Sustainable Agriculture Through Compost Tea: Production, Application, and Impact on Horticultural Crops
Abstract
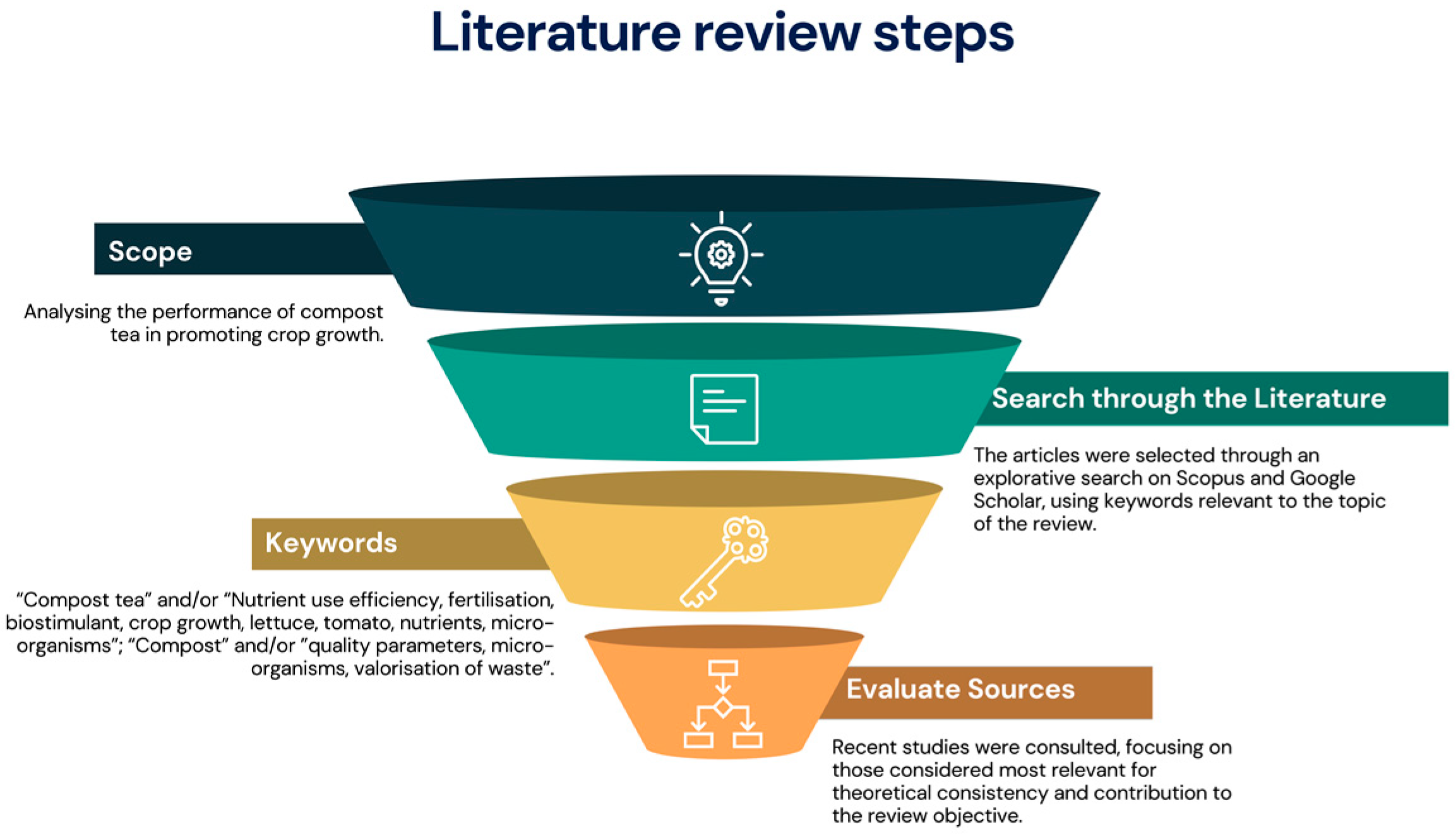
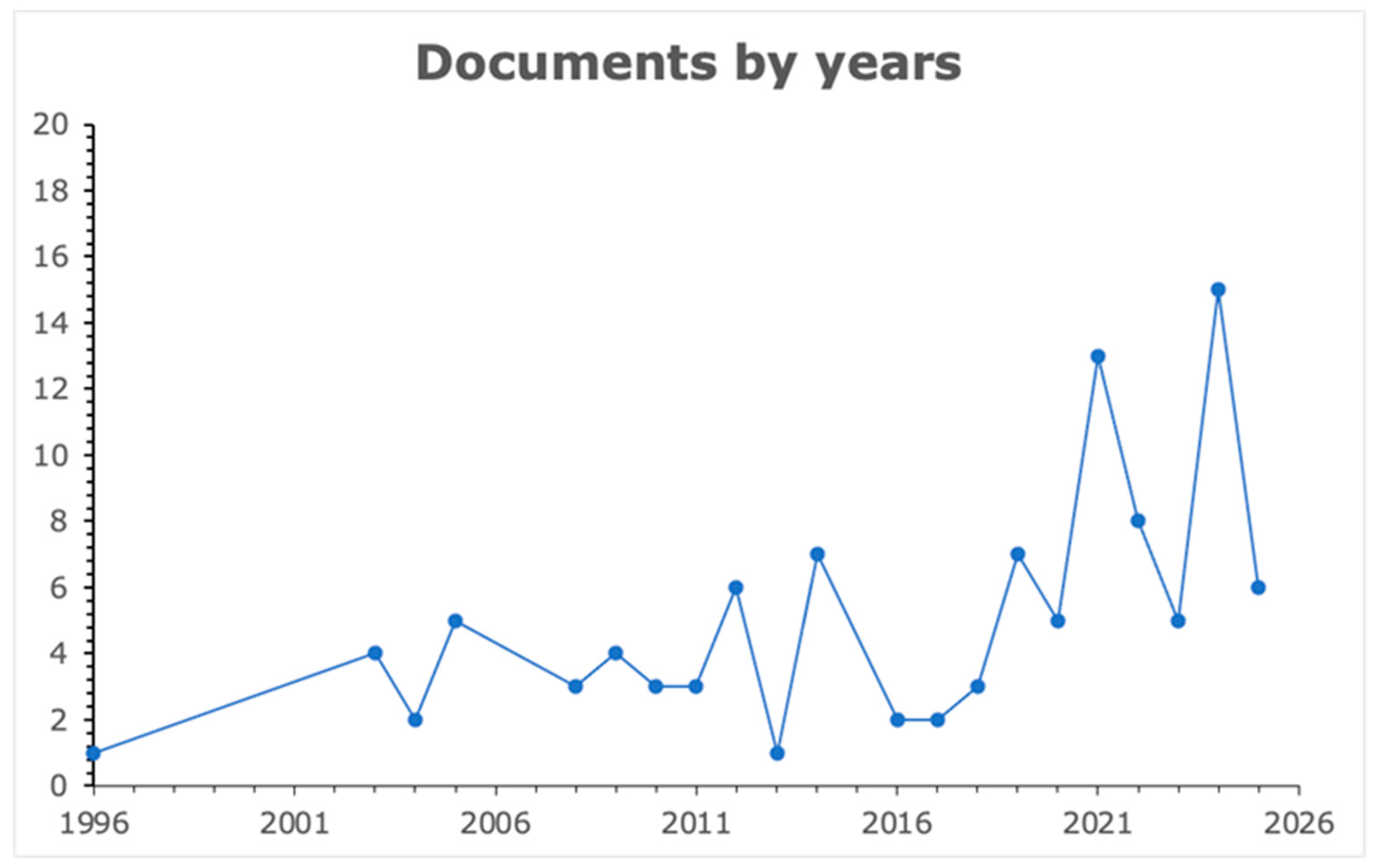
1. Introduction
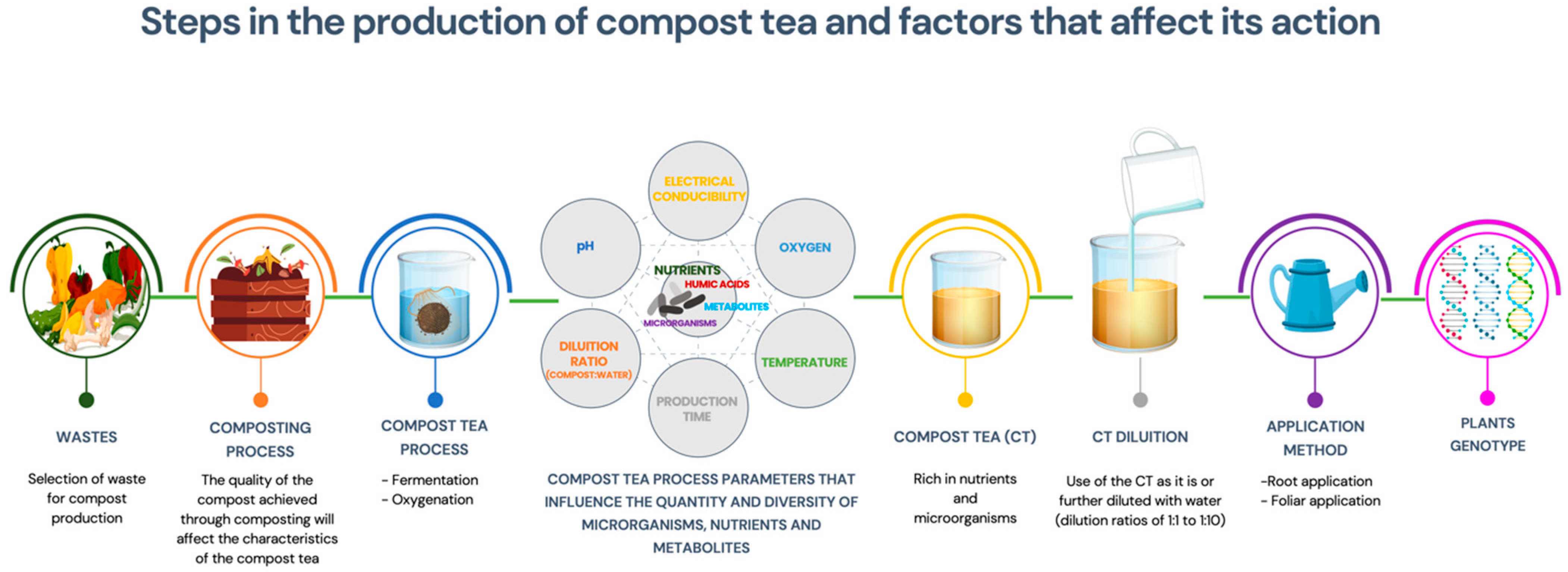
2. Compost Tea: What It Is and How to Produce It
3. From Compost-to-Compost Tea
3.1. Waste Used for Compost Production
3.2. Compost Quality Indicators
3.2.1. Physical Indicators
3.2.2. Chemical Indicators
3.2.3. Biological Indicators
3.3. Compost Tea: Compost/Water Ratio
pH, Brewing Time, Temperature and Oxygenation
3.4. Compost Tea: Application Methods
4. Opportunities and Threats of Compost Tea as a Biostimulant
5. How Does Compost Tea Improve Crop Growth?
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Curadelli, F.; Alberto, M.; Uliarte, E.M.; Combina, M.; Funes-Pinter, I. Meta-analysis of yields of crops fertilized with compost tea and anaerobic digestate. Sustainability 2023, 15, 1357. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; da Silva, E.C.; de Lima, B.H.; Fernandes, G.C.; Thiengo, C.C.; Bernardes, J.V.S.; Jalal, A.; da Silva Oliveira, C.E.; dos Santos, G.D. Unveiling contribution and fate of nitrogen with 15N techniques affected by microbial co-inoculation on field-grown maize: A novel approach to optimize N-fertilizer use efficiency. Plant Physiol. Biochem. 2024, 217, 109261. [Google Scholar] [CrossRef]
- Adzawla, W.; Setsoafia, E.D.; Setsoafia, E.D.; Amoabeng-Nimako, S.; Atakora, W.K.; Camara, O.; Jemo, M.; Bindraban, P.S. Fertilizer use efficiency and economic viability in maize production in the Savannah and transitional zones of Ghana. Front. Sustain. Food Syst. 2024, 8, 1340927. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Khan, M.N.; Sial, T.A.; Ali, A.; Wahid, F. Impact of Agricultural Wastes on Environment and Possible Management Strategies. In Frontier Studies in Soil Science; Springer: Berlin/Heidelberg, Germany, 2024; pp. 79–108. [Google Scholar]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Yin Chan, K.; Donovan, N.J.; Saleh, F.; Orr, L.; Barchia, I. Agronomic and economic benefits of green-waste compost for peri-urban vegetable production: Implications for food security. Nutr. Cycl. Agroecosyst. 2018, 111, 155–173. [Google Scholar] [CrossRef]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef]
- Khair Biek, S.; Khudur, L.S.; Ball, A.S. Challenges and Remediation Strategies for Per-and Polyfluoroalkyl Substances (PFAS) Contamination in Composting. Sustainability 2024, 16, 4745. [Google Scholar] [CrossRef]
- Baum, C.; Eichler-Löbermann, B.; Hrynkiewicz, K. Impact of organic amendments on the suppression of Fusarium wilt. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Springer: Berlin/Heidelberg, Germany, 2015; pp. 353–362. [Google Scholar]
- Garg, J.; Rakshit, A. Compost Tea: An Emerging Nature-Based Supplement Strengthening Options for Durable Agriculture. J. Soil Sci. Plant Nutr. 2024, 24, 8075–8098. [Google Scholar] [CrossRef]
- Bouchtaoui, E.M.; Haouas, A.; Dababat, A.A.; Lahlali, R.; Benali, A.; Fahr, M.; Smouni, A.; Azim, K.; Liu, Z.; Li, J. Exploring mechanisms of compost-mediated suppression of plant pathogens: A critical review. Appl. Soil Ecol. 2024, 203, 105644. [Google Scholar] [CrossRef]
- Yin, J.; Wang, J.; Zhao, L.; Cui, Z.; Yao, S.; Li, G.; Yuan, J. Compost tea: Preparation, utilization mechanisms, and agricultural applications potential—A comprehensive review. Environ. Technol. Innov. 2025, 38, 104137. [Google Scholar] [CrossRef]
- Szekely, I.; Jijakli, M.H. Bioponics as a promising approach to sustainable agriculture: A review of the main methods for producing organic nutrient solution for hydroponics. Water 2022, 14, 3975. [Google Scholar] [CrossRef]
- Proietti, P.; Calisti, R.; Gigliotti, G.; Nasini, L.; Regni, L.; Marchini, A. Composting optimization: Integrating cost analysis with the physical-chemical properties of materials to be composted. J. Clean. Prod. 2016, 137, 1086–1099. [Google Scholar] [CrossRef]
- Matouk, A.; EL-Hadidi, Y.; Tharwat, A.; Khafagy, S. Production of Compost Tea from Farm Wastes. J. Soil Sci. Agric. Eng. 2017, 8, 323–329. [Google Scholar] [CrossRef]
- Pergola, M.; Maffia, A.; Carlucci, G.; Persiani, A.; Palese, A.M.; Zaccardelli, M.; Altieri, G.; Celano, G. An environmental and economic analysis of strawberry production in southern Italy. Agriculture 2023, 13, 1705. [Google Scholar] [CrossRef]
- Ingham, E.R. The Compost Tea Brewing Manual; Unisun Communications: Corvallis, OR, USA, 2000. [Google Scholar]
- Palmer, A.; Evans, K.; Metcalf, D. Characters of aerated compost tea from immature compost that limit colonization of bean leaflets by Botrytis cinerea. J. Appl. Microbiol. 2010, 109, 1619–1631. [Google Scholar] [CrossRef]
- Palese, A.M.; Pane, C.; Villecco, D.; Zaccardelli, M.; Altieri, G.; Celano, G. Effects of organic additives on chemical, microbiological and plant pathogen suppressive properties of aerated municipal waste compost teas. Appl. Sci. 2021, 11, 7402. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- St. Martin, C. Potential of compost tea for suppressing plant diseases. CABI Rev. 2015, 1–38. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Verrillo, M.; Salzano, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Bioactivity and antimicrobial properties of chemically characterized compost teas from different green composts. Waste Manag. 2021, 120, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Scheuerell, S.; Mahaffee, W. Compost tea: Principles and prospects for plant disease control. Compost. Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Diánez, F.; Marín, F.; Santos, M.; Gea, F.J.; Navarro, M.J.; Piñeiro, M.; González, J.M. Genetic analysis and in vitro enzymatic determination of bacterial community in compost teas from different sources. Compost. Sci. Util. 2018, 26, 256–270. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259. [Google Scholar] [CrossRef]
- St. Martin, C.; Dorinvil, W.; Brathwaite, R.; Ramsubhag, A. Effects and relationships of compost type, aeration and brewing time on compost tea properties, efficacy against Pythium ultimum, phytotoxicity and potential as a nutrient amendment for seedling production. Biol. Agric. Hortic. 2012, 28, 185–205. [Google Scholar]
- Tóthné Bogdányi, F.; Boziné Pullai, K.; Doshi, P.; Erdős, E.; Gilián, L.D.; Lajos, K.; Leonetti, P.; Nagy, P.I.; Pantaleo, V.; Petrikovszki, R. Composted municipal green waste infused with biocontrol agents to control plant parasitic nematodes—A review. Microorganisms 2021, 9, 2130. [Google Scholar] [CrossRef]
- Sharma, A.; Soni, R.; Soni, S.K. From waste to wealth: Exploring modern composting innovations and compost valorization. J. Mater. Cycles Waste Manag. 2024, 26, 20–48. [Google Scholar] [CrossRef]
- Martínez-Yáñez, M.G.; Silva-Ortega, C.O.; Hernández-Aranda, V.A.; Vallejo-Pérez, M.R.; Alcalá-Briseño, R.; Vega-Manriquez, D.X.; Aguilar-Benítez, G.; Jarquin-Gálvez, R.; Lara-Ávila, J.P. Analysis of bacterial microbiota of aerated compost teas and effect on tomato growth. Microb. Ecol. 2023, 86, 959–972. [Google Scholar] [CrossRef]
- St. Martin, C.C.; Rouse-Miller, J.; Barry, G.T.; Vilpigue, P. Compost and compost tea microbiology: The “-omics” era. In Biology of Composts; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–30. [Google Scholar]
- Seddigh, S.; Kiani, L. Evaluation of different types of compost tea to control rose powdery mildew (Sphaerotheca pannosa var. rosae). Int. J. Pest Manag. 2018, 64, 178–184. [Google Scholar] [CrossRef]
- Pilla, N.; Tranchida-Lombardo, V.; Gabrielli, P.; Aguzzi, A.; Caputo, M.; Lucarini, M.; Durazzo, A.; Zaccardelli, M. Effect of compost tea in horticulture. Horticulturae 2023, 9, 984. [Google Scholar] [CrossRef]
- Shaban, H.; Fazeli-Nasab, B.; Alahyari, H.; Alizadeh, G.; Shahpesandi, S. An Overview of the Benefits of Compost tea on Plant and Soil Structure. Adv. Biores. 2015, 6, 154–158. [Google Scholar]
- Ngakou, A.; Koehles, H.; Ngueliaha, H. The role of cow dung and kitchen manure composts and their non-aerated compost teas in reducing the incidence of foliar diseases of Lycopersicon esculentum (Mill). Int. J. Agric. Res. Innov. Technol. (IJARIT) 2014, 4, 88–97. [Google Scholar] [CrossRef]
- Marín, F.; Santos, M.; Diánez, F.; Carretero, F.; Gea, F.J.; Yau, J.A.; Navarro, M.J. Characters of compost teas from different sources and their suppressive effect on fungal phytopathogens. World J. Microbiol. Biotechnol. 2013, 29, 1371–1382. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.; Calvo, L.; Morán, A. The influence of aeration system, temperature and compost origin on the phytotoxicity of compost tea. Compost. Sci. Util. 2009, 17, 127–139. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.; Hue, N.V.; Paull, R.E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 2012, 148, 138–146. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Gómez-Sánchez, M.Á.; Pérez-Sánchez, R.; Morales-Corts, M.R. Garden Waste Compost Tea: A Horticultural Alternative to Promote Plant Growth and Root Traits in Tomato (Solanum lycopersicum L.) Plants. Horticulturae 2023, 9, 1127. [Google Scholar] [CrossRef]
- Abdel-Haleem, E.-S.; Farrag, H.M.; Abeer, B.; Abdelrasheed, K.G. Combined use of compost, compost tea, and vermicompost tea improves soil properties, and growth, yield, and quality of (Allium cepa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12565. [Google Scholar]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. Are compost teas an effective nutrient amendment in the cultivation of strawberries? Soil and plant tissue effects. J. Sci. Food Agric. 2009, 89, 390–397. [Google Scholar] [CrossRef]
- Bonanomi, G.; Idbella, A.; Amoroso, G.; Iacomino, G.; Gherardelli, M.; De Sio, A.; Saccocci, F.; Abd-ElGawad, A.M.; Moreno, M.; Idbella, M. Agronomic impacts of chemically and microbiologically characterized compost tea in Mediterranean volcanic soils. Front. Plant Sci. 2025, 16, 1524884. [Google Scholar] [CrossRef]
- Hirzel, J.; Cerda, F.; Millas, P.; France, A. Compost tea effects on production and extraction of nitrogen in ryegrass cultivated on soil amended with commercial compost. Compost. Sci. Util. 2012, 20, 97–104. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Jain, M.S.; Jambhulkar, R.; Kalamdhad, A.S. Biochar amendment for batch composting of nitrogen rich organic waste: Effect on degradation kinetics, composting physics and nutritional properties. Bioresour. Technol. 2018, 253, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, Y.A.Y.; Shi, Z.-J.; Sun, S.-S.; Luo, Y.-S.; Yang, X.; Hou, W.-T.; Wang, R.-L. An assessment of composting conditions, humic matters formation and product maturity in response to different additives: A meta-analysis. J. Clean. Prod. 2022, 366, 132953. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic bacteria and their thermozymes in composting processes: A review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Amran, M.A.; Norhashim, N.A.; Mohamad-Fauzi, N.; Peng-Hui, F.; Hui-Wen, L.; Kai-Lin, Y.; Jiale, L.; Chian-Yee, M.G.; Jing-Yi, L. Food waste composting and microbial community structure profiling. Processes 2020, 8, 723. [Google Scholar] [CrossRef]
- Shan, Y.N.; Chen, J.H.; Wang, L.; Li, F.; Fu, X.H.; Le, Y.Q. Influences of adding easily degradable organic waste on the minimization and humification of organic matter during straw composting. J. Environ. Sci. Health Part B 2013, 48, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, M.; Alkoaik, F.; Abdel-Razzak, H.; Ibrahim, M.; Fulleros, R.; Shady, M.; Abdel-Ghany, A. Evaluation of tomato waste compost stability and maturity using CIELAB color indicator. J. Plant Nutr. 2020, 43, 1427–1437. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.; Roig, A.; Paredes, C.; Bernal, M. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Vázquez, M.; Sen, R.; Soto, M. Physico-chemical and biological characteristics of compost from decentralised composting programmes. Bioresour. Technol. 2015, 198, 520–532. [Google Scholar] [CrossRef]
- Chung, W.J.; Chang, S.W.; Chaudhary, D.K.; Shin, J.; Kim, H.; Karmegam, N.; Govarthanan, M.; Chandrasekaran, M.; Ravindran, B. Effect of biochar amendment on compost quality, gaseous emissions and pathogen reduction during in-vessel composting of chicken manure. Chemosphere 2021, 283, 131129. [Google Scholar] [CrossRef]
- Mussa, S.; Farhan, M.; Ahmad, S.; Zahra, K.; Kanwal, A.; Khan, Q.F.; Afzaal, M.; Wahid, A.; Sarker, P.K.; El-Sheikh, M.A. Exploring the utility of different bulking agents for speeding up the composting process of household kitchen waste. Sci. Rep. 2025, 15, 2488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.H.; Huang, M.C.; Lu, M.F.; Chou, Y.J.; Lin, J.J.M. Assessment of degree of maturity of compost produced by different kitchen waste composting methods. Adv. Mater. Res. 2013, 652, 1642–1651. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chanyasak, V.; Kubota, H. Carbon/organic nitrogen ratio in water extract as measure of composting degradation. J. Ferment. Technol. 1981, 59, 215–219. [Google Scholar]
- Bernal, M.; Sanchez-Monedero, M.; Paredes, C.; Roig, A. Carbon mineralization from organic wastes at different composting stages during their incubation with soil. Agric. Ecosyst. Environ. 1998, 69, 175–189. [Google Scholar] [CrossRef]
- Noor, R.S.; Shah, A.N.; Tahir, M.B.; Umair, M.; Nawaz, M.; Ali, A.; Ercisli, S.; Abdelsalam, N.R.; Ali, H.M.; Yang, S.H. Recent Trends and Advances in Additive-Mediated Composting Technology for Agricultural Waste Resources: A Comprehensive Review. ACS Omega 2024, 9, 8632–8653. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Aparna, C.; Saritha, P.; Himabindu, V.; Anjaneyulu, Y. Techniques for the evaluation of maturity for composts of industrially contaminated lake sediments. Waste Manag. 2008, 28, 1773–1784. [Google Scholar] [CrossRef]
- Jimenez, E.I.; García, V.P. Determination of maturity indices for city refuse composts. Agric. Ecosyst. Environ. 1992, 38, 331–343. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Gautam, R.K.; Navaratna, D. Humic Substances: Its Toxicology, Chemistry and Biology Associated. In Humic Substances; InTech Open: Rijeka, Croatia, 2021; p. 97. [Google Scholar]
- Mahler, C.F.; Dal Santo Svierzoski, N.; Bernardino, C.A.R. Chemical Characteristics of Humic Substances in Nature; InTech Open: Rijeka, Croatia, 2021. [Google Scholar]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers. Biorefin. 2021, 13, 5445–5468. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, J.; Zhang, X.; Gao, X.; Yin, J.; Wang, G.; Li, J.; Li, G.; Cui, Z.; Yuan, J. Applicability and limitation of compost maturity evaluation indicators: A review. Chem. Eng. J. 2024, 489, 151386. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Coosemans, J.; Deprins, K.; Swings, J. Microbiological aspects of biowaste during composting in a monitored compost bin. J. Appl. Microbiol. 2003, 94, 127–137. [Google Scholar] [CrossRef]
- Gea, T.; Barrena, R.; Artola, A.; Sánchez, A. Monitoring the biological activity of the composting process: Oxygen uptake rate (OUR), respirometric index (RI), and respiratory quotient (RQ). Biotechnol. Bioeng. 2004, 88, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Pane, C.; Palese, A.M.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Enhancing sustainability of a processing tomato cultivation system by using bioactive compost teas. Sci. Hortic. 2016, 202, 117–124. [Google Scholar] [CrossRef]
- Lu, S.; Pentico, D.; Castro, R.; Dinh, S.; Love, J.J.; Larom, D.L.; Pérez, R.L.; Liu, C. Effect of ultraviolet light exposure and compost tea supplementation on growth, antioxidant activities, and microbiome of hydroponically grown mustard greens. ACS Agric. Sci. Technol. 2022, 2, 521–533. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Zhao, Q.; Liu, X.; Deng, H.; Li, Z. Short-chain fatty acid producers in compost tea as affected by brewing time and aeration condition. J. Soils Sediments 2023, 23, 3096–3107. [Google Scholar] [CrossRef]
- Raza, A.; Ali, Z.; Ali, S.A. Standardization of protocol for the formulation of compost tea and its efficacy study on potato. Pure Appl. Biol. (PAB) 2023, 12, 1044–1055. [Google Scholar] [CrossRef]
- Vail, D.C.; Hernández, D.L.; Velis, E.; Wills, A. Compost tea production methods affect soil nitrogen and microbial activity in a northern highbush blueberry system. Agroecol. Sustain. Food Syst. 2020, 44, 1370–1383. [Google Scholar] [CrossRef]
- Islam, M.; Yaseen, T.; Traversa, A.; Kheder, M.B.; Brunetti, G.; Cocozza, C. Effects of the main extraction parameters on chemical and microbial characteristics of compost tea. Waste Manag. 2016, 52, 62–68. [Google Scholar] [CrossRef]
- Brinton, W.; Storms, P.; Evans, E.; Hill, J. Compost teas: Microbial hygiene and quality in relation to method of preparation. Biodynamics 2004, 2, 36–45. [Google Scholar]
- Larkin, R.P. Relative effects of biological amendments and crop rotations on soil microbial communities and soilborne diseases of potato. Soil Biol. Biochem. 2008, 40, 1341–1351. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-Gizawy, E.; Geries, L. Effect of compost extract, N2-fixing bacteria and nitrogen levels applications on soil properties and onion crop. Arch. Agron. Soil Sci. 2015, 61, 185–201. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Subramanian, S.; Smith, D.L. Phytomicrobiome coordination signals hold potential for climate change-resilient agriculture. Front. Plant Sci. 2020, 11, 634. [Google Scholar] [CrossRef]
- Li, J.; Lardon, R.; Mangelinckx, S.; Geelen, D. A practical guide to the discovery of biomolecules with biostimulant activity. J. Exp. Bot. 2024, 75, 3797–3817. [Google Scholar] [CrossRef]
- Morales-Corts, M.R.; Pérez-Sánchez, R.; Gómez-Sánchez, M.Á. Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soilborne pathogens. Sci. Agric. 2018, 75, 400–409. [Google Scholar] [CrossRef]
- Mengesha, W.; Gill, W.; Powell, S.; Evans, K.; Barry, K. A study of selected factors affecting efficacy of compost tea against several fungal pathogens of potato. J. Appl. Microbiol. 2017, 123, 732–747. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Boix, A.; De Cara, M.; Trillas, I.; Avilés, M.; Tello, J. Grape marc compost tea suppressiveness to plant pathogenic fungi: Role of siderophores. Compost. Sci. Util. 2006, 14, 48–53. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pane, C.; Villecco, D.; Palese, A.M.; Celano, G. Compost tea spraying increases yield performance of pepper (Capsicum annuum L.) grown in greenhouse under organic farming system. Ital. J. Agron. 2018, 13, 229–234. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Sami, R.; Al-Mushhin, A.A.; Ali, M.M.E.; El-Desouky, H.S.; Ismail, K.A.; Khalil, R.; Zewail, R.M. Impacts of effective microorganisms, compost tea, fulvic acid, yeast extract, and foliar spray with seaweed extract on sweet pepper plants under greenhouse conditions. Plants 2021, 10, 1927. [Google Scholar] [CrossRef]
- Abd-Alrahman, H.A.; Aboud, F.S. Response of sweet pepper plants to foliar application of compost tea and dry yeast under soilless conditions. Bull. Natl. Res. Cent. 2021, 45, 119. [Google Scholar] [CrossRef]
- Villecco, D.; Pane, C.; Ronga, D.; Zaccardelli, M. Enhancing sustainability of tomato, pepper and melon nursery production systems by using compost tea spray applications. Agronomy 2020, 10, 1336. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Celano, G.; Zaccardelli, M. Effects of compost tea treatments on productivity of lettuce and kohlrabi systems under organic cropping management. Ital. J. Agron. 2014, 9, 153–156. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Pérez-Sánchez, R.; Gómez-Sánchez, M.Á.; Morales-Corts, M.R. Compost tea as biostimulant: Promoting tomato root development. Chem. Proc. 2022, 10, 57. [Google Scholar] [CrossRef]
- Abubaker, S.; Qrunfleh, I.; Shatnawi, M.; Ammari, T.G.; Hasan, H.; Al-Tawaha, A.R.M. The effect of compost tea on some growth and yield parameters and soil chemical properties of greenhouse tomato (Solanum lycopersicum L.). Ecol. Eng. Environ. Technol. 2024, 25, 362–370. [Google Scholar] [CrossRef]
- Girshe, L.; Mengistu, H.; Tadesse, T. The effects of rate and method of aerated compost tea application on yield and yield component of tomato (Lycopersicon esculentum Mill.) at Burusa, South Western Ethiopia. Int. J. Multidiscip. Curr. Res. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- De Corato, U. Compost and Compost Tea from On-Farm Composted Agro-Wastes Improve the Sustainability of Horticultural Organic Cropping Systems. In Agri-Based Bioeconomy; CRC Press: Boca Raton, FL, USA, 2021; pp. 143–162. [Google Scholar]
- Formisano, L.; Miras-Moreno, B.; Ciriello, M.; Zhang, L.; De Pascale, S.; Lucini, L.; Rouphael, Y. Between light and shading: Morphological, biochemical, and metabolomics insights into the influence of blue photoselective shading on vegetable seedlings. Front. Plant Sci. 2022, 13, 890830. [Google Scholar] [CrossRef]
- Carrascosa, A.; Pascual, J.A.; López-García, Á.; Romo-Vaquero, M.; De Santiago, A.; Ros, M.; Petropoulos, S.A.; Alguacil, M.D.M. Effects of inorganic and compost tea fertilizers application on the taxonomic and functional microbial diversity of the purslane rhizosphere. Front. Plant Sci. 2023, 14, 1159823. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Min, T.; Ru, S.; Li, J. Response of cotton root growth and rhizosphere soil bacterial communities to the application of acid compost tea in calcareous soil. Appl. Soil Ecol. 2022, 177, 104523. [Google Scholar] [CrossRef]
- Lazcano, C.; Domínguez, J. The use of vermicompost in sustainable agriculture: Impact on plant growth and soil fertility. Soil Nutr. 2011, 10, 187. [Google Scholar]
- Atiyeh, R.; Lee, S.; Edwards, C.; Arancon, N.; Metzger, J. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.d.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Ali, O.A. Role of humic substances and compost tea in improvement of endogenous hormones content, flowering and yield and its components of faba bean (Vicia faba L.). Ann. Agric. Sci. Moshtohor 2015, 53, 373–384. [Google Scholar]
- El-Din, A.A.E.; Hendawy, S. Effect of dry yeast and compost tea on growth and oil content of Borago officinalis plant. Res. J. Agric. Biol. Sci. 2010, 6, 424–430. [Google Scholar]
- Li, W.; Liu, Y.; Chai, X.; He, J.; Liu, C.; Li, J. Advantages of compost tea: Promotion of nitrogen influx into the fruit and improvement of fruit nitrogen metabolism in tomato. Plant Physiol. Biochem. 2024, 216, 109184. [Google Scholar] [CrossRef]
- Xu, D.; Raza, W.; Yu, G.; Zhao, Q.; Shen, Q.; Huang, Q. Phytotoxicity analysis of extracts from compost and their ability to inhibit soil-borne pathogenic fungi and reduce root-knot nematodes. World J. Microbiol. Biotechnol. 2012, 28, 1193–1201. [Google Scholar] [CrossRef]
- Xu, D.-B.; Wang, Q.-J.; Wu, Y.-C.; Yu, G.-H.; Shen, Q.-R.; Huang, Q.-W. Humic-like substances from different compost extracts could significantly promote cucumber growth. Pedosphere 2012, 22, 815–824. [Google Scholar] [CrossRef]
- Eudoxie, G.; Grogan, K.; Beckford, M.; Martin, M. Compost tea influence on lettuce (Lactuca sativa L.) root architecture. In Proceedings of the International Symposium on Growing Media, Soilless Cultivation, and Compost Utilization in Horticulture 1266, Portland, OR, USA, 20–25 August 2017; pp. 79–88. [Google Scholar]

| Waste Material | CT Brewing Process | (dS m−1) | ppm | Reference | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compost-to-Water Ratio | Production Time | Areated or Not | Temperature (°C) | pH | EC | NO3− | P2O5 | K2O | SO42− | Ca | Mg | Na | Cl | Humic Acids | Fe | Mn | Cu | Zn | B | ||
| Banana leaves | 1000 g compost:17 L water (tap water) | 18 h | Aerated | - | 6.62 | 4.19 | 92.5 | 80.7 | 47.03 | - | 443.7 | 150.1 | 28.55 | - | - | - | - | 0.05 | 0.39 | - | [33] |
| 27 h | Aerated | - | 6.5 | 4.47 | 116.2 | 89.3 | 38.24 | - | 439.5 | 240.1 | 19.05 | - | - | - | - | 0.06 | 0.52 | - | |||
| 36 h | Aerated | - | 6.57 | 3.9 | 132.7 | 83.9 | 42.64 | - | 436.5 | 129.2 | 20.99 | - | - | - | - | 0.05 | 0.35 | - | |||
| 56 h | Not areated | - | 6.12 | 4.46 | 368 | 104.4 | 40.44 | - | 151 | 105 | 21.53 | - | - | - | - | 0.03 | 0.08 | - | |||
| 112 h | Not areated | - | 6.43 | 4.86 | 204.6 | 107.5 | 60.22 | - | 167 | 146.8 | 26.38 | - | - | - | - | 0.04 | 0.08 | - | |||
| 168 h | Not areated | - | 6.18 | 4.58 | 352.9 | 104.6 | 45.93 | - | 250.8 | 174 | 21.38 | - | - | - | - | 0.04 | 0.08 | - | |||
| Cattle manure | 1:10 (w:v with tap water) | 48 h | Areated | 10 | 9.1 | 10.5 | - | - | - | - | - | - | - | - | - | - | - | 1.12 | 1.66 | - | [39] |
| 20 | 9.1 | 11.5 | - | - | - | - | - | - | - | - | - | - | - | 1.43 | 1.93 | - | |||||
| 30 | 8.9 | 12.5 | - | - | - | - | - | - | - | - | - | - | - | 2.05 | 2.91 | - | |||||
| 10 days | Not areated | 10 | 9.1 | 11 | - | - | - | - | - | - | - | - | - | - | - | 1.68 | 2.46 | - | |||
| 20 | 8.8 | 12.5 | - | - | - | - | - | - | - | - | - | - | - | 2.08 | 3.14 | - | |||||
| 30 | 8.4 | 14.7 | - | - | - | - | - | - | - | - | - | - | - | 2.17 | 2.01 | - | |||||
| Chicken manure-based termophilic compost | 1:10 (v:v) | 12 h | Areated | - | 7.3 | 22.2 | 289.2 | - | - | - | 152.6 | 138.3 | - | - | 94.9 | - | - | - | - | - | [40] |
| Chicken manure-based vermicompost (aged) | - | 6.8 | 3.4 | 137.9 | - | - | - | 59.6 | 61.6 | - | - | 464.8 | - | - | - | - | - | ||||
| Chicken manure-based vermicompost (fresh) | - | 6.9 | 1.4 | 39.6 | - | - | - | 38.7 | 33.3 | - | - | 435.3 | - | - | - | - | - | ||||
| Commercial compost (Almagro, Ciudad Real province, Spain, LOT 2010/02) | 1:4 (w:v) | 14 days | Aerated | 25 | 7.88 | 5.44 | 193 | 14.2 | 2247 | 353 | 1758 | 128 | 292 | 174 | - | 64.59 | 2.69 | 0.52 | 2.32 | - | [38] |
| Commercial compost (Almagro, Ciudad Real province, Spain, LOT 2010/02) | Not areated | 25 | 8.24 | 5.34 | 76 | 14.9 | 1892 | 626 | 290 | 92 | 215 | 184 | - | 0.22 | 0.57 | 0.16 | 0.17 | - | |||
| Commercial compost (La Manchela, Albacete province, Spain, LOT 2010/04) | Aerated | 25 | 7.79 | 9.36 | 168 | 11.2 | 2626 | 2723 | 967 | 272 | 585 | 177 | - | 5.91 | 0.98 | 0.12 | 0.58 | - | |||
| Commercial compost (La Manchela, Albacete province, Spain, LOT 2010/04) | Not areated | 25 | 8.19 | 9.18 | 155 | 12.7 | 2467 | 2241 | 819 | 491 | 529 | 170 | - | 0.05 | 1.19 | 0.01 | 0.81 | - | |||
| Green and pruning waste consisting of a mixture of grass cuttings and pruning debris | 1:5 (v:v) | 5 days | Areated | 20 | 7.32 | 1.22 | 3200 | 102 | 3840 | 28 | 79 | 150 | - | - | 190 | - | - | - | - | - | [41] |
| 15 days | 20 | 7.33 | 1.48 | 3300 | 368 | 4123 | 31 | 110 | 135 | - | - | 179 | - | - | - | - | - | ||||
| 5 days | 15 | 7.16 | 1.2 | 2741 | 61.4 | 2851.2 | 20 | 280 | 20 | - | - | 100.3 | - | - | - | - | - | ||||
| 5 days | 20 | 7.16 | 1.46 | 4700 | 105 | 4039 | 12 | 26 | 138 | - | - | 179 | - | - | - | - | - | ||||
| 15 days | 20 | 7.12 | 1.43 | 3934 | 315 | 4016 | 13 | 20 | 128 | - | - | 183 | - | - | - | - | - | ||||
| 5 days | 15 | 7.11 | 1.23 | 3050 | 56.2 | 2782.2 | 15 | 101 | 22 | - | - | 98.3 | - | - | - | - | - | ||||
| Green waste termophilic compost | 1:10 (v:v) | 12 h | Areated | - | 7.8 | 3.3 | 8.4 | - | - | - | 48.7 | 21.2 | - | - | 556.5 | - | - | - | - | - | [40] |
| Lawn clippings | 1000 g compost:17 L water (tap water) | 18 h | Aerated | - | 5.16 | 3.64 | 145.7 | 110.9 | 17.37 | 361.7 | 68.6 | 9.68 | - | - | - | - | 0.46 | 0.54 | - | [29] | |
| 27 h | - | 5.13 | 3.73 | 140.1 | 106 | 11.87 | 516.4 | 75.6 | 9.7 | - | - | - | - | 0.046 | 0.62 | - | |||||
| 36 h | - | 5.07 | 4.04 | 126.1 | 109.1 | 17.37 | 553.2 | 74.2 | 7.47 | - | - | - | - | 0.046 | 0.76 | - | |||||
| 56 h | Not areated | - | 6.08 | 3.85 | 319.8 | 104.2 | 16.27 | 436.6 | 43.8 | 14.5 | - | - | - | - | 0.06 | 0.97 | - | ||||
| 112 h | - | 5.97 | 3.88 | 93.2 | 126.7 | 9.68 | 488.1 | 78.5 | 14.5 | - | - | - | - | 0.05 | 1.04 | - | |||||
| 168 h | - | 6.02 | 3.75 | 223 | 123.8 | 16.27 | 504.2 | 83 | 2.95 | - | - | - | - | 0.05 | 0.75 | - | |||||
| Mixture of horticultural crop residues, tree leaves and field crop waste with poultry litter and cow dung | 1:10 (p:v) (Tap water) | 7 days | Areated | - | 7.93 | 2.93 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [42] |
| Mixture of sheep manure, beef manure and sheep litter composed mainly of straw (year 2004) | 1:10 (w:v) | 72 h | Not areated | - | 7.31 | - | - | - | - | - | 23.4 | 13 | 13.1 | - | - | 9.3 | 0.82 | 0.05 | 0.38 | 0.11 | [43] |
| Mixture of sheep manure, beef manure and sheep litter composed mainly of straw (year 2005) | - | 6.74 | - | - | - | - | - | 6.58 | 6.64 | 13.1 | - | - | 0.47 | 0.008 | 0.006 | 0.006 | 0.31 | ||||
| Mixture of sheep manure, beef manure and sheep litter composed mainly of straw (year 2006) | - | 6.68 | - | - | - | - | - | 41.5 | 35.7 | 29.2 | - | - | 0.82 | 0.11 | 0.06 | 0.08 | 0.38 | ||||
| Stimol-C® (the organic feedstock consists of mixed plant materials, straw and composted cow manure from biological farms) | 2:100 (p:v with tap water) | 48 h | Aerated | - | 6.4 | 1.37 | 1.4 | 18.4 | - | 170 | - | - | - | - | - | 2.3 | - | - | - | - | [44] |
| Vitafert commercial compost | 13:100 | 24 h | Aerated | - | 6.96 | 3.38 | 199 | - | - | - | 437.4 | 39.5 | - | - | - | 0.05 | - | 0.09 | 0.03 | 2.35 | [45] |
| Bacteria | ||
|---|---|---|
| Phyla | Genus | References |
| Actinobacteria | Micrococcus | [9] |
| Bacillota | Bacillus | [9,28,32,72] |
| Weissella | [32] | |
| Brevibacillus | [73] | |
| Staphylococcus | [9] | |
| Proteobacteria | Pseudomonas | [9,32,72,73] |
| Burkholderia | [9] | |
| Comamonas | [28,73] | |
| Pseudomonadota | Sphingobium | [28,32,73] |
| Azotobacter | [32] | |
| Firmicutes | Lactobacillus | [9,28,32] |
| Clostridium | [28,32,73] | |
| Crop | Growing Condition | Growing Media | Method of Brewing | Compost Material Used | Compost Tea Production Process Specifications | Application Method | Effect of Compost Tea | References |
|---|---|---|---|---|---|---|---|---|
| Bell pepper (Capsicum annuum L.) | Greenhouse | Loamy soil | Aerated water extraction | Waste of artichokes, wood chips, fennel and escarole | The duration of the fermentation process was one week, the compost tea obtained was refrigerated and stored at 4 °C. 1:5 compost–water dilution ratio | Foliar application | The application of compost tea singificantly improved the physiological status of treated plants and yield in two different years. Specifically, the treated plants were characterized by a higher number of fruits. | [89] |
| Sand soil | Vegetable waste | The duration of the fermentation process was one week. Compost–water dilution ratio of 1:10. | Root application | In the two-year experiment, the application of compost tea detemined a significant increase in yield and quality parameters (vitamin C and carotenoid content). | [90] | |||
| Peat-based substrate | The compost tea brew was extracted with a compost–water dilution ratio of 1:10. | Foliar application | Compared with control plants, the application of compost tea significantly increased vegetative growth, biometric parameters (length, diameter and fresh weight), total yield and plant nutritional status (N, P and K). | [91] | ||||
| Waste of tomato, escarole, wood chips, artichoke and fennel | The duration of the fermentation process was one week. Dilution ratio used was 1:5 compost–water. | Root and foliar application | Compared to control, compost tea application stimulated root growth of treated bell pepper seedlings by recording a significant increase in root length (+8%). | [92] | ||||
| Kohlrabi (Brassica oleracea) | Greenhouse | Loam soil | Aerated water extraction | Waste of artichoke and fennel | The duration of the fermentation process was one week, the compost tea obtained was refrigerated stored at 4 °C. 1:5 Compost–water dilution ratio. | Foliar application | Compared to the control, the use of compost tea provided a significant increase in commercial yield (+32%) that is attributable to an improvement in the physiological status of treated plants. | [93] |
| Lettuce (Lactuca sativa L.) | Greenhouse | Coconut beat and Peat-based substrate | Aerated water extraction | Rice straw and Hinoki cypress bark | The duration of the fermentation process was 96 h at room temperature. Compost–water dilution ratio 1:10. | Root and foliar application | Regardless of the dose, application of aerated compost tea to the root zone increased shoot and root growth and lettuce yield. | [28] |
| Loam soil | Waste of artichoke and fennel | The duration of the fermentation process was one week, the compost tea obtained was refrigerated and stored at 4 °C. 1:5 compost–water dilution ratio. | Root application | The use of compost tea positively influenced the physiological status of plants (significant increase in SPAD index) leading to an increase in commercial yield (+24%) compared to the control. | [93] | |||
| Melon (Cucumis melo L.) | Greenhouse | Peat-based substrate | Aerated water extraction | Waste of tomato, escarole, wood chips, artichoke and fennel | The duration of the fermentation process was one week. Dilution ratio used was 1:5 compost–water. | Root and foliar application | Compost tea application improved growth parameters (number of leaves and fresh biomass) of melon seedlings. | [92] |
| Pak choi (Brassica rapa) | Greenhouse | Peat-perlite | Aerated water extraction | Chicken manure, aged chicken manure, green waste and food waste | The compost tea brew was extracted with a compost–water dilution ratio of 1:10. | Root application | The different types of compost tea significantly differed in total nutrient, phytohormone and beneficial microorganism contents. The use of compost tea obtained from chicken manure-based vermicompost significantly influenced plant growth and development due to higher nitrogen and gibberellin contents. | [40] |
| Tomato (Solanum lycopersicum L.) | NT | Agar | Aerated water extraction | Green waste | The compost tea brew was extracted with a compost–water dilution ratio of 1:6. | Seed application | Application of compost tea significantly improved lateral root number and primary root length. Analyses performed on the compost tea used revealed relevant humic acid, nitrogen and amino acid contents. | [94] |
| Greenhouse | Peat-based substrate | Pruning waste (branches and leaves of cypresses, willows and poplars). | The compost tea brew was extracted with a compost–water dilution ratio of 1:5. The fermentation period lasted 15 days at constant temperature (20 °C). | Root and foliar application | The application of compost tea increased the number of leaves, plant height and dry weight of plants compared with the control. | [41] | ||
| Soil | Vegetable and animal wastes | The compost tea brew was extracted with a compost–water dilution ratio of 1:5. | Composted tea extract significantly improved the main parameters of vegetative growth (number of stem internodes, plant height and number of leaves), marketable yield and average fruit weight. | [95] | ||||
| Poultry manure, dried cow dung, green leaves, ash, top soil and dried crop residue | The duration of the fermentation process was 72 h. Compost–water dilution ratio 1:5. | Regardless of the mode of application, compost tea positively influenced the yield of tomato plants through a general improvement in nutritional status. However, the best results were recorded following root application. | [96] | |||||
| Peat-based substrate | Waste of tomato, escarole, wood chips, artichoke and fennel | The duration of the fermentation process was one week. Dilution ratio used was 1:5 compost–water. | Compared to control, compost tea application significantly increased root length (+9%) and stem diameter (+12%) of treated tomato seedlings. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campana, E.; Ciriello, M.; Lentini, M.; Rouphael, Y.; De Pascale, S. Sustainable Agriculture Through Compost Tea: Production, Application, and Impact on Horticultural Crops. Horticulturae 2025, 11, 433. https://doi.org/10.3390/horticulturae11040433
Campana E, Ciriello M, Lentini M, Rouphael Y, De Pascale S. Sustainable Agriculture Through Compost Tea: Production, Application, and Impact on Horticultural Crops. Horticulturae. 2025; 11(4):433. https://doi.org/10.3390/horticulturae11040433
Chicago/Turabian StyleCampana, Emanuela, Michele Ciriello, Matteo Lentini, Youssef Rouphael, and Stefania De Pascale. 2025. "Sustainable Agriculture Through Compost Tea: Production, Application, and Impact on Horticultural Crops" Horticulturae 11, no. 4: 433. https://doi.org/10.3390/horticulturae11040433
APA StyleCampana, E., Ciriello, M., Lentini, M., Rouphael, Y., & De Pascale, S. (2025). Sustainable Agriculture Through Compost Tea: Production, Application, and Impact on Horticultural Crops. Horticulturae, 11(4), 433. https://doi.org/10.3390/horticulturae11040433









