Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Plant Materials and Biostimulant Treatments During the Seedling Stage
2.3. Fertilizer Treatments After Transplanting
2.4. Growth Measurements
2.5. Statistical Analysis
3. Results
3.1. Plant Height and Mini Bulb Parameters
3.2. Fresh Weight Parameters
3.3. Dry Weight Parameters
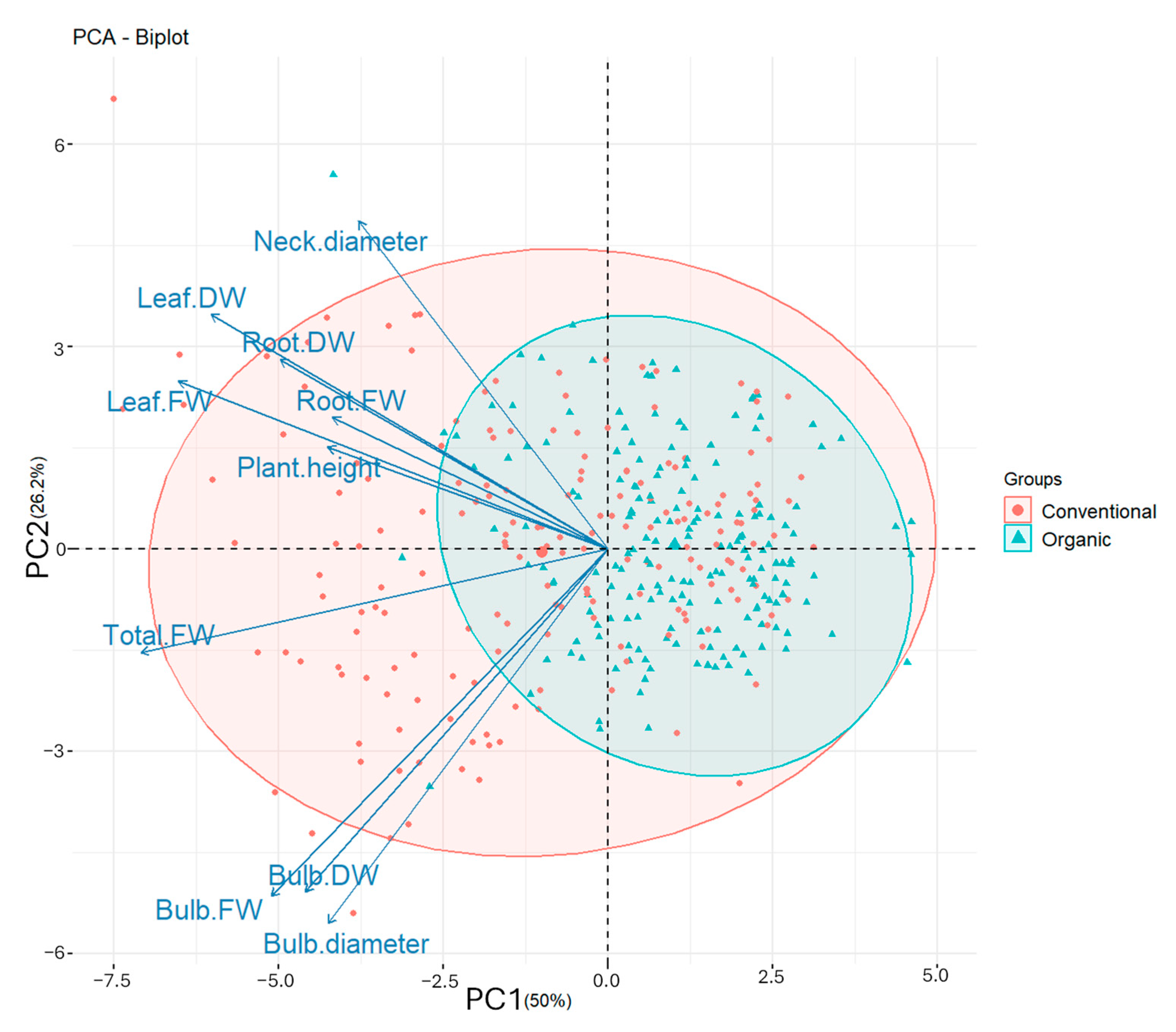
3.4. Principal Component Analysis
4. Discussion
4.1. Multiple Applications of Seaweed Extract May Be Necessary
4.2. Different Microbial Biostimulants Have Cultivar-Specific Effects on Onion Plant Growth
4.3. Organic Fertilizer Enhanced Microbial Biostimulant Benefits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.; Delisle-Houde, M.; Nguyen, T.T.A.; Dorais, M.; Tweddell, R.J. Effect of Biostimulants on Leafy Vegetables (Baby Leaf Lettuce and Batavia Lettuce) Exposed to Abiotic or Biotic Stress under Two Different Growing Systems. Agronomy 2023, 13, 879. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial Biostimulants as Response to Modern Agriculture Needs: Composition, Role and Application of These Innovative Products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The Potential Role of Microbial Biostimulants in the Amelioration of Climate Change-Associated Abiotic Stresses on Crops. Front. Microbiol. 2022, 12, 829099. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review Report on the Role of Bioproducts, Biopreparations, Biostimulants and Microbial Inoculants in Organic Production of Fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Werner, G.D.A.; Kiers, E.T. Order of Arrival Structures Arbuscular Mycorrhizal Colonization of Plants. New Phytol. 2015, 205, 1515–1524. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed Extract: Biostimulator of Plant Defense and Plant Productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Tuhy, Ł.; Chojnacka, K. Plant Growth Biostimulants Based on Different Methods of Seaweed Extraction with Water. BioMed Res. Int. 2016, 2016, 5973760. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological Responses to Humic Substances as Plant Growth Promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, R.; Thoma, G.; Matlock, M.D. Environmental Sustainability of Fruit and Vegetable Production Supply Chains in the Face of Climate Change: A Review. Sci. Total Environ. 2019, 650, 2863–2879. [Google Scholar] [CrossRef]
- Wu, H.; MacDonald, G.K.; Galloway, J.N.; Zhang, L.; Gao, L.; Yang, L.; Yang, J.; Li, X.; Li, H.; Yang, T. The Influence of Crop and Chemical Fertilizer Combinations on Greenhouse Gas Emissions: A Partial Life-Cycle Assessment of Fertilizer Production and Use in China. Resour. Conserv. Recycl. 2021, 168, 105303. [Google Scholar] [CrossRef]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Li, H.; Yue, H.; Li, L.; Liu, Y.; Zhang, H.; Wang, J.; Jiang, X. Seed Biostimulant Bacillus Sp. MGW9 Improves the Salt Tolerance of Maize during Seed Germination. AMB Express 2021, 11, 74. [Google Scholar] [CrossRef]
- Silva, E.G.; Takata, W.H.S.; Frias, A.G.; Goto, R.; Nobuyoshi, N. Biostimulant Action on Quality of Tomato Seedlings Produced in Different Types of Trays. Acta Hortic. 2019, 1249, 227–232. [Google Scholar] [CrossRef]
- Mendez, A.; Martinez, S.; Leal, A.; Hernandez, A.; Garcia, J.; Sanchez, M. Synergism of Microorganisms and Seaweed Extract on Vegetative Growth, Yield and Quality of Cucumber Fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 12888. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Kulkarni, M.G.; Stirk, W.A.; Gupta, S.; Finnie, J.F.; van Staden, J. Interactions Between Microorganisms and a Seaweed-Derived Biostimulant on the Growth and Biochemical Composition of Amaranthus hybridus L. Nat. Prod. Commun. 2020, 15, 1934578X20934228. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-Ul-Hye, M.; Khan, R.I.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The Effects of Biostimulants, Biofertilizers and Water-Stress on Nutritional Value and Chemical Composition of Two Spinach Genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef] [PubMed]
- Pannacci, E.; Baratta, S.; Falcinelli, B.; Farneselli, M.; Tei, F. Mugwort (Artemisia vulgaris L.) Aqueous Extract: Hormesis and Biostimulant Activity for Seed Germination and Seedling Growth in Vegetable Crops. Agriculture 2022, 12, 1329. [Google Scholar] [CrossRef]
- Novello, G.; Cesaro, P.; Bona, E.; Massa, N.; Gosetti, F.; Scarafoni, A.; Todeschini, V.; Berta, G.; Lingua, G.; Gamalero, E. The Effects of Plant Growth-Promoting Bacteria with Biostimulant Features on the Growth of a Local Onion Cultivar and a Commercial Zucchini Variety. Agronomy 2021, 11, 888. [Google Scholar] [CrossRef]
- Yildirim, E.; Dursun, A.; Güvenc, I.; Kumlay, A.M. THE EFFECTS OF DIFFERENT SALT, BIOSTIMULANT AND TEMPERATURE LEVELS ON SEED GERMINATION OF SOME VEGETABLE SPECIES. Acta Hortic. 2002, 579, 249–253. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Sharma, R. Effect of Different Concentration of Commercial Seaweed Liquid Extract of Ascophylum Nodosum on Germination of Onion (Allium cepa L.). Int. J. Sci. Res. 2017, 6, 1488–1491. [Google Scholar] [CrossRef]
- Muhİe, S.; Özdamar, C.; Gökdaş, Z.; Njİe, E.S.; Memİş, N.; Demİr, I. Effect of Solid Matrix Priming with Seaweed Extract on Germination and Seedling Performance of Onion Seeds under Abiotic. Black Sea J. Agric. 2020, 3, 233–238. [Google Scholar]
- Szczepanek, M.; Wszelaczyńska, E.; Pobereżny, J.; Ochmian, I. Response of Onion (Allium cepa L.) to the Method of Seaweed Biostimulant Application. Acta Sci. Pol. Hortorum Cultus 2017, 16, 113–122. [Google Scholar]
- Tinna, D.; Garg, N.; Sharma, S.; Pandove, G.; Chawla, N. Utilization of Plant Growth Promoting Rhizobacteria as Root Dipping of Seedlings for Improving Bulb Yield and Curtailing Mineral Fertilizer Use in Onion under Field Conditions. Sci. Hortic. 2020, 270, 109432. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G. Microalgae Associated to Humic Acid as a Novel Biostimulant Improving Onion Growth and Yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Taylor, A.; Pereira, N.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Bending, G.D. Growth and Nutritional Responses to Arbuscular Mycorrhizal Fungi Are Dependent on Onion Genotype and Fungal Species. Biol. Fertil. Soils 2015, 51, 801–813. [Google Scholar] [CrossRef]
- Zhang, Q.; Masabni, J.; Niu, G. Organic Fertilizer Type and Dose Affect Growth, Morphological and Physiological Parameters, and Mineral Nutrition of Watermelon Seedlings. PeerJ 2024, 12, e16902. [Google Scholar] [CrossRef]
- Xue, L.L.; Shen, C.C. The Sustainable Development of Organic Agriculture: The Role of Wellness Tourism and Environmental Restorative Perception. Agriculture 2022, 12, 197. [Google Scholar] [CrossRef]
- Bergstrand, K.J. Organic Fertilizers in Greenhouse Production Systems—A Review. Sci. Hortic. 2022, 295, 110855. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Yong, J.W.H. Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming. Biology 2021, 11, 41. [Google Scholar] [CrossRef]
- Tarantino, E.; Disciglio, G.; Frabboni, L.; Libutti, A.; Gatta, G.; Gagliaridi, A.; Tarantino, A. Effects of Biostimulant Application on Quali- Quantitative Characteristics of Cauliflower, Pepper and Fennel Crops under Organic and Conventional Fertilization. Int. J. Agric. Biosyst. Eng. 2015, 9, 712–716. [Google Scholar]
- Liu, J.; Zhang, Q.; Masabni, J.; Niu, G. Low Nitrogen Availability in Organic Fertilizers Limited Organic Watermelon Transplant Growth. Horticulturae 2024, 10, 1140. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X.; Li, Y. Correction: Effects of Long-Term Fertilization on Soil Organic Carbon Mineralization and Microbial Community Structure. PLoS ONE 2019, 14, e0216006. [Google Scholar] [CrossRef]
- Molla, M.A.Z.; Chowdhury, A.A.; Islam, A.; Hoque, S. Microbial Mineralization of Organic Phosphate in Soil. Plant Soil 1984, 78, 393–399. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Zhang, Q.; Masabni, J.; Niu, G. Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars. Horticulturae 2024, 10, 800. [Google Scholar] [CrossRef]
- Burnett, S.E.; Peterson, B.J. Propagation of Herbaceous and Woody Perennials in Submist and Overhead Mist Systems. J. Environ. Hortic. 2022, 40, 164–169. [Google Scholar] [CrossRef]
- Ali, A.; Niu, G.; Masabni, J.; Ferrante, A.; Cocetta, G. Integrated Nutrient Management of Fruits, Vegetables, and Crops through the Use of Biostimulants, Soilless Cultivation, and Traditional and Modern Approaches—A Mini Review. Agriculture 2024, 14, 1330. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; van Staden, J. Abscisic Acid, Gibberellins and Brassinosteroids in Kelpak®, a Commercial Seaweed Extract Made from Ecklonia Maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Stirk, W.A.; Arthur, G.D.; Lourens, A.F.; Novák, O.; Strnad, M.; Staden, J. van Changes in Cytokinin and Auxin Concentrations in Seaweed Concentrates When Stored at an Elevated Temperature. J. Appl. Phycol. 2004, 16, 31–39. [Google Scholar] [CrossRef]
- van Iersel, M. Auxins Affect Posttransplant Shoot and Root Growth of Vinca Seedlings. Hortscience 1998, 33, 1210–1214. [Google Scholar] [CrossRef]
- Keller, C.P. Leaf Expansion in Phaseolus: Transient Auxin-induced Growth Increase. Physiol. Plant. 2007, 130, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Stirk, W.A.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Interactive Effects of Plant Growth-Promoting Rhizobacteria and a Seaweed Extract on the Growth and Physiology of Allium cepa L. (Onion). J. Plant Physiol. 2021, 262, 153437. [Google Scholar] [CrossRef]
- Hussain, H.I.; Kasinadhuni, N.; Arioli, T. The Effect of Seaweed Extract on Tomato Plant Growth, Productivity and Soil. J. Appl. Phycol. 2021, 33, 1305–1314. [Google Scholar] [CrossRef]
- de Araújo, V.L.V.P.; Junior, M.A.L.; de Souza Júnior, V.S.; de Araújo Filho, J.C.; Fracetto, F.J.C.; Andreote, F.D.; de Araujo Pereira, A.P.; Júnior, J.P.M.; do Rêgo Barros, F.M.; Fracetto, G.G.M. Bacteria from Tropical Semiarid Temporary Ponds Promote Maize Growth under Hydric Stress. Microbiol. Res. 2020, 240, 126564. [Google Scholar] [CrossRef]
- Pokluda, R.; Ragasová, L.N.; Jurica, M.; Kalisz, A.; Komorowska, M.; Niemiec, M.; Caruso, G.; Gąstoł, M.; Sekara, A. The Shaping of Onion Seedlings Performance through Substrate Formulation and Co-Inoculation with Beneficial Microorganism Consortia. Front. Plant Sci. 2023, 14, 1222557. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H.; Whalen, J.K. Carbon-Rich Organic Fertilizers to Increase Soil Biodiversity: Evidence from a Meta-Analysis of Nematode Communities. Agric. Ecosyst. Environ. 2016, 232, 199–207. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Springer International Publishing: Cham, Switzerland, 2021; Volume 2, pp. 1–20. [Google Scholar]
- Qin, H.; Lu, K.; Strong, P.J.; Xu, Q.; Wu, Q.; Xu, Z.; Xu, J.; Wang, H. Long-Term Fertilizer Application Effects on the Soil, Root Arbuscular Mycorrhizal Fungi and Community Composition in Rotation Agriculture. Appl. Soil Ecol. 2015, 89, 35–43. [Google Scholar] [CrossRef]
- Poulaki, E.G.; Tjamos, S.E. Bacillus Species: Factories of Plant Protective Volatile Organic Compounds. J. Appl. Microbiol. 2023, 134, lxad037. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, N.; Qiu, M.; Feng, H.; Vivanco, J.M.; Shen, Q.; Zhang, R. Enhanced Rhizosphere Colonization of Beneficial Bacillus Amyloliquefaciens SQR9 by Pathogen Infection. FEMS Microbiol. Lett. 2014, 353, 49–56. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bolan, N.; Farrell, M.; Sarkar, B.; Sarker, J.R.; Kirkham, M.B.; Hossain, M.Z.; Kim, G.H. Role of Cultural and Nutrient Management Practices in Carbon Sequestration in Agricultural Soil, 1st ed.; Elsevier Inc.: New York, NY, USA, 2021; Volume 166, ISBN 9780128245873. [Google Scholar]
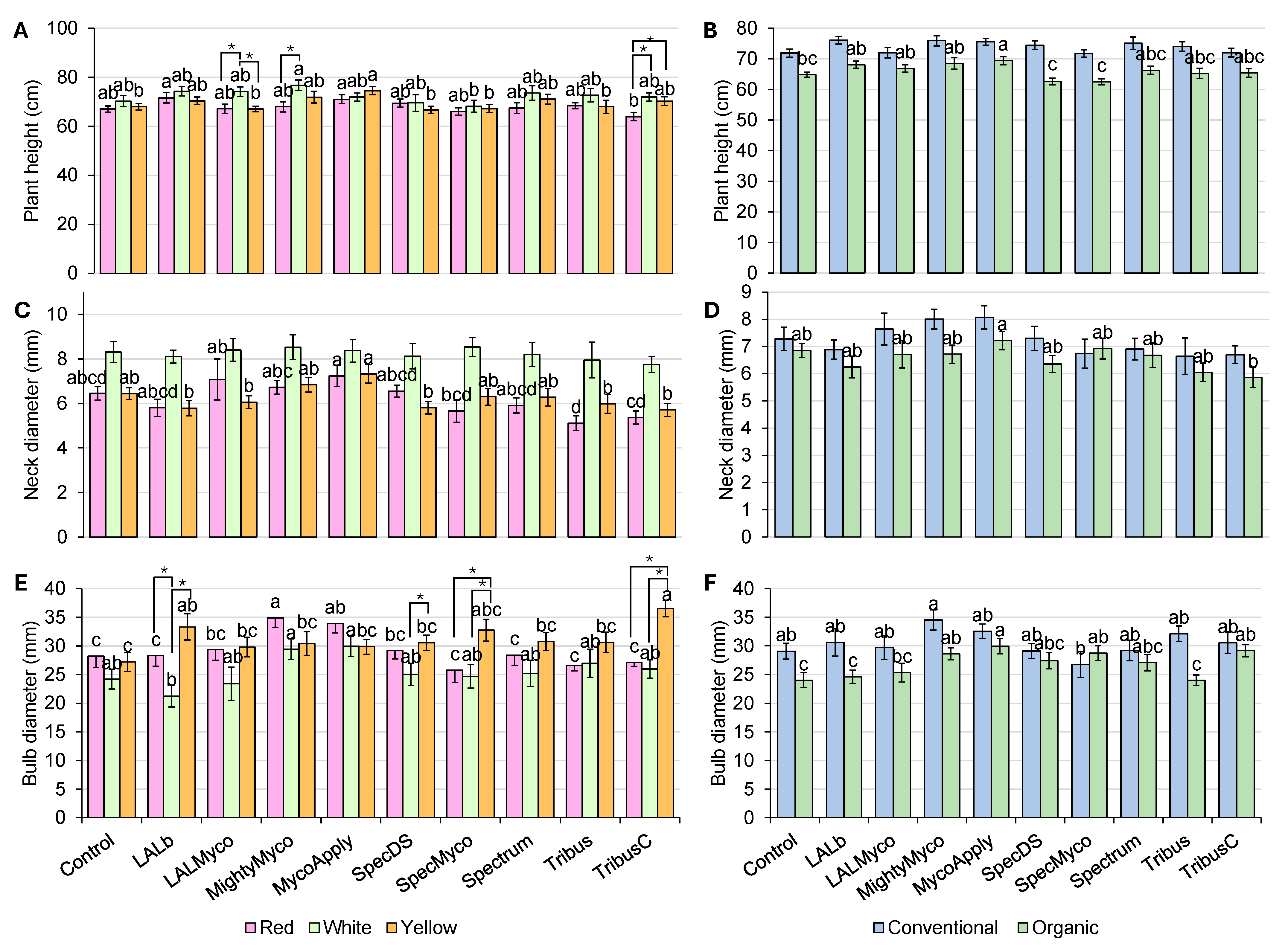
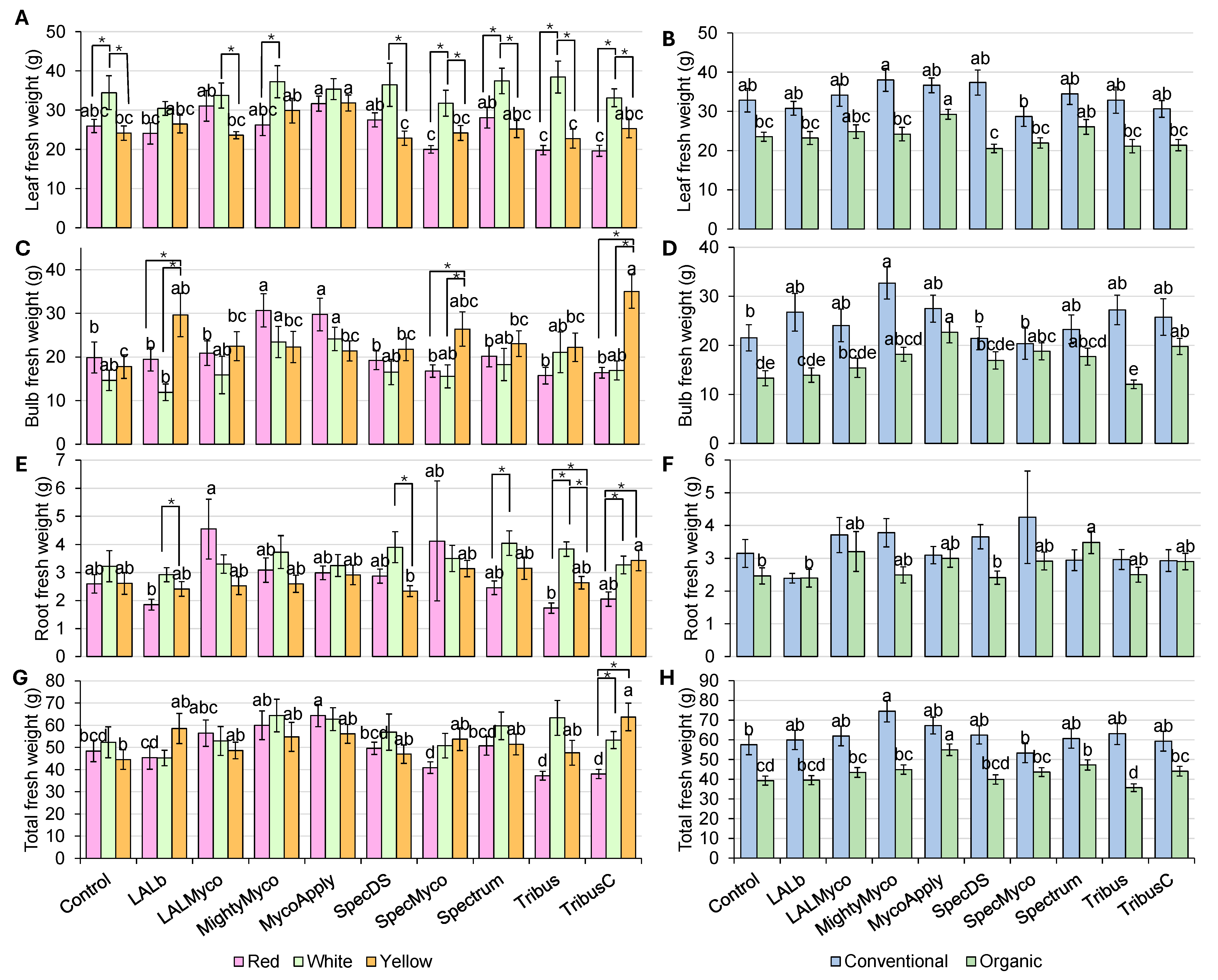
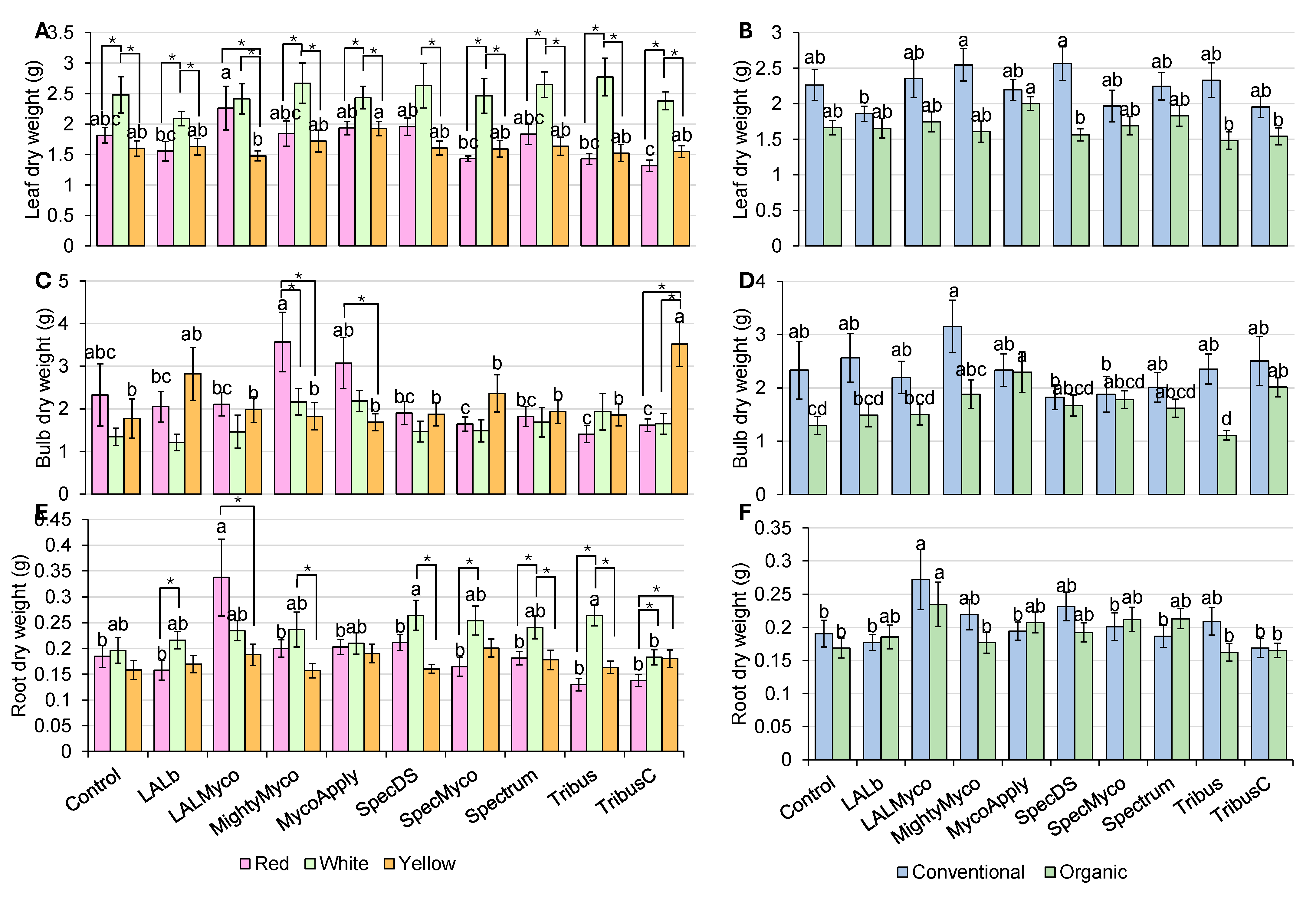
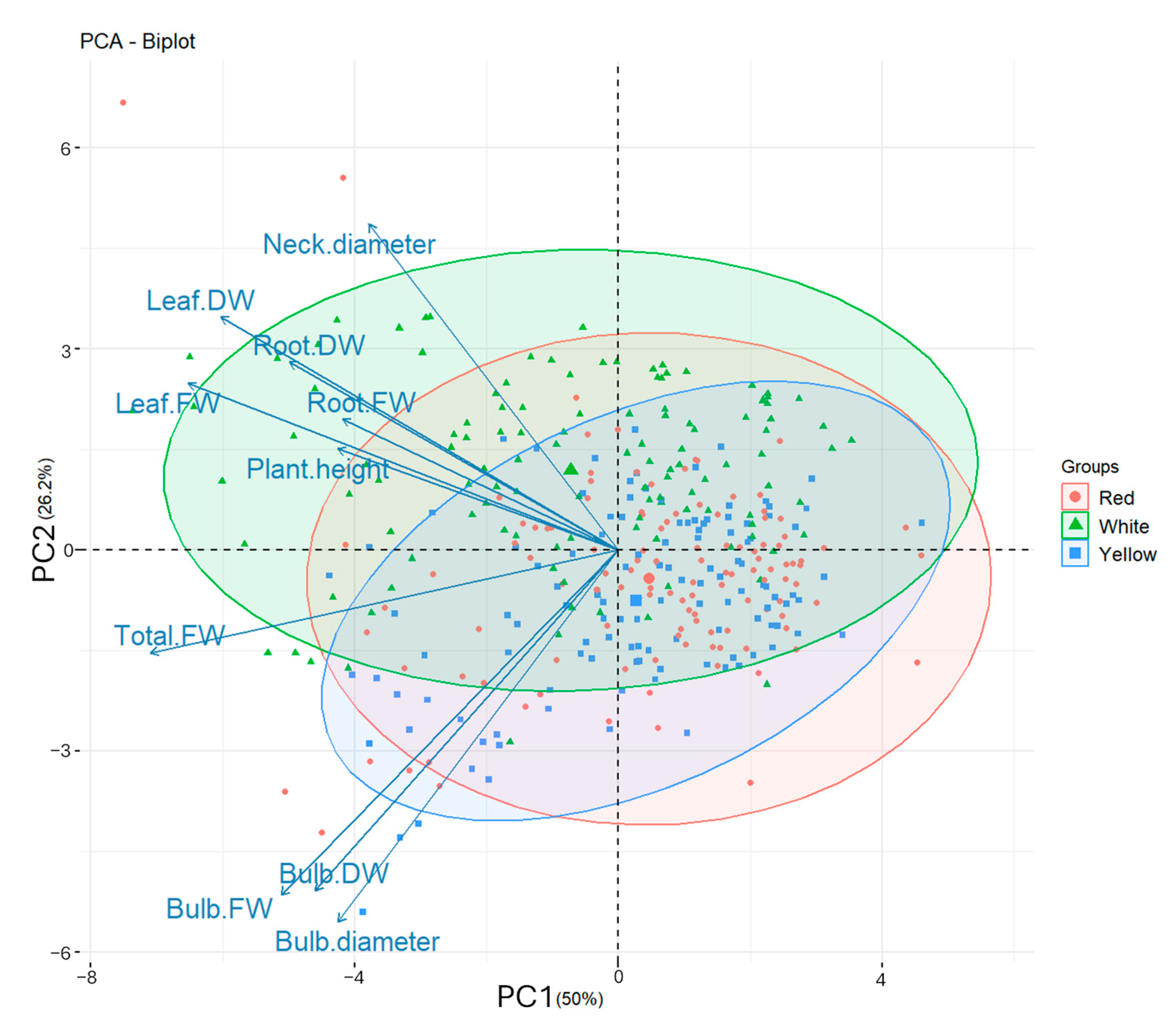

| Plant Height | Neck Diameter | Bulb Diameter | Leaf FW | Bulb FW | Root FW | Total FW | Leaf DW | Bulb DW | Root DW | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | *** | *** | *** | *** | *** | ** | ** | *** | *** | *** |
| Micro | *** | ** | ** | *** | ** | NS | ** | * | ** | ** |
| Fertilizer | *** | *** | *** | *** | *** | * | *** | *** | *** | NS |
| Cultivar × Micro | * | NS | ** | * | *** | NS | *** | NS | *** | *** |
| Cultivar × Fert | ** | NS | * | ** | ** | NS | * | * | * | NS |
| Micro × Fert | NS | NS | * | NS | * | NS | NS | * | ** | NS |
| Cultivar × Micro × Fert | *** | NS | NS | ** | * | NS | ** | * | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Liu, J.; Jeong, S.J.; Masabni, J.; Niu, G. Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects. Horticulturae 2025, 11, 402. https://doi.org/10.3390/horticulturae11040402
Zhang Q, Liu J, Jeong SJ, Masabni J, Niu G. Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects. Horticulturae. 2025; 11(4):402. https://doi.org/10.3390/horticulturae11040402
Chicago/Turabian StyleZhang, Qianwen, Jun Liu, Sang Jun Jeong, Joseph Masabni, and Genhua Niu. 2025. "Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects" Horticulturae 11, no. 4: 402. https://doi.org/10.3390/horticulturae11040402
APA StyleZhang, Q., Liu, J., Jeong, S. J., Masabni, J., & Niu, G. (2025). Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects. Horticulturae, 11(4), 402. https://doi.org/10.3390/horticulturae11040402








