress on the Physiological Characteristics and Active Components of Ginseng Under Different Soil Moisture Conditions. Horticulturae 2025, 11, 526. https://doi.org/10.3390/horticulturae11050526
Liu J, Jin H, Wang Y, Liu X, Xu Y, Yang H. The Effects of Low-Temperature Stress on the Physiological Characteristics and Active Components of Ginseng Under Different Soil Moisture Conditions. Horticulturae. 2025; 11(5):526. https://doi.org/10.3390/horticulturae11050526
Chicago/Turabian StyleLiu, Jiao, Hongyan Jin, Yingping Wang, Xiaoying Liu, Yonghua Xu, and He Yang. 2025. "The Effects of Low-Temperature Stress on the Physiological Characteristics and Active Components of Ginseng Under Different Soil Moisture Conditions" Horticulturae 11, no. 5: 526. https://doi.org/10.3390/horticulturae11050526
APA StyleLiu, J., Jin, H., Wang, Y., Liu, X., Xu, Y., & Yang, H. (2025). The Effects of Low-Temperature Stress on the Physiological Characteristics and Active Components of Ginseng Under Different Soil Moisture Conditions. Horticulturae, 11(5), 526. https://doi.org/10.3390/horticulturae11050526






