Abstract
Water scarcity can negatively affect crop yield, posing a significant threat to global food security, such as drought. Plant growth-promoting rhizobacteria (PGPR), either as single strains or synthetic communities (SynComs), has shown promise in alleviating drought stress in various plant species. In this study, we examined the effects of water limitation on Salvia officinalis and the potential of a SynCom composed of five phosphate-solubilizing, auxin-producing, and/or nitrogen-fixing Gram-negative bacteria to enhance plant growth and drought tolerance. Plant growth, morphology, physiology, and leaf metabolomic profiles were assessed using a combination of physiological measurements and LC-MS untargeted metabolomics. Mild water stress induced a conservative water-use strategy in S. officinalis, characterized by increased root-to-shoot ratio and altered leaf morphology, without compromising photosynthetic performance. SynCom inoculation under well-watered conditions elicited drought-like responses, including transient reductions in stomatal conductance. Leaf metabolomic analysis revealed that inoculation influenced the abundance of several metabolites, including biogenic amines and dipeptides, under both irrigation regimes. Notably, drought stress and SynCom inoculation increased histamine and α-ketoglutaric acid levels, highlighting potential impacts on food quality. Under reduced irrigation, inoculation further modulated leaf morphology and biomass allocation, promoting thicker leaves and increased root biomass allocation. These results demonstrate the ability of the SynCom to modulate plant physiology and metabolism in response to both optimal and reduced irrigation, potentially enhancing drought resilience without directly improving growth. The study also highlights the complex interactions among microbial inoculation, plant stress responses, and leaf metabolite profiles, emphasizing the importance of considering the effects on the production of bioactive compounds when developing microbial inoculants for edible plants.
1. Introduction
Plant growth-promoting rhizobacteria (PGPR), whether utilized as individual strains or consortia, represent a promising biotechnological strategy for enhancing agricultural sustainability. This approach reduces both the environmental and economic costs associated with conventional fertilization [1]. These beneficial microbes establish intricate interactions with host plants, offering a valuable strategy to improve plant productivity under both optimal and stressful conditions [2,3,4,5,6]. PGPR employ a variety of mechanisms to promote plant growth, primarily through enhanced nutrient acquisition and modulation of plant hormone signaling, thereby mitigating stress perception. Nutrient availability is increased through processes such as nitrogen fixation [7,8,9], solubilization of inorganic phosphate [10], and the production of high-affinity iron-chelating molecules known as siderophores [11,12]. Furthermore, PGPR can influence root architecture, improving water and nutrient uptake [13] by stimulating root elongation and lateral root development [14,15,16,17,18,19]. Beyond nutrient acquisition, PGPR significantly impact plant physiology by modulating phytohormone levels. Microbial auxins, particularly indole-3-acetic acid (IAA), regulate plant growth in over 50% of PGPR species [20]. Some rhizobacteria also produce 1-aminocyclopropane-1-carboxylate deaminase (ACCD), an enzyme metabolizing ACC, the precursor to the stress hormone ethylene [21]. This activity reduces ethylene biosynthesis, alleviating its inhibitory effects on plant growth [13,22,23]. The complex interplay between ACC and IAA is further highlighted by the observation that IAA can upregulate ACC synthase, the enzyme responsible for ACC production [21]. PGPR also influence abscisic acid (ABA) levels, with some strains inducing ABA production to reduce stomatal conductance and water loss under stress [24,25,26], while others decrease ABA concentrations, thus mitigating the negative impacts of stomatal closure on plant growth [27,28].
Synthetic microbial communities (SynComs), defined as rationally assembled microbial consortia, provide a simplified yet powerful platform for studying and replicating the complex interactions observed in natural microbiomes [29]. These reduced-complexity systems, often comprising a limited number (1–10) of selected microbial strains with documented plant growth-promoting activity, hold great promise for advancing agricultural practices [30,31,32]. The strategic design of SynComs aims to enhance plant growth by increasing the abundance of functionally redundant microbial populations to amplify specific PGP traits like nitrogen fixation or phosphate solubilization, thereby enhancing nutrient bioavailability and combining strains with diverse PGP functionalities to address multiple plant needs simultaneously [3,33]. The isolation and characterization of novel PGPR are essential for expanding the microbial resources available for diverse environments and plant species and deepening our understanding of plant–microbe interactions. This is particularly crucial as microorganisms are susceptible to the same environmental stresses as plants, and laboratory results may not always translate effectively to field applications [34]. For instance, the survival of inoculated PGPR, especially Gram-negative non-spore-forming bacteria, is a significant concern due to their sensitivity to factors such as desiccation, pH fluctuations, and temperature changes [35]. While protective formulations like encapsulated products have been proposed [36,37], these can increase production costs. Therefore, SynComs, engineered with strains selected for specific PGP traits and stress tolerance, offer a promising alternative for developing effective inoculants that promote plant growth even under adverse environmental conditions, such as drought.
Water scarcity can negatively affect crop yield, posing a significant threat to global food security, such as drought [38,39]. The effect of water stress on plant performance can be reconducted into a series of morphological, physiological, and biochemical changes that aim at endorsing plant survival under water scarcity [40]. These include modifications in root and leaf architecture to enhance water uptake and reduce water losses, accumulation of metabolites (sugars, polyols, amino acids) to adjust osmotic pressure, dissipation of excess energy, and synthesis of metabolites to mitigate oxidative stress arising from the accumulation of reactive oxygen species (ROS) due to an impaired consumption between energy production (NADPH-H+ and ATP) in the light reaction and consumption in the Calvin cycle [39,41,42,43,44]. While inoculation with plant growth-promoting rhizobacteria (PGPR), either as single strains or SynComs, has shown promise in alleviating drought stress in various plant species, the reported effects on biomass accumulation, plant architecture, photosynthetic efficiency, and metabolic profiles are variable and often context-dependent (see Abou Jaoudé et al. [34] and references therein). Besides, plants, particularly medicinal species, exposed to abiotic stress can represent a promising source for discovering novel pharmaceuticals. This potential stems from significant qualitative and quantitative alterations in their secondary metabolite profiles [45]. In a meta-analysis aiming at evaluating the effect of drought stress on medicinal plants, Tan and Gören [46] showed that a moderate reduction in water availability can increase essential oil content in medicinal plants, without modifying essential oil yield. Therefore, the targeted application of drought has been proposed as a strategy to improve the quality of medicinal plants [41]. Investigating the impact of drought stress on medicinal plant physiology is, therefore, crucial for developing adaptive strategies that can mitigate the adverse effects of water scarcity, among which is the use of PGPR. This is essential to maintain the productivity of medicinal plant agriculture in the face of evolving climatic conditions.
In this study, we employed a multidisciplinary approach to evaluate the effects of a SynCom composed of five selected salt- and drought-tolerant Gram-negative bacterial strains positive for some PGP traits on Salvia officinalis L., a medicinal and aromatic shrub native to the Mediterranean region. While Salvia species generally exhibit some tolerance to water deficit, drought conditions can still negatively impact their physiological processes [42,47,48]. Therefore, the SynCom was inoculated in plants grown under optimal and sub-optimal irrigation. This study assessed the efficacy of the SynCom in promoting growth and drought tolerance in S. officinalis. We investigated the functional integration of the microbial consortium with the host plant by analyzing architectural, ecophysiological, and metabolic responses. Furthermore, leaf metabolomic analysis provided insights into the impact of drought stress on some nutritional aspects of this edible plant species.
2. Materials and Methods
2.1. Microbiome Extraction and Isolation of Strains with In Vitro Plant Growth-Promoting (PGP) Potential
Soil samples were collected in the saltern Margherita di Savoia Nature Reserve (41°22′33″ N, 16°05′28″ E; Apulia, Italy) during the summer. This area is characterized by high biodiversity and selective pressures such as constant salinity, high summer temperatures, and drought, typical of Mediterranean coastal ecosystems. Rhizospheric soil samples were extracted at a 0–10 cm depth at the base of the two herbaceous halophytes Halimione portulacoides L. and Limonium narbonense Mill (Figure 1a). For each species, three individual plants were selected. Soil samples from each species were pooled and sieved at 2 mm. A soil subsample (5 g) for each species was inserted in a tube with peptone water (peptone 1 g L−1, NaCl 0.5 g L−1) (1:5 w/v) in sterile conditions and shaken for 15 min at 150 rpm at room temperature. The suspension was serially diluted in peptone water. A total 100 µL of the dilution 10−3 was plated on a selective culture medium used to isolate and enumerate inorganic phosphate (P) solubilizers (Pikovskaya agar: yeast extract 0.5 g L−1, glucose 10 g L−1, (NH4)2SO4 0.5 g L−1, MgSO4·7H2O 0.1 g L−1, Ca3(PO4)2 5 g L−1, KCl 0.2 g L−1, MnSO4·2H2O 0.002 g L−1, FeS-O4·7H2O 0.02 g L−1, bromophenol blue 0.4% (w/v) stock solution in ethanol 10 mL l−1, agar 15 g L−1) (Figure 1b) [49]. Microbial phosphate solubilization is indicated by a halo zone formation around the colonies, signifying the breakdown of tricalcium phosphate (Ca3(PO4)2), the insoluble phosphate source present in the medium. The plates were incubated for 72 h at 30 °C. Ten and seven distinguishable morphotypes forming spatially separated colonies were identified among the culturable bacteria detected in H. portulacoides and L. narborense rhizosphere, respectively. The colonies belonging to each morphotype were sampled with a sterile pin and deposited on Luria Bertani Agar (LBA: tryptone 10 g L−1, yeast extract 5 g L−1, NaCl 5 g L−1, agar 15 g L−1) plates [50]. The plates were incubated at 30 °C for 24 h. To assess the purity of the isolated strains, each distinct morphotype was subcultured onto LBA plates using a streak plate technique. The plates were incubated at 30 °C for 24 h. Single colonies of each morphotype were then sampled with a sterile pin and deposited on Ashby Agar (mannitol 20 g L−1, K2HPO4 0.2 g L−1, NaCl 0.2 g L−1, MgSO4·7H2O 0.2 g L−1, K2SO4 0.1 g L−1, CaCO3 5 g L−1, agar 15 g L−1) (Figure 1b). Ashby is a specialized growth medium devoid of nitrogen (N), used for isolating and cultivating bacteria capable of utilizing atmospheric N as a source of this macro-element [51]. Ashby plates were incubated for 72 h at 30 °C. Moreover, the cells were used to inoculate 10 mL LB broth within 150 mL Erlenmeyer flask, incubated overnight at 30 °C with shaking at 180 rpm. Late exponentially growing cultures exhibiting an optical density at 600 nm (OD600) between 3 and 5 were utilized to inoculate 25 mL of LB broth supplemented with 4 mM tryptophan. The initial OD600 of each culture was standardized to 0.2. Cultures were grown in triplicate within 250 mL Erlenmeyer flasks maintained at 30 °C with agitation at 180 rpm. Following a 24 h incubation period, 10 mL aliquots were sampled from each culture for the quantification of indole auxins (Figure 1b). Quantification of indole auxins in spent culture media was performed using a colorimetric assay based on the Salkowski reaction, as described by Patten and Glick [52]. Briefly, cultures were subjected to centrifugation at 8000 rpm for 10 min using a Thermo Fisher Scientific multispeed centrifuge (Waltham, MA, USA) to separate the cell pellet from the supernatant. A 1 mL aliquot of the supernatant, after filter sterilization (0.22 μm pore size) and appropriate dilution, was reacted with 2 mL of Salkowski’s reagent (composed of 0.5 M FeCl3 and 35% v/v HClO4). Following a 20 min incubation period at ambient temperature, the absorbance of the colored solution was measured at 530 nm. Indole auxin concentrations were determined by comparison to a standard curve generated using known concentrations of pure indole-3-acetic acid (IAA; Sigma-Aldrich, Milan, Italy). Data were expressed as μg IAA equivalents (IAAequ) per mL of liquid culture.
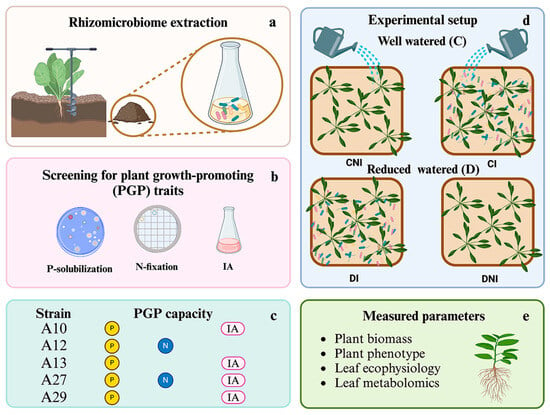
Figure 1.
Experimental setup. (a) Microbiome extraction from the rhizosphere of halophytes. (b) Bacterial isolation and testing for plant growth-promoting (PGP) traits. (c) Characterization of PGP activities of the selected strains. P: phosphate solubilization (Pikovskaya agar plates), N: nitrogen fixation (Ashby agar plates), IA: production of indole auxins (LB broth amended with 4 mM tryptophan). (d) Treatments applied to S. officinalis: well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) plants. (e) Parameters analyzed on S. officinalis plants.
Chemicals were of the highest purity commercially available and were purchased from Carlo Erba (Milan, Italy). Reagents for culture media were from Difco (Becton Dickinson, BD Italia, Milan, Italy), whereas salts for culture media and buffers were from Sigma-Aldrich (Milan, Italy).
Of the seventeen P-solubilizing bacteria, five strains (A10, A12, and A13 from H. portulacoides; A27 and A29 from L. narborense) were selected to create a SynCom; among these, four produced indole auxins and two were N-fixers (Figure 1c). Indole auxins’ production was, on average, equal to 7.6 µg of IAAequ mL−1 in A10 and A13, 6.7 µg of IAAequ mL−1 in A27, and 21.9 µg of IAAequ mL−1 in A29.
2.2. Molecular Identification of Culturable Bacteria with In Vitro PGP Capacity
The cells of the five isolates were pelleted by centrifugation of the LB liquid culture, and DNA was extracted using the DNeasy PowerSoil Pro kit (Qiagen, Hilden, Germany) based on manufacturer protocol. DNA purity and quantity were examined using Qubit fluorometers (Thermo Fischer, Waltham, MA, USA). The DNA was used as a template for amplification of the 16 S rRNA gene. Universal primer 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1389R (5′-ACGGGCGGTGTGTACAAG-3′) were used to generate amplicons (about 1400 bp) that were cloned into the pGEM-Teasy vector (Promega, Madison, WI, USA) and sequenced using BioFab commercial service (Rome, Italy). All 16S rRNA sequences from isolates with the same morphotype were identical, and only sequences that could be shown to be derived from independent templates were analyzed.
2.3. Nucleotide Sequence Accession Numbers
Sequences from independent templates/clones and amplicon libraries were deposited in GenBank under the following accession numbers: PV265484 (A10); PV265486 (A12); PV265487 (A13); PV265511 (A27); PV265518 (A29).
2.4. Plant Experimental Setup
Seeds of Salvia officinalis L. (096503BOLS, Semillas Batlle S.A., Molins de Rei, Barcelona, Spain) were surface sterilized by immersion for 10 min in sodium hypochlorite (NaClO) solution (50% v/v) amended with Twin 20 (0.025% v/v). Following sanitization, the seeds were rinsed 10 times with 2 mL of sterile deionized water. Sterilized seeds were sown on a sterile substrate composed of two parts peat and one part vermiculite, in a germination chamber maintained at 26 °C with a photon flux density of 200 µmol photons m−2s−1 for eleven days. Twenty seedlings of uniform size and number of leaves were then selected and divided into four groups composed of five plants (n = 5). Each group of five seedlings was transplanted into a 10 L growth pot containing 7.5 l of a sterile peat, perlite, and vermiculite substrate (2:1:1). The four pots were saturated with water three days before the plant transplanting. After transplanting, each seedling was irrigated with 125 mL of sterilized deionized water, to which was added 0.2% (v/v) 0.2 µm filter-sterilized nutrient solution A (B’cuzz, Atami B.V., Rosmalen, The Netherlands)—containing K2O 4.7%, CaO 3.8%, MgO 1.3%, SO3 0.11%, Fe 0.04%, and N 4.9% (calcium and ammonium nitrate salts), or 0.2% (v/v) filtered nutrient solution B (B’cuzz, Atami B.V., Rosmalen, The Netherlands)—containing P2O5 4.1%, K2O 5.71%, B 0.01%, Mn 0.03%, Mo 0.001%, and Zn 0.039%. The pots were placed in a grow tent (Mars Hydro EU, Ginsheim-Gustavsburg, Germany) equipped with a Mars Hydro Smart FC 3000 Samsung LED Grow Light powered by Samsung LM301B LED. Salvia officinalis plantlets were grown under common conditions used for the indoor sage cultivation [42,53,54]. The temperature was set to 26 °C, and the ventilation system (DF150A, Inline Duct Fan, Mars Hydro EU, Ginsheim-Gustavsburg, Germany) guaranteed an air exchange in the tent. The photon flux density was fixed to 400 μmol photon m−2 s−1 and the photoperiod to 16/8 h (light/dark). The air humidity was set at 60%. The pots were assigned to one of four treatments in a 2 × 2 factorial design. Two pots (optimal water regime, C) were irrigated once a week with a volume of nutrient solution sufficient to restore the relative water content (RWC) to above 95% (field capacity). To induce a mild water stress, the remaining two pots (reduced water regime, D) received a progressively decreasing volume of nutrient solution throughout the experiment (Figure 1d). The RWC changes in D treatment was monitored by weekly weighting the pots. The RWC in the D treatment decreased from 95% to 55% by the end of the experiment. The two pots belonging to each water treatment were differently subjected to inoculation: C and D non-inoculated plants (CNI and DNI, respectively) were not treated with the SynCom (see Section 2.5), while control- and drought-inoculated plants (CI and DI, respectively) were inoculated with the consortium of isolated bacteria (Figure 1d).
2.5. Inoculum Preparation
For inoculum preparation, 15 mL of pre-cultures of the five selected strains were grown in LB broth, supplemented with 4 mM tryptophan. Following an 18 h incubation period at 30 °C, the pre-cultures were combined and appropriately diluted to achieve an optical density (OD) of 1.2, corresponding to a cell concentration of 8 × 108 colony forming units (CFUs) mL−1. OD was measured at 600 nm using a spectrophotometer (Agilent Cary 100; Agilent Technologies Inc., Santa Clara, CA, USA); CFUs were determined on LB agar after incubation at 30 °C for 24 h, utilizing the serial dilution and spread plate methods. The diluted microbial culture mixture (cells and exometabolites) was used to inoculate plants in CI and DI. The first inoculation was performed on DAT0 (day after treatment 0). After two weeks, a second inoculum was prepared using the same method, and inoculation of the same plants and treatments was performed on DAT15. In both instances, inoculated plants received 18 mL of the diluted microbial mixture per plant (108 CFU 100 mL−1 of peat), while control and non-inoculated drought plants received 18 mL of sterile water. The inoculum was applied to the soil around the base of the plant stem in the vicinity of the root system.
2.6. Physiological Measurements
Leaf ecophysiological measurements (stomatal conductance, gs; quantum yield of photosystem II, ΦPSII; electron transport rate, ETR) were taken at DAT7, 14, 21, and 28 on the first fully expanded leaf from the apical bud of each plant for each treatment using an LI-600 porometer (Li-Cor, Lincoln, OR, USA) with a flow rate of 150 μmol s−1.
On DAT28, the canopy reflectance was measured in the 350–2500 nm range and collected at 1.0 nm intervals using a spectroradiometer (ASD FieldSpec®3, Analytical Spectral Devices, Boulder, CO, USA). Hyperspectral reflectance data were acquired from two discrete leaf cohorts per plant within each treatment. Ten spectral measurements were obtained from a recently expanded leaf located in the upper canopy, reflecting post-treatment physiological adaptations. Simultaneously, ten spectral measurements were acquired from a mature leaf in the lower canopy, serving as a baseline proxy for pre-treatment physiological status. Reflectance spectral signatures were derived by averaging data replicates and, subsequently, several vegetation indices were calculated to assess the physiological state of plants under experimental drought and inoculation conditions. These indices, based on reflectance at specific wavelengths, provide insights into various aspects of plant physiology. Specifically, indices related to plant water status included the Photochemical Reflectance Index (PRI), sensitive to changes in plant water status [55]; the Moisture Stress Index (MSI), related to plant water stress based on leaf water potential [56]; and the Water Index, sensitive to changes in leaf water content [57]. Additionally, the Normalized Water Index, a normalized version of the WI, was calculated to minimize the influence of factors other than water content, such as leaf structure and background reflectance, as it is significantly associated with water relations parameters and soil water potential [58]. The ratio of WI/NWI was also determined to further evaluate water stress. Indices related to chlorophyll content included the Green Coverage Index (GCI), used to estimate leaf chlorophyll content in various plant species [59], and the Green Chlorophyll Index (CI green), which—along with the Normalized Difference Infrared Index (NDII)—provides metrics indicative of changes in plant canopy chlorophyll and water content, respectively. The Optimized Soil-Adjusted Vegetation Index (OSAVI) was calculated to assess vegetation vigor [60]. Finally, the Canopy Response Salinity Index (CRSI), developed by Scudiero et al. [61], was included to assess potential correlations with soil salinity.
2.7. Plant Biomass and Phenotype
On DAT28, plants were harvested and separated into leaf, (not lignified) branch, and root fractions. The fresh weight of each fraction was recorded (RFW: root fresh weight; BFW: branch fresh weight; LFW: leaf fresh weight). Total fresh weight (TFW) was calculated as the sum of RFW, BFW, and LFW. The leaves were arranged on a flatbed scanner for digital image acquisition. ImageJ 1.53t software (Wayne Rasband and contributors, National Institutes of Health, Bethesda, MD, USA) was employed to analyze the images, determining total leaf area (TLA) and number of leaves (LN) per plant. Average leaf surface area was then calculated (ALA = TLA/LN). To determine dry weights and the leaf water content (LWC), all roots and a leaf subsample per plant were subjected to oven drying at 105 °C until constant weight, using a Sartorius MA 100 moisture analyzer (Göttingen, Germany) operating in standard mode. Total root dry weight (RDW) was determined by weighting roots after desiccation. Total leaf dry weight (LDW) was estimated by establishing the relationship between fresh and dry weight for a subsample of leaves from each plant belonging to the four treatments (LDWCNI = LFWCNI × 0.115 + 0.0209, R2 = 0.8679; LDWCI = LFWCI × 0.1273 − 0.0074, R2 = 0.9960; LDWDNI = LFWDNI × 0.14690.0053; R2 = 0.9815; LDWDI = LFWDI × 0.1705 − 0.0228; R2 = 0.9674). The same relations were used to estimate the branch dry weight (BDW). Total plant dry weight (TDW) was calculated as the sum of RDW and aboveground biomass (AGB = BDW + LDW). Biomass allocation was assessed by calculating the root-to-shoot ratio (R/S) as the ratio of estimated RDW to AGB. Average leaf biomass (ALB) was then calculated as (LDW/LN). Leaf mass per area (LMA) was determined as the ratio of leaf dry weight to total leaf area.
2.8. Leaf Untargeted Metabolomics
Leaf samples from the elemental analysis cohort were pooled, weighed, and metabolites for the LC-MS analysis were extracted by sonication using a modified protocol by Bansal et al. [62]. In short, 1 g of dried leaf powder was transferred to a 15 mL tube, and 9 mL ethanol:water (1:1, v/v) was added to the sample. After sonication for 30 min, the samples were centrifuged (12,000 rpm for 10 min). The supernatants were collected, filtered (0.22 µm), and stored for further analysis. The LC-MS analysis was performed by using an ACQUITY I-Class PLUS UPLC System (Waters, Milford, MA, USA) coupled to an ACQUITY RDa mass spectrometer (Waters, Milford, MA, USA) equipped with an ESI probe in positive and negative ion modes to perform reversed-phase chromatography. All samples (2 µL) were analyzed in positive and negative ion modes in triplicate injections. An Acquity Premier HSS T3 column with VanGuard FIT (2.1 mm × 150 mm, 1.8 μm, 1/pK—Waters; Milford, MA, USA) was used for metabolite separation. LC separations were achieved at a column temperature of 40 °C and a flow rate of 0.25 mL/min. The temperature of the autosampler was 6 °C. A 0–100% linear gradient of solvent A (ddH2O, 0.1% formic acid) to B (acetonitrile, 0.1% formic acid) was employed and set as follows: 100% A and 0% B from zero to 6 min, 95–5% A–B from 6 to 9 min, 60–40% A–B from 9 to 14 min, 5–95% A–B from 14 to 17.10 min, 100–0% A–B from 17.10 to 19 min. The ACQUITY RDa mass spectrometer was used in a full scan with fragmentation mode to detect small-mass molecules (50–800 m/z). The scan rate was 5 Hz in positive and negative polarity, and the cone voltage was 20 and 40 V, respectively, in positive and negative polarity. The fragmentation voltage was 60 to 80 V and 40 to 40 V, respectively, in positive and negative polarity. The absorbance read was made at a wavelength of 254 nm. The capillary voltage was set at 1.0 kV in positive and 0.8 kV in negative polarity, and a default capillary desolvation temperature of 550 °C was used.
The software UNIFI Scientific Information System (vers. 3.3.0, Waters Corp., Milford, MA, USA) analyzed the data, which were processed for peak picking, alignment, and normalization. Compound identification was performed using the UNIFI library searching algorithm. The resulting data matrices, including the component name, the expected retention time, the observed retention time, the observed m/z, the mass error, and the detector count were then exported for further statistical analysis. Data were first corrected by removing the molecules identified in the blank. Moreover, data were filtered according to the difference between observed and expected retention times, and all molecules showing a difference higher than 5 and lower than -5 min were discarded. Moreover, the analysis did not consider the metabolite when the mass error was greater than 5 mDa.
2.9. Statistical Analysis
To test the effect of the SynCom inoculation and drought treatment on biomass production, plant phenotype, and ecophysiological responses, a t-test was performed on the website www.socscistatistics.com (accessed on 22 January 2025). Statistically significant differences were reported for p ≤ 0.05.
For leaf metabolites, peak intensity values were normalized to account for variations in total molecule abundance between treatments. Data were normalized by dividing each molecule’s abundance by the total abundance of all molecules in that treatment. Data were analyzed by using the software Metaboanalyst 6.0, available at www.metaboanalyst.ca/ (accessed on 22 January 2025). Peak intensity values were filtered by the relative standard deviation to increase the power of data. An abundance filter was applied based on median intensity value. Data were then normalized by a median, transformed using a square root transformation, and auto-scaled. Principal component and hierarchical cluster analysis were produced by using the same software.
3. Results
3.1. Molecular Characterization of Culturable Bacteria
The five isolates composing the SynCom were characterized to the genus/species level using 16S rRNA gene sequencing (see Section 2). The strains A10 and A27 were taxonomically identified as Pseudomonas sp. A10 had more than 97% 16S rRNA gene sequence similarity with Pseudomonas koreensis species, while strain A27 had 96% 16S rRNA gene sequence similarity with Pseudomonas putida species (Table 1). The strain A12 was taxonomically identified as Achromobacter sp., with more than 99% 16S rRNA gene sequence similarity with Achromobacter spanius species (Table 1). The strain A13 was identified as belonging to the genus Pantoea, with 97% 16S rRNA gene sequence similarity with Pantoea agglomerans (Table 1). The strain A29 was identified as belonging to the genus Duffyella, with more than 99% 16S rRNA gene sequence similarity with Duffyella gerundensis (Table 1).

Table 1.
Molecular identification of the PGPR isolates using 16s rRNA gene sequencing and sequences analysis.
3.2. Effect of Drought and SynCom Inoculation on Biomass Production and Plant Structure
Sage biomass production was not affected by the water regime or by inoculation with the SynCom.
In non-inoculated plants (CNI and DNI), the average total plant biomass at DAT28 was 1.15 ± 0.1 g and 1.51 ± 0.4 g in well-irrigated and low-irrigated conditions, respectively (Figure 2). No significant differences were observed in aboveground (1.01 ± 0.2 g) or belowground (0.32 ± 0.13 g) biomass (Figure 2).
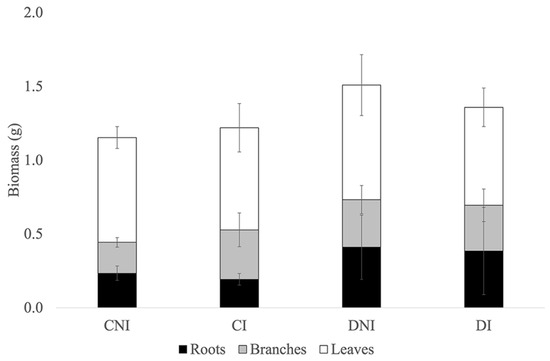
Figure 2.
Effect of drought and SynCom inoculation on sage biomass. The histogram illustrates the root (black), branch (gray), and leaf (white) dry weight repartition in well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) plants at DAT28. Data represent means ± standard errors (n = 5).
The reduced water availability significantly altered the phenotype of the sage plants. Under low water supply (DNI), plants exhibited a higher branch-to-total aboveground biomass ratio (BDW/AGB, 0.29 ± 0.01 g/g) and a lower leaf-to-total aboveground biomass ratio (LDW/AGB, 0.71 ± 0.03 g/g) compared to well-irrigated plants (CNI, 0.23 ± 0.03 g/g and 0.77 ± 0.03 g/g, respectively; p < 0.05; Table 2). This shift in biomass allocation was further reflected in a significantly higher root-to-shoot ratio (R/S, 0.40 ± 0.04 g/g) in plants grown under reduced irrigation compared to their well-irrigated counterparts (0.24 ± 0.03 g/g; p < 0.001; Table 2). While total (TLA) and average leaf area (ALA), as well as the number of leaves (LN), remained unaffected by water availability, the average leaf biomass (ALB) and leaf mass per area (LMA) were significantly higher in low-irrigated plants (0.020 ± 0.003 g/g and 47.3 ± 4.3 g/m2, respectively) compared to the control (0.015 ± 0.002 g/g, p < 0.05 and 38.6 ± 2.5, p < 0.05, respectively; Table 2). In contrast, plants grown under reduced water supply exhibited significantly lower leaf water content (84.8 ± 0.6%) compared to well-watered plants (87.3 ± 0.6%; p < 0.001; Table 2).

Table 2.
Effect of drought and SynCom inoculation on S. officinalis phenotype. Branch (BDW) and leaf biomass (LDW) to aboveground biomass (AGB = BDW + LDW), belowground-to-aboveground biomass ratio (root-to-shoot ratio, R/S), total leaf area per plant (TLA), number of leaves per plant (NL), average leaf area per plant (ALA), average leaf biomass per plant (ALB), leaf mass per area (LMA), and leaf water content (LWC) measured in well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) sages at DAT28.
Under non-limiting water conditions, inoculation with the SynCom did not significantly modify total plant biomass (CI, 1.22 ± 0.3 g), aboveground biomass (1.03 ± 0.3 g), or below-ground biomass (0.19 ± 0.039 g) compared to non-inoculated plants (CNI, Figure 2). However, plant phenotype changed in inoculated plants, although these changes were less pronounced than those observed in plants subjected to reduced water supply. Inoculated sage plants exhibited a significant shift in aboveground biomass allocation, with a higher proportion of biomass allocated to branches (0.30 ± 0.03 g/g; p < 0.05) and a lower proportion to leaves (0.70 ± 0.03 g/g; p < 0.05) compared to the aboveground biomass allocation in non-inoculated plants (Table 2).
Inoculation modulated the biomass allocation and leaf traits in plants subjected to reduced irrigation (DI). Under low water supply, inoculation significantly increased the ratio between below- and aboveground biomass (+83%; p < 0.001; Table 2) and decreased the branch-to-total aboveground biomass ratio (0.28 ± 0.04 g/g) compared to well-watered plants (CI, 0.30 ± 0.03; p < 0.01; Table 2). Conversely, inoculation under reduced water supply increased the average leaf biomass (0.028 ± 0.010 g; p < 0.05) and the leaf mass per area (51.1 ± 9.3 g/m2; p < 0.05) and decreased the leaf water content (85 ± 0.8%; p < 0.01) compared to well-watered controls (0.019 ± 0.004 g, 38.9 ± 2.6 g/m2, 87.8 ± 0.3%, respectively; Table 2).
Despite the observed alterations in plant and leaf structure induced by water deficit conditions, stomatal conductance (gs), quantum yield of photosystem II (ΦPSII), and electron transport rate (ETR) remained consistent across all measurement dates in water-stressed sages (DNI) compared to well-watered controls (CNI, Table 3).

Table 3.
Effect of drought and SynCom inoculation on S. officinalis ecophysiology. Stomatal conductance, the quantum yield of photosystem II, and electron transport rate measured in well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) sages at DAT7, DAT14, DAT21, and DAT28.
Inoculation with the SynCom significantly impacted stomatal conductance and photosynthetic activity in sage plants under non-water limiting conditions (CI). Under non-water limiting conditions, inoculated plants exhibited a significant reduction in stomatal conductance at DAT7 (0.38 ± 0.06 mol H2O m−2 s−1; p < 0.05) and DAT14 (0.42 ± 0.02 mol H2O m−2 s−1; p < 0.05) compared to non-inoculated controls (CNI, 0.51 ± 0.02 mol H2O m−2 s−1 and 0.60 ± 0.06 mol H2O m−2 s−1, respectively; Table 3). This effect was transient, however, as no differences were observed at DAT21 and DAT28 (Table 3). Inoculation also led to a significant decrease (p < 0.05) in the quantum yield of photosystem II at DAT7 and DAT14 (0.71 ± 0.01 and 0.69 ± 0.02, respectively) compared to non-inoculated plants (0.74 ± 0.01 and 0.73 ± 0.01, respectively); this effect was not observed at the later time points (Table 3). While no significant changes in electron transport rate were detected at DAT7, DAT14, and DAT21, a pronounced reduction was observed in inoculated plants at DAT28 (74.2 ± 6.4 µmol photon m−2 s−1) compared to non-inoculated plants (166.3 ± 13.3 µmol photon m−2 s−1; p < 0.01; Table 3).
The analysis of the ecophysiological parameters revealed that SynCom inoculation influenced the stomatal conductance in sage plants under water-limited conditions (DI). Specifically, inoculation significantly increased stomatal conductance at DAT14 (0.68 ± 0.08 mol H2O m−2 s−1; p < 0.01), while it decreased at DAT28 (0.21 ± 0.05 mol H2O m−2 s−1; p < 0.01) compared to well-watered plants (CI, 0.42 ± 0.02 and 0.51 ± 0.05 mol H2O m−2 s−1, respectively; Table 3). Inoculation under reduced water supply increased the quantum yield of photosystem II at DAT7 (0.74 ± 0.01; p < 0.05), DAT14 (0.73 ± 0.01; p < 0.05), DAT28 (0.74 ± 0.01; p < 0.05) compared to well-watered plants (0.71 ± 0.01, 0.69 ± 0.02, and 0.70 ± 0.01, respectively; Table 3). The electron transport rate measured in inoculated plants grown under reduced water supply only differed from well-watered plants at DAT7, being higher under low irrigation (+19.3%, p < 0.05; Table 3).
While individual vegetation indices did not exhibit significant variations across treatments, the Principal Component Analysis (PCA) effectively elucidated the complex interplay between physiological indices, growth parameters, and drought-related responses. The PCA revealed that the first two principal components (PCs) accounted for a substantial 69.6% of the total variance, with PC1 explaining 38.6% and PC2 explaining 31.0%, demonstrating their efficacy in capturing the primary patterns within the dataset. Notably, the PCA biplot (Figure 3) clearly differentiated treatments based on water availability and inoculation status. Specifically, PC1 primarily distinguished plants subjected to water stress from those under well-irrigated conditions. This suggests that PC1 effectively represents the gradient of water availability, with higher values correlating with water-stressed conditions. Conversely, PC2 primarily separated inoculated plants from non-inoculated plants within their respective water availability groups, indicating that PC2 effectively captured the influence of inoculation. PC1 was positively associated with growth-related parameters such as total plant biomass, above- and belowground biomass, branch biomass, and drought indicators like the Moisture Stress Index (MSI) and the Canopy Response Salinity Index (CRSI). PC2, on the other hand, was positively associated with chlorophyll-related indices (GCI, CI green, and PRI-y) and stress markers (MSI).
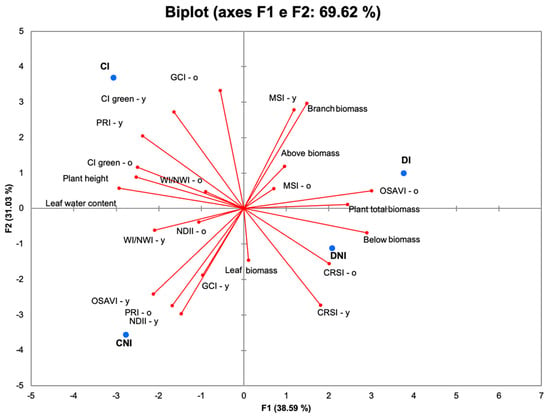
Figure 3.
Principal Component Analysis based on plant architecture and leaf reflectance parameters measures in mature (-o) and in young (-y), well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) S. officinalis at DAT28.
Reduced water availability was positively associated with branch and root biomass, and with the Moisture Stress Index (MSI) and Canopy Response Salinity Index (CRSI).
3.3. Effect of Drought and SynCom Inoculation on the Leaf Metabolome
A comprehensive untargeted metabolomic analysis of the leaf metabolome of S. officinalis cultivated under optimal and water deficit conditions was conducted to evaluate the effect of the SynCom inoculation. This analysis revealed 153 metabolites whose abundance varied among inoculated and non-inoculated plants under both water conditions. Among the 153 metabolites, 78 were characterized by a detector count (DC) > 1000. A comparative analysis of leaf molecules with DC > 1000 was conducted using Principal Component Analysis (PCA). The study revealed that the first two principal components (PC) accounted for 73% of the total variance, with PC1 and PC2 contributing 42.3% and 30.7%, respectively (Figure S1). The PCA demonstrated that the leaf metabolomic profile of plants grown under well-watered conditions separates from plants grown under water deficit along the PC1 coordinate (Figure S1). Differently from plants grown under water deficit, under non-limiting water conditions, the PCA demonstrated a clear separation along PC2 between inoculated and non-inoculated plants (Figure S1).
Among the 78 metabolites characterized by a DC > 1000, 57 were common to all treatments (Figure 4). Metabolic profiling of leaf tissue across the four treatment groups revealed distinct alterations in metabolite abundance. Sages grown under the different treatments were distinctly enriched in exclusive molecules, except for non-inoculated plants grown under reduced irrigation, which did not show the presence of any exclusive leaf molecule (Figure 4). The reduced-water irrigation treatment changed the leaf metabolomic profile. N-acetylglucosamine (NAG) was only found under reduced water availability, independently of inoculation (Figure 4). In non-inoculated plants, two molecules (anserine and leucic acid) were exclusively found in well-irrigated plants (Figure 4). Moreover, pantothenic acid (Vit. B5) was only found in well-watered sage, independently of inoculation (Figure 4). Inoculation increased the number of exclusive leaf metabolites under both optimal (citicoline, flavin mononucleotide, glycyl-proline, nicotinamide) and reduced (AABA, carnosine, hydroxylysine, methionine) irrigation (Figure 4). Finally, the leaves of inoculated plants showed the presence of alanyl-glutamine and quinic acid, independently of the irrigation treatment (Figure 4).
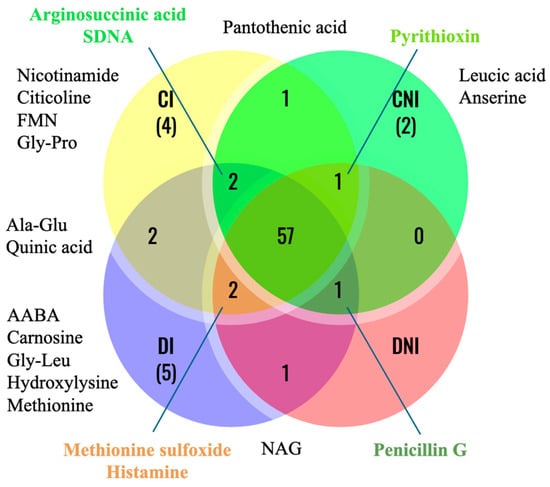
Figure 4.
Venn diagram of leaf metabolites. The diagram shows the number of metabolites with a detector count > 1000 found in the leaves of well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) S. officinalis at DAT28. Unique metabolites in each treatment are reported in black. Metabolites shared in three out of four treatments are reported in colors.
Specific metabolites were significantly depleted in response to the treatment conditions. Methionine sulfoxide and histamine were found in the leaves of plants subjected to all treatments except in non-inoculated plants grown under optimal irrigation (Figure 4). Arginosuccinic acid and SDMA were not detected in the leaf metabolome of non-inoculated plants subjected to reduced irrigation. Moreover, inoculation in well-irrigated plants depleted the leaf metabolome in penicillin G, while under reduced water availability, inoculation depleted the leaf metabolome in pyrithioxin (Figure 4).
In addition to the exclusive molecules, reduced irrigation induced a significant increase in the relative abundance of 3-phenyllactic acid and lactose compared to well-irrigated sages (Figure 5a and Figure S2). Differently, reduced irrigation significantly decreased the relative abundance of several amino acids and derivatives (arginine, asparagine, homoarginine, citrulline) citric, and glucuronic acids (Figure 5a and Figure S2). Under well-watered conditions, inoculation with the SynCom increased the relative abundance of α-ketoglutaric acid, 3-phenyllactic acid, and lactose and reduced the abundance of glucuronic acid, guanine, and nicotinamide riboside compared to non-inoculated plants (Figure 5b and Figure S2). Under reduced water availability, inoculation increased the relative abundance of glucuronic acid, guanine, guanosine, and methionine sulfoxide compared to non-inoculated plants (Figure 5c and Figure S2). On the contrary, the SynCom reduced the relative abundance of lactose.

Figure 5.
Impact of reduced water availability and SynCom inoculation on the leaf metabolomic profile (detector count > 1000) of S. officinalis. The plots show the fold change variation in the abundance of leaf metabolites of (a) non-inoculated plants grown under reduced irrigation (DNI) vs. well-watered sages (CNI), (b) well-watered and inoculated plants (CI) vs. non-inoculated sages (CNI), and (c) plants grown under reduced water supply, inoculated (DI) vs. non-inoculated (DNI). Statistical significance was measured using an unpaired analysis, in which FCs are calculated as the ratios between two group means. In the plot, the y-axis displays the logarithm (base 2) of the fold change (Log2 Fold Change). The red and blue dots indicate the significantly (p < 0.05) increased and decreased metabolites, respectively. The gray dots indicate metabolites showing non-statistically significant differences between the conditions.
Most of these molecules (guanine, histamine, glycyl-proline, nicotinamide, citicoline, and flavin mononucleotide) were also among the ten most important features determining significant separation among treatments, together with uridine, leucine, cysteine, and aspartic acid (Figure S1).
4. Discussion
Salvia officinalis is a characteristic species of the Mediterranean basin, a region known for its semi-arid soils, decreasing water availability, and periods of extremely high air temperatures and irradiance [63,64]. Although Salvia species generally tolerate water scarcity, severe drought can negatively impact their growth [42,47,48]. This study aimed to evaluate the response of sage to reduced water supply, the inoculation of a synthetic community (SynCom) composed of five Gram-negative, phosphate-solubilizing bacteria capable of producing auxin, fixing nitrogen, or both, and the effect of the SynCom on promoting growth under mild water stress conditions.
4.1. Sage Response to Reduced Water Supply
In the experimental setup utilized in this study, we did not observe a decrease in plant biomass (Figure 2); however, we did find a reduction in leaf water content in plants subjected to limited water supply (Table 2). Leaf water content represents the balance between the water transported to the leaves and the transpiration rate, reflecting the water retention capacity of plants under adverse conditions. As Ullah et al. [65] reported, this parameter can be used as a useful index to identify the water status of crops. A decrease in leaf water content under a reduced water supply usually causes a concomitant increase in leaf temperature, which provokes stomatal closure and a reduction in the CO2 assimilation rate, negatively affecting the photosynthetic process [66,67]. This is in accordance with studies reporting reductions in stomatal conductance, net photosynthesis, and leaf area in S. officinalis under drought stress [68]. However, the mild water stress applied in our study did not affect photosynthetic parameters under reduced water availability (Table 3). Our data indicate that sage undergoes phenotypic modifications to tolerate mild reductions in water availability. Plant architecture adapts to drought conditions by modifying biomass allocation, particularly the root-to-shoot ratio (R/S, +64.1% under reduced water availability) and crown composition, which includes branch and leaf biomass (T/AGB, +26.5% under reduced water availability; L/AGB, −7.7% under reduced water availability; see Table 2).
The increased biomass allocation to the roots is a common response to water shortage. This finding aligns with the research conducted by Abate et al. [69], which emphasized the importance of root hydraulics in drought resistance within Salvia species. Moreover, the authors proposed that increased root biomass allocation enhances the accumulation of reserves, which is essential for post-drought recovery. A decreased proportion (−29.5%) of leaf-to-branch biomass characterized aboveground sage architecture under reduced water availability compared to well-watered plants (Table 2). The biomass distribution between leaves and branches reflects the biochemical and hydraulic architecture characteristics of leaves and plant configuration in specific environments [70]. Indeed, de Andrés et al. [71], investigating the spatiotemporal variation of functional traits along water availability gradients in evergreen and deciduous Mediterranean oaks, reported a positive association between the Standardized Precipitation Evapotranspiration Index and the branch-to-leaf mass ratio. This association suggests enhanced drought resistance and resilience. Savi et al. [63] observed that Salvia species exhibit reduced leaf hydraulic conductance at water potential thresholds typically associated with mesophytic plants, implying a conservative water-use strategy in line with our observations. Moreover, leaf traits also changed in response to the reduced water supply, with the average leaf biomass and leaf mass per area higher than untreated sage (Table 2). The increase in leaf mass per area is considered a potential adaptation to stressful environments, such as those characteristics of Mediterranean climates, and is associated with increased leaf thickness and density [72,73]. Minimizing the transpiring surface area, or the ratio of transpiring surface area to biomass, such as through decreased leaf size or number, or increased leaf thickening or shedding, can further enhance water conservation and optimize water use.
4.2. Sage Response to SynCom Inoculation Under Non-Limiting Water Supply
Under non-limiting water supply conditions, inoculation with the SynCom comprising bacterial strains with phosphate solubilization and auxin production capacities did not induce significant variations in the biomass produced by S. officinalis, at the epigeal nor the hypogeal level (Figure 2). This result indicates that, apart from PGP traits, no plant growth promotion occurs without a synergistic plant–PGPR interaction, or that this interaction can affect the plant in different ways and should be analyzed by integrating multiple approaches [34]. Similar results were reported by Nunes Tiepo et al. [74], who did not detect variations in the dry biomass of roots and stems in Trema micrantha and Cariniana estrellensis under optimal water supply conditions and inoculated with Azospirillum brasilense Ab-V5, Bacillus sp., Azomonas sp., and Azorhizophillus sp. Nevertheless, our study clearly distinguished between inoculated and non-inoculated plants when analyzing eco-physiological parameters (Figure 3) and leaf metabolome profiles (Figure 4 and Figure 5). The clustering of CI and CNI along the negative axis of PC1, with their separation along PC2, indicates that inoculation influenced plant physiology under well-irrigated conditions, potentially enhancing growth and chlorophyll-related indices (GCI, CI green, PRI; Figure 3). Thus, assessing plant growth promotion solely based on increases in biomass is insufficient for capturing the complexity of plant–microbe interactions. Interestingly, inoculation also resulted in a reduction in the leaf-to-branch biomass ratio (−39.4%; Table 2), coinciding with a transient decrease in stomatal conductance at DAT7 and DAT14 (Table 3), which was not observed in non-inoculated plants under reduced water availability. These findings suggest a temporary microbial influence on plant water relations, acting on Salvia similarly to drought. In agreement with these findings, the plants subjected to reduced water supply and those inoculated with the SynCom and grown under non-limiting water supply shared specific metabolites (histamine, methionine sulfoxide, lactose, α-ketoglutaric acid, anserine, isoleucine, leucic acid, 3-phenyllactic acid, and glucuronic acid), significantly varying in their relative abundance (Figure 5a,b). Histamine was only detected in inoculated plants under well-watered conditions while its abundance increased in plants grown under reduced water conditions compared to well-watered sages (fold change = 5.2). α-ketoglutaric acid abundance increased (fold change = 3.2 and 6.3) and anserine decreased (fold change = 0.21 and 0.24) in non-inoculated sages subjected to water limitation and in inoculated sage grown under well-watered conditions, respectively (Figure 5a,b). Histidine serves as a precursor in the synthesis of α-ketoglutaric acid, a metabolite capable of mitigating oxidative stress by eliminating reactive oxygen species [75], which, under stress, accumulates early compared to the other TCA cycle metabolites [76]. Histidine is also a common biosynthetic precursor to histamine and anserine. The decarboxylation of histidine forms histamine in a reaction catalyzed by the enzyme histidine decarboxylase [77]. In plants, histamine modulates stomatal aperture, stimulates cytokinin synthesis, and enhances water and oxygen uptake, promoting growth and development [78]. Anserine, a histidine-containing dipeptide, also functions as a multi-faceted stress protectant, bolstering plant resilience against drought, salinity, and cold stress [78,79]. The elevated levels of histidine and other metabolites associated with histidine metabolism, including histamine, carnosine, and aspartate, observed in SynCom-inoculated plants and those subjected to mild drought conditions, suggest that biogenic amines may function as neurotransmitters in S. officinalis. This finding provides the first evidence, obtained from parallel experiments, that histamine is involved in the cellular perception of drought stress and the interaction between plants and microorganisms in sage, as well as in other species [80,81].
Analysis of the relative abundance of leaf metabolites in inoculated and non-inoculated plants also revealed a significant increase in the abundance of methionine sulfoxide and lactose in response to water deficit and SynCom inoculation. Methionine sulfoxide is recognized as a redox marker, reflecting the oxidative status of the cell [82]. Methionine, cysteine, tyrosine, and tryptophan represent a sensitive target of reactive oxygen species (ROS) under stress conditions, undergoing oxidation [82,83]. This oxidation results in the formation of methionine sulfoxide, concomitant with a reduction in methionine’s biological activity [84]. Wang et al. [85] observed elevated methionine sulfoxide levels in Medicago sativa under drought stress, potentially attributable to a disruption in the methionine-to-spermine conversion pathway. Similarly to our findings, the relative abundance of lactose, in contrast to other sugars and polyalcohols that typically accumulate under stress, decreased in response to salinity and drought in Cynodon dactylon [86]. This reduction in the relative abundance of lactose, particularly under water-limiting conditions, has been associated with heightened stress levels, potentially linked to increased ROS production. Safronov et al. [87] found an increase in the relative abundance of lactose and glucuronic acid in the leaves of the drought-tolerant species Phoenix dactylifera (date palm) subjected to drought stress, suggesting the activation of cell wall-related metabolic pathways, which could indirectly influence membrane composition by affecting the availability of precursors for membrane lipid or protein synthesis, with the synthesis and remodeling of the cell walls being membrane-related processes [88]. Moreover, in this study, we observed a decrease in the relative abundance of the branched-chain amino acids leucine and isoleucine, commonly found in membrane-spanning protein domains [89], in drought and SynCom inoculation treatments. In Arabidopsis seedlings, the accumulation of leucic acid has been associated with reduced root growth [90]. Thus, while SynCom inoculation under well-watered conditions did not significantly alter S. officinalis biomass, it triggered notable physiological and metabolic shifts, including transient reductions in stomatal conductance and leaf-to-branch ratio, alongside changes in key metabolite levels indicative of a drought-like response and the activation of pathways related to cell wall maintenance, redox homeostasis, and energy production, ultimately suggesting a complex interplay between the inoculated microbiome and plant stress responses, even in the absence of water deficit.
SynCom inoculation under non-limiting water irrigation also produced exclusive molecules. Nicotinamide, present only in inoculated plants, is a component of the coenzymes NADH and NADPH involved in many enzymatic cell redox reactions. The main source of nicotinamide in plant cells seems to be NAD, from which nicotinamide can be released because of NAD glycohydrolase [91] or poly-(ADP-ribose) polymerase (PADPRP) activity. Nicotinamide induces a pronounced and lasting increase in reduced and oxidized glutathione; improves the accumulation of secondary metabolites, such as anthocyanins and alkaloids; and induces a lasting increase in the activity of the key enzyme of the phenylpropanoid pathway, phenylalanine amide-lyase (PAL) [92]. Interestingly, this molecule was not detected in non-inoculated plants, and concomitantly, a significant decrease in the content of nicotinamide riboside (a metabolite related to nicotinamide, both forms of vitamin B3 and involved in the synthesis of NADH and NADPH coenzymes) was observed in inoculated leaf extracts compared to non-inoculated ones. At the metabolic level, nicotinamide riboside is a precursor of nicotinamide; thus, an increase in nicotinamide and a decrease in nicotinamide riboside may indicate increased activity of enzymes that convert nicotinamide riboside to nicotinamide. This suggests that the plant has likely increased its ability to produce energy for growth, development, or stress response. Among the mechanisms capable of favoring a more remarkable synthesis of nicotinamide and nicotinamide riboside precursors is the release of phytohormones, such as auxins, which the inoculated strains have been shown to produce. In this context, it is also possible to explain the significant increase in the relative abundance of flavin mononucleotide in inoculated plants compared to non-inoculated plants. Flavin mononucleotide is an active form of vitamin B2 (riboflavin) and acts as an essential cofactor in numerous cellular processes, playing a crucial role in the electron transport chain. Consequently, an increase in this molecule suggests a greater capacity of the plant to produce energy. The nucleotide citicoline is one of the exclusive molecules whose production was induced by PGPR inoculation and is an intermediate in lipid synthesis produced from choline phosphate in glycerophospholipid metabolism. Citicoline can be converted into betaine, providing the cell with osmotic protection [93]. Citicoline upregulation has been found in halophilic microalgae under salinity stress [94] and was also found to increase in sweet corn inoculated with the biocontrol endophytes Bacillus subtilis R31, suggesting a crucial role in stress adaptation induced by inoculation.
In contrast to all other treatments, the leaves of inoculated plants grown under non-limiting water availability exhibited a significant reduction in the amount of penicillin G (Figure 5, panel b). Studies utilizing the trunk injection method have demonstrated that this antibiotic is transported to the leaves through systemic translocation via the plant’s vascular system [95]. Several factors may contribute to the low levels of penicillin G observed in the leaves of inoculated plants. Under conditions that promote the growth of filamentous fungi capable of producing penicillin G (i.e., well-watered plants), the SynCom can (1) antagonize fungal growth, leading to a decrease in the production of this metabolite; (2) produce enzymes that catalyze the degradation of penicillin G, thereby reducing its concentration in the soil; and (3) inhibit or diminish the uptake and transport of penicillin G by the plant. Conversely, in conditions where fungal growth is restricted (e.g., low water irrigation), this effect is not evident due to the already low levels of penicillin G (Figure 5, panel c).
After stomatal closure, the reduction in intercellular CO2 concentration can limit the efficiency of biochemical processes within the leaf [96]. In our study, a compensation for the reduction in stomatal conductance in inoculated plants can be hypothesized, which can be explained by higher carboxylation rates or a reduction in losses due to exudation or respiration, considering that plant biomass was not negatively affected by the treatment. Vitale et al. [97] observed in spinach plants (Spinacia oleracea L.) that, regardless of the light quality used, treatment with PGPB induced a significantly higher RuBisCO concentration compared to uninoculated plants, producing a compensation in the reduction of the observed stomatal conductance. Despite a significant decrease in the quantum yield of photosystem II under water stress (Table 3), the observed values remained within the normal range (0.70 to 0.80) reported for plants grown under optimal conditions, suggesting that the photosynthetic apparatus was not compromised.
4.3. Sage Response to SynCom Inoculation Under Limiting Water Supply
Similarly to the observations under optimal water supply, inoculation did not affect sage biomass production in plants subjected to water restriction (Figure 2). Nevertheless, microbial inoculation modulated biomass allocation and leaf morphology in response to water availability. Under reduced water availability, inoculation significantly increased root biomass allocation (+83%; Table 2). Moreover, inoculated plants under reduced water supply exhibited a 47.5% increase in average leaf biomass and a 31.2% increase in leaf mass per area compared to their well-watered counterparts (Table 2). These effects were more pronounced than those observed in non-inoculated plants under drought stress, where increases in average leaf biomass and leaf mass per area were 34.5% and 22.7%, respectively (Table 2). Strains from the same species occurring in the microbial SynCom utilized in this study were inoculated into plants grown under water or osmotic stress conditions. In soybean plants subjected to salt stress, P. koreensis MU2 significantly reduced sodium ion influx, lipid peroxidation, and proline accumulation [98]. These findings were associated with elevated expression levels of salt-tolerance genes, which likely mitigate osmotic stress by activating antioxidant biosynthetic pathways and promoting osmolyte production. Similarly, inoculation with P. koreensis S4T10 enhanced drought tolerance in A. thaliana by modulating the ABA pathway [99]. Moreover, inoculation with Achromobacter sp. FB-14, which demonstrated in vitro potential for ACCD activity, synthesis of indole compounds, and phosphate solubilization at NaCl concentrations of up to 100 mM in the culture medium, was reported to alleviate salt stress in rice, enhancing plant growth and upregulating the expression of stress-responsive genes at NaCl concentrations of up to 50 mM [100]. The expression of genes associated with stress response, reactive oxygen species detoxification, ethylene biosynthesis, ad salicylic acid, and jasmonate signaling was found in two chickpea cultivars subjected to drought stress in the presence of the PGPR Pseudomonas putida MTCC527 [101].
Unlike inoculated plants grown under optimal soil moisture conditions, stomatal conductance did not exhibit significant variations following inoculation under water restriction compared to non-inoculated plants, with the sole exception of DAT28, in which stomatal conductance was reduced by half (0.21 mol H2O m−2 s−1 in DI; Table 3). A lower net reduction in stomatal conductance was also observed in non-inoculated plants subjected to limited water availability (0.49 mol H2O m−2 s−1 in DNI) and inoculated plants under non-limiting water conditions (0.51 mol H2O m−2 s−1 in CI; -18.7%), both compared to non-inoculated plants (0.63 mol H2O m−2 s−1 in CNI; Table 3). Sage is classified as an anisohydric species, which means it maintains open stomata and high photosynthetic rates for extended periods. While this strategy can be beneficial under sufficient water availability and moderate stress conditions, it may jeopardize plant survival during severe drought conditions [102]. The reduction in stomatal conductance induced by the microorganisms, which was not accompanied by worsening electron transport rate or quantum yield of photosystem II, likely indicates a microbial-induced mechanism of water conservation rather than a direct negative impact on photosynthetic machinery. These results suggest that the observed reduction in stomatal conductance in inoculated sage plants under water restriction, potentially mediated by similar mechanisms of stress modulation as seen in other plant–microbe interactions, represents a beneficial microbial-induced water conservation strategy rather than a detrimental effect on photosynthetic processes.
Modifications in leaf metabolomics were observed in plants grown under reduced water availability and inoculated with the SynCom compared to non-inoculated sage (Figure 5c). The peptide class varied significantly in response to SynCom inoculation, producing an accumulation of dipeptides at the leaf level. Proteinogenic dipeptides are primarily products of protein degradation, and dipeptide levels respond to changes in the environment, often in a dipeptide-specific manner. What determines this specificity is currently unknown; what probably contributes is the activity of the various peptidases that detach the terminal dipeptide from longer peptides [103]. A decrease in the foliar content of dipeptides was observed in a non-drought-tolerant maize cultivar subjected to water stress; conversely, the concentration of dipeptides increased in the tolerant cultivar [104]. In a study that evaluated the effect of vegetable protein hydrolysates on tomato plants subjected to water stress, Leporino et al. [105] reported greater stress tolerance determined by the accumulation of dipeptides at the leaf level. The authors suggested that the mitigation of water stress exerted by dipeptides containing glucogenic amino acids was linked to their use as a carbon source for plant growth under limiting conditions for photosynthesis. These molecules can represent a resource for the generation of carbon skeletons, useful in the production of pyruvate (a major product of protein degradation) and, subsequently, of glucose through gluconeogenesis when the plant’s carbon resources are scarce [106]. The plant can absorb or release these molecules through the root systems. Among the dipeptides detected at the leaf level, alanyl-glutamine is noteworthy, a clinically and nutritionally significant molecule [107]. Moreover, while in well-watered inoculated plants, the concentration of methionine sulfoxide alone was observed compared to non-inoculated plants, and the concentration of both compounds increased under water deficit conditions. An increase in methionine levels following PGPR inoculation is associated with greater tolerance to water stress in chickpea plants, potentially contributing to the ROS scavenging mechanism and, thus, maintaining the functionality of the photosynthetic apparatus [83]. Foliar application of methionine has been reported to improve the antioxidant activity of superoxide dismutase, catalase, and peroxidase under drought stress and improve chlorophyll content and stomatal conductance [108].
Furthermore, foliar application of methionine has been reported to increase plant growth. It reduced oxidative stress in Vigna unguiculata grown under water deficit conditions [109], demonstrating the positive role played by the amino acid on plant stress tolerance. In addition, the phenolic quinic acid showed notable increases in the inoculated plants, particularly under reduced water availability. Quinic acid is an antioxidant, and its concentration has been observed to increase after inoculation with PGPR in Salicornia europaea and Brassica rapa, enhancing the plant’s tolerance to abiotic and biotic stress [110]. Moreover, the increase in the histidine-derived dipeptide carnosine observed in this study has been reported as being an important antioxidant substance that can inhibit the production of ROS by scavenging free radicals [79].
Together with the observed changes in the profile of leaf metabolites, the clustering of DNI and DI along the positive axis of PC1, coupled with their separation along PC2, suggests that inoculation, while not completely mitigating the impact of drought, induced distinct physiological changes in stressed plants (Figure 3). The accumulation of specific metabolic markers suggest that SynCom inoculation may enhance drought tolerance in sage by modulating key metabolic pathways related to stress response, carbon metabolism, and antioxidant defense, warranting further investigation into the underlying molecular mechanisms.
5. Conclusions
This study reveals the remarkable plasticity of S. officinalis in response to water limitation, encompassing adaptive shifts in biomass allocation, leaf morphology, and metabolic profiles. While mild water stress did not impair photosynthetic performance, it induced a conservative water-use strategy. Interestingly, inoculation with the microbial SynCom under well-watered conditions elicited physiological and metabolic responses mirroring those observed under drought stress, including a reduced leaf-to-branch biomass ratio; transient decreases in stomatal conductance; and altered abundances of specific metabolites such as histamine, α-ketoglutaric acid, and anserine. These shared responses suggest that inoculation triggers a drought-like acclimation, potentially involving stomatal regulation and mitigation of oxidative stress. The metabolites exhibiting similar trends under both drought and inoculation represent potential markers for drought-related responses in sage. Furthermore, the SynCom enhanced drought tolerance under water-limiting conditions, likely by modulating key metabolic pathways associated with stress response, carbon metabolism, and antioxidant defense. These findings underscore the intricate interplay between plant-associated microorganisms and plant stress responses, highlighting the potential of microbial inoculants to improve drought resilience in S. officinalis. Future research should prioritize elucidating the specific molecular mechanisms underlying these beneficial interactions, including identifying the microbial factors responsible for triggering drought-like responses, investigating the role of shared metabolites in mediating drought tolerance, exploring the impact of the SynCom on root architecture and hydraulic conductivity, and assessing the long-term effects of inoculation under field conditions. Elucidating the interplay between plant–microbe interactions, drought stress responses, and the production of bioactive compounds is crucial for developing targeted microbial inoculants that enhance drought resilience in S. officinalis without compromising its safety for human consumption.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11040374/s1, Figure S1: Principal component analysis (PCA) plot based on the average Euclidean distances of the leaf metabolomic dataset and the ten most important features determining significant differences among the treatments. The two major principal components explain 73.1% of the total variance. The different colors indicate the groups: well-irrigated (C) and reduced water-supplied (D), inoculated (I), and non-inoculated (NI) S. officinalis at DAT28.; Figure S2: Heatmaps and dendrograms showing clustering of analytes found in S. officinalis leaves grown under well-irrigated (C) and reduced water-supplied (D) conditions, inoculated (I) and non-inoculated (NI) on DAT28.
Author Contributions
Conceptualization, R.A.J. and M.R.; methodology, R.A.J., E.B., R.B. and M.R.; validation, R.A.J., A.G.F. and M.R.; formal analysis, R.A.J. and M.R.; investigation, R.A.J., A.B., I.B., F.L., A.G.F. and M.R.; resources, M.R.; data curation, R.A.J., F.L., E.B., A.G.F. and M.R.; writing—original draft preparation, R.A.J.; writing—review and editing, R.A.J., R.B., E.B., F.L., A.G.F. and M.R.; visualization, R.A.J.; supervision, M.R.; project administration, M.R.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022).
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge Waters (Waters, Milford, MA, USA) for their invaluable contribution in providing the ACQUITY I-Class PLUS UPLC System coupled to the ACQUITY RDa mass spectrometer used in this research.
Conflicts of Interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest. The funders had no role in the design of the study, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Song, C.; Jin, K.; Raaijmakers, J.M. Designing a home for beneficial plant microbiomes. Curr. Opin. Plant Biol. 2021, 62, 102025. [Google Scholar]
- Berruto, C.A.; Demirer, G.S. Engineering agricultural soil microbiomes and predicting plant phenotypes. Trends Microbiol. 2024, 29, 858–873. [Google Scholar]
- Khan, S.T. Consortia-based microbial inoculants for sustaining agricultural activities. Appl. Soil Ecol. 2022, 176, 104503. [Google Scholar]
- Pérez-Izquierdo, L.; Zabal-Aguirre, M.; González-Martínez, S.C.; Buée, M.; Verdú, M.; Rincón, A.; Goberna, M. Plant intraspecific variation modulates nutrient cycling through its below ground rhizospheric microbiome. J. Ecol. 2019, 107, 1594–1605. [Google Scholar]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [PubMed]
- Fürnkranz, M.; Wanek, W.; Richter, A.; Abell, G.; Rasche, F.; Sessitsch, A. Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J. 2008, 2, 561–570. [Google Scholar]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar]
- Lorenzi, A.S.; Bonatelli, M.L.; Chia, M.A.; Peressim, L.; Quecine, M.C. Opposite sides of Pantoea agglomerans and its associated commercial outlook. Microorganisms 2022, 10, 2072. [Google Scholar] [CrossRef]
- Raymond, N.S.; Gómez-Muñoz, B.; van der Bom, F.J.T.; Nybroe, O.; Jensen, L.S.; Müller-Stöver, D.S.; Oberson, A.; Richardson, A.E. Phosphate-solubilising microorganisms for improved crop productivity: A critical assessment. New Phytol. 2021, 229, 1268–1277. [Google Scholar]
- Kumawat, K.C.; Sharma, P.; Sirari, A.; Singh, I.; Gill, B.S.; Singh, U.; Saharan, K. Synergism of Pseudomonas aeruginosa (LSE-2) nodule endophyte with Bradyrhizobium sp. (LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World J. Microbiol. Biotechnol. 2021, 35, 47. [Google Scholar]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR mediated alterations in root traits: Way toward sustainable crop production. Front. Sutain. Food Syst. 2021, 4, 18230. [Google Scholar]
- Mantelin, S.; Desbrosses, G.; Larcher, M.; Tranbarger, T.J.; Cleyet-Marel, J.C.; Touraine, B. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta 2006, 223, 591–603. [Google Scholar] [PubMed]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Bellini, C.; Kowalczyk, M.; Touraine, B. The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 2010, 232, 1455–1470. [Google Scholar] [PubMed]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial- mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar]
- Wang, B.; Mei, C.; Seiler, J.R. Early growth promotion and leaf level physiology changes in Burkholderia phytofirmans strain PsJN inoculated switchgrass. Plant Physiol. Biochem. 2015, 86, 16–23. [Google Scholar]
- Bisht, S.; Singh, S.; Singh, M.; Sharma, J.G. Augmentative role of Piriformospora indica fungus and plant growth promoting bacteria in mitigating salinity stress in Trigonella foenum-graecum. J. Appl. Biol. Biotechnol. 2022, 10, 85–94. [Google Scholar]
- Khalid, A.; Arshad, M.; Zahir, Z. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol. 2004, 96, 473–480. [Google Scholar]
- Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene, ACC, and the plant growth-promoting enzyme ACC deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Singh, U.B.; Saghir Khan, M.; Singh, P.; Kumar, R.; Narian Singh, R. Bacterial ACC deaminase: Insights into enzymology, biochemistry, genetics, and potential role in amelioration of environmental stress in crop plants. Front. Microbiol. 2023, 14, 1132770. [Google Scholar]
- Teo, H.M.; Aziz, A.; Wahizatul, A.A.; Bhubalan, K.; Siti, N.M.S.; Muhamad, S.C.I.; Lee Chuen, N. Setting a plausible route for saline soil-based crop cultivations by application of beneficial halophyte-associated bacteria: A review. Microorganisms 2022, 10, 657. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Latif Khan, A.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Barquero, M.; Poveda, J.; Laureano-Marıń, A.M.; Ortiz-Liébana, N.; Brañas, J.; González-Andrés, F. Mechanisms involved in drought stress tolerance triggered by rhizobia strains in wheat. Front. Plant Sci. 2022, 13, 1036973. [Google Scholar]
- Marín, O.; Gonzalez, B.; Poupin, M.J. From microbial dynamics to functionality in the rhizosphere: A systematic review of the opportunities with synthetic microbial communities. Front. Plant Sci. 2021, 12, 650609. [Google Scholar] [CrossRef]
- Vanegas, J.; Uribe-Vélez, D. Selection of mixed inoculants exhibiting growth-promoting activity in rice plants from undefined consortia obtained by continuous enrichment. Plant Soil 2014, 375, 215–227. [Google Scholar] [CrossRef]
- Azizi, S.; Tabari, M.; Abad, A.R.F.N.; Ammer, C.; Guidi, L.; Bader, M.K.-F. Soil inoculation with beneficial microbes buffers negative drought effects on biomass, nutrients, and water relations of common myrtle. Front. Plant Sci. 2022, 13, 892826. [Google Scholar]
- Mehnaz, S.; Baig, D.N.; Lazarovits, G. Genetic and phenotypic diversity of plant growth promoting rhizobacteria isolated from sugarcane plants growing in Pakistan. J. Microbiol. Biotechnol. 2010, 20, 1614–1623. [Google Scholar] [PubMed]
- Compant, S.; Hanna Faist, A.S.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar]
- Abou Jaoudé, R.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. A plant’s perception of growth-promoting bacteria and their metabolites. Front. Plant Sci. 2024, 14, 1332864. [Google Scholar]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar]
- Chaparro-Rodríguez, M.; Estrada-Bonilla, G.; Rosas-Pérez, J.; Gómez-Álvarez, M.; Cruz-Barrera, M. Hydrogel capsules as new approach for increasing drying survival of plant biostimulant gram-negative consortium. Appl. Microbiol. Biotech. 2023, 107, 6671–6682. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [PubMed]
- Kumar, M.; Kumar Patel, M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Tan, U.; Gören, H.K. Comprehensive evaluation of drought stress on medicinal plants: A meta-analysis. PeerJ 2024, 12, e17801. [Google Scholar]
- Khodadadi, F.; Ahmadi, F.S.; Talebi, M.; Matkowski, A.; Szumny, A.; Afshari, M.; Rahimmalek, M. Metabolic and transcriptomic approaches of chitosan and water stress on polyphenolic and terpenoid components and gene expression in Salvia abrotanoides (Karl.) and S. yangii. Int. J. Mol. Sci. 2023, 24, 15426. [Google Scholar] [CrossRef]
- Li, J.; Mei, X.; Zhang, J.; Song, Z.; Wang, S.; Chen, W.; Wang, J. Effects of potassium application on growth and root metabolism of Salvia miltiorrhiza under drought stress. Agronomy 2023, 13, 2796. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiol. 1948, 17, 362–370. [Google Scholar]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Ashby, S.F. Some observations on the assimilation of atmospheric nitrogen by a free-living soil organism. Azotobacter chroococcum of Beijerinck. J. Agric. Sci. 1907, 2, 35–51. [Google Scholar]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, Y.-H.; Han, F.-X.; Chen, X.; Wu, F.-S.; Liu, Q.; Ma, W.-Z.; Zhang, Y.-Q. Transcriptome sequencing and metabolome analysis reveal the molecular mechanism of Salvia miltiorrhiza in response to drought stress. BMC Plant Biol. 2024, 24, 446. [Google Scholar] [CrossRef]
- Mohammadi-Cheraghabadi, M.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; Rashidi-Monfared, S.; Mokhtassi-Bidgoli, A. Improving water deficit tolerance of Salvia officinalis L. using putrescine. Sci. Rep. 2021, 11, 21997. [Google Scholar]
- Yang, X.; Liu, S.; Liu, Y.; Ren, X.; Su, H. Assessing shaded-leaf effects on photochemical reflectance index (PRI) for water stress detection in winter wheat. Biogeoscience 2019, 16, 2937–2947. [Google Scholar] [CrossRef]
- Cohen, W. Response of vegetation indices to changes in three measures of leaf water stress. Eng. Remote Sens. 1991, 57, 195–202. [Google Scholar]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Gutierrez, M.; Reynolds, M.P.; Klatt, A.R. Association of water spectral indices with plant and soil water relations in contrasting wheat genotypes. J. Exp. Bot. 2010, 61, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Scudiero, E.; Skaggs, T.H.; Corwin, D.L. Regional scale soil salinity evaluation using Landsat 7, western San Joaquin Valley, California, USA. Geoderma Reg. 2014, 2, 82–90. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Syeed, R.; Mohsin, M.; Nafees, A.; Dewir, Y.H.; Mendler- Drienyovszki, N. Integrated GC-MS and UPLC-ESI-QTOF-MS based untargeted metabolomics analysis of in vitro raised tissues of Digitalis purpurea L. Front. Plant Sci. 2024, 15, 1433634. [Google Scholar]
- Savi, T.; Marin, M.; Luglio, J.; Petruzzellis, F.; Mayr, S.; Nardini, A. Leaf hydraulic vulnerability protects stem functionality under drought stress in Salvia officinalis. Funct. Plant Biol. 2016, 43, 370–379. [Google Scholar] [PubMed]
- Armada, E.; Roldán, A.; Azcon, R. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb. Ecol. 2013, 67, 410–420. [Google Scholar]
- Ullah, S.; Skidmore, A.K.; Groen, T.A.; Schlerf, M. Evaluation of three proposed indices for the retrieval of leaf water content from the mid-wave infrared (2–6 μm) spectra. Agric. For. Meteorol. 2013, 171, 65–71. [Google Scholar]
- Arad, S.M.; Mizrahi, Y.; Richmond, A.E. Leaf water content and hormone effects on ribonuclease activity. Plant Physiol. 1973, 52, 510–512. [Google Scholar]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hort. Forest. Biotech. 2011, 15, 173–177. [Google Scholar]
- Grisafi, F.; Oddo, E.; Maggio, A.; Panarisi, A.; Panarisi, M. Morpho-physiologic traits in two sage taxa grown under different irrigation regime. Chem. Eng. Trans. 2017, 58, 697–702. [Google Scholar]
- Abate, E.; Azzarà, M.; Trifilò, P. When water availability is low, two mediterranean Salvia species rely on root hydraulics. Plants 2021, 10, 1888. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Fan, R.; Zhong, Q.; Cheng, D. Scaling relationships of twig biomass allocation in Pinus hwangshanensis along an altitudinal gradient. PLoS ONE 2017, 12, e0178344. [Google Scholar]
- de Andrés, E.G.; Serra-Maluquer, X.; Gazol, A.; Olano, J.M.; García-Plazaola, J.I.; Fernández-Marín, B.; Camarero, J.J. Constrained trait variation by water availability modulates radial growth in evergreen and deciduous Mediterranean oaks. Agric. For. Meteorol. 2024, 346, 109884. [Google Scholar]
- Niinemets, Ü. Research review. Components of leaf dry mass per area–thickness and density–alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47. [Google Scholar]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar]
- Nunes Tiepo, A.; Fernandes Hertel, M.; Santos Rocha, S.; Kikuchi Calzavara, A.; Luiz, A.; De Oliveira, M.; Pimenta, J.A.; Oliveira, H.C.; Bianchini, E.; Stolf-Moreira, R. Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol. Biochem. 2018, 130, 277–288. [Google Scholar]
- Song, F.; Zhang, G.; Li, H.; Ma, L.; Yang, N. Comparative transcriptomic analysis of Stenotrophomonas sp. MNB17 revealed mechanisms of manganese tolerance at different concentrations and the role of histidine biosynthesis in manganese removal. Ecotoxicol. Environ. Saf. 2022, 244, 114056. [Google Scholar] [PubMed]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar]
- Darvas, Z.; Falus, A. Histidine decarboxylase (HDC) enzyme and gene. In Histamine: Biology and Medical Aspects; SpringMed: Budapest, Hungary, 2021; pp. 31–42. [Google Scholar]
- Shi, Y.; Jin, Z.; Wang, J.; Zhou, G.; Wang, F.; Peng, Y. 5-Aminolevulinic acid (5-ala)-induced drought resistance in maize seedling root at physiological and transcriptomic levels. Int. J. Mol. Sci. 2024, 25, 12963. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Li, W.H.; Zhang, J.; Li, H.E.; Chen, C.S.; Liu, X.Q. Progress in investigations of histidine dipeptide and its functions. Food Res. Devel. 2022, 43, 213–218. [Google Scholar]
- Roshchina, V.V. Biogenic amines in plant cell at norma and stress: Probes for dopamine and histamine. In Emerging Plant Growth Regulators in Agriculture; Aftab, T., Naeem, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 357–376. [Google Scholar]
- Akula, R.; Mukherjee, S. New insights on neurotransmitters signaling mechanisms in plants. Plant Sign. Behav. 2020, 15, 1737450. [Google Scholar]
- Akter, S.; Khan, M.S.; Smith, E.N.; Flashman, E. Measuring ROS and redox markers in plant cells. RSC Chem. Biol. 2021, 2, 1384–1401. [Google Scholar]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar]
- Ali, Q.; Athar, H.U.; Haider, M.Z.; Shahid, S.; Aslam, N.; Shehzad, F.; Naseem, J.; Ashraf, R.; Ali, A.; Hussain, S.M. Role of amino acids in improving abiotic stress tolerance to plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–204. [Google Scholar]
- Wang, W.; Kang, W.; Shi, S.; Liu, L. Physiological and metabolomic analyses reveal the mechanism by which exogenous spermine improves drought resistance in alfalfa leaves (Medicago sativa L.). Front. Plant Sci. 2024, 15, 1466493. [Google Scholar]
- Ye, T.; Shi, H.; Wang, Y.; Yang, F.; Chan, Z. Contrasting proteomic and metabolomic responses of bermudagrass to drought and salt stresses. Front. Plant Sci. 2024, 7, 1694. [Google Scholar]
- Safronov, O.; Kreuzwieser, J.; Haberer, G.; Alyousif, M.S.; Schulze, W.; Al-Harbi, N.; Arab, L.; Ache, P.; Stempfl, T.; Kruse, J.; et al. Detecting early signs of heat and drought stress in Phoenix dactylifera (date palm). PLoS ONE 2017, 12, e0177883. [Google Scholar]
- Liu, Z.; Persson, S.; Sánchez-Rodríguez, C. At the border: The plasma membrane–cell wall continuum. J. Exp. Bot. 2015, 66, 1553–1563. [Google Scholar] [PubMed]
- Binder, S. Branched-chain amino acid metabolism in Arabidopsis thaliana. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0137. [Google Scholar]
- Bauer, S.; Mekonnen, D.W.; Geist, B.; Lange, B.; Ghirardo, A.; Zhang, W.; Schäffner, A.R. The isoleucic acid triad: Distinct impacts on plant defense, root growth, and formation of reactive oxygen species. J. Exp. Bot. 2020, 71, 4258–4270. [Google Scholar]
- Taguchi, H.; Nishitani, H.; Okumura, K.; Shimabayashi, Y.; Iwai, K. Biosynthesis and metabolism of trigonelline in Lemna paucicostata 151. Agric. Biol. Chem. 1989, 5, 2867–2871. [Google Scholar]
- Berglund, T.; Ohlsson, A.B.; Rydstrom, J. Nicotinamide increases glutathione and anthocyanin in tissue culture of Catharanthus roseus. J. Plant Physiol. 1993, 141, 596–600. [Google Scholar]
- Lin, Y.; Wang, W.; Li, J.; Ai, Y.; Wang, H.; Han, Y.; Wang, W.; Hou, W. Unlocking the antibacterial activity and mechanisms of ε-poly-l-Lysine and perilla combination against Pseudomonas fluorescens: Insights from non-targeted metabolomic analyses. LWT 2024, 208, 116572. [Google Scholar]
- Jiang, J.; Zhu, S.; Zhang, Y.; Sun, X.; Hu, X.; Huang, H.; Ren, L. Integration of lipidomic and transcriptomic profiles reveals novel genes and regulatory mechanisms of Schizochytrium sp. in response to salt stress. Biores. Tech. 2019, 294, 122231. [Google Scholar]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.; Jones, D.; Kolawole, O.O.; van Bruggen, A.H. Effects and side effects of penicillin injection in huanglongbing affected grapefruit trees. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Francesca, S.; Lorenz, C.; Arena, C. Plant-Growth promoting microbes change the photosynthetic response to light quality in spinach. Plants 2023, 12, 1149. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Lee, K.-E.; Kang, S.-M.; Dhungana, S.K.; Bhusal, N.; Lee, I.-J. The Halotolerant Rhizobacterium—Pseudomonas koreensis MU2 enhances inorganic silicon and phosphorus use efficiency and augments salt stress tolerance in soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef]
- Kalleku, J.N.; Ihsan, S.; Al-Azzawi, T.N.I.; Khan, M.; Hussain, A.; Chebitok, F.; Das, A.K.; Moon, Y.-S.; Mun, B.-G.; Lee, I.-J.; et al. Halotolerant Pseudomonas koreensis S4T10 mitigate salt and drought stress in Arabidopsis thaliana. Physiol. Plant. 2024, 176, e14258. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shah, A.A.; Basit, F.; Noman, M.; Zubair, M.; Ahmed, T.; Naqqash, T.; Manzoor, I.; Maqsood, A. Achromobacter sp. FB-14 harboring ACC deaminase activity augmented rice growth by upregulating the expression of stress-responsive CIPK genes under salinity stress. Braz. J. Microbiol. 2020, 51, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Sign. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Minen, R.I.; Thirumalaikumar, V.P.; Skirycz, A. Proteinogenic dipeptides, an emerging class of small-molecule regulators. Curr. Opin. Plant Biol. 2023, 75, 102395. [Google Scholar] [CrossRef]
- Yang, L.; Fountain, J.C.; Ji, P.; Ni, X.; Chen, S.; Lee, R.D.; Kemerait, R.C.; Guo, B. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotech. J. 2018, 16, 1616–1628. [Google Scholar] [CrossRef]
- Leporino, M.; Rouphael, Y.; Bonini, P.; Colla, G.; Cardarelli, M. Protein hydrolysates enhance recovery from drought stress in tomato plants: Phenomic and metabolomic insights. Front. Plant Sci. 2024, 15, 1357316. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, P.J.; Astley, H.M.; Parsley, K.; Aubry, S.; Williams, B.P.; Menard, G.N.; Craddock, C.P.; Nunes-Nesi, A.; Fernie, A.R.; Hibberd, J.M. Arabidopsis uses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nat. Comm. 2015, 6, 6659. [Google Scholar]
- Su, X.; Li, Y.; Zhang, Y.; Han, S. Efficacy of alanyl glutamine in nutritional support therapy for patients with sepsis: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e24861. [Google Scholar] [PubMed]
- Maqsood, M.F.; Shahbaz, M.; Kanwal, S.; Kaleem, M.; Shah, S.M.R.; Luqman, M.; Iftikhar, I.; Zulfiqar, U.; Tariq, A.; Naveed, S.A.; et al. Methionine promotes the growth and yield of wheat under water deficit conditions by regulating the antioxidant enzymes, reactive oxygen species, and ions. Life 2022, 12, 969. [Google Scholar] [CrossRef]
- Merwad, A.R.M.; Desoky, E.S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hort. 2018, 228, 132–144. [Google Scholar]
- Kim, T.J.; Hwang, Y.J.; Park, Y.J.; Lee, J.S.; Kim, J.K.; Lee, M.H. Metabolomics Reveals Lysinibacillus capsici TT41-induced metabolic shifts enhancing drought stress tolerance in kimchi cabbage (Brassica rapa L. subsp. pekinensis). Metabolites 2024, 14, 87. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).