Effects of Different LED Light Quality Combinations on Nutritional Quality and Physiological Characteristics of Celery
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
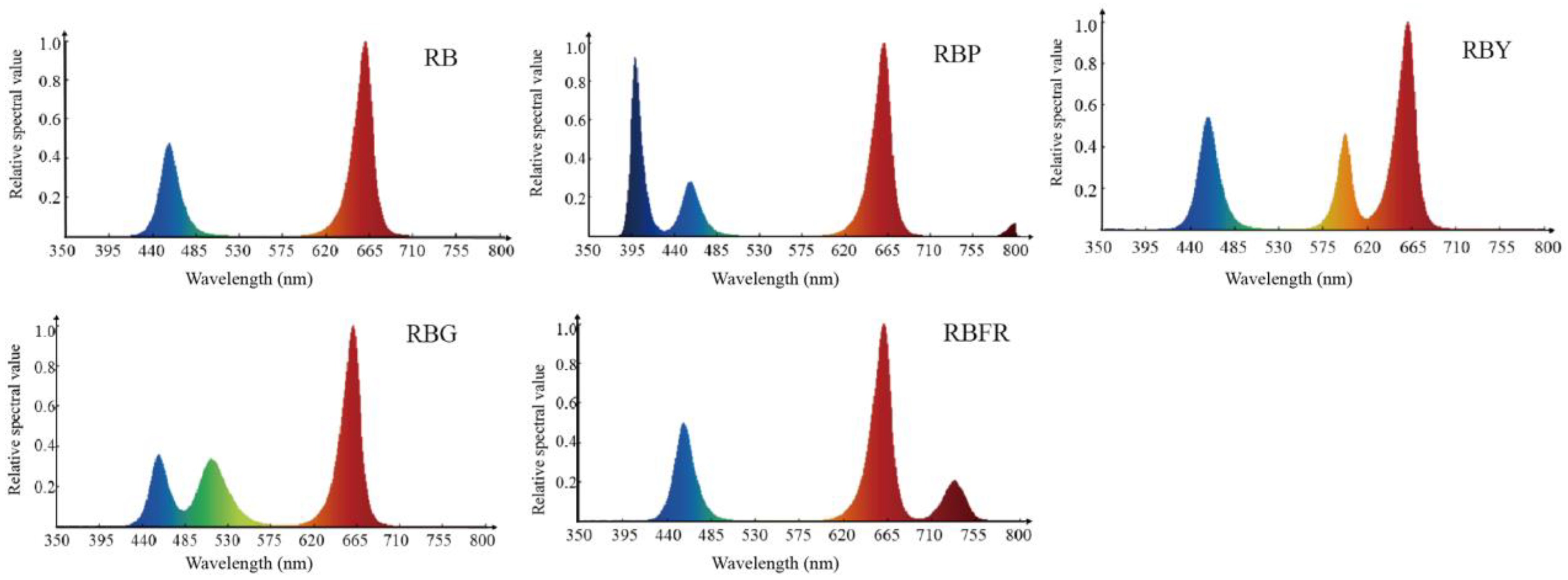
2.2. Experimental Treatments
2.3. Cultivation Conditions
2.4. Determination of Plant Growth and Morphology
2.5. Measurement of Photosynthetic Parameters
2.6. Determination of Nutritional Quality Indicators
2.7. Determination of Mineral Element Content
2.8. Determination of Flavonoid-Related Enzyme Activities
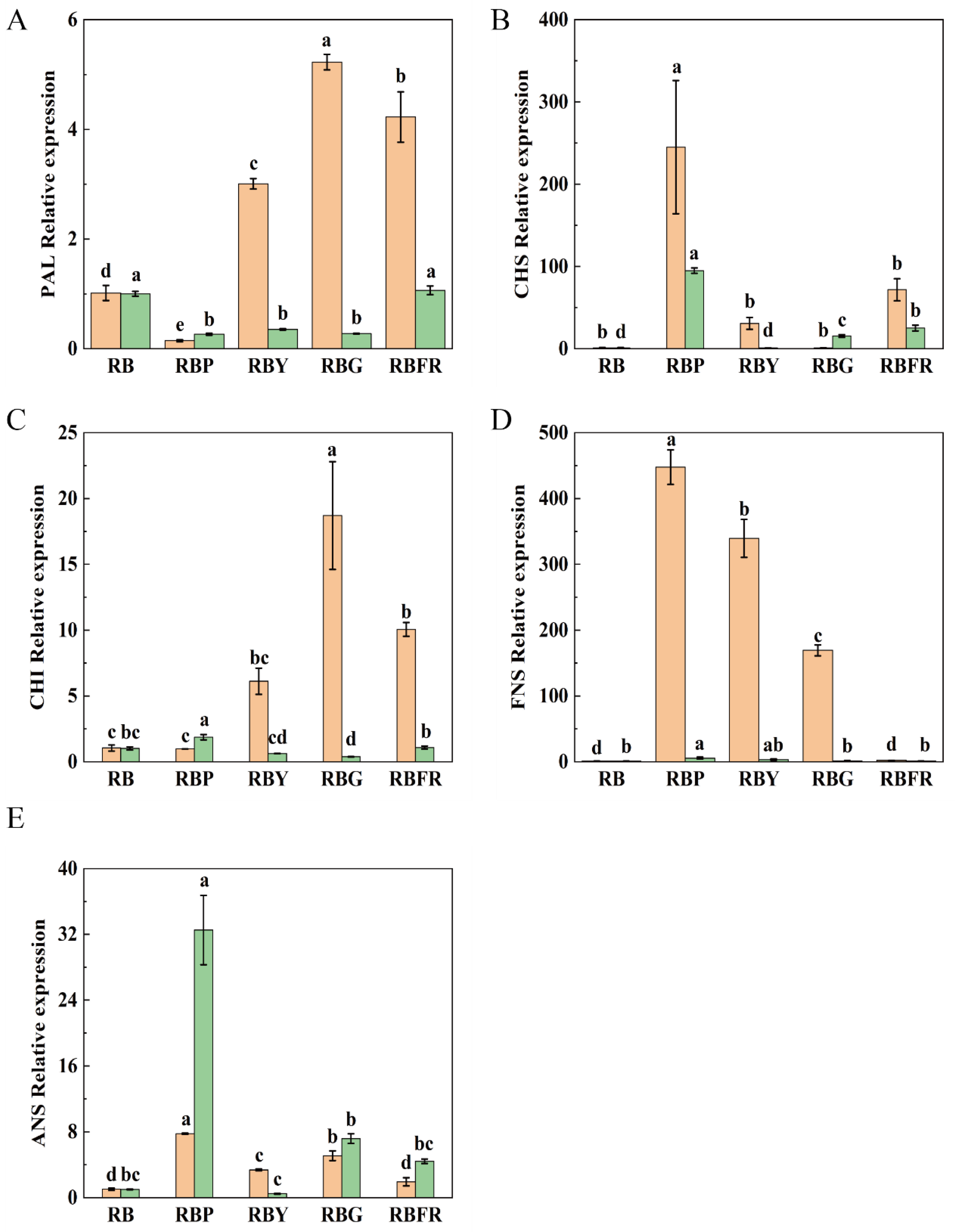
2.9. Expression Analysis of Key Flavonoid-Related Genes
2.10. Statistical Analysis
3. Results and Analysis
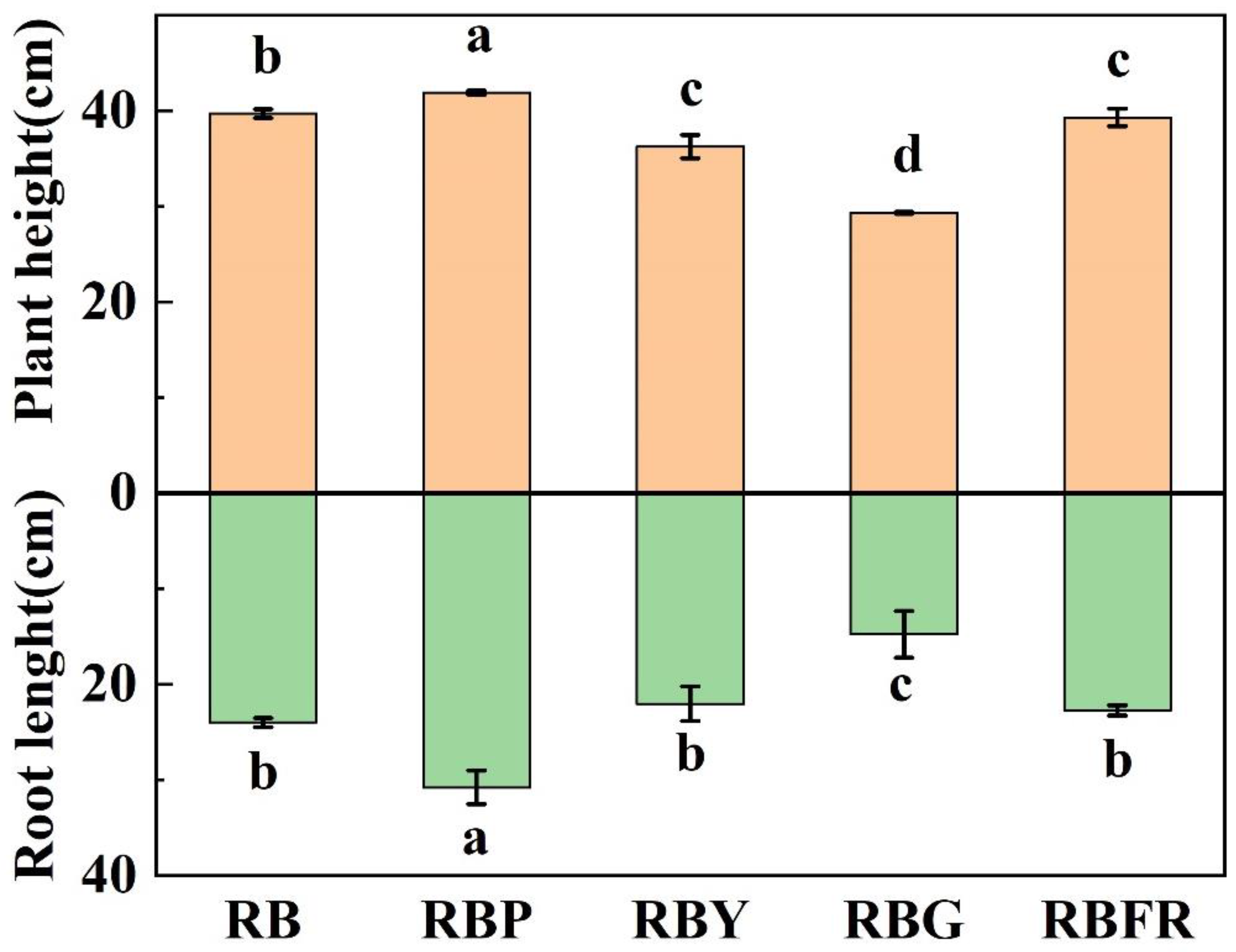
3.1. Effects of LED Combined Light on Growth and Morphological Characteristics of Celery
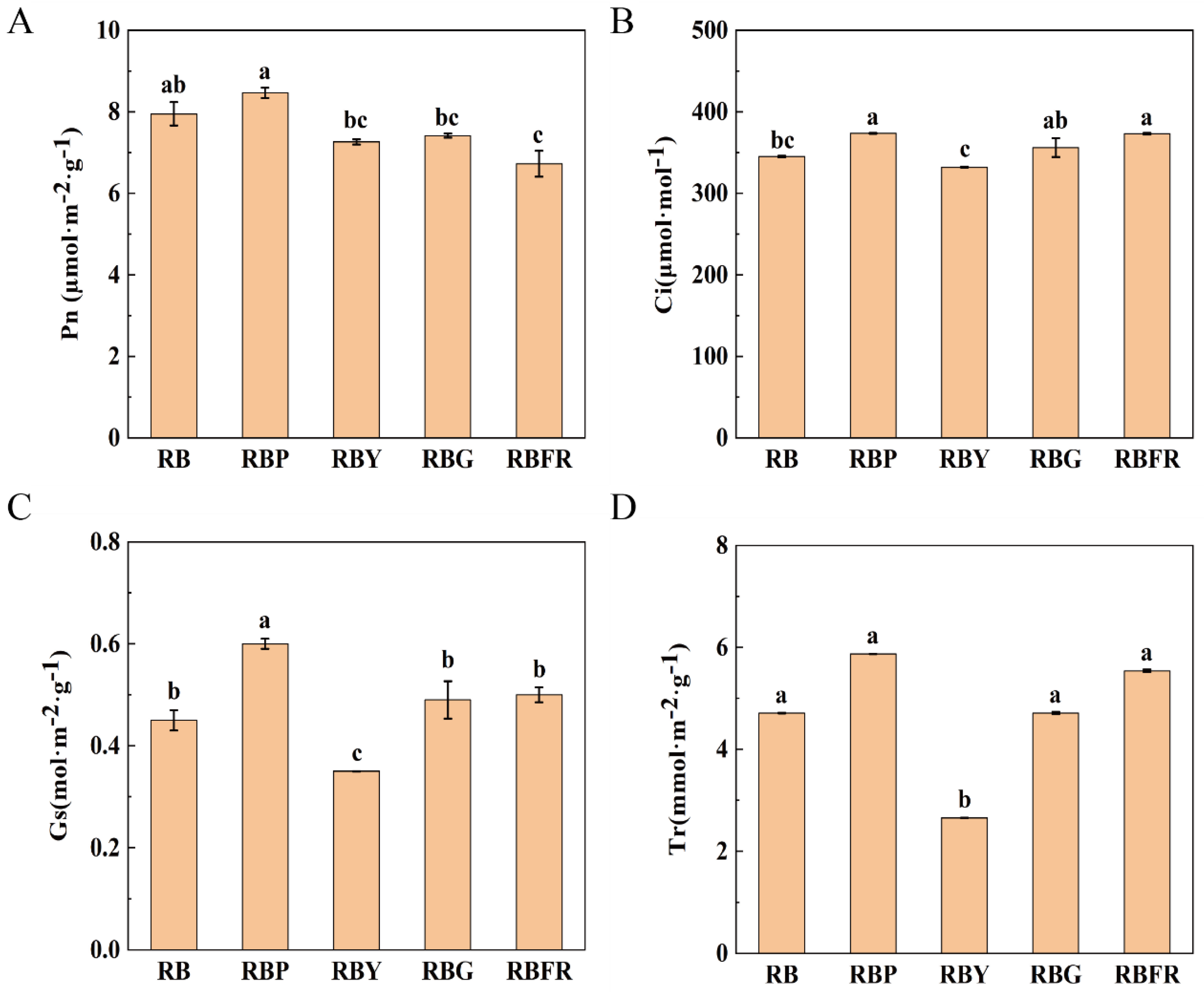
3.2. Effect of LED Combined Light on Photosynthetic Parameters of Celery
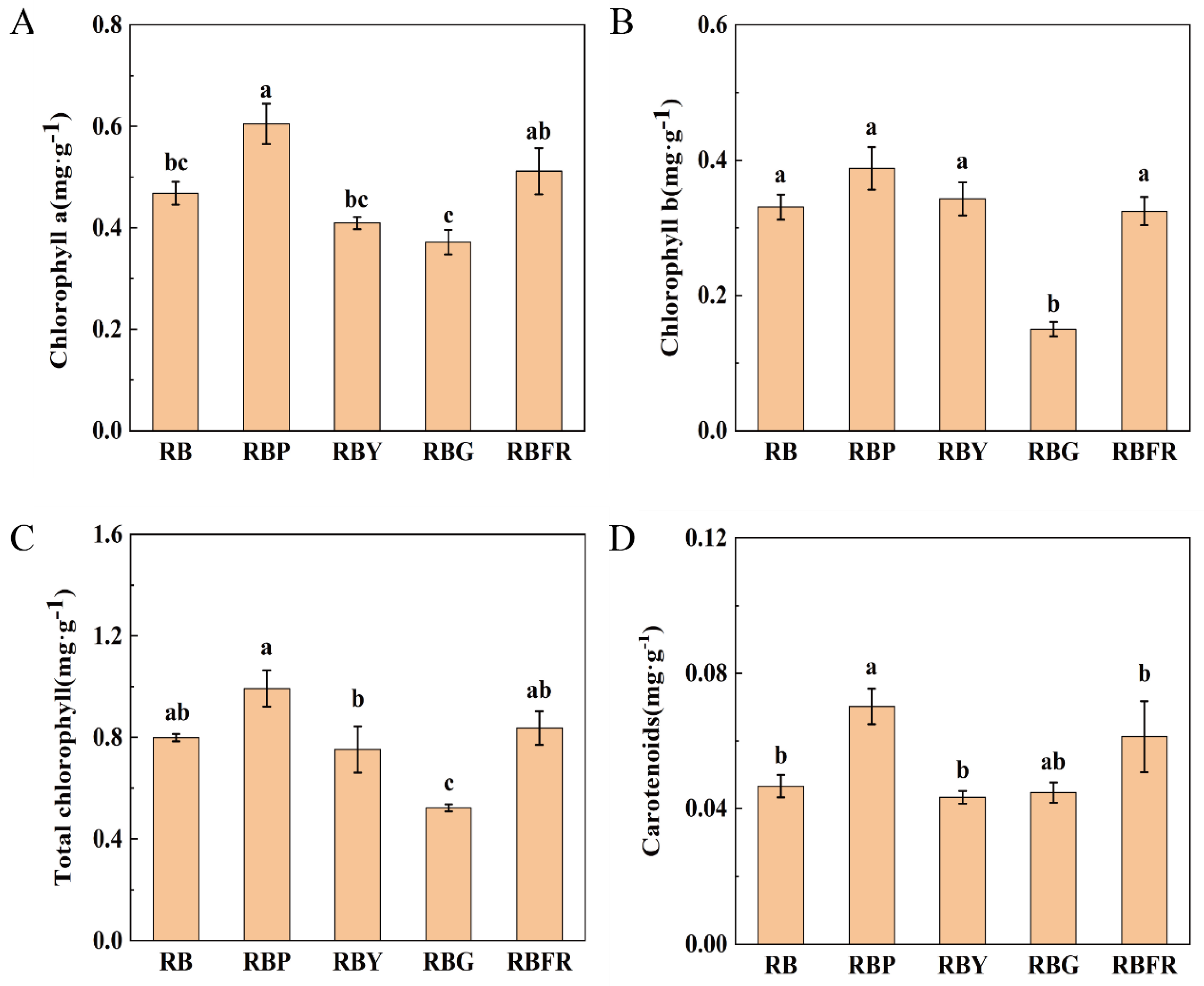
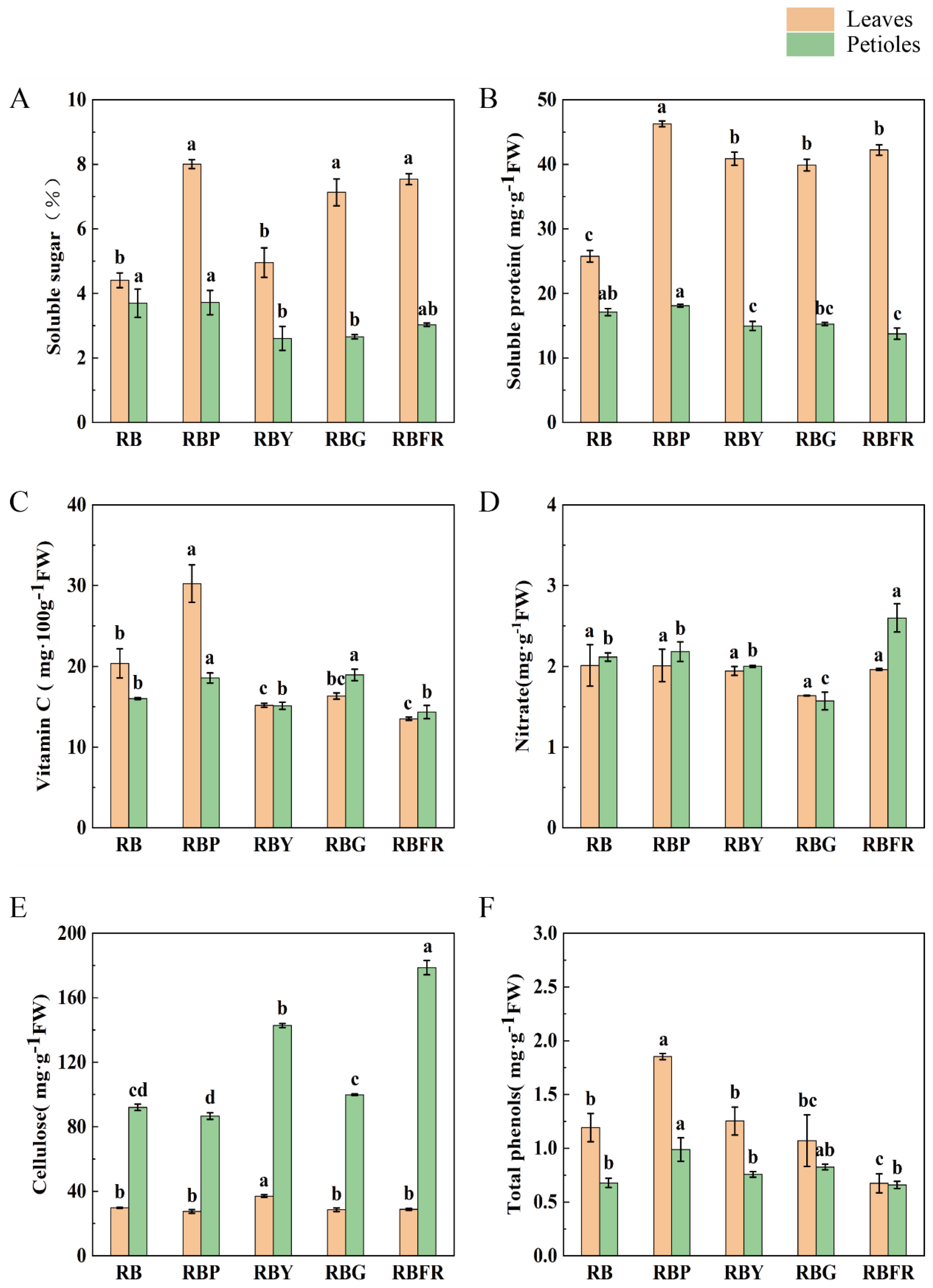
3.3. The Effect of LED Combined Light on the Nutritional Quality of Celery
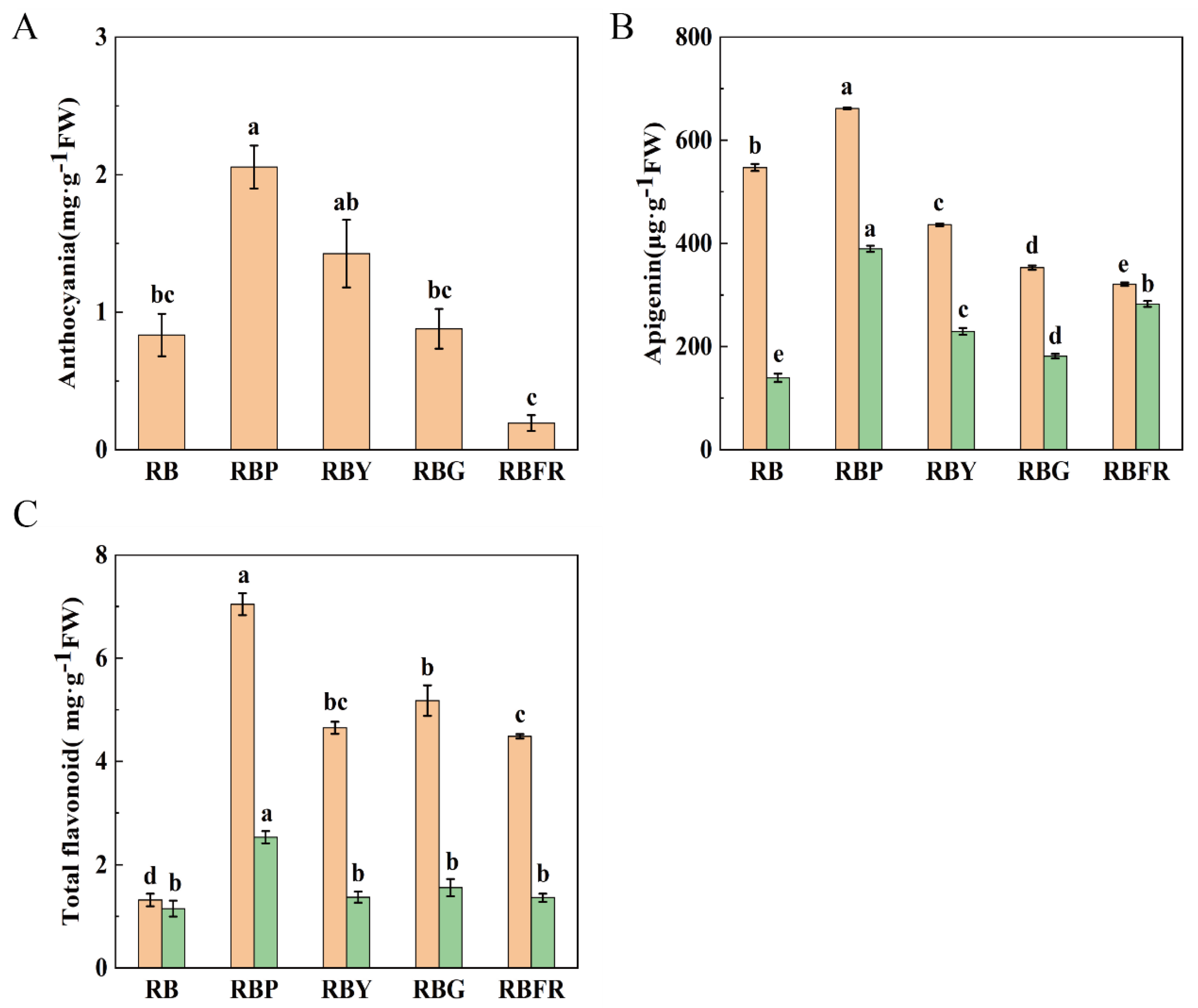
3.4. The Effect of LED Combined Light on Flavonoids in Celery
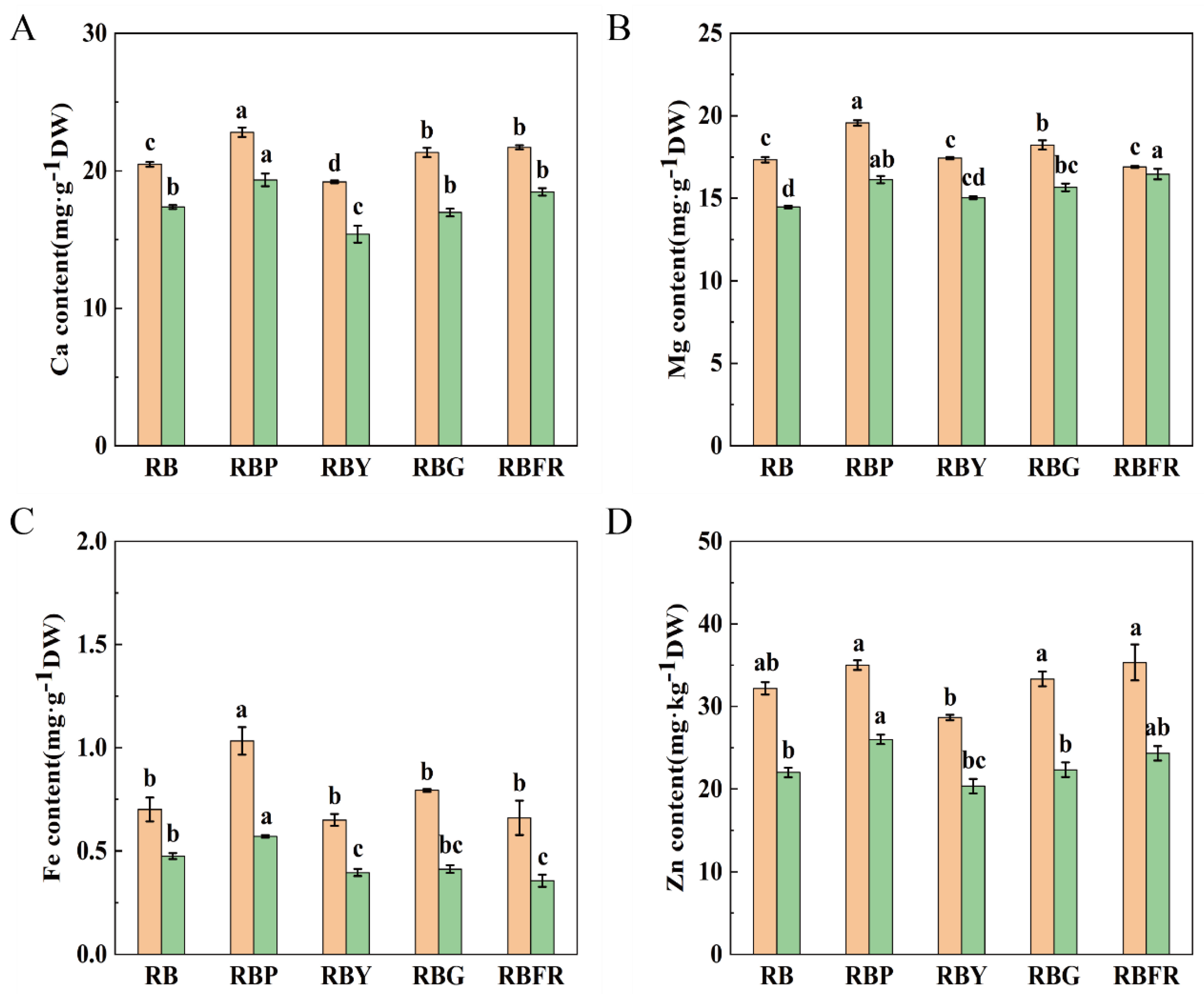
3.5. Effect of LED Combined Light on Mineral Elements of Celery
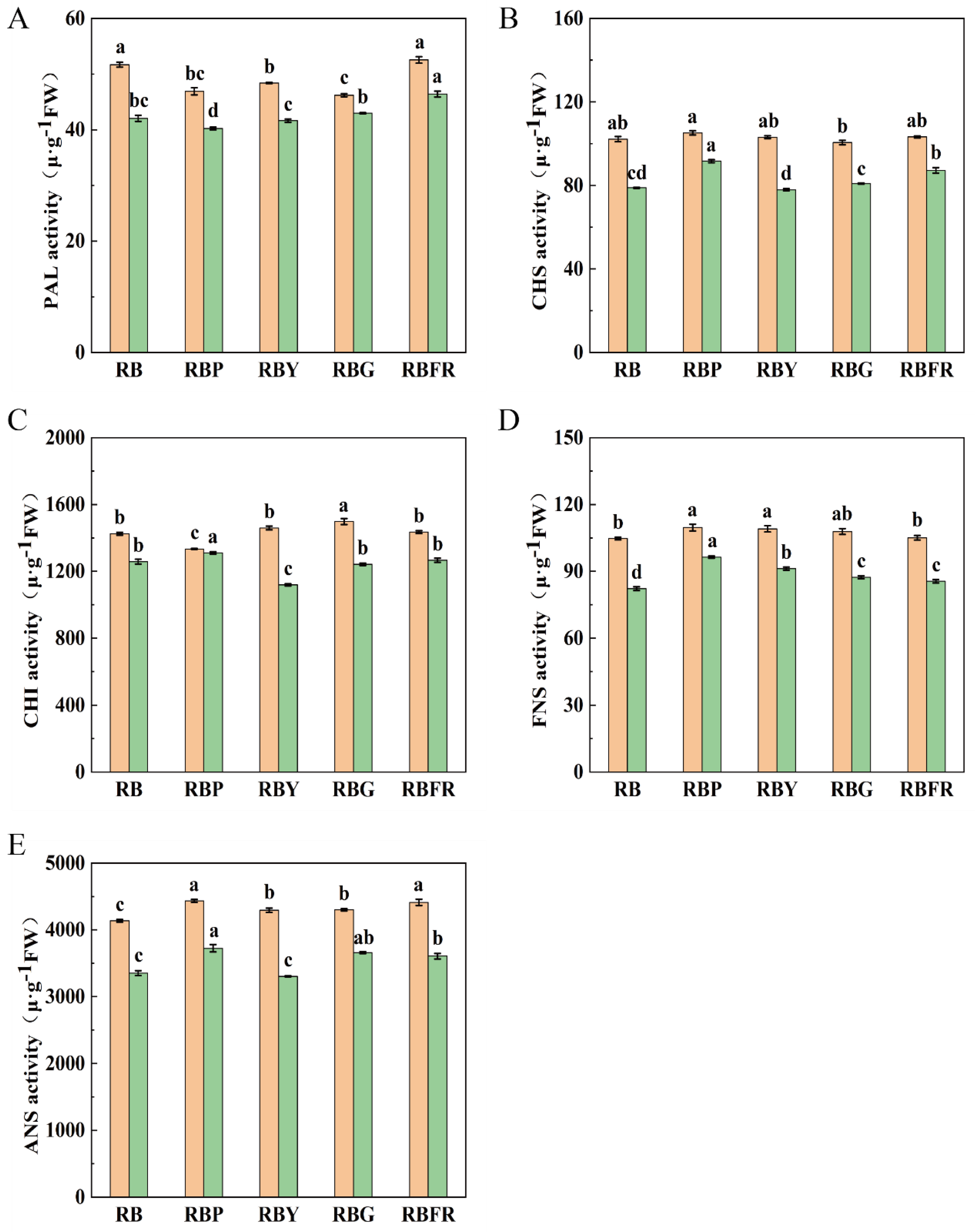
3.6. Flavonoid-Related Enzyme Activities
3.7. Expression Analysis of Key Flavonoid-Related Genes
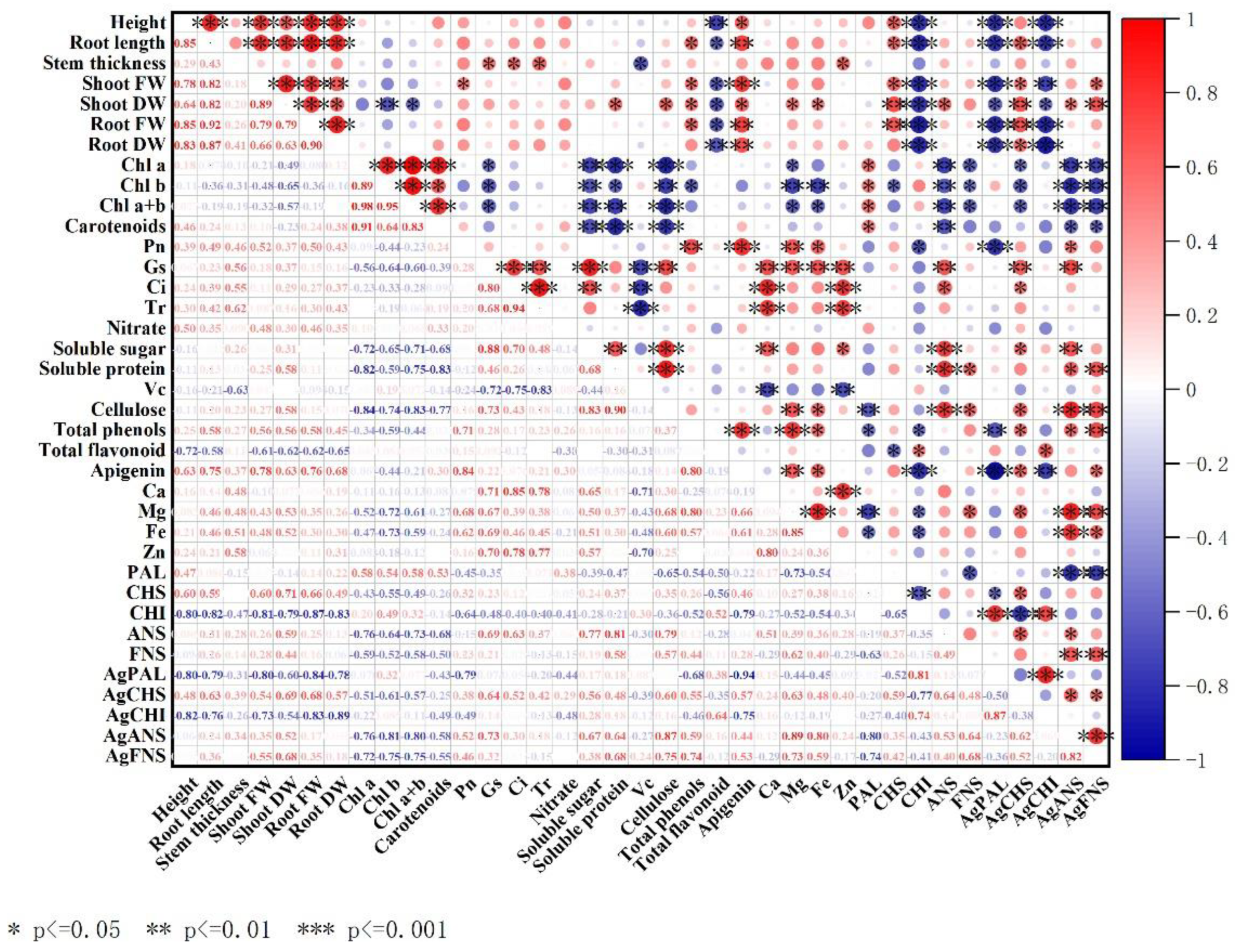
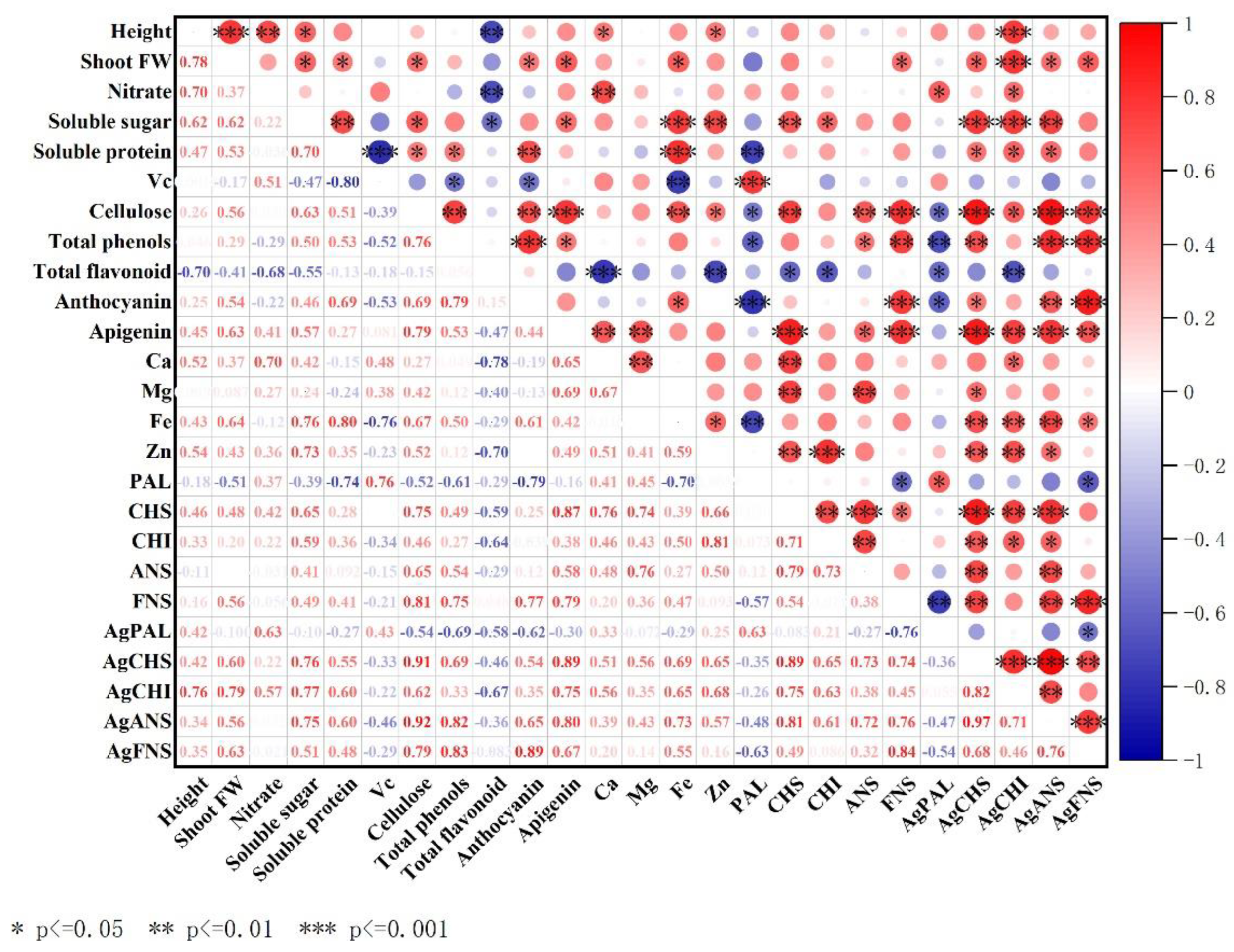
3.8. Correlation Analysis of Celery Leaves and Petioles
4. Discussion
4.1. Effects of Different LED Light Quality Combinations on Celery Growth
4.2. Effects of Different LED Light Quality Combinations on Celery Quality
4.3. Effects of Different LED Light Qualities on Plant Photosynthetic Properties
4.4. Effect of Different LED Light Quality Combinations on Mineral Elements in Celery
4.5. Effects of Different LED Light Qualities on Flavonoid Compound Synthesis-Related Enzymes and Gene Expression
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kooti, W.; Daraei, N. A Review of the Antioxidant Activity of Celery (Apium graveolens L). J. Evid. Based Complement. Altern. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.L.; Li, M.Y.; Jiang, Q.; Xu, Z.S.; Liu, J.X.; Xiong, A.S. CeleryDB: A genomic database for celery. Database 2018, 2018, bay070. [Google Scholar] [CrossRef]
- Sowbhagya, H.B. Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Veisi, A.; Ahangarpour, A.; Fathi Moghaddam, H. The effect of hydro-alcoholic celery (Apiumgraveolens) leaf extract on cardiovascular parameters and lipid profile in animal model of hypertension induced by fructose. Avicenna J. Phytomedicine 2015, 5, 203–209. [Google Scholar]
- Singh, M.; Nara, U.; Kaur, K.; Rani, N.; Jaswal, R. Genetic, genomic and biochemical insights of celery (Apium graveolens L.) in the era of molecular breeding. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100420. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Z.G.; Cui, Y.L.; Shao, Y.; Tao, Y.D.; Mei, L.J. Apigenin from daily vegetable celery can accelerate bone defects healing. J. Funct. Foods 2019, 54, 412–421. [Google Scholar] [CrossRef]
- Tang, Y.K.; Mao, R.X.; Guo, S.S. Effects of LED spectra on growth, gas exchange, antioxidant activity and nutritional quality of vegetable species. Life Sci. Space Res. 2020, 26, 77–84. [Google Scholar] [CrossRef]
- Hammock, H.A.; Kopsell, D.A.; Sams, C.E. Application timing and duration of LED and HPS supplements differentially influence yield, nutrient bioaccumulation, and light use efficiency of greenhouse basil across seasons. Front. Plant Sci. 2023, 14, 1174823. [Google Scholar] [CrossRef]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light emitting diode (LED) lights for the improvement of plant performance and production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Gourrierec, J.L.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Kohler, A.E.; Lopez, R.G. Duration of light-emitting diode (LED) supplemental lighting providing far-red radiation during seedling production influences subsequent time to flower of long-day annuals. Sci. Hortic. 2021, 281, 109956. [Google Scholar] [CrossRef]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of Light-Emitting Diodes for Improving the Nutritional Quality and Bioactive Compound Levels of Some Crops and Medicinal Plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Demir, K.; Sarıkamış, G.; Çakırer Seyrek, G. Effect of LED lights on the growth, nutritional quality and glucosinolate content of broccoli, cabbage and radish microgreens. Food Chem. 2023, 401, 134088. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Chen, Y.H.; Huang, L.H. A sustainable vegetable supply chain using plant factories in Taiwanese markets: A Nash–Cournot model. Int. J. Prod. Econ. 2014, 152, 49–56. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Zhang, N.M.; Chen, J.Y.; Zhou, X.J.; Molokeev, M.S.; Guo, C.F. The Vis-NIR multicolor emitting phosphor Ba4Gd3Na3(PO4)6F2: Eu2+, Pr3+ for LED towards plant growth. J. Ind. Eng. Chem. 2018, 65, 411–417. [Google Scholar] [CrossRef]
- Sun, T.H.; Wang, P.; Rao, S.; Zhou, X.S.; Wrightstone, E.; Lu, S.; Yuan, H.; Yang, Y.; Fish, T.; Thannhauser, T.; et al. Co-chaperoning of chlorophyll and carotenoid biosynthesis by ORANGE family proteins in plants. Mol. Plant 2023, 16, 1048–1065. [Google Scholar] [CrossRef]
- Zou, H.C.; Li, C.Z.; Zhang, A.Y.; Zhang, X.P.; Chen, X.D.; Wang, F.Q.; Yan, Y.Y.; Zhang, S.A. Light environment control for reducing energy loss and increasing crop yield in plant factories. Sol. Energy 2024, 268, 112281. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.T.; Song, S.W.; Su, W.; Hao, Y.W.; Liu, H.C. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2020, 345, 128727. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhang, N.N.; Wang, Y.X.; Khan, A.B.I.D.; Ma, S.; Bai, X.; Zeng, Q.; Pan, Q.M.; Li, B.H.; Zhang, L.G. Blue light induces leaf color change by modulating carotenoid metabolites in orange-head Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Integr. Agric. 2023, 22, 3296–3311. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Monostori, I.; Heilmann, M.; Kocsy, G.; Rakszegi, M.; Ahres, M.; Altenbach, S.B.; Szalai, G.; Pál, M.; Toldi, D.; Simon-Sarkadi, L.; et al. LED Lighting—Modification of Growth, Metabolism, Yield and Flour Composition in Wheat by Spectral Quality and Intensity. Front. Plant Sci. 2018, 9, 605. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Xie, C.Y.; Tang, J.; Xiao, J.X.; Geng, X.; Guo, L.P. Purple light-emitting diode (LED) lights controls chlorophyll degradation and enhances nutraceutical quality of postharvest broccoli florets. Sci. Hortic. 2022, 294, 110768. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.F.; Wei, M.; Shi, Q.H.; Yang, F.J.; Wang, X.F. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; Kooten, O.V.; Harbinson, J.; Ieperen, W.V. Plasticity of photosynthesis after the ‘red light syndrome’in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Liu, M.X.; Xu, Z.G.; Yang, Y.; Feng, Y.J. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell, Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar]
- Silva, T.D.; Batista, D.S.; Fortini, E.A.; Castro, K.M.; Felipe, S.H.S.; Fernandes, A.M.; Sousa, R.M.J.; Chagas, K.; Silva, J.V.S.D.; Correia, L.N.F.; et al. Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J. Photochem. Photobiol. B 2020, 203, 111761. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Stomatal conductance of lettuce grown under or exposed to different light qualities. Ann. Bot. 2004, 94, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; He, Q.Q.; Pan, Q.M.; Feng, Y.Z.; Shi, Y.; Li, G.Z.; Zhang, Y.; Liu, Y.L.; Khan, A. Blue-green light treatment enhances the quality and nutritional value in postharvest Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Chem. X 2024, 24, 102004. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.M.; Hu, D.W.; Yu, J.; Liu, H. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock out”. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2021, 291, 110561. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef]
- Son, K.H.; Lee, J.H.; Oh, Y.; Kim, D.; Oh, M.M.; In, B.C. Growth and Bioactive Compound Synthesis in Cultivated Lettuce Subject to Light-quality Changes. HortScience 2017, 52, 584–591. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.Z.; Yang, W.X.; Shang, X.L.; Fu, X.X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobiol. B. 2018, 179, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, L.C.; Li, Y.L.; Yang, Q.C.; Guo, W.Z. Alternating red and blue irradiation affects carbohydrate accumulation and sucrose metabolism in butterhead lettuce. Sci. Hortic. 2022, 302, 111177. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, J.; Xie, F.J.; Zhou, J.; Sun, Y.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Lin, Y.X.; et al. Combined evaluation of agronomic and quality traits to explore heat germplasm in celery (Apium graveolens L.). Sci. Hortic. 2023, 317, 112039. [Google Scholar] [CrossRef]
- Li, H.Q.; Sun, H.; Li, M.Z. Prediction of soluble sugar content in cabbage by near infrared spectrometer. Spectrosc. Spectr. Anal. 2018, 38, 3058–3063. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Communications in soil science and plant analysis rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Chen, G.; Mo, L.; Li, S.; Zhou, W.T.; Wang, H.; Liu, J.X.; Yang, C.M. Separation and determination of reduced vitamin C in polymerized hemoglobin-based oxygen carriers of the human placenta. Artif. Cells Nanomed. Biotechnol. 2015, 43, 152–156. [Google Scholar] [CrossRef]
- Antia, B.S.; Akpan, E.J.; Okon, P.A.; Umoren, I.U. Nutritive and anti-nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pakistan J. Nutr. 2006, 5, 166–168. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Rsponse to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Li, Y.Y.; Mao, K.; Zhao, C.; Zhao, X.Y.; Zhang, H.L.; Shu, H.Y.; Hao, Y.J. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef]
- Tan, G.F.; Ma, J.; Zhang, X.Y.; Xu, Z.S.; Xiong, A.S. AgFNS overexpression increase apigenin and decrease anthocyanins in petioles of transgenic celery. Plant Sci. 2017, 263, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yu, L.; Xu, S.; Gu, W.H.; Zhu, W.M. Apigenin accumulation and expression analysis of apigenin biosynthesis relative genes in celery. Sci. Hortic. 2014, 165, 218–224. [Google Scholar] [CrossRef]
- Xie, Y.D.; Su, L.H.; He, Z.Q.; Zhang, J.W.; Tang, Y. Selenium Inhibits Cadmium Absorption and Improves Yield and Quality of Cherry Tomato (Lycopersicon esculentum) Under Cadmium Stress. J. Soil Sci. Plant Nutr. 2021, 21, 1125–1133. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Q.; Shen, Z.G.; Xia, K.; Cui, J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 2014, 73, 227–235. [Google Scholar] [CrossRef]
- Kim, D.; Moon, T.; Kwon, S.; Hwang, I.; Son, J.E. Supplemental inter-lighting with additional far-red to red and blue light increases the growth and yield of greenhouse sweet peppers (Capsicum annuum L.) in winter. Hortic. Environ. Biotechnol. 2022, 64, 83–95. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef]
- Gao, W.; He, D.X.; Ji, F.; Zhang, S.; Zheng, J.F. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 2–15. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.Z.; Zhang, W.Z.; Wang, L.S.; Li, Z.M.; Liu, H. Synergistic effect between green light and nitrogen concentration on nitrate primary metabolism in lettuce (Lactuca sativa L.). Sci. Hortic. 2024, 13, 328. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.H.; Zhou, Q.; Qu, Y.H. Growth, nutritional quality, and energy use efficiency in two lettuce cultivars as influenced by white plus red versus red plus blue LEDs. Int. J. Agric. Biol. Eng. 2020, 13, 33–40. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, W.K. Substituting green light for partial red light promoted the growth and quality, and regulated the nitrogen metabolism of Medicago sativa grown under red-blue LEDs. Environ. Exp. Bot. 2024, 220, 105623. [Google Scholar] [CrossRef]
- Huner, N.P.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Lillo, C. Light regulation of nitrate reductase in green leaves of higher plants. Physiol. Plant. 1994, 90, 616–620. [Google Scholar] [CrossRef]
- Cheng, C.L.; Acedo, G.N.; Cristinsin, M.; Conkling, M.A. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc. Natl. Acad. Sci. USA 1992, 89, 1861–1864. [Google Scholar] [CrossRef]
- Sivasankar, S.; Rothstein, S.; Oaks, A. Regulation of the Accumulation and Reduction of Nitrate by Nitrogen and Carbon Metabolites in Maize Seedlings. Plant Physiol. 1997, 114, 583–589. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.W.; Liu, W.K.; Ding, Y.T.; Zhang, Q.S.; Wang, S.R. Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. cv. “Yidali”) in Plant Factory. Plants 2023, 12, 4147. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Wang, Y.; Yang, Q.C.; Lu, C.G. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Smirnoff, N. Chapter 4—Vitamin C: The Metabolism and Functions of Ascorbic Acid in Plants. Adv. Bot. Res. 2011, 59, 107–177. [Google Scholar]
- Geng, M.J.; Feng, X.M.; Wu, X.X.; Tan, X.Y.; Liu, Z.N.; Li, L.J.; Huang, Y.Y.; Teng, F.; Li, Y. Encapsulating vitamins C and E using food-grade soy protein isolate and pectin particles as carrier: Insights on the vitamin additive antioxidant effects. Food Chem. 2023, 418, 135955. [Google Scholar] [CrossRef]
- Qian, H.M.; Liu, T.Y.; Deng, M.D.; Miao, H.Y.; Cai, C.X.; Shen, W.S.; Wang, Q.M. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.C.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef]
- Zhuang, L.; Huang, G.Q.; Li, X.D.; Xiao, J.X.; Guo, L.P. Effect of different LED lights on aliphatic glucosinolates metabolism and biochemical characteristics in broccoli sprout. Food Res. Int. 2022, 154, 111015. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.D.; Jang, D.C.; Tran, T.T.H.; Nguyen, Q.T.; Kim, I.S.; Hoang, T.L.H.; Vu, N.T. Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. [Google Scholar] [CrossRef]
- Dong, T.Y.; Zhang, P.A.; Hakeem, A.; Liu, Z.J.; Su, L.Y.; Ren, Y.H.; Fang, J.G. Integrated transcriptome and metabolome analysis reveals the physiological and molecular mechanisms of grape seedlings in response to red, green, blue, and white LED light qualities. Environ. Exp. Bot. 2023, 213, 105441. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Hsu, M.H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.A.; Aparicio-Fernández, X.; Ávila-Sosa, R.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C.E. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018, 135, 19–26. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y.B. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Dou, H.J.; Niu, G.H.; Gu, M.M. Photosynthesis, Morphology, Yield, and Phytochemical Accumulation in Basil Plants Influenced by Substituting Green Light for Partial Red and/or Blue Light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Oh, H.E.; Yoon, A.; Park, Y.G. Red Light Enhances the Antioxidant Properties and Growth of Rubus hongnoensis. Plants 2021, 10, 2589. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Li, W.F.; Mao, J.; Li, W.; Zuo, C.W.; Zhao, X.; Dawuda, M.M.; Shi, X.Y.; Chen, B.H. Synthesis of light-inducible and light-independent anthocyanins regulated by specific genes in grape ‘Marselan’ (V. vinifera L.). PeerJ 2019, 7, e6521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zheng, X.T.; Sun, B.Y.; Peng, C.L.; Chow, W.S. Over-expression of the CHS gene enhances resistance of Arabidopsis leaves to high light. Environ. Exp. Bot. 2018, 154, 33–43. [Google Scholar] [CrossRef]
| Primer Names | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| AgACTIN | CTTCCTGCCATATATGATTGG | GCCAGCACCTCGATCTTCATG |
| AgPAL | TGATGCAGGGGAAGCCTGAATTTA | TGAGGACCAAGCCACTGAGGAGAT |
| AgCHS | GGGCCTTACCTTCCATCTTCTTAA | GGTCGCTCGCATTTTTTCTTCCTT |
| AgCHI | CACTTGTCATTCCTTCTTCCTTGC | AACTGGTCTGCCGTCTTGCCCTTC |
| AgFNS | AAGGCGGCTTTACTATCTCCACTC | CACCTAGCACCATTAACTTCTCAC |
| AgANS | CTCTTTCCTCCTCGTACCTTTGCT | CTTCGGTGTTCTTATGTTCCCCTG |
| Treatment | Shoot FW (g) | Shoot DW (g) | Root FW (g) | Root DW (g) | Stem Thickness (mm) |
|---|---|---|---|---|---|
| RB | 29.71 ± 0.095 b | 2.15 ± 0.010 cd | 11.93 ± 0.491 b | 1.39 ± 0.043 ab | 13.54 ± 0.214 ab |
| RBP | 36.96 ± 1.949 a | 3.98 ± 0.227 a | 14.35 ± 0.848 a | 1.57 ± 0.065 a | 15.17 ± 0.110 a |
| RBY | 30.72 ± 1.081 b | 2.93 ± 0.103 b | 11.09 ± 0.107 b | 1.19 ± 0.026 bc | 11.37 ± 1.892 b |
| RBG | 21.32 ± 1.139 c | 1.65 ± 0.153 d | 7.49 ± 0.388 c | 0.89 ± 0.160 c | 13.21 ± 0.703 ab |
| RBFR | 27.54 ± 1.372 b | 2.66 ± 0.199 bc | 11.08 ± 0.595 b | 1.28 ± 0.086 ab | 13.22 ± 0.464 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Tang, L.; Chu, Q.; Lu, Y.; Su, L.; Cheng, S.; He, Z.; Zhou, X. Effects of Different LED Light Quality Combinations on Nutritional Quality and Physiological Characteristics of Celery. Horticulturae 2025, 11, 524. https://doi.org/10.3390/horticulturae11050524
Liu K, Tang L, Chu Q, Lu Y, Su L, Cheng S, He Z, Zhou X. Effects of Different LED Light Quality Combinations on Nutritional Quality and Physiological Characteristics of Celery. Horticulturae. 2025; 11(5):524. https://doi.org/10.3390/horticulturae11050524
Chicago/Turabian StyleLiu, Kaiyue, Li Tang, Qianwen Chu, Yingyi Lu, Lihong Su, Shaobo Cheng, Zhongqun He, and Xiaoting Zhou. 2025. "Effects of Different LED Light Quality Combinations on Nutritional Quality and Physiological Characteristics of Celery" Horticulturae 11, no. 5: 524. https://doi.org/10.3390/horticulturae11050524
APA StyleLiu, K., Tang, L., Chu, Q., Lu, Y., Su, L., Cheng, S., He, Z., & Zhou, X. (2025). Effects of Different LED Light Quality Combinations on Nutritional Quality and Physiological Characteristics of Celery. Horticulturae, 11(5), 524. https://doi.org/10.3390/horticulturae11050524




