Abstract
Synthetic seed technology is an innovative in vitro technique that provides improved storage capabilities for vegetative propagules. Its success mostly depends on the encapsulation matrix’s composition and the encapsulation procedure. The present study focuses on optimizing an encapsulation protocol for short-term storage and germplasm exchange using micro-cuttings of Salix tetrasperma. Among the different synthetic seed types evaluated, double-layered synthetic seeds (DLSs) exhibited the highest re-growth (93.6%) on MS medium supplemented with meta-Topolin (mT) (5.0 µM) and α-naphthalene acetic acid (NAA) (0.5 µM) after 8 weeks of culture. Viability assessment of non-embryogenic synthetic seeds during low-temperature storage (4 °C) demonstrated the enhanced resilience of double-layered synthetic seeds (DLSs) compared to their single-layered (SLS) counterparts. Following acclimatization in Soilrite®-filled cups, 82% of the plantlets were successfully established in a greenhouse after four weeks. The increased activity and concentration of antioxidants in DLS-derived plantlets suggest the potential role of the extra layer of alginate in mitigating the effects of low-temperature stress during storage. SCoT molecular analysis confirmed the genetic integrity of the synthetic seed-derived plants.
1. Introduction
Salix tetrasperma Roxb., commonly known as Indian willow, is a deciduous tree native to tropical and subtropical regions of South Asia, including India. While it is primarily adapted to warm climates, the species demonstrates resilience to diverse environmental conditions and can also thrive in moist soils of temperate zones [1]. This species is highly valued for its esthetics, quality timber, and potential as a biofuel source. Additionally, it exhibits medicinal properties, including analgesic, anti-inflammatory, antipyretic, and CNS activities [2,3,4]. Aspirin, the widely used non-steroidal anti-inflammatory drug (NSAID), was developed from salicin, one of the key phytoconstituents derived from Salix species [5,6].
However, the quality and quantity of the plant products vary with genotypes. Therefore, propagation of only the elite genotypes is essential for effectively exploiting the potential of multipurpose trees like S. tetrasperma. In vitro techniques provide an effective alternative to conventional methods to regenerate improved genotypes [7,8]. While considerable progress has been made in the micro-propagation of such species, storage and transport of the vegetative propagules remain challenging as transit across international boundaries is often met with unforeseen delays.
Synthetic seeds encapsulate small vegetative propagules within a gel matrix with various supplements, simulating natural seeds, but for producing genetically similar plants [9,10,11]. Encapsulation increases the shelf life of the vegetative propagules by several folds, saving both money and the space required for such exchanges [12,13,14,15]. However, various factors, including the selection of explant, experimental conditions, and the inclusion or exclusion of nutrients and growth regulators in the encapsulating matrix, significantly impact its success. Moreover, easy recovery/germination of synthetic seeds is as important as their storage. Often, complicated protocols require expertise and specific conditions, making their use redundant. It is therefore imperative to develop synthetic seeds that require the minimum possible intervention during storage and recovery.
Selecting appropriate encapsulation methods and matrix compositions is a fundamental prerequisite for establishing a reliable synthetic seed production protocol. Micro-cuttings (nodal segments) are the most successfully used propagules for synthetic seed production [16] in several medicinal plant species, such as Plumbago rosea [17], Decalepis salicifolia [18], Allamanda cathartica [19], Satureja khuzistanica [20], and Ruta chalepensis [21].
While single-layered synthetic seeds (SLSs) have been widely employed, limitations such as dehydration, nutrient instability, and vulnerability to abiotic stress have often hindered their effectiveness during storage and germination [22]. Double-layered synthetic seeds (DLSs) are proposed to address these issues by providing an additional layer of alginate, which may enhance structural integrity, improve nutrient retention, and mitigate the oxidative stress induced by low-temperature storage [23]. Recent studies have demonstrated that the multi-layered approach in encapsulation can modulate the controlled release of nutrients and growth regulators while ensuring better protection against environmental stresses [24].
Sodium alginate, a commonly used encapsulation material for synthetic seeds, has been shown to influence various physiological responses during storage [25]. Strategies such as the use of encapsulation materials with antioxidative properties and the incorporation of exogenous antioxidants have been explored, although their effectiveness has been inconsistent across different plant systems [26]. Previous research has focused on the ability of the encapsulation medium to provide physical protection and moisture retention, but its biochemical interactions with plant tissues, particularly in mitigating oxidative stress, remain poorly understood [27]. Oxidative stress is characterized by the overproduction of ROS, which can lead to cellular damage if not adequately scavenged by antioxidative enzymes such as superoxide dismutase (SOD) and catalase (CAT). These enzymes are pivotal in mitigating oxidative damage and maintaining cellular homeostasis [28,29]. Additionally, proline, an important osmoprotectant, accumulates under stress conditions and plays a critical role in protecting plants against free radical-induced damage [30]. Understanding the differential accumulation of antioxidative enzymes and proline in synseeds encapsulated with sodium alginate can provide insights into its potential role in alleviating oxidative stress during cold storage.
The present study aims to optimize the encapsulation protocol using DLSs for Salix tetrasperma, proposing that the improved structural and functional integrity of DLSs overcomes the limitations of SLSs and enhances storage viability and regeneration potential. Moreover, this study further aims to explore the impact of sodium alginate coatings on oxidative stress markers, particularly on antioxidative enzyme activity and proline accumulation, to enhance the viability and performance of non-embryogenic synseeds under cold storage conditions.
2. Materials and Methods
2.1. Collection of Explant
In vitro cultures of S. tetrasperma were established on MS medium [31] using nodal explants from greenhouse-maintained healthy trees. The cultures were kept in a controlled culture room set at 26 ± 2 °C, with relative humidity (RH) between 55 and 60%, and exposed to a spectral flux of 35–40 μmol m−2 s−1 of photosynthetically active radiation (460–700 nm) for 12 h each day. The cultures underwent regular transfers onto fresh medium at every 4-week interval. Healthy nodal segments (0.5 cm) were carefully obtained from the in vitro cultures under aseptic conditions and used for encapsulation experiments.
2.2. Single-Layer Encapsulation
Different concentrations of sodium alginate (0, 1, 2, 3, 4, and 5% w/v) supplemented with activated charcoal (2 g L−1; Supplementary Figure S1) and calcium chloride (CaCl2·2H2O) solution (0, 50, 75, 100, 125, and 150 mM) were prepared using MS liquid medium for encapsulation and complexation. The pH of both solutions was adjusted to 5.8 before autoclaving at 121 °C for 20 min. Nodal segments were mixed with sodium alginate solution and then dropped into the CaCl2·2H2O solution to achieve encapsulation. The droplets containing the explants were left in the solution for at least 20 min for complete polymerization. Thereafter, calcium alginate beads containing nodal segments were retrieved and rinsed thrice with autoclaved distilled water (Figure 1).
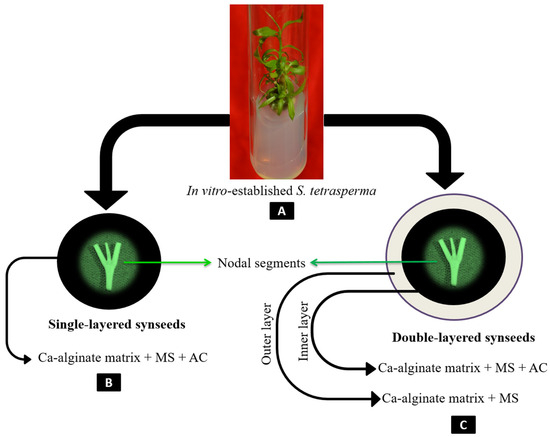
Figure 1.
Schematic representation of the encapsulation process for the preparation of single-layered and double-layered synseeds of S. tetrasperma. The process begins with in vitro-established nodal segments of S. tetrasperma (A). The nodal segments are encapsulated in a Ca-alginate matrix to form single-layered synseeds (B), which consist of a Ca-alginate matrix supplemented with MS medium and activated charcoal (AC). For double-layered synseeds (C), the nodal segments are first encapsulated in an inner layer of Ca-alginate matrix supplemented with MS medium, followed by an outer layer of Ca-alginate matrix supplemented with MS medium and AC.
2.3. Double Layer Encapsulation
Double-layered synthetic seeds (DLSs) were formed by submerging single-layered synthetic seeds (SLSs) again into the Na2-alginate solution. These beads were placed in a solution containing 100 mM CaCl2 for 30 min, followed by 3–4 washes with sterile double-distilled water. The resulting double-layered beads, approximately 6–7 mm in diameter (Figure 1), were placed on filter paper in Petri dishes for 5–7 min to absorb excess moisture.
2.4. Growth Media and Culture Conditions
Encapsulated nodal segments were placed on MS medium [31] augmented with different combinations of cytokinin (mT, BA, and Kn) and auxin (NAA). Following shoot emergence, the cultures were transferred to 100 mL culture flasks containing the same medium to facilitate continued growth. The media for all experiments contained 3% (w/v) sucrose and 0.8% (w/v) agar, with 5.8 pH, adjusted before autoclaving at 121 °C for 20 min. Cultures were maintained at 24 ± 2 °C under a 16/8 h light/dark cycle, with a light intensity of 50 μmol m−2 s−1.
2.5. Rooting and Acclimatization
The fully grown microshoots (3.0 to 4.0 cm) were collected and transferred to a rooting medium containing ½ MS augmented with IBA at concentrations of 0.5, 1.0, 1.5, or 2.0 µM. After 4 weeks, the rooting percentage, length, and number of roots per shoot were recorded.
Healthy rooted plantlets were transferred to plastic cups filled with sterile Soilrite. These potted plantlets were enclosed in transparent polyethene bags to maintain high humidity and were watered every 2 days for 3 weeks. Following this acclimation period, the plants were transplanted into pots filled with regular garden soil and were kept in a greenhouse under normal conditions.
2.6. Effect of Low-Temperature Storage on Encapsulated and Non-Encapsulated Synthetic Seeds
A series of encapsulated and non-encapsulated nodal segments were placed on Petri dishes containing water and agar medium and stored at 4 °C. At six different instances (0 to 8 weeks), stored samples were assessed for their regeneration potential. Following each storage period, both explant types were cultured on MS medium supplemented with various hormonal combinations to facilitate their transformation into plantlets. The quantification of shoot and root development percentages in both encapsulated and non-encapsulated nodal segments was conducted after an 8-week culture period on the regeneration medium.
2.7. Biochemical Studies
Leaf samples were collected from plantlets derived from encapsulated synthetic seeds (SLSs and DLSs) as well as non-encapsulated nodal segments. The synthetic seeds were subjected to different durations of storage at 4 °C. Subsequently, after 4 weeks of culture, leaf samples were extracted from in vitro cultures to evaluate various biochemical attributes, including the estimation of superoxide dismutase (SOD), catalase (CAT), and proline levels.
2.8. Superoxide Dismutase (SOD)
Superoxide dismutase (SOD; EC 1.15.1.1) activity was assessed following the method outlined by Dhinsa et al. [32], with minor modifications. Briefly, the total reaction volume was adjusted to 3 mL to accommodate the cuvette size used in the spectrophotometer, and the reaction mixture was maintained at 25 °C using a temperature-controlled water bath to ensure consistent enzyme activity measurements. Additionally, the riboflavin concentration was altered to 0.01 mM to optimize the reaction for our specific plant tissue extracts. The light exposure time for inducing the photochemical reaction was standardized to 10 min based on preliminary trials to ensure reproducibility and consistency in results. The enzyme activity was measured and expressed as U g−1 FW.
2.9. Catalase (CAT)
Catalase (CAT; EC 1.11.1.6) activity was determined following the protocol outlined by Aebi [33], with minor modifications. Briefly, the total reaction volume was reduced to 2 mL to match the cuvette size of the spectrophotometer, and the phosphate buffer pH was adjusted to 7.0 to better suit the plant tissue extracts. The assay was performed at a controlled temperature of 25 °C to ensure consistent enzyme activity measurements. Additionally, the hydrogen peroxide concentration was modified to 10 mM to optimize the reaction conditions, and a 2 min pre-incubation step was included to stabilize the enzyme extract before initiating the reaction. The activity was measured and expressed as m mol H2O2 g−1 FW.
2.10. Estimation of Proline
The determination of proline content followed the protocol outlined by Bates et al. [34]. Briefly, 0.5 g of fresh leaf sample was homogenized in 10 mL of 3% aqueous sulfosalicylic acid. Subsequently, 2 mL of the extract was added with an equivalent volume of ninhydrin and glacial acetic acid, followed by heating the solution at 100 °C. The reaction was halted using an ice bath, and proline was isolated using 4 mL of toluene. The absorbance of the toluene layer was measured at 520 nm using a UV–Visible spectrophotometer (Shimadzu, Japan). The quantification of proline was determined and represented as mg per gram of fresh weight (FW).
2.11. SCoT Analysis
The genomic DNA was extracted from fresh leaves of plants derived from synthetic seeds and the donor plant. An adapted Cetyltrimethylammonium bromide (CTAB) DNA isolation method based on the Doyle and Doyle protocol [35] was employed for genetic analysis. Briefly, 250 mg of leaf tissue was ground to a fine powder using liquid nitrogen. The tissue was then mixed with an extraction buffer containing 2% CTAB, 100 mM Tris-HCl (pH 8.0), 20 mM EDTA, and 1.4 M NaCl. The sample was incubated at 65 °C for 30 min with occasional shaking to facilitate cell lysis. Subsequently, an additional step, proteinase-K treatment and the use of a phenyl–chloroform–isoamyl alcohol mixture, was added for phase separation, followed by centrifugation. The aqueous phase containing the DNA was then precipitated with cold isopropanol and incubated at −20 °C for 30 min. The DNA pellet was washed with 70% ethanol, briefly dried, and then resuspended in 50 µL of TE buffer (Tris-HCl and EDTA). DNA quality and quantity assessments were carried out using a Nanodrop™ Spectrophotometer (Implen, Munich, Germany), measuring absorbance at 260 nm and 280 nm. For genetic fidelity assessment, the PCR-based SCoT (Start codon targeted) technique [36] with ten specific SCoT (Start codon targeted) primers was utilized. PCR reactions were conducted in a 20 µL volume, involving 2 µL of 10× buffer, 1.2 µL of 25 mM MgCl2, 0.4 µL of 10 mM dNTPs, 0.2 µL of Taq DNA polymerase (1 U), 1 µL of primer (1 U), 1.5 µL of DNA (25 ng/µL), and 14.2 µL of deionized water. The amplification of PCR reactions was carried out for 45 cycles at different temperature intervals. Each cycle consisted of a denaturation step at 94 °C for 5 min, an annealing step at 55 °C for 1 min, and elongation at 72 °C for 1 min. Finally, an extension was allowed for 10 min at 72 °C. The PCR products amplified using SCoT primers were resolved by gel electrophoresis on a 1% agarose gel. The gel was run in 1× TBE buffer at 60 V for 2 h and stained with ethidium bromide for visualization of the DNA fragments. Finally, photography was performed using a UV gel documentation system (Bio-Rad, Hercules, CA, USA). Only well-defined and reproducible bands were scored, whereas faint DNA bands with low visual intensity that could not be clearly distinguished as present or absent were considered ambiguous and were excluded from scoring. The experiment was replicated three times to ensure reproducibility and significance in confirming genetic fidelity.
2.12. Statistical Analysis
The experimental setup was carried out following a completely randomized design (CRD), with each experiment being replicated three times and a minimum of 20 replicates per treatment. Statistical analysis was performed through one-way Analysis of Variance (ANOVA) using SPSS version 23 (Chicago, IL, USA). Variations among means were examined through Duncan’s multiple range test (DMRT) at a significance level of p < 0.05. The findings are expressed as the mean ± standard error (SE).
3. Results and Discussion
The formulation of the gel matrix significantly influences the conversion efficacy of encapsulated tissue. The current study involved the production of both single-layered synthetic seeds (SLSs) and double-layered synthetic seeds (DLSs) using different alginate matrix compositions. Moreover, to ensure significant shoot induction and the conversion of encapsulated beads into plantlets, an evaluation was carried out to compare the influence of phytohormones in gel matrices with their effects in the conversion media.
3.1. Effect of Sodium Alginate and Calcium Chloride Concentrations on Bead Formation
The variations in the concentrations of the gel matrix and gel complexation resulted in distinct attributes of the Ca-alginate encapsulating nodal segments, including differences in texture, firmness, shape, and transparency, which influenced the shoot emergence response. These differences became more pronounced with increasing variations in concentrations. The results in the current study identified that a composition of 3.0% (w/v) Na-alginate and 100 mM CaCl2 yielded the optimal beads, characterized by a spherical shape, good texture, transparency, and ease of handling (Figure 2A,B; Table 1 and Table 2). Reduced concentrations of Na-alginate (1.0–2.0%) and CaCl2 (50 mM) required an extended polymerization duration and yielded fragile beads that were tough to handle. Conversely, higher concentrations of Na-alginate (4.0–5.0%) and CaCl2 (125–150 mM) resulted in hard, easily manageable beads, but the increased hardness resulted in a significant delay and reduction in shoot induction (Figure 2A,B; Table 1 and Table 2). Moreover, the graphical plots demonstrating negative second-order quadratic trends and the corresponding optimal conditions for sodium alginate concentration and calcium chloride concentration are represented in the Supplementary Materials (Figures S2 and S3). Similar results have been reported by Javed et al. [12] in Erythrina variegate, Khanam et al. [19] in Allamanda cathartica, Behera et al. [37] in Hedychium coronarium, and Siddique et al. [38] in Capparis decidua.
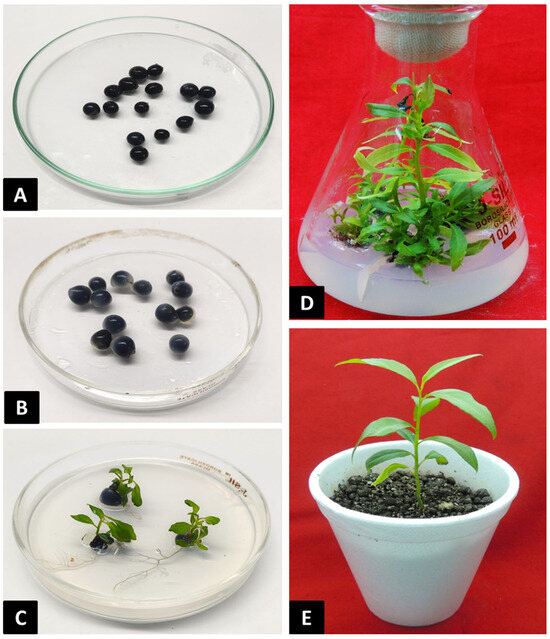
Figure 2.
Different types of synthetic seeds and their in vitro germination. (A) Single-layered synthetic seeds prepared by using 3% Na2-alginate and 100 mM CaCl2. (B) Double-layered synthetic seeds with an inner layer with activated charcoal and outer layer without activated charcoal. (C) In vitro germination of encapsulated synthetic seeds. (D) Proliferation of in vitro cultures on MS medium supplemented with mT (5.0 µM) and NAA (0.5 µM). (E) Acclimatization of synthetic seed-derived plantlets on Soilrite.

Table 1.
Effect of different concentrations of sodium alginate with optimal concentration of calcium chloride (100 mM) on formation of synseeds after 4 weeks of culture on MS medium.

Table 2.
Effect of calcium chloride at different concentrations with sodium alginate (3%) on formation of synseeds after 4 weeks of culture on MS medium.
The optimal concentration of Na-alginate and CaCl2 required for the smooth and healthy encapsulation of micro-cuttings varies from species to species [39]. Hatzilazarou et al. [40] and Singh et al. [41] reported a 2.5% best concentration, whereas Kumar and Thomas [42] in Clitoria ternatea and Mahdavi et al. [43] in Phalaenopsis orchid found 4% sodium alginate solution optimal for encapsulation. Similarly, Khan et al. [13] and Qahtan et al. [44] emphasized the significance of a well-suited combination of Na-alginate and CaCl2 in facilitating optimal ion exchange involving Na+ and Ca2+ for the gel complexation process. Insufficient doses of sodium alginate compositions proved incompatible, as they experienced reduced gelling capability upon exposure to higher temperatures during autoclaving [45,46].
3.2. Effect of Complexing Time on Bead Formation
The nature and shape of beads are chiefly governed by the extent of duration for the proper exchange of sodium ions with calcium ions, leading to the generation of insoluble calcium alginate beads. Therefore, the optimal duration required for effective ion exchange was investigated. Various time intervals (10, 20, 30, 40, or 50 min) were assessed to determine the appropriate duration for thorough ion exchange. Smooth and round beads were obtained with 3% (w/v) Na2-alginate and 100 mM CaCl2 solution complexed for 30 min, achieving a 51.6% conversion response (Table 3). Moreover, a graphical plot demonstrating negative second-order quadratic trends and the corresponding optimal conditions for complexing time are represented in the Supplementary Materials (Figure S4). Exposure durations less than 30 min showed inadequate polymerization of the beads, whereas exposure for more than the optimal duration resulted in harder beads.

Table 3.
Effect of complexing time on bead formation for optimal concentration of calcium chloride (100 mM) and sodium alginate (3%) after 4 weeks of incubation.
3.3. Effect of Media Composition on Conversion of SLSs and DLSs into Plantlets
The concentration and type of plant growth regulators play a crucial role in the regeneration capacity and conversion frequency of synthetic seeds. Both types of non-embryogenic synthetic seeds, (i.e., SLSs and DLSs) were cultures on various conversion media containing half- or full-strength MS nutrients alone or supplemented with different concentrations of plant growth regulators. The conversion rates of the encapsulated explant varied with the germination media.
The application of full-strength MS media resulted in a better conversion frequency (46.9% for SLSs and 52.6% for DLSs) of synthetic seeds as compared to the half-strength MS medium, which recorded only 38.2% for the SLS and 46.3% for the DLS conversion rate after 8 weeks of incubation (Table 4; Figure 2A–D). Full-strength MS media contains a higher concentration of nutrients, which are critical in supporting the growth and development of encapsulated explants [47]. Nutrients such as nitrogen, phosphorus, and micronutrients play vital roles in cell division, tissue differentiation, and overall plant development [48]. These nutrients provide the necessary support for the successful conversion and regeneration of synthetic seeds into viable plantlets [49]. In the current study, the combination of mT and NAA was identified as the most effective combination for inducing high conversion rates of both SLS and DLS synthetic seeds. Among all the combinational treatments, MS media augmented with mT (5.0 µM) and NAA (0.5 µM) exhibited the maximum number of shoots, with a 93.6% conversion rate in DLSs and 87.3% conversion rate in SLSs, after 8 weeks of incubation. The highest number of 7.2 ± 0.13 shoots per bead was recorded in DLSs, whereas 6.4 ± 0.12 shoots per bead were demonstrated in SLSs (Table 4; Figure 2A–D). The auxin and cytokinin combination is known to synergistically promote shoot initiation and growth by influencing cellular division and differentiation pathways [50]. Such synergistic interactions between plant growth regulators have been extensively studied and validated by Khanam et al. [19] in Allamanda cathartica. Optimizing the parameters of synthetic seed media composition and PGR concentration is vital for advancing the scalability and efficiency of synthetic seed production. The ability to achieve higher conversion rates with specific hormone combinations and media compositions ensures the successful regeneration of plants with superior growth rates.

Table 4.
Effect of MS media augmented with different concentrations of plant growth regulators on conversion of encapsulated nodal segments of S. tetrasperma after 8 weeks of culture.
The results demonstrated that the conversion frequency of non-embryogenic synthetic seeds is significantly impacted by factors such as the number of encapsulated layers, activated charcoal (AC), and the media composition. Among the two synthetic seed types examined, DLSs showed the highest conversion rate (93.6%), followed by SLS synthetic seeds (87.3%), suggesting differing levels of suitability among them (Table 4; Figure 2A–D). Our results are in agreement with Maruyama et al. [51], who reported superior efficacy of DLS in Guazuma crinite, Cidrella odorata, and Jacaranda mimosaefolia. The positive impact of AC on DLS germination could be attributed to its capacity to absorb unwanted exudates and detrimental by-products, as well as its increased root induction [20,52]. Additionally, Balaji et al. [53] proposed that charcoal disrupts the alginate bead and aids in the retention of nutrients within the encapsulated capsule, with their gradual release to support the developing synthetic seeds.
The simultaneous development of roots and shoots was observed within a single medium, resulting in healthy root growth. However, for rooting of individual shoots, the micro-shoots were transplanted on ½ MS medium augmented with various IBA concentrations. Among these concentrations, the highest percentages of rooting, 84% and 70%, were achieved with optimal IBA concentrations of 1.0 µM and 1.5 µM, respectively.
3.4. Low-Temperature Storage of Encapsulated and Non-Encapsulated Beads
Retention of regeneration capacity over a prolonged storage period is a significant and desirable characteristic of synthetic seeds. Therefore, the conversion rate of encapsulated and non-encapsulated beads was assessed after different storage periods (0–8 weeks). Among different storage durations, the maximum response of 66.7% and 58.6% was observed for DLSs and SLSs, respectively, after 4 weeks of storage (Table 5). The optimal duration for conversion response was determined using regression analysis, represented in the Supplementary Materials (Figure S5). The non-encapsulated beads, however, showed a rapid fall in the conversion rate during storage. The conversion rate declined to a mere 33.7% after 4 weeks of storage; during extended storage, a sharp decline was recorded.

Table 5.
Effect of encapsulation of nodal segments of S. tetrasperma on regeneration percentage on MS + mT (5.0 µM) + NAA (0.5 µM) stored at 4 °C for different time durations.
While the reduction in conversion rate in the non-encapsulated explants could be attributed to dehydration and starvation of the tissue, in encapsulated tissues it is protected by the alginate matrix from desiccation and starvation during the storage period [51,54]. Similar findings have been reported previously by other researchers, including Fatima et al. [55] in Withania somnifera, Naz et al. [56] in Althaea officinalis, and Rodrigues et al. [18] in Decalepis salicifolia. Encapsulated nodal segments, whether SLSs or DLSs, retained viability even after 6-weeks of storage. Comparatively, DLSs demonstrated greater resilience for prolonged storage, as indicated by the higher conversion rates observed after 4 and 8 weeks of cold storage. The extended survival of DLSs compared to SLSs could be due to the supplementary nutritive layer, providing enhanced nourishment and protection against desiccation during prolonged storage [13,57,58].
3.5. Acclimatization
Hardening plantlets to an autotrophic state from heterotrophic, controlled conditions before transplantation is crucial to ensure better survivability. The plants derived from synthetic seeds were assessed employing various potting substrates during acclimatization. Among the various potting materials, the highest survival rate (82%) was observed in plantlets grown on Soilrite, while garden soil had the lowest survival rate (50%) after 4 weeks of acclimatization (Figure 3). Soilrite has been the most preferred substrate for acclimatization among vermiculite and garden soil, and has been reported to facilitate improved root development during acclimatization [59].
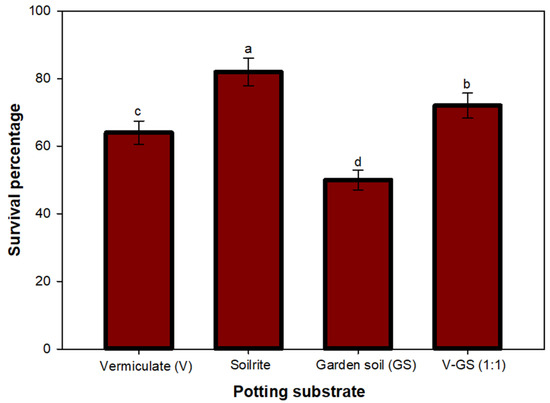
Figure 3.
Effect of potting substrate on survival rate of of synseed-derived plantlets. The value of bars represent mean ± standard error (SE). Means sharing the same letter are not significantly different (p ≤ 0.05) using Duncan’s multiple range test.
3.6. Biochemical Studies
A significant change in the storage temperature causes excessive production of electrons in the mitochondria, which may consequently trigger the production of reactive oxygen species (ROS) [60]. In response to excess reactive oxygen species (ROS) accumulation, plants engage in synthesizing/activating antioxidative enzymes (SOD and CAT) and osmoprotectants such as proline. These elements regulate and maintain ROS levels, mitigating damage to biomolecules and ensuring cellular redox balance [61,62]. During 4 weeks of cold storage, a significant increase in proline accumulation was observed in synthetic seed-raised plantlets. However, storage beyond this period did not result in a substantial rise in proline content.
The stagnation in the activity/concentration of SOD, CAT, and proline content after 6 and 8 weeks of storage implies potential limitations in the protective mechanisms against prolonged low-temperature stress. Furthermore, a significant finding was the enhanced activity of leaf antioxidants and proline levels in plants raised from encapsulated nodal segments compared to exposed nodal segments. The maximum increase in SOD, CAT, and proline accumulation resulted in DLS-raised plantlets (Figure 4 and Figure 5). Double coatings of Ca-alginate around nodal segments in DLSs effectively mitigated oxidative stress by sustaining elevated antioxidant and proline activity during cold storage [63]. Moreover, alginate coatings act as a physical barrier, reducing gas permeability to the encapsulated propagules, thereby lowering their respiration rates. The higher SOD, CAT, and proline concentrations in the SLSs might be linked to the activated charcoal component, known for its capacity to irreversibly adsorb phenolic exudates [38]. Activated charcoal possesses a high surface area and volume, with fine pores that enable the absorption of phenolic compounds influencing plant growth. Previous studies by Akhtar et al. [64] demonstrated its efficacy in mitigating oxidative browning in Rosa centifolia.
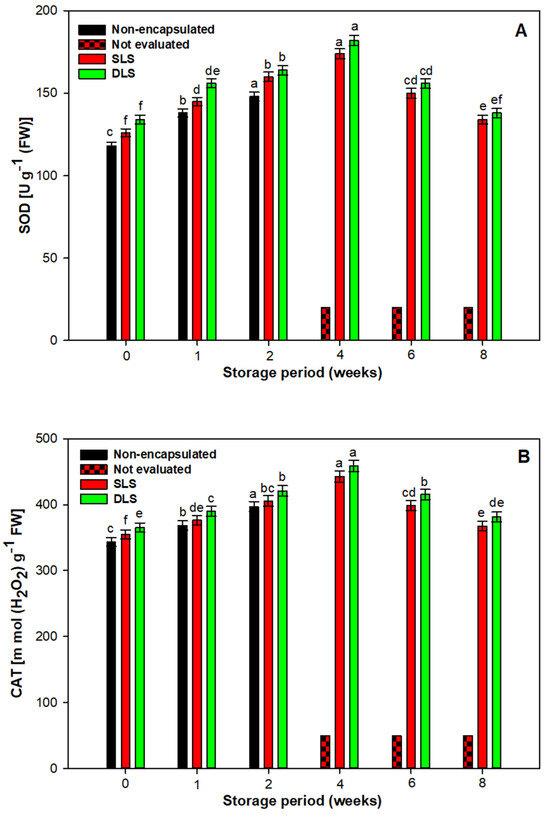
Figure 4.
Effect of different durations of cold (4 °C) storage on antioxidant enzyme activity during acclimatization of S. tetrasperma plantlets. (A) Superoxide dismutase (SOD); (B) catalase (CAT). The value of bars represent mean ± standard error (SE). Means sharing the same letter are not significantly different (p ≤ 0.05) using Duncan’s multiple range test. Abbreviations: SLSs= single-layered synthetic seeds; DLSs = double-layered synthetic seeds.
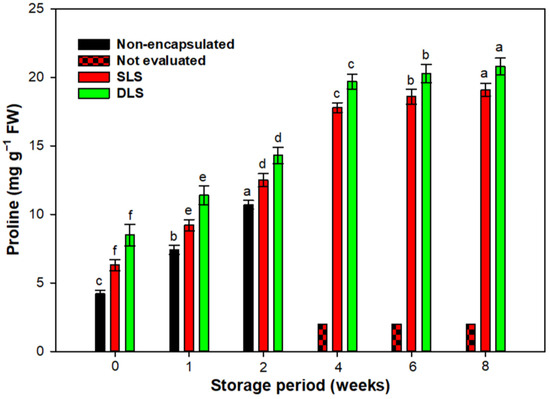
Figure 5.
Effect of different durations of cold (4 °C) storage on the accumulation of proline content during the acclimatization of S. tetrasperma plantlets. The value of bars represent mean ± standard error (SE). Means sharing the same letter are not significantly different (p ≤ 0.05) using Duncan’s multiple range test. Abbreviations: SLSs= single-layered synthetic seeds; DLSs = double-layered synthetic seeds.
3.7. Evaluation of Genetic Stability Using SCoT Molecular Markers
The potential drawback of in vitro propagation is the occurrence of genetic variations between regenerants and the mother plant [65]. Therefore, it becomes necessary to ascertain the genetic stability of the plantlets obtained from prolonged in vitro conditions. We validated the genetic integrity of synthetic seed-derived plantlets using SCoT primers, which resulted in distinct and reproducible bands ranging from 500 to 2500 base pairs (bp). Among the various monomorphic banding profiles, the SCoT8 primer generated the highest (09) band count, whereas the SCoT5 primer exhibited a lower (06) band count (Table 6; Figure 6). The DNA fingerprint revealed consistent banding patterns between parental and in vitro-raised plantlets. Furthermore, no morphological variation or polymorphism was observed among the regenerants.

Table 6.
Start codon targeted (SCoT) primers used to assess genetic fidelity of mother plant and in vitro-raised plantlets of Salix tetrasperma.
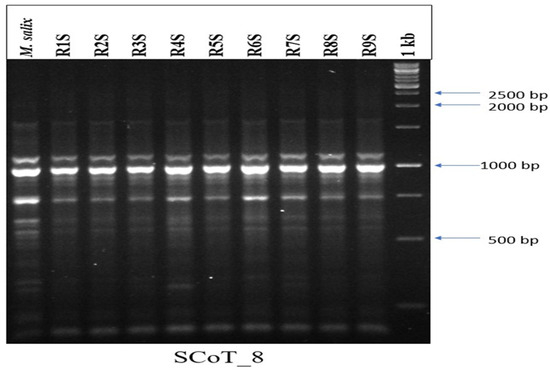
Figure 6.
Start codon targeted (SCoT) banding pattern generated with SCoT8 primer among regenerated plants of Salix tetrasperma. M. Salix = mother plant. R1S to R9S = randomly selected synseed-derived plants.
A monomorphic pattern in SCoT analysis indicates stability because it reflects uniformity in the genetic markers across different samples or conditions. This uniformity suggests that the genetic regions being analyzed are conserved and unaffected by environmental factors, mutations, or other external influences. Such stability is often associated with critical or conserved genomic regions, which may play a vital role in maintaining the structural and functional integrity of the genome.
Therefore, we can be sure that our encapsulation and recovery protocols yield true-to-type plants. PCR-based DNA fingerprinting to ascertain genetic uniformity has also been used by various authors, including Fatima et al. [55] in Withania somnifera, Javed et al. [12] in Erythrina variegate, Kader et al. [66] in Azadirachta indica, and Asadi et al. [20] in Satureja khuzistanic.
4. Conclusions
The present study offers a valuable approach for producing S. tetrasperma synthetic seeds using nodal segments and their conversion to plantlets, which was significantly influenced by factors such as the number of encapsulated layers, AC, and the composition of the growth medium. The DLS were more effective in maintaining tissue viability at higher rates even after 8 weeks of low-temperature storage. Furthermore, plantlets recovered from double-layered synthetic seeds showed elevated levels of antioxidants and proline accumulation, suggesting a beneficial effect of alginate coatings in mitigating oxidative stress. SCoT analysis demonstrated a monomorphic banding pattern in plants recovered from encapsulated segments, emphasizing the stability and integrity of germplasm. The established protocol demonstrates the potential for conservation, short-term storage, and germplasm exchange for this economically important tree species over long distances. Future research could focus on optimizing encapsulation procedures and assessing synthetic seed performance under field conditions, including seedling survival, growth rates, and adaptability in different conditions. Moreover, investigating the genetic stability of synthetic seeds across multiple generations, would provide valuable insights for their broader application in sustainable agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11050486/s1, Figure S1: Effect of different concentration of activated charcoal on conversion of encapsulated nodal segments of S. tetrasperma after 8 weeks of culture. Figure S2: Regression analysis showing effect of different concentrations of Na-alginate with optimal concentration of CaCl2 (100 mM) on formation of synseeds after 4 weeks of culture. Figure S3: Regression analysis showing effect of calcium chloride at different concentrations with sodium alginate (3%) on formation of synseeds after 4 weeks of culture. Figure S4: Regression analysis showing effect of complexing time on bead formation for optimal concentration of CaCl2 and Na-alginate after 4 weeks of incubation. Figure S5: Regression analysis showing effect of encapsulation of nodal segments of S. tetrasperma on regeneration percentage on optimal media stored at 4 °C for different time durations.
Author Contributions
Z.A.R.: conceived the study, collected the data, and drafted the original manuscript. W.A., F.M.H. and M.N.K.: writing—reviewing and editing. S.B.J.: supervised and edited the final version of the manuscript and figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions made in this study are incorporated in the article/Supplementary Materials. Additional inquiries can be directed to the corresponding author.
Acknowledgments
The authors extend their sincere appreciation to the Researchers Supporting Project number (RSP2025R729), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors affirm that the research was carried out without any commercial or financial relationships that could be perceived as a potential conflict of interest.
References
- Tienaho, J.; Reshamwala, D.; Sarjala, T.; Kilpeläinen, P.; Liimatainen, J.; Dou, J.; Viherä-Aarnio, A.; Linnakoski, R.; Marjomäki, V.; Jyske, T. Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities—The Bioactive Properties of 16 Clones. Front. Bioeng. Biotechnol. 2021, 9, 797939. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; El-Sayed, A.; Fikrey, E. Bioactive Secondary Metabolites from Salix tetrasperma Roxb. Z. Fur Naturforschung. C 2012, 67, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Virupaksha, J.H.; Nadendla, R.R.; Kumar, S.M.; Kavya, S. Effect of Salix tetrasperma Roxburgh Leaf Extracts on Central Nervous System Activities. Res. J. Pharm. Biol. Chem. Sci. 2017, 7, 2060–2064. [Google Scholar]
- Prashith Kekuda, T.; Vinayaka, K.; Raghavendra, H. Ethnobotanical Uses, Phytochemistry, and Biological Activities of Salix tetrasperma Roxb. (Salicaceae)—A Review. J. Med. Plants 2017, 5, 201–206. [Google Scholar]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, Pharmacology, and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef] [PubMed]
- Gligorić, E.; Igić, R.; Čonić, B.S.; Kladar, N.; Teofilović, B.; Grujić, N. Chemical Profiling and Biological Activities of “Green” Extracts of Willow Species (Salix L., Salicaceae): Experimental and Chemometric Approaches. Sustain. Chem. Pharm. 2023, 32, 100981. [Google Scholar] [CrossRef]
- Khan, M.I.; Anis, M. Modulation of In Vitro Morphogenesis in Nodal Segments of Salix tetrasperma Roxb. through the Use of TDZ, Different Media Types, and Culture Regimes. Agrofor. Syst. 2012, 86, 95–103. [Google Scholar] [CrossRef]
- Kordrostami, M.; Mafakheri, M.; Al-Khayri, J.M. Date Palm (Phoenix dactylifera L.) Genetic Improvement via Biotechnological Approaches. Tree Genet. Genomes 2022, 18, 26. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. Production of Synthetic Seeds for Hybrid Cymbidium Using Protocorm-Like Bodies. J. Fruit Ornam. Plant Res. 2012, 20, 135–146. [Google Scholar] [CrossRef]
- Standardi, A.; Micheli, M. Encapsulation of In Vitro-Derived Explants: An Innovative Tool for Nurseries. Methods Mol. Biol. 2013, 11013, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Nongdam, P. Development of Synthetic Seed Technology in Plants and Its Applications: A Review. Int. J. Curr. Sci. 2016, 19, 86–101. [Google Scholar]
- Javed, S.B.; Alatar, A.A.; Anis, M.; Faisal, M. Synthetic Seeds Production and Germination Studies for Short-Term Storage and Long-Distance Transport of Erythrina variegata L.: A Multipurpose Tree Legume. Ind. Crops Prod. 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Khan, S.A.; Verma, P.; Rahman, L.U.; Parasharami, V. Synthetic Seed-Mediated Synchronized Multiplication Under In Vitro Conditions: An Efficient Technique for Conservation, Multiplication, and Storage of Medicinal Plants. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Abbas, M.K.; Alhasan, A.S. Production of Synthetic Seeds in Vegetable Crops: A Review. IOP Conf. Series Earth Environ. Sci. 2022, 1060, 012099. [Google Scholar] [CrossRef]
- Ahmad, A.; Ansari, F.A.; Anis, M.; Khan, A.S. Micropropagation of Pterocarpus marsupium Roxb. Through Synthetic Seeds and Its Novel Antibiofilm Activities Against ESKAPE Pathogens. Ind. Crops Prod. 2023, 198, 116681. [Google Scholar] [CrossRef]
- Gantait, S.; Kundu, S.; Ali, N.; Sahu, N.C. Synthetic Seed Production of Medicinal Plants: A Review on Influence of Explants, Encapsulation Agent, and Matrix. Acta Physiol. Plant. 2015, 37, 98. [Google Scholar] [CrossRef]
- Prakash, A.V.; Nair, D.S.; Alex, S.; Soni, K.B.; Viji, M.M.; Reghunath, B.R. Calcium Alginate Encapsulated Synthetic Seed Production in Plumbago rosea L. for Germplasm Exchange and Distribution. Physiol. Mol. Biol. Plants 2018, 24, 963–971. [Google Scholar] [CrossRef]
- Rodrigues, V.; Kumar, A.; Gokul, S.; Verma, R.S.; Rahman, L.U.; Sundaresan, V. Micropropagation, Encapsulation, and Conservation of Decalepis salicifolia, a Vanillin Isomer Containing Medicinal and Aromatic Plant. In Vitro Cell. Dev. Biol. Plant 2020, 56, 526–537. [Google Scholar] [CrossRef]
- Khanam, M.N.; Javed, S.B.; Ahmad, N.; Anis, M. Encapsulation of Nodal Segments of Allamanda cathartica for Short-Term Storage and Germplasm Exchange. Plant Cell Tissue Organ Cult. 2021, 145, 435–443. [Google Scholar] [CrossRef]
- Asadi, R.; Abdollahi, M.R.; Moosavi, S.S.; Mirzaie-Asl, A. Alginate Encapsulation of Micro-Cuttings in Endangered Satureja khuzistanica Species: A Promising Method for Obtaining Genetically Stable Plants with High Rosmarinic Acid Content. Plant Cell Tissue Organ Cult. 2022, 151, 307–320. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Alatar, A.A.; Faisal, M. In Vitro Regeneration, Phytochemical Profiling, and Antioxidant Activity in Ruta chalepensis Plants Established from Alginate Encapsulated Synthetic Seeds. S. Afr. J. Bot. 2023, 161, 575–585. [Google Scholar] [CrossRef]
- Rai, M.K.; Asthana, P.; Singh, S.K.; Jaiswal, V.S.; Jaiswal, U. The Encapsulation Technology in Fruit Plants—A Review. Biotechnol. Adv. 2009, 27, 671–679. [Google Scholar] [CrossRef]
- Sharma, S.; Shahzad, A.; Parveen, S. Synthetic Seed Technology for Short-Term Storage and Propagation of Vitex Species: A Review. In Vitro Cell. Dev. Biol. Plant 2013, 49, 507–515. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.; Zheng, H.; Li, J.; Wu, X.; Xu, J.; Zhen, Z.; Du, C. The Application of Encapsulation Technology in the Food Industry: Classifications, Recent Advances, and Perspectives. Food Chem. X 2024, 13, 101240. [Google Scholar] [CrossRef] [PubMed]
- Szopa, D.; Mielczarek, M.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Chojnacka, K.; Witek-Krowiak, A. Encapsulation Efficiency and Survival of Plant Growth-Promoting Microorganisms in an Alginate-Based Matrix—A Systematic Review and Protocol for a Practical Approach. Ind. Crops Prod. 2022, 181, 114846. [Google Scholar] [CrossRef]
- Ma, Y. Seed Coating with Beneficial Microorganisms for Precision Agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Hu, D.; Ma, G.; Wang, Q.; Yao, J.; Wang, Y.; Pritchard, H.W.; Wang, X. Spatial and Temporal Nature of Reactive Oxygen Species Production and Programmed Cell Death in Elm (Ulmus pumila L.) Seeds during Controlled Deterioration. Plant Cell Environ. 2012, 35, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Poór, P.; Ördög, A.; Czékus, Z.; Borbély, P.; Takács, Z.; Kovács, J.; Tari, I. Regulation of the Key Antioxidant Enzymes by Developmental Processes and Environmental Stresses in the Dark. Biol. Plant. 2018, 62, 201–210. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dhinsa, P.S.; Plumb-Dhinsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, J.D. Rapid Determination of Proline for Water Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 39–40. [Google Scholar]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Behera, S.; Rout, K.K.; Panda, P.C.; Naik, S.K. Production of Non-Embryogenic Synthetic Seeds for Propagation and Germplasm Transfer of Hedychium coronarium J Koenig. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100271. [Google Scholar] [CrossRef]
- Siddique, I.; Bukhari, N.A.W. Synthetic Seed Production by Encapsulating Nodal Segment of Capparis decidua (Forsk.), In Vitro Regrowth of Plantlets and Their Physio-Biochemical Studies. Agrofor. Syst. 2018, 92, 1711–1719. [Google Scholar] [CrossRef]
- Alatar, A.A.; Ahmad, N.; Javed, S.B.; Abdel-Salam, E.M.; Basahi, R.; Faisal, M. Two-Way Germination System of Encapsulated Clonal Propagules of Vitex trifolia L.: An Important Medicinal Plant. J. Hortic. Sci. Biotechnol. 2017, 92, 175–182. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Kostas, S.; Nendou, T.; Economou, A. Conservation, Regeneration, and Genetic Stability of Regenerants from Alginate-Encapsulated Shoot Explants of Gardenia jasminoides Ellis. Polymers 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Tejovathi, G.; Ahuja, A.; Tiwari, S. Production of Synthetic Seed from Encapsulated Nodal Segments of Giloe (Tinospora cordifolia Willd.). In Research Developments in Science and Technology; BP International: Hooghly, West Bengal, India, 2022; Volume 2, pp. 156–170. [Google Scholar]
- Kumar, G.K.; Thomas, T.D. High-Frequency Somatic Embryogenesis and Synthetic Seed Production in Clitoria ternatea Linn. Plant Cell Tissue Organ Cult. 2012, 110, 141–151. [Google Scholar] [CrossRef]
- Mahdavi, Z.; Daylami, S.D.; Fadavi, A.; Vahdati, K. Artificial Seed Production of Phalaenopsis Orchid: Effect of Encapsulation Materials, Temperature, Light Spectra, and Storage Period. Plant Cell Tissue Organ Cult. 2023, 155, 797–808. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Abdel-Salam, E.M.; Alatar, A.A.; Wang, Q.C.; Faisal, M. An Introduction to Synthetic Seeds: Production, Techniques, and Applications. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Springer: Cham, Switzerland, 2019; pp. 1–20. [Google Scholar]
- Gantait, S.; Mitra, M. Applications of Synthetic Seed Technology for Propagation, Storage, and Conservation of Orchid Germplasms. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Springer: Cham, Switzerland, 2019; pp. 301–321. [Google Scholar]
- Ekinci, H.; Çiftçi, Y.Ö.; Nadarajan, J. Medium- and Long-Term Conservation of Ornamental Plants Using Synthetic Seed Technology. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Springer: Cham, Switzerland, 2019; pp. 259–281. [Google Scholar]
- Ghanbarali, S.; Abdollahi, M.R.; Zolnorian, H.; Moosavi, S.S.; Seguí-Simarro, J.M. Optimization of the Conditions for Production of Synthetic Seeds by Encapsulation of Axillary Buds Derived from Minituber Sprouts in Potato (Solanum tuberosum). Plant Cell Tiss. Organ Cult. 2016, 126, 449–458. [Google Scholar] [CrossRef]
- de Andrade, S.A.L.; de Oliveira, V.H.; Mazzafera, P. Metabolomics of Nutrient-Deprived Forest Trees. In Monitoring Forest Damage with Metabolomics Methods; Springer: Cham, Switzerland, 2024; pp. 235–265. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial Seeds: Principle, Aspects, and Applications. Agronomy 2017, 7, 71. [Google Scholar] [CrossRef]
- Raspor, M.; Motyka, V.; Kaleri, A.R.; Ninković, S.; Tubić, L.; Cingel, A.; Ćosić, T. Integrating the Roles for Cytokinin and Auxin in de Novo Shoot Organogenesis: From Hormone Uptake to Signaling Outputs. Int. J. Mol. Sci. 2021, 22, 8554. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, E.; Kinoshita, I.; Ishii, K.; Shigenaga, H.; Ohra, K.; Saito, A. Alginate-Encapsulation Technology for the Propagation of the Tropical Forest Trees: Cedrela odorata L., Guazuma crinita Mart., and Jacaranda mimosaefolia D. Don. Silvae Genet. 1997, 46, 17–23. [Google Scholar]
- Jayaprakash, C.M.; Divakaran, M.; Madhusoodanan, P.V. Role of Activated Charcoal in the Micropropagation and Conservation of Celastrus paniculatus Willd. Med. Plants-Int. J. Phytomedicines Relat. Ind. 2022, 14, 448–455. [Google Scholar] [CrossRef]
- Balaji, R.; Sriram, S.; Vigneshwaran, K.; Bharathi, A. Micropropagation of Acmella calva (DC.) RK Jansen (Asteraceae) via Encapsulation of In Vitro Nodal Explants. Plant Cell Tissue Organ Cult. 2016, 127, 1–9. [Google Scholar] [CrossRef]
- Largia, M.J.V.; Pandian, S.K.; Ramesh, M. Genetic Fidelity Assessment of Encapsulated In Vitro Tissues of Bacopa monnieri after Six Months of Storage Using ISSR and RAPD Markers. Turk. J. Bot. 2013, 37, 1008–1017. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, N.; Anis, M.; Ahmad, I. An Improved In Vitro Encapsulation Protocol, Biochemical Analysis, and Genetic Integrity Using DNA-Based Molecular Markers in Regenerated Plants of Withania somnifera L. Ind. Crops Prod. 2013, 50, 468–477. [Google Scholar] [CrossRef]
- Naz, R.; Anis, M.; Alatar, A.A.; Ahmad, A.; Naaz, A. Nutrient Alginate Encapsulation of Nodal Segments of Althaea officinalis L., for Short-Term Conservation and Germplasm Exchange. Plant Biosyst. 2018, 152, 1256–1262. [Google Scholar] [CrossRef]
- Benson, E.E.; Harding, K.; Ryan, M.; Petrenko, A.; Petrenko, Y.; Fuller, A. Alginate Encapsulation to Enhance Biopreservation Scope and Success: A Multidisciplinary Review of Current Ideas and Applications in Cryopreservation and Non-Freezing Storage. CryoLetters 2018, 39, 14–38. [Google Scholar]
- Kocak, M.; Sevindik, B.; Izgu, T.; Tutuncu, M.; Mendi, Y.Y. Synthetic Seed Production of Flower Bulbs. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Springer: Cham, Switzerland, 2019; pp. 283–299. [Google Scholar]
- Javed, S.B.; Anis, M. Cobalt-Induced Augmentation of In Vitro Morphogenic Potential in Erythrina variegata L.: A Multipurpose Tree Legume. Plant Cell Tissue Organ Cult. 2015, 120, 463–474. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B. Reactive Oxygen Species Involved in Regulating Fruit Senescence and Fungal Pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.R.; Anis, M. Changes in Activity of Antioxidant Enzymes and Photosynthetic Machinery during Acclimatization of Micropropagated Cassia alata L. Plantlets. In Vitro Cell. Dev. Biol. Plant 2014, 50, 601–609. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Lycopersicon esculentum under Low-Temperature Stress: An Approach toward Enhanced Antioxidants and Yield. Environ. Sci. Pollut. Res. 2015, 22, 14178–14188. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Alatar, A.A. Synthetic Seeds; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Akhtar, G.; Jaskani, M.J.; Sajjad, Y.; Akram, A. Effect of Antioxidants, Amino Acids, and Plant Growth Regulators on In Vitro Propagation of Rosa centifolia. Iran. J. Biotechnol. 2016, 14, 51. [Google Scholar] [CrossRef]
- Javed, S.B.; Alatar, A.A.; Anis, M.; El-Sheikh, M.A. In Vitro Regeneration of Coral Tree from Three Different Explants Using Thidiazuron. HortTechnology 2019, 29, 946–951. [Google Scholar] [CrossRef]
- Kader, A.; Sinha, S.N.; Ghosh, P. A Strategy for Development of Genetically Stable Synthetic Seeds of Azadirachta indica A. Juss. (Neem) Suitable for In Vitro Storage. Plant Cell Tissue Organ Cult. 2022, 151, 47–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).