Biometric and Biochemical Responses to Salt in Solanum dasyphyllum, a Potential Donor of Tolerance for Eggplant
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Procedures
2.3. Soil Electrical Conductivity
2.4. Biochemical Assays
2.4.1. Ion Content
2.4.2. Photosynthetic Pigments
2.4.3. Osmolyte Contents
2.4.4. Oxidative Stress Markers
2.4.5. Antioxidant Compounds
2.4.6. Antioxidant Enzyme Activities
2.5. Statistical Analysis
3. Results
3.1. Growth Measurements
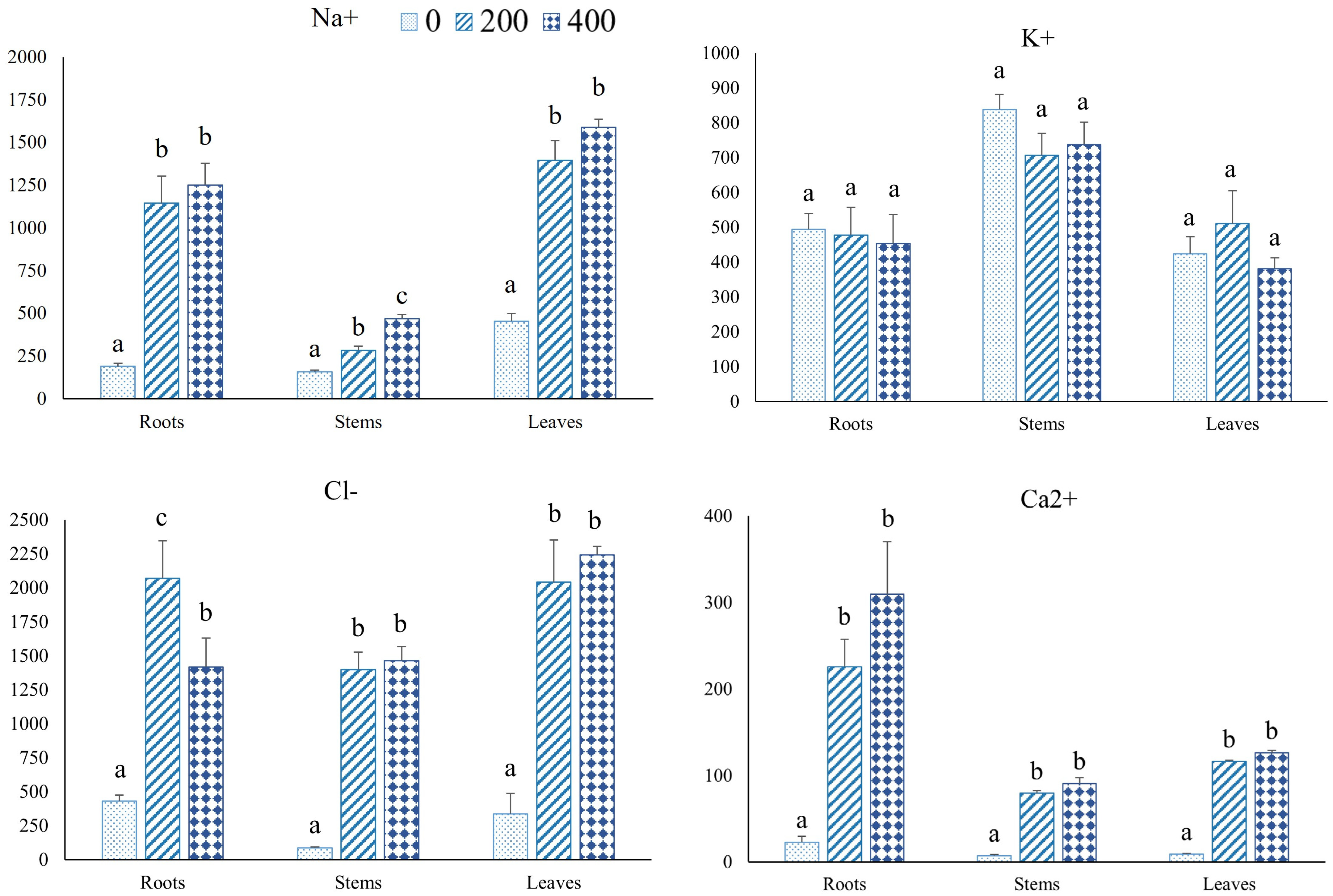
3.2. Ion Contents
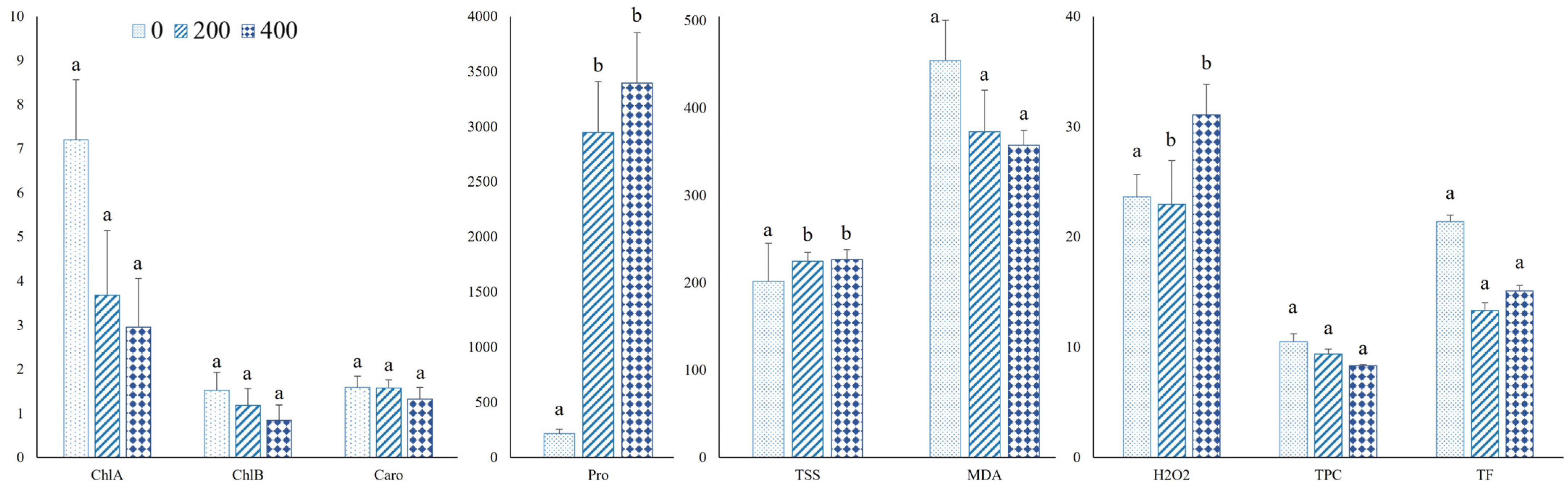
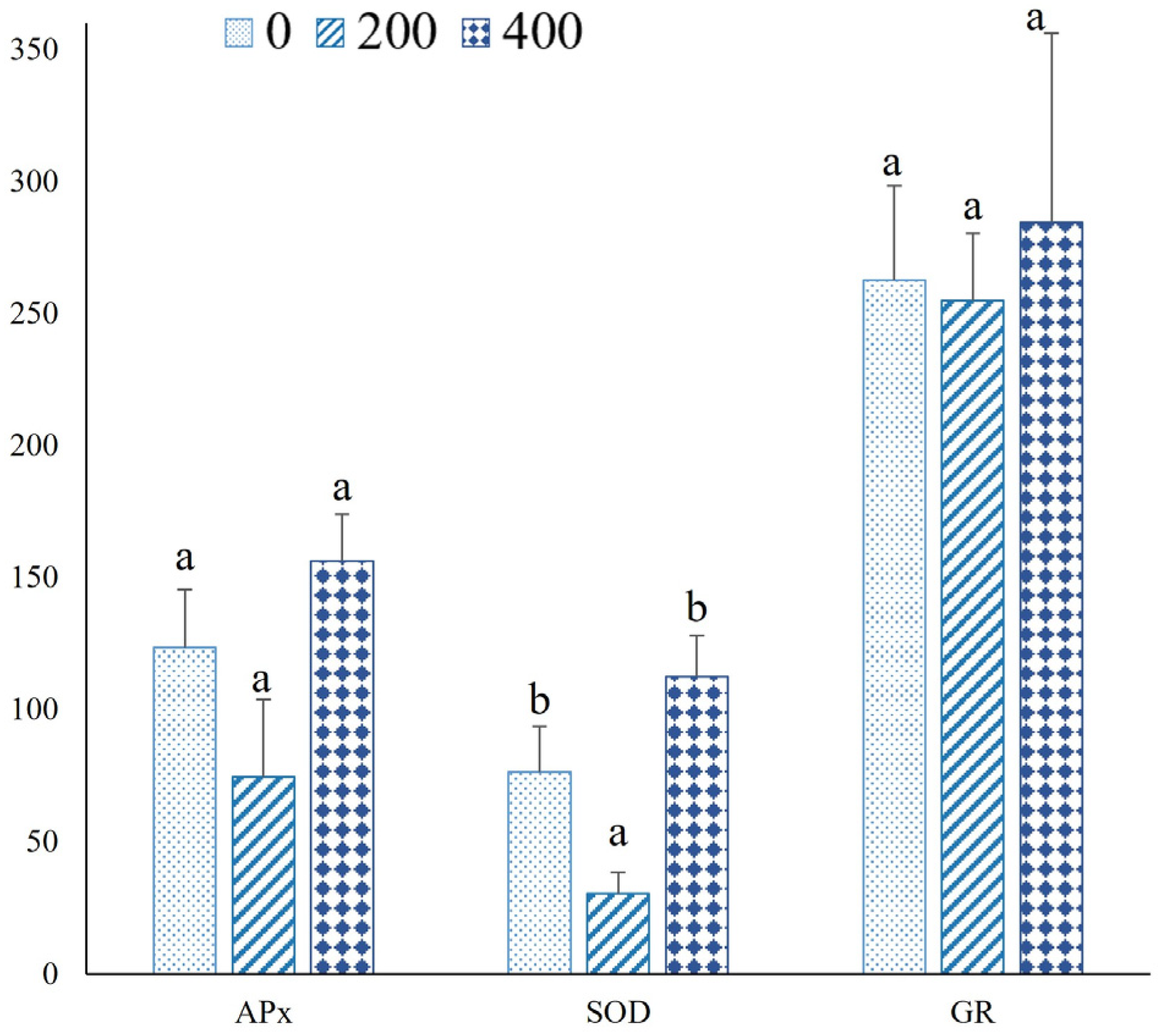
3.3. Biochemical Measurements
3.4. Meta-Analysis of Salinity Response in Solanum Species
4. Discussion
4.1. Previous Available Information About S. dasyphyllum
4.2. Growth Performance of S. dasyphyllum Under Salt Stress
4.3. Impact of Salt Stress on Photosynthetic Pigments
4.4. Ion Accumulation and Comparison with Other CWRs
4.5. Osmoprotectants and Oxidative Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Organization Corporate Statistical Database. Available online: https://www.fao.org/statistics/es/ (accessed on 10 March 2024).
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative Analysis of the Responses to Water Stress in Eggplant (Solanum melongena) Cultivars. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, A.B.; Kouassi, K.B.A.; Sylla, Z.; Plazas, M.; Fonseka, R.M.; Kouassi, A.; Fonseka, H.; N’guetta, A.S.P.; Prohens, J. Genetic Parameters of Drought Tolerance for Agromorphological Traits in Eggplant, Wild Relatives, and Interspecific Hybrids. Crop. Sci. 2021, 61, 55–68. [Google Scholar] [CrossRef]
- Plazas, M.; Vilanova, S.; Gramazio, P.; Rodríguez-Burruezo, A.; Fita, A.; Herraiz, F.J.; Ranil, R.; Fonseka, R.; Niran, L.; Fonseka, H.; et al. Interspecific Hybridization between Eggplant and Wild Relatives from Different Genepools. J. Am. Soc. Hortic. Sci. 2016, 141, 34–44. [Google Scholar] [CrossRef]
- Gramazio, P.; Yan, H.; Hasing, T.; Vilanova, S.; Prohens, J.; Bombarely, A. Whole-Genome Resequencing of Seven Eggplant (Solanum melongena) and One Wild Relative (S. incanum) Accessions Provides New Insights and Breeding Tools for Eggplant Enhancement. Front. Plant Sci. 2019, 10, 1220. [Google Scholar] [CrossRef]
- Rakha, M.; Namisy, A.; Chen, J.R.; El-Mahrouk, M.E.; Metwally, E.; Taha, N.; Prohens, J.; Plazas, M.; Taher, D. Development of Interspecific Hybrids between a Cultivated Eggplant Resistant to Bacterial Wilt (Ralstonia solanacearum) and Eggplant Wild Relatives for the Development of Rootstocks. Plants 2020, 9, 1405. [Google Scholar] [CrossRef]
- Gisbert, C.; Prohens, J.; Raigón, M.D.; Stommel, J.R.; Nuez, F. Eggplant Relatives as Sources of Variation for Developing New Rootstocks: Effects of Grafting on Eggplant Yield and Fruit Apparent Quality and Composition. Sci. Hortic. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Knapp, S.; Vorontsova, M.S.; Prohens, J. Wild Relatives of the Eggplant (Solanum melongena L.: Solanaceae): New Understanding of Species Names in a Complex Group. PLoS ONE 2013, 8, e57039. [Google Scholar] [CrossRef]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing Capacity, Chlorogenic Acid Content and Biological Activity in a Collection of Scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) Eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef]
- Vorontsova, M.S.; Stern, S.; Bohs, L.; Knapp, S. African Spiny Solanum (Subgenus Leptostemonum, Solanaceae): A Thorny Phylogenetic Tangle. Bot. J. Linn. Soc. 2013, 173, 176–193. [Google Scholar] [CrossRef]
- Vorontsova, M.; Knapp, S. A New Species of Solanum (Solanaceae) from South Africa Related to the Cultivated Eggplant. PhytoKeys 2012, 8, 1. [Google Scholar] [CrossRef]
- Kouassi, B.; Prohens, J.; Gramazio, P.; Kouassi, A.B.; Vilanova, S.; Galán-Ávila, A.; Herraiz, F.J.; Kouassi, A.; Seguí-Simarro, J.M.; Plazas, M. Development of Backcross Generations and New Interspecific Hybrid Combinations for Introgression Breeding in Eggplant (Solanum melongena). Sci. Hortic. 2016, 213, 199–207. [Google Scholar] [CrossRef]
- Brenes, M.; Pérez, J.; González-Orenga, S.; Solana, A.; Boscaiu, M.; Prohens, J.; Plazas, M.; Fita, A.; Vicente, O. Comparative Studies on the Physiological and Biochemical Responses to Salt Stress of Eggplant (Solanum melongena) and Its Rootstock S. torvum. Agriculture 2020, 10, 328. [Google Scholar] [CrossRef]
- Plazas, M.; González-Orenga, S.; Nguyen, H.T.; Morar, I.M.; Fita, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Growth and Antioxidant Responses Triggered by Water Stress in Wild Relatives of Eggplant. Sci. Hortic. 2022, 293, 110685. [Google Scholar] [CrossRef]
- Plazas, M.; Gramazio, P.; Vilanova, S.; Kouassi, A.B.; Fonseka, R.M.; Rakha, M.; García-Fortea, E.; Mangino, G.; Kouassi, K.B.A.; Fonseka, H.; et al. Introgression Breeding from Crop Wild Relatives in Eggplant Landraces for Adaptation to Climate Change. Crop Wild Relat. 2020, 12, 32–36. [Google Scholar]
- Villanueva, G.; Vilanova, S.; Plazas, M.; Prohens, J.; Gramazio, P. Transcriptome Profiles of Eggplant (Solanum melongena) and Its Wild Relative S. dasyphyllum under Different Levels of Osmotic Stress Provide Insights into Response Mechanisms to Drought. Curr. Plant Biol. 2023, 33, 100276. [Google Scholar] [CrossRef]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, Á.; Prohens, J.; Plazas, M. Physiological and Biochemical Responses to Salt Stress in Cultivated Eggplant (Solanum melongena L.) and in S. insanum L., a Close Wild Relative. Agronomy 2020, 10, 651. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.R.; Pereira-Dias, L.; Soler, S.; López-Serrano, L.; Alonso, D.; Calatayud, Á.; Díez, M.J. Adaptation to Water and Salt Stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme. Agronomy 2020, 10, 1169. [Google Scholar] [CrossRef]
- Zayova, E.; Philipov, P.; Nedev, T.; Stoeva, D. Response of in Vitro Cultivated Eggplant (Solanum melongena L.) to Salt and Drought Stress. AgroLife Sci. J. 2017, 6. Available online: https://agrolifejournal.usamv.ro/index.php/agrolife/article/view/686 (accessed on 10 March 2024).
- Shabala, S. Salinity and Programmed Cell Death: Unravelling Mechanisms for Ion Specific Signalling. J. Exp. Bot. 2009, 60, 709–712. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. ISBN 978-3-319-96189-7. [Google Scholar]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of Plant Response to Salt and Drought Stress and Their Alteration by Rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Hesami, A.; Bazdar, L.; Shahriari, M.H. Effect of Soil Salinity on Growth, Proline, and Some Nutrient Accumulation in Two Genotypes Seedlings of Ziziphus Spina-christi (L.) Willd. Commun. Soil Sci. Plant Anal. 2020, 51, 804–815. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Ortega-Albero, N.; González-Orenga, S.; Vicente, O.; Rodríguez-Burruezo, A.; Fita, A. Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species. Plants 2023, 12, 295. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Murata, N. Revised Scheme for the Mechanism of Photoinhibition and Its Application to Enhance the Abiotic Stress Tolerance of the Photosynthetic Machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, Metabolomics, and Ionomics Perspectives of Salinity Tolerance in Halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Gramazio, P.; Prohens, J.; Plazas, M.; Mangino, G.; Herraiz, F.J.; Vilanova, S. Development and Genetic Characterization of Advanced Backcross Materials and an Introgression Line Population of Solanum incanum in a S. melongena Background. Front. Plant Sci. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Ranil, R.H.G.; Niran, H.M.L.; Plazas, M.; Fonseka, R.M.; Fonseka, H.H.; Vilanova, S.; Andújar, I.; Gramazio, P.; Fita, A.; Prohens, J. Improving Seed Germination of the Eggplant Rootstock Solanum torvum by Testing Multiple Factors Using an Orthogonal Array Design. Sci. Hortic. 2015, 193, 174–181. [Google Scholar] [CrossRef]
- Weimberg, R. Solute Adjustments in Leaves of Two Species of Wheat at Two Different Stages of Growth in Response to Salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.K.; Taulavuori, K.; Laine, K. Comparison of Two Methods Used to Analyse Lipid Peroxidation from Vaccinium myrtillus (L.) during Snow Removal, Reacclimation and Cold Acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin-Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinache Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Connell, J.P.; Mullet, J.E. Pea Chloroplast Glutathione Reductase: Purification and Characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef]
- Ortega-Albero, N.; González-Orenga, S.; Rodríguez-Burruezo, A.; Fita, A. Growth variability in Solanum melongena and two related species under salt stress. In Proceedings of the 18th EUCARPIA Meeting on Genetics and Breeding of Capsicum and Eggplant, Plovdiv, Bulgaria, 8–21 September 2023; p. 81, Book of Abstracts. [Google Scholar]
- Prohens, J.; Whitaker, B.D.; Plazas, M.; Vilanova, S.; Hurtado, M.; Blasco, M.; Gramazio, P.; Stommel, J.R. Genetic Diversity in Morphological Characters and Phenolic Acids Content Resulting from an Interspecific Cross between Eggplant, Solanum melongena, and Its Wild Ancestor (S. incanum). Ann. Appl. Biol. 2013, 162, 242–257. [Google Scholar] [CrossRef]
- Hannachi, S.; Bahrini, I.; Ibrahim, N.I.; Abdelgadir, A.; Siddiqui, H.A. NaCl Affects Lipids Perodixation and Oxygen Free Radicals Scavenging Machinery in Callus Tissues of a Cultivared (Solanum macrocarpon L.) and a Wild Eggplant (Solanum dasyphyllum L.). Adv. Life Sci. Int. Q. J. Biol. Sci. 2021, 8, 396–405. [Google Scholar]
- de Paz, J.M.; Visconti, F.; Rubio, J.L. Spatial Evaluation of Soil Salinity Using the WET Sensor in the Irrigated Area of the Segura River Lowland. J. Plant Nutr. Soil Sci. 2011, 174, 103–112. [Google Scholar] [CrossRef]
- de Paz, J.M.; Visconti, F.; Zapata, R.; Sánchez, J. Integration of Two Simple Models in a Geographical Information System to Evaluate Salinization Risk in Irrigated Land of the Valencian Community, Spain. Soil Use Manag. 2004, 20, 333–342. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, M.; Kim, S.R.; Ryu, H.; Cho, Y.G. Insights into Genomics of Salt Stress Response in Rice. Rice 2013, 6, 27. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; De Boer, G.J.; et al. Genetic Components of Root Architecture Remodeling in Response to Salt Stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of Leaf Gas Exchange Attributes, Photosynthetic Pigments and Antioxidant Enzymes in NaCl-Stressed Cotton (Gossypium hirsutum L.) Seedlings to Exogenous Glycine Betaine and Salicylic Acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef]
- David-Rogeat, N.; Broadley, M.R.; Stavridou, E. Heat and Salinity Stress on the African Eggplant F1 Djamba, a Kumba Cultivar. Front. Plant Sci. 2024, 15, 1323665. [Google Scholar] [CrossRef]
- Shaheen, S.; Naseer, S.; Ashraf, M.; Akram, N.A. Salt Stress Affects Water Relations, Photosynthesis, and Oxidative Defense Mechanisms in Solanum melongena L. J. Plant Interact. 2013, 8, 85–96. [Google Scholar] [CrossRef]
- Shahbaz, M.; Mushtaq, Z.; Andaz, F.; Masood, A. Does Proline Application Ameliorate Adverse Effects of Salt Stress on Growth, Ions and Photosynthetic Ability of Eggplant (Solanum melongena L.)? Sci. Hortic. 2013, 164, 507–511. [Google Scholar] [CrossRef]
- Li, J.; Gao, Z.; Zhou, L.; Li, L.; Zhang, J.; Liu, Y.; Chen, H. Comparative Transcriptome Analysis Reveals K+ Transporter Gene Contributing to Salt Tolerance in Eggplant. BMC Plant Biol. 2019, 19, 67. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Mekawy, A.M.M.; Ueda, A.; Saneoka, H. Salinity-Induced Expression of HKT May Be Crucial for Na+ Exclusion in the Leaf Blade of Huckleberry (Solanum scabrum Mill.), but Not of Eggplant (Solanum melongena L.). Biochem. Biophys. Res. Commun. 2015, 460, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and Salt-Stress Signaling in Plants: Shedding Light on SOS Pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]




| S. melongena | Primary Genepool | Secondary Genepool | Tertiary Genepool | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEL1 | MEL5 | S. insanum | S. dasyphyllum | S. aethiopicum | S. torvum | |||||||
| 200 mM | 400 mM | 200 mM | 400 mM | 200 mM | 400 mM | 200 mM | 400 mM | 200 mM | 400 mM | 200 mM | 300 mM | |
| LFW | 60 | 30 | 60 | 30 | 58 | 30 | 57 | 30 | 60 | 33 | 40 | 30 |
| RFW | 130 | 79 | 119 | 86 | 108 | 68 | 102 | 62 | 74 | 49 | 45 | 40 |
| RL | 70 | 56 | 92 | 70 | 94 | 68 | 100 | 66 | 95 | 81 | 80 | 60 |
| Na+ leaves | 312 | 299 | 293 | 418 | 524 | 617 | 308 | 351 | 468 | 517 | 1101 | 835 |
| Na+ roots | 215 | 297 | 443 | 505 | 745 | 954 | 605 | 660 | 645 | 605 | 522 | 429 |
| K+ leaves | 116 | 326 | 49 | 61 | 88 | 147 | 121 | 90 | 162 | 185 | 56 | 75 |
| K+ roots | 45 | 64 | 79 | 99 | 106 | 84 | 97 | 92 | 63 | 105 | 62 | 59 |
| Cl− leaves | 331 | 324 | 356 | 488 | 981 | 1133 | 604 | 664 | 413 | 463 | 875 | 649 |
| Cl− roots | 195 | 224 | 300 | 322 | 509 | 686 | 479 | 328 | 379 | 323 | 337 | 371 |
| ChlA | 117 | 133 | 109 | 77 | 205 | 123 | 51 | 41 | 109 | 120 | 64 | 74 |
| ChlB | 81 | 80 | 160 | 83 | 138 | 60 | 77 | 55 | 108 | 109 | 59 | 58 |
| Caro | 89 | 94 | 141 | 92 | 112 | 55 | 99 | 84 | 64 | 67 | 66 | 92 |
| Pro | 524 | 1061 | 578 | 1028 | 817 | 13,610 | 1362 | 1568 | 369 | 1082 | 3676 | 4282 |
| TPC | 84 | 83 | 100 | 100 | 100 | 100 | 89 | 79 | 100 | 100 | 82 | 132 |
| Refs. | Ortega-Albero et al. (2023) [42] | Ortega-Albero et al. (2023) [25] | Ortega-Albero et al. (2023) [42] | Brenes et al. (2020) [13] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Albero, N.; González-Orenga, S.; Vicente, O.; Rodríguez-Burruezo, A.; Fita, A. Biometric and Biochemical Responses to Salt in Solanum dasyphyllum, a Potential Donor of Tolerance for Eggplant. Horticulturae 2025, 11, 405. https://doi.org/10.3390/horticulturae11040405
Ortega-Albero N, González-Orenga S, Vicente O, Rodríguez-Burruezo A, Fita A. Biometric and Biochemical Responses to Salt in Solanum dasyphyllum, a Potential Donor of Tolerance for Eggplant. Horticulturae. 2025; 11(4):405. https://doi.org/10.3390/horticulturae11040405
Chicago/Turabian StyleOrtega-Albero, Neus, Sara González-Orenga, Oscar Vicente, Adrián Rodríguez-Burruezo, and Ana Fita. 2025. "Biometric and Biochemical Responses to Salt in Solanum dasyphyllum, a Potential Donor of Tolerance for Eggplant" Horticulturae 11, no. 4: 405. https://doi.org/10.3390/horticulturae11040405
APA StyleOrtega-Albero, N., González-Orenga, S., Vicente, O., Rodríguez-Burruezo, A., & Fita, A. (2025). Biometric and Biochemical Responses to Salt in Solanum dasyphyllum, a Potential Donor of Tolerance for Eggplant. Horticulturae, 11(4), 405. https://doi.org/10.3390/horticulturae11040405










