Abstract
The outdoor cultivation of true morels has been successfully commercialized in China in recent years. However, unstable yields make it a high-risk business. A lack of understanding of the morel life cycle has led to chaotic spawn production processes, further affecting cultivation. In this study, the life cycle of Morchella sextelata, the most widely cultivated species of true morels, was characterized. A disproportion in the two mating-type idiomorphs, MAT1-1 and MAT1-2, was observed in the mycelia during vegetative growth, successive subcultures, and different parts of the fruiting body. Homokaryotic hyphae were found to dominate the mycelia and fruiting body of M. sextelata through the separation and detection of protoplast-regenerated single strains. The findings suggest that two homokaryotic hyphae with different mating types fuse to form heterokaryotic hyphae just before ascospore production in the life cycle of M. sextelata. The observed disproportion of mating-type idiomorphs is a primary reason for strain degeneration during spawn production. This study offers new insights into the life cycle of M. sextelata, particularly the role of mating-type distribution, which may inform strategies for improving the artificial cultivation of true morels.
1. Introduction
True morels are globally prized for their exceptional culinary qualities. For many years, wild collection served as the primary source of these prized edible mushrooms [1,2,3,4]. Meanwhile, artificial cultivation of morels has been attempted for more than 100 years [5]. The first commercial cultivation of true morels was reported by Ower in 1982 [6]; however, cultivation was abandoned in 2008 due to low yield and high contamination [2]. The invention and application of exogenous nutrition bag technology in true morel cultivation made commercial cultivation a reality [7]. By using this technology, in recent years, the outdoor cultivation of true morels was successfully commercialized in China, which became the major country for true morel cultivation and exportation [2,8,9]. The selection of the proper cultivar is another key element for the successful commercial cultivation of true morels in China. The species cultivated by Ower was suggested to be Morchella rufobrunnea; however, Morchella sextelata and Morchella importuna are the most cultivated species in China. Commercial cultivation of morels keeps expanding in China, and the cultivation area was about 7000 to 9000 ha in 2019 in China (The 5th National Morchella Conference in 2020). The cultivation of Morchella is a high-risk business due to the instability of the yield, ranging from 7620 kg per ha to zero [2].
One of the main reasons leading to the reduction of production is the lower spawn quality of Morchella. Initially adopting cultivation techniques developed primarily for basidiomycetes, growers propagated wild Morchella tissues on PDA medium and selected high-quality mycelia. After successive artificial domestication and successful pilot-scale trials, the optimized strains were applied to commercial production. However, due to the time-consuming process, demanding laboratory requirements, and inconsistent strain quality, most professional growers now source spawn exclusively from specialized commercial suppliers. The sclerotial formation was considered one of the standards for evaluating spawn quality [10]. Sclerotium formation is regulated by the coordination of multiple factors, including environmental conditions (temperature, moisture, and light), nutritional parameters (C/N ratio, Fe3+ levels, etc.), biological mediators (oxidative stress, etc.), and genetic determinants (scl1-scl4 operon, MAPK signaling pathway, etc.) [11,12]. Reactive oxygen species is one of the main inducing factors of sclerotial formation in morels [10]. However, sclerotium is not the solution to all problems in Morchella seed degeneration. Understanding the life cycle, especially the mating system, is also important for seed breeding and strain improvements in fungi [13,14]. A comprehensive understanding of the life cycle of Morchella species, as with other edible fungi, is fundamental for advancing their artificial cultivation technologies.
The mating types of mushroom fungi, including Ascomycetes and Basidiomycetes, were typically classified into homothallism (selfing) and heterothallism (self-in-compatibility). Most mushroom fungi are heterothallic species [15,16]. In heterothallic Ascomycetes, the mating group to which a strain belongs is generally determined as two mating types at a single locus. Genetic breeding of sexual reproduction occurs only between strains of the opposite mating type, which is called heterothallism [14]. The mating type of true morels was an obstacle until Volk and Leonard proposed the heterothallic life cycle of Morchella based on cytological observations [17]. The ascospores were homokaryotic, which could germinate to homokaryotic mycelia. Two compatible homokaryotic mycelia meet each other, and then they develop the fruiting process. In addition, the conidium and sclerotium produce during the vegetative growth process. The genetic basis of the heterothallism life cycle of morels was unknown until Chai et al. and Du et al. reported the heterothallic life cycle of black morel species by cloning the mating-type genes at the same time [8,9,18]. Chai et al. [8] characterized the mating-type locus, MAT1, of Morchella importuna, Mel-20, and Morchella sextelata based on genome sequencing data and PCR analysis, confirming the heterothallic life cycle of morels at the gene level. In the meantime, Du et al. [9] investigated the mating systems and life cycles of fourteen black morel species based on the morel genome information, and all fourteen morel species were found to be heterothallic.
Genomic studies have made enormous contributions to our understanding of the life cycle of morels. However, many questions remain to be answered, especially for the commercial cultivar. The main question is when the plasmogamy happens in true morels’ life cycle. For most basidiomycetes, somatic cell fusion is sufficient for mating. Homokaryotic hyphae containing different mating-type genes fuse (plasmogamy) when they meet each other to form strain GL0053 with two detectable nuclear genotypes hyphae. Karyogamy happens just before meiosis. Heterokaryotic hyphae of Basidiomycetes mushroom with tetrapolar elongate without karyogamy through a special clamp connection structure and are used as the seed for mushroom cultivation. For bipolar mating mushrooms and tetrapolar mating mushrooms, heterokaryotic hyphae usually grow faster than homokaryons [19]. Volk and Leonard hypothesize that morels (Morchella spp.) may employ a hyphal anastomosis mechanism similar to Basidiomycetes to form heterokaryons, a genetic characteristic that could potentially enhance the colonies’ adaptability to environmental changes [17]. However, Du’s research team demonstrated that heterokaryons only transiently form during a specific developmental phase preceding meiosis and ascospore production [9]. The indirect evidence for this is that type II mating gene distribution is dominant for natural morels. The sterile tissue has one MAT allele, while the fertile tissue harbors both mating types. Gene analysis results also proved that the fertile and sterile tissue of cultivated strains always exhibited both MAT alleles [8]. Moreover, unlike most edible Basidiomycetes, the heterokaryotic mycelia of Ascomycetes (including morels) lack clamp connections. This structural distinction makes it challenging to differentiate homokaryotic from heterokaryotic states in Morchella species. Critically, neither of these two hypotheses has been substantiated by direct experimental evidence. Resolving these questions would not only elucidate Morchella’s life cycle but also advance the development of stable inocula for morel cultivation. In the present study, the distribution of two mating-type idiomorphs during the vegetative growth and successive subculture of mycelia were determined. The mating type of the single hypha in mycelia and the fruiting body was also investigated by protoplast separation and regeneration. This study is an important complement to the life cycle of Morchella species.
2. Materials and Methods
2.1. Chemicals and Strains
Mannitol, dextrose, maltose, yeast extract, Congo red, and agar were purchased from Sangon Biotech Co. Ltd. (Shanghai, China). DAPI (4′,6-diamidino-2-phenylindole) was purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). Lywallzyme was purchased from the Guangdong Institute of Microbiology (Guangzhou, China). All other reagents used in this study were of analytical purity and commercially available.
Morchella sextelata GL0053 was isolated from Sichuan province and provided by Shandong Provincial Key Laboratory of Applied Mycology (Qingdao, China). Mycelia were routinely grown at 20 ± 2 °C in 120 mm diameter PDA (extract from 200 g potato, 20 g dextrose, 20 g agar) media as previous described [20]. Mycelial plugs (5 mm diameter) taken from the periphery of actively growing colonies were used to inoculate the center of a 120 mm plate with a new PDA medium [21]. During the 2018–2020 production seasons, our laboratory successfully cultivated M. sextelata strain GL0053 in vegetable plastic greenhouses in Qingdao, China. The cultivation protocol involved inoculating pre-cultured solid spawn into polypropylene bag substrates containing the following optimized formulation (dry weight basis): hardwood sawdust (40%), cottonseed hulls (25%), wheat bran (15%), rice husks (5%), corn flour (5%), gypsum (1%), sucrose (1%), and calcium carbonate (0.5%). Through standardized fruiting management protocols, maintaining temperature at 18–22 °C and relative humidity at 85%, we consistently obtained mature fruiting bodies within 60–75 days post-inoculation.
2.2. Homokaryotic Mycelia Separation and Mating Type Determination
Homokaryotic strains were separated from strain GL0053 with two detectable nuclear genotypes of strain GL0053. These may also contain heterokaryotic strains that cannot be stabilized. Mycelia of strain M. sextelata GL0053 were ground using a manual grinder, diluted with PBS buffer (pH 7.4), and spread on the PDA plates. The single colonies regenerated on the PDA plate were picked and inoculated into new PDA media, respectively.
Mating types of parent and isolated strains were determined by PCR approach using genomic DNA as the template, and the partial sequence of MAT1-1-1 and MAT1-2-1 genes were amplified, respectively. The genomic DNA of mycelia and fruiting body of morels was extracted using EZNA Fungal DNA Mini Kit (Omega Bio-Tek, Inc., Dayton, OH, USA) according to the instructions of the kit. Primers for amplification of MAT1-1-1 gene are mat1-F (5′-CCAGGAGGGTCGGTATTTCA-3′) and mat1-R (5′-TTGATAAAATCTTCGAGAGTA-3′). Primers for amplification of MAT1-2-1 gene are mat2-F (5′-ATGGGTCATATCAATCAGA-3′) and mat2-R (5′-GGCGAACAGCCTTCATCTCT-3′). PCR reactions were performed using Taq polymerase (Tiangen Biotech Co., Ltd., Beijing, China). The reaction mixture was subjected to the following thermal cycles: one cycle at 95 °C for 5 min; 35 cycles (95 °C, 30 s; 52 °C, 30 s; 72 °C, 48 s); and a final extension at 72 °C for 5 min. Agarose gel (1%) electrophoresis was performed to analyze the PCR product. The obtained homokaryotic strains were inoculated into a PDA slant medium and stored at 4 °C.
2.3. Determining the Mating Type of the Hypha by Protoplast Isolation
To determine the distribution of homokaryons and heterokaryons in the mycelia and fruiting body tissues of M. sextelata, protoplasts were isolated and regenerated into single colonies. Strain GL0053 was inoculated into a 120 mm potato dextrose broth (PDB) plate using a 5 mm cork borer for sample collection and incubated for 2 days. The cultured mycelia were washed three times with 0.6 mol/L NaCl to remove impurities, and the remaining liquid buffer was absorbed using sterilized filter papers. The fruiting body of strain GL0053 was harvested and washed with sterilized water thrice. The tissues from different parts of the fruiting body were cut into 1 mm × 1 mm blocks. Then, 0.1 g mycelia or tissue was transferred to a sterilized 2 mL centrifugation tube with 1 mL of sterilized lywallzyme solution (15 mg/mL, dissolved in 0.6 mol/L NaCl), and the tube was gently shaken at 30 °C for 3.5 h. The mycelia remnants were removed by filtration through absorbent cotton and then filtered through a 300 mesh screen to obtain protoplast suspension. The suspended protoplasts were precipitated at 600× g for 10 min. Then, the protoplasts were washed twice with the osmotic stabilizer (0.6 mol/L NaCl). The final protoplast pellet was suspended in 1 mL osmotic stabilizer, and the density of protoplasts was measured using a hemacytometer. According to the density of protoplasts, the re-suspended protoplasts were diluted with osmotic stabilizer to 104/mL. The protoplast suspension (100 μL) was mixed with 500 μL liquid regeneration medium and spread on the solid regeneration media plate. The plate was rotated to evenly distribute the protoplast suspension on the surface of the regeneration medium. Regeneration media contains (per liter) 15 g soluble starch, 10 g dextrose, 5 g maltose, 5 g yeast extract, 1 g KH2PO4, 0.5 g MgSO4, 31.15 g NaCl, and 20 g agar. The plate was cultivated at 20 °C for 3 to 4 days in darkness, and the single colony in the plate was picked up carefully and transferred to a new PDA plate with cellophane and cultured at 20 °C for 3–4 days. The mating type of the isolated strains was determined by PCR reaction as mentioned above.
2.4. Microscopic Observation
The sterilized coverslips were inserted into the edge of the mycelia grown on the PDA plate, and the mycelia that grew on the coverslips were used for confocal laser scanning microscope (CLSM) observation. The cell wall was stained by 1.0% (w/v) Congo red (dissolved in ddH2O), and DNA was stained using DIPA (10 μg/mL diluted with PBS buffer, pH 7.4). The mycelium was firstly immersed into DIPA for 30 min in darkness. The dye was allowed to run off, and a drop of Congo red was added on the coverslip for 1 min. The stained mycelia were washed with 1 × PBS three times. The CLSM TCS SP5 II (Leica Microsystems GmbH, Bensheim, Germany) was used to observe the morphology and structure of mycelia on the coverslip.
2.5. Quantification of Mycelia of Different Mating Types by Quantitative PCR (qPCR)
qPCR was used to quantify the distribution of two different mating-type idiomorphs, and the genomic DNA was used as the template. The primers for quantification of MAT1-1-1 gene are M1Q-F (5′-ATCAAGGGACGCTTACATC-3′) and M1Q-R (5′-CACAGTCCCAATAGCAAAG-3′), and primers for quantification of MAT1-2-1 gene are M2Q-F (5′-CCTCGTCCTATGAATGCGTAA-3′) and M2Q-R (5′-TTGAGGGATAAATGAGCAAC-3′). qPCR was performed in a volume of 15 μL on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific Inc., Waltham, MA, USA) with ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China) and primers. Threshold cycle (CT) values given by the software were normalized to the quantity of MAT1-1-1 and MAT1-2-1, respectively. Melt curve analyses were performed to validate the specificity of the PCR amplicons. Mating type distribution was calculated according to a 2-ΔΔCT method. The quantification of mycelia with MAT1-1-1 or mycelia with MAT1-2-1 gene was determined using standard curve methods. Briefly, the mycelia of strain GL0053-M1 and GL0053-M2 were mixed with different ratios of 1:10; 1:2, 1:1, 2:1, and 10:1, and the genomic DNA of the mixed mycelia was extracted. qPCR was performed with this genomic DNA as the template, and the Tm values of MAT1-1-1 gene and Tm MAT1-2-1 gene were used to construct a standard curve. All the experiments were repeated at least three times.
2.6. Determination of Mycelia Type Composition in Plates and Fruiting Body of Morels
To determine the composition of two mating-type idiomorphs in different parts of mycelia, the 4-day-old mycelia culture on a PDA plate with cellophane was divided into three equal parts according to the radius: the inner part, the middle part, and the outer part. There was no overlap between the three parts. Mycelia from different parts were cut out from cellophane, and the composition of the two mycelia mating types was determined using qPCR as mentioned above.
The composition of two mating-type idiomorphs during successive subcultures on the PDA plate was determined. The first generation of the mycelia used in this process was isolated from the fruiting body of the M. sextelata, which was inoculated in the center of a PDA plate with cellophane. The plates were cultured at 20 °C for 2–3 days until the mycelia covered the whole plate. For the subculture, a mycelia plug (5 mm diameter) was cut from the margin of an old agar culture and placed in the center of the new medium. Mycelia of the 1st, 5th, 10th, 15th, and 20th generations were scratched from the plates, and the composition of the two mating-type idiomorphs was determined using qPCR as mentioned above.
To determine the composition of the two mating-type idiomorphs in different parts of the M. sextelata fruiting body, three different parts (inside of pileus, outside of, and stipe) were cut from the fresh mature fruiting body of M. sextelata GL0053. The genomic DNA of fruiting body tissues was extracted using EZNA Fungal DNA Mini Kit (Omega Bio-Tek, Norcross, GA, USA), and the composition of the two mycelia mating types was determined using qPCR as mentioned above.
2.7. Measurement of Mycelium Growth Rate
The 3-day-old cultures of homokaryotic strains (strain GL0053-M1 and strain GL0053-M2) and multinucleated body (strain GL0053) grown on PDA plate were inoculated on the new PDA plate using a 5 mm perforator, respectively. Each group had five replicates, the mycelia diameter was measured in turn every 12 h, and the growth rate (cm/day) was calculated.
2.8. Statistical Analysis
All experiments were performed with at least three replicates. The mycelia in the plate experiments in this study were of the 1st or 2nd generation if not indicated specifically. Statistic differences between each group were analyzed by t-test for two independent means performed using Pandas software (2.1.3 version), and the plots were drawn using ggplot2 software (3.4.3 version).
3. Results
3.1. Structure of Two Mating-Type Hyphae
M. sextelata strain GL0053 was isolated from the inside tissue of M. sextelata fruiting body pileus. Both mating-type genes were detected in strain GL0053. Strains GL0053-M1 and GL0053-M2 were isolated from the mycelia of strain GL0053, and the mating types of strain GL0053-M1 and GL0053-M2 were determined by PCR. Strain GL0053-M1 only contains the MAT1-1-1 gene, while strain GL0053-M2 only contains the MAT1-2-1 gene. The hypha structure of strain GL0053-M1 (Figure 1a) and GL0053-M2 (Figure 1b) was observed to evaluate the difference of cell structure between two mating-type strains. The homokaryotic hyphae have clear septa, and multiple nuclei in one hyphal cell. There was no visible difference between the two hyphae.
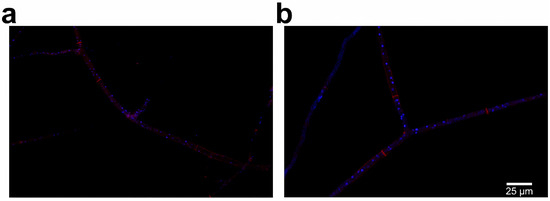
Figure 1.
Hyphae of homokaryotic strains with DAPI and Congo red staining observed under laser confocal fluorescence microscope: (a) hyphae of strain GL0053-M1 with MAT1-1 mating type; (b) hyphae of strain GL0053-M2 with MAT1-2 mating type.
3.2. Distribution of Two Mating-Type Mycelia in Vegetative Growth
Detecting two mating-type genes based on PCR results is one of the key means to evaluate the quality of the Morchella spawn during the production process. However, uneven distribution of two mating-type mycelia may also affect the quality of spawn, which could not be detected by normal PCR. In the present study, qPCR technology was used to detect the distribution of two mating-type idiomorphs in different areas of mycelia that grew on PDA plate and during the successive subculture processes.
Both mating-type genes were detected in all three parts of the mycelia in the PDA plate. Figure 2 shows that the proportion of the MAT1-1-1 gene is larger than that of the MAT1-2-1 gene in all three parts. The ratios of MAT1-1-1/MAT1-2-1 in the inner part, middle part, and outer part of the mycelia were 1.74, 1.11, and 1.07, respectively, while there were no statistical differences in the ratios of MAT1-1-1/MAT1-2-1 between the three parts of the mycelia in the plate.
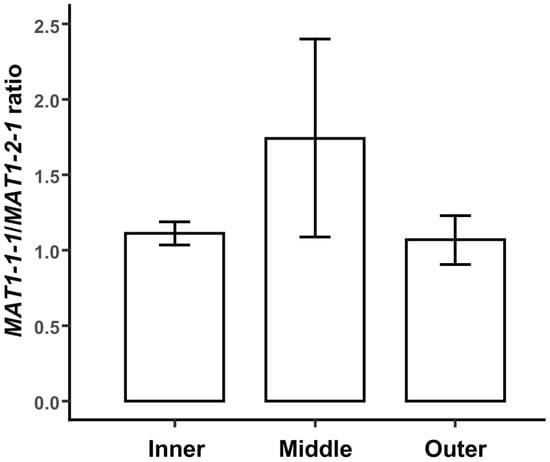
Figure 2.
The distribution of two mating-type hyphae in different parts of M. sextelata mycelia grown in PDA plates. Each value is the mean of five parallel replicates ± SD.
The quality of edible mushroom spawn can decrease during successive subcultures. To evaluate the stability of distribution of MAT1-1-1 and MAT1-2-1 genes during successive subcultures, the two genes were quantitatively detected in the mycelia of the 1st, 5th, 10th, 15th, and 20th generations using qPCR. Both mating-type genes were detected in all five samples, indicating that the two types of idiomorphs were not lost during successive subcultures within 20 generations. As with the results from different parts of mycelia on the PDA plate, the proportion of the MAT1-1-1 gene was larger than that of the MAT1-2-1 gene in all generations. Furthermore, the ratio of MAT1-1-1/MAT1-2-1 increased with the increase of generation (Figure 3). Idiomorphs with MAT1-1-1 mating type dominated during the vegetative growth of mycelia on PDA plates.
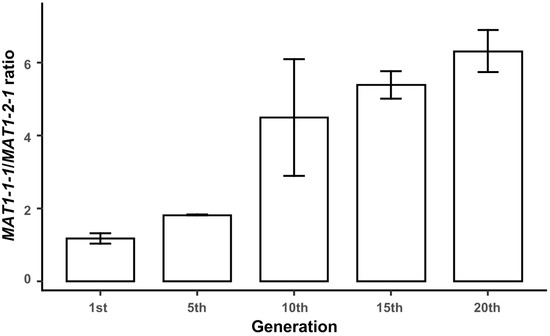
Figure 3.
Proportion of two mating-type hyphae during continuous subculture on PDA plate. Each value is the mean of five parallel replicates ± SD.
3.3. Growth of Strain GL0053 with Two Detectable Nuclear Genotypes and Homokaryotic Strains in PDA Plates
To investigate if the disproportion between MAT1-1-1 and MAT1-2-1 was caused by the different growth rates of the two mating-type mycelia, the growth rates of strains GL0053, GL0053-M1, and GL0053-M2 on PDA plates were measured. The growth curves of strains GL0053-M1 and GL0053-M2 were almost the same. The growth rate of strain GL0053 was lower than those of strain GL0053-M1 and strain GL0053-M2 during the first 12 h, while it showed almost the same growth rate later (Figure 4a). Growth rate based on colony diameters is shown in Figure 4b. The results show that the growth rate of GL0053 was significantly lower than those of GL0053-M1 and GL0053-M2 between 0 h to 15 h, while there was no significant difference in growth rate between the three strains after 15 h. These findings suggest that the observed fluctuations in hyphal ratio may result from differential growth rates between the two mating-type hyphae during distinct developmental phases.
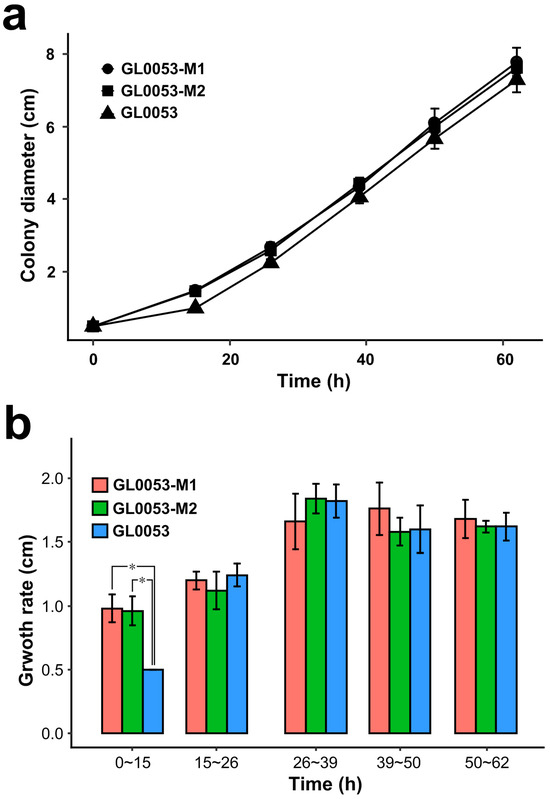
Figure 4.
Growth measurement of GL0053, GL0053-M1, and GL0053-M2 on the plate. (a) The diameters of three strains grown on PDA plates. (b) The growth rate of three strains grown on PDA plates. Each value is the mean of five parallel replicates ± SD. *: p < 0.05.
3.4. Distribution of Two Mating Types in Different Parts of the Cultivated Fruiting Body of Morels
Figure 5 showed the distribution of two mating-type hyphae in three different parts of the fruiting body of cultivated M. sextelata: the stipe, the inside of pileus, and the outside of pileus. Both mating-type genes were detected in all the tested samples; however, the ratio of MAT1-1-1/MAT1-2-1 is quite different among different samples. Overall, after the removal of outliers, the idiomorphs of MAT1-1-1 were dominant in the stipe area, while the ratios of MAT1-1-1/MAT1-2-1 in the pileus area were close to 1:1. However, for every single sample from the pileus area, one mating type gene always predominated in the sample.
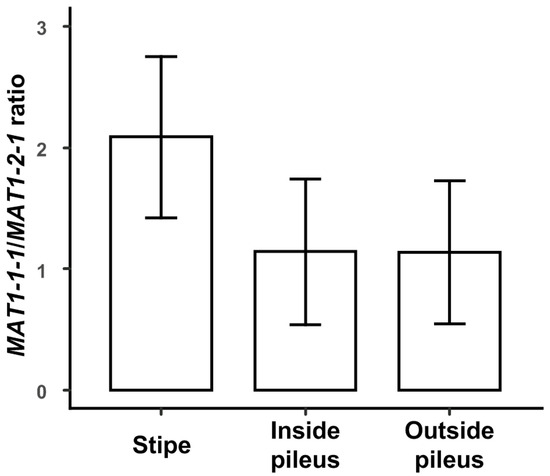
Figure 5.
Distribution of two mating types in different parts of the mature cultivated fruiting body of M. sextelata. Each value is the mean of nine parallel replicates ± SD.
3.5. Hyphae Isolation and Protoplasts Isolation
To further investigate the proportion of homokaryotic hyphae in the vegetative growth and fruiting process of M. sextelata, protoplasts from strain GL0053 mycelia grown on PDA plate and fruiting bodies were prepared and regenerated by spreading onto regeneration media plates after dilution. Single colonies on regeneration media plates were picked up carefully and inoculated into the new PDA plate for each colony. The mating type of the regenerated colony was detected by PCR. A total of 69 regenerated single colonies were obtained from mycelia grown on PDA plates, among which 25 were MAT1-1-type strains, 23 were MAT1-2-type strains, and 21 were double-mating-type strains (Figure 6a). Thirty-two regenerated single colonies were obtained from the stipe of mature M. sextelata mature fruiting body, among which 16 were MAT1-1-type strains, 10 were MAT1-2-type strains, and 6 were double-mating-type strains (Figure 6b). Twenty-seven and eight regenerated single colonies were obtained from inside and outside pileus of mature M. sextelata mature fruiting body, respectively, among which 13 and 2 were MAT1-1-type strains, 11 and 2 were MAT1-2-type strains, and 3 and 4 were double-mating-type strains, respectively (Figure 6b).
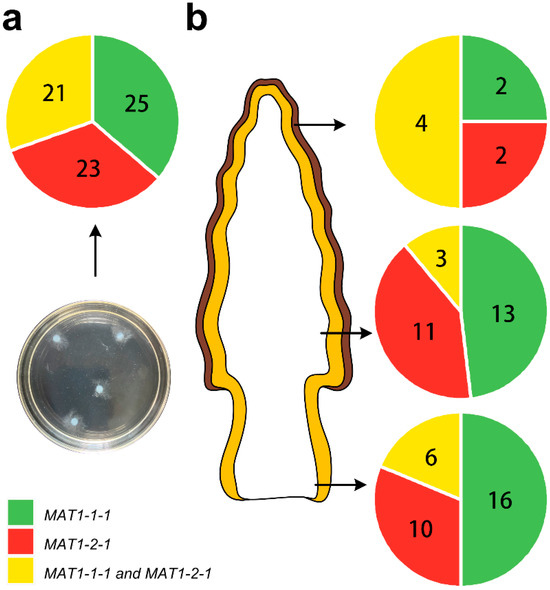
Figure 6.
Distribution of homokaryotic and heterokaryotic hyphae in vegetative growth and fruiting process of M. sextelata. (a) The protoplasts of hyphae were prepared and regenerated, and the mating type of the isolated single colony was detected by PCR. (b) The distributions of different mating-type hyphae are presented with different colors as indicated.
4. Discussions
The instability of spawn, also known as strain degeneration, is one of the important reasons that make the production of true morels a high-risk economic endeavor. Tissue isolation and subculture technologies are one of the major methods used to obtain and keep the producing spawn for edible mushroom cultivation, which has been mastered by most of the growers. The area between stipe and pileus is the primary choice for tissue isolation; however, success does not always occur in the case of true morels. Detecting the mating types of the producing spawn by PCR, which ensures the existence of both mating-type idiomorphs, is one of the main techniques seed companies can use to maintain the quality of the spawn. Such detection methods are generally not required during spawn production for most edible Basidiomycete mushrooms. This raises an important question: What are the consequences if the ratio between the two mating-type idiomorphs (undetectable by conventional PCR methods) fluctuates during M. spawn production? To date, however, the proportion of the two mating-type idiomorphs in the life cycle of true morels, especially during spawn production, is still not very clear. In the present study, we detected the proportion of two mating-type idiomorphs in different parts of the true morels’ fruiting body. Mycelia with both mating-type genes could be detected in all detected tissues, although the proportion was not simply 1:1, as shown in Figure 5. Indeed, the disproportion of the two mating types in the fruiting body was more unstable than is presented in Figure 5. We analyzed the ratio of MAT1-1-1/MAT1-2-1 in the small, middle, and mature fruiting body of M. sextelata, and the ratio was larger than 50 in the middle fruiting body. In some cases, the ratio of MAT1-1-1/MAT1-2-1 in stipe was larger than 1000, while it was less than 0.004 in the pileus area. Therefore, tissue isolation from the disproportionate part of the fruiting body may lead to the disproportion of two mating-type mycelia in the spawn, which may be one of the causes of the degeneration of the true morels’ spawn.
The disproportion of the two mating types could not explain the easy loss of one mating-type idiomorph during spawn production. To find out, the proportion of two mating types during vegetative growth was measured. The disproportion of the two mating idiomorphs could be observed in almost all tested mycelia on PDA plates. The ratio of MAT1-1-1/MAT1-2-1 increased with the increasing of subculture generations. The results indicated that the disproportion of two mating-type hyphae by subculture was one of the causes of the degeneration of the true morels’ spawn.
As detected, the M. sextelata hypha has a multi-nucleus cell structure. The disproportion of two mating-type idiomorphs may be due to the different replication speeds of the two mating dikaryotic/heterokaryotic types’ cell nucleus in the heterokaryotic cells, and it may also due to the coexistence of and the disproportion between two mating-type homokaryotic hyphae in the tested samples. To be more exact, plasmogamy may not happen during the vegetative growth of M. sextelata. Firstly, the growth rate of GL0053, GL0053-M1, and GL0053-M2 was compared. As we know, for both bipolar mating mushrooms and tetrapolar mating mushrooms, heterokaryotic mycelia usually grow faster than homokaryons [19]. The results indicated that GL0053 did not show a higher growth rate than the homokaryotic strains. Furthermore, the mating type of single cells was detected by protoplast preparation, regeneration, and single colony isolation. Most (73.4%) of the isolated colonies were homokaryotic strains, while only 26.6% were heterokaryotic strains. Considering the isolation process could not strictly ensure the picked colony was a single colony, the proportion of homokaryotic strains would be higher than we detected. The results confirmed our hypothesis that (at least most of) M. sextelata mycelia did not fuse to form heterokaryotic mycelia. In the cross-mating culture, mycelia with the mating type of MAT1-1 may have similar tip growth to that of MAT1-2 and have stronger branching growth than that of MAT1-2-1.
The mating type of single cells in the fruiting body was also detected, and 81.3% and 88.9% of the isolated strain were homokaryotic strains in the stipe and inside pileus, respectively. The proportion in the outside cap was 50%. All the ascospores could not be removed from this tissue; therefore, the ratio of the outside cap has reference value only. Still, the results confirmed that two mating-type mycelia grow independently without plasmogamy throughout most of the life cycle. Even if there is plasmogamy during the vegetative growth or fruiting process, the heterokaryotic cells are temporary, unstable, and easy to separate into homokaryotic cells during cell division and growth. Only when M. sextelata produces ascospores do the cells with different mating types fuse, followed by nuclei fusion, meiosis, and haploid spore formation. The plasmogamy has more to do with the stimulation of ascocarp formation than obtaining vegetative growth advantages.
These results lead to another question: If heterokaryotic hyphae are not the main phase of the life cycle, what is the role of the two mating-type hyphae in the morels’ life cycle? Du et al. mentioned that, besides ascospores, MAT1-1-1 is more common in the stipe than MAT1-2-1 in natural populations, indicating a different competitive advantage for two-mating-type strains [8]. The temporary heterokaryon mycelia cannot be excluded, and the plasmogamy may be temporary to stimulate the fruiting body formation. The role of two-mating-type strains in the fruiting process of true morels needs more investigation.
Strain degeneration after successive subculture in mushroom spawn leads to a decrease in yield, fruiting time, and stress resistance, or worse, a complete incapability of the fruiting process [22,23,24,25]. The reasons for strain degeneration include genetic mutation, epigenetic factors, and environmental factors [23,25]. Strain degeneration is more common in non-tetrapolar species, such as Agaricus bisporus, Volvariella volvacea, and especially for mushrooms from Ascomycetes, such as Cordyceps militaris and Morchella. The subcultured C. militaris strains degenerated beginning at the third generation, had incomplete fruiting bodies at the fourth generation, and completely lost the ability to produce fruiting bodies in the fifth generation [24]. Interestingly, the missing MAT1-2-1 idiomorph was also reported in another Ascomycetes edible mushroom, Cordyceps militaris. However, the dynamic change of MAT1-1-1 and MAT1-2-1 was not reported [26]. The results suggested that life cycles are one of the main factors that influence the stability of mushroom spawn. The detailed nuclear behavior during meiosis and ascospore genesis of Morchella has been reported by He et al. [27]. Yuan et al. [28] elucidated the asexual reproduction processes of Morchella. The life cycle of Morchella from basidiospore to fruiting process has been reported in detail by Du et al. [9]. However, the processes from plasmogamy to karyogamy in the Morchella life cycle are still not very clear. Our study partially filled this gap.
The use of a self-compatible strain is uncommon during mushroom production and unfriendly to breeding for species of the tetrapolar mating system. However, considering the unique life cycle of morels, generating inbred lines may be a good choice for stable true morel seed breeding. A mutation that generated an HD1-HD2 gene fusion in C. cinereus generated a self-compatibility and constitutive sexual development strain. Therefore, an artificial transformation of the MAT1-2-1 gene into homokaryotic hypha containing MAT1-1-1 might produce a homothallic strain. In addition, spawn production by tissue isolation is not recommended for Morchella strains, while keeping the two mycelia mating types separate and mixing them in specific proportions during spawn production is a preferred method. However, this approach is currently subject to research.
5. Conclusions
In the life cycle of Morchella sextelata, two mixed homokaryons with different mating types grow together and independently without cell fusion during the vegetative growth and fruiting process. It is only before the production of ascospores that two homokaryons fuse to form the heterokaryon, followed by the formation of ascus. It can be inferred from this study that the disproportion of two mycelia mating types is the main reason for the degeneration of the spawn obtained by tissue separation. The increased disproportion during successive subcultures leads to strain degeneration during subculture or spawn production. qPCR or half-quantitative PCR is better technology for spawn quality measurement than normal PCR. Therefore, the development of breeding techniques for self-compatible strains or the exploration of separate preservation methods for homokaryotic strains should be investigated.
Author Contributions
D.Z.: writing—original draft, writing—review and editing, methodology, software, visualization, supervision, formal analysis. J.W.: software, methodology, formal analysis, supervision, visualization, writing—original draft, writing—review and editing. X.L.: writing—review and editing, investigation. X.G. and Y.Z.: writing—review and editing, resources. W.L., L.X. and M.L.: writing—review and editing, data curation. H.Y.: conceptualization, writing—review and editing, funding acquisition, validation, writing—original draft, project administration. X.Y.: conceptualization, validation, funding acquisition, project administration, writing—review and editing, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Shandong Provincial Natural Science Foundation, China (ZR2024MC127); Key R&D Program of Shandong Province, China (2022LZGC023); and Shandong Edible Fungus Agricultural Technology System (SDAIT-07-02).
Institutional Review Board Statement
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability Statement
All the data are presented in the paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Huo, W.; Feng, Z.; Hu, S.; Cui, L.; Qiao, T.; Dai, L.; Qi, P.; Zhang, L.; Liu, Y.; Li, J. Effects of polysaccharides from wild morels on immune response and gut microbiota composition in non-treated and cyclophosphamide-treated mice. Food Funct. 2020, 11, 4291–4303. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, H.; Süfer, Ö.; Attar, Ş.H.; Bozok, F.; Baktemur, G.; Büyükalaca, S.; Kafkas, N.E. Total phenolics, antioxidant activities and fatty acid profiles of six Morchella species. Int. J. Food Sci. Tech. 2021, 58, 692–700. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Kang, Z.; Wu, Y.; Xing, Y.; Yang, Y. Antioxidant and anti-tumour activity of triterpenoid compounds isolated from Morchella mycelium. Arch. Microbiol. 2020, 202, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Masaphy, S. Biotechnology of morel mushrooms: Successful fruiting body formation and development in a soilless system. Biotechnol. Lett. 2010, 32, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Ower, R.D. Notes on the development of the morel ascocarp: Morchella esculenta. Mycologia 1982, 74, 142–144. [Google Scholar] [CrossRef]
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J.; et al. Multi-omic analyses of exogenous nutrient bag decomposition by the black morel Morchella importuna reveal sustained carbon acquisition and transferring. Environ. Microbiol. 2019, 21, 3909–3926. [Google Scholar] [CrossRef]
- Chai, H.; Chen, L.; Chen, W.; Zhao, Q.; Zhang, X.; Su, K.; Zhao, Y. Characterization of mating-type idiomorphs suggests that Morchella importuna, Mel-20, and M. sextelata are heterothallic. Mycol. Prog. 2017, 16, 743–752. [Google Scholar] [CrossRef]
- Du, X.H.; Zhao, Q.; Xia, E.H.; Gao, L.Z.; Richard, F.; Yang, Z.L. Mixed-reproductive strategies, competitive mating-type distribution and life cycle of fourteen black morel species. Sci. Rep. 2017, 7, 1493. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Z.; Dong, H.; Dong, C. Reactive oxygen species induce sclerotial formation in Morchella importuna. Appl. Microbiol. Biot. 2018, 102, 7997–8009. [Google Scholar] [CrossRef]
- Amir, R.; Levanon, D.; Hadar, Y.; Chet, I. Factors affecting translocation and sclerotial formation in Morchella esculenta. Exp. Mycol. 1995, 19, 61–70. [Google Scholar] [CrossRef]
- Kanwal, H.K.; Reddy, M.S. The effect of carbon and nitrogen sources on the formation of sclerotia in Morchella spp. Ann. Microbiol. 2012, 62, 165–168. [Google Scholar] [CrossRef]
- Brown, A.J.; Casselton, L.A. Mating in mushrooms: Increasing the chances but prolonging the affair. Trends Genet. 2001, 17, 393–400. [Google Scholar] [CrossRef]
- Pöggeler, S. Mating-type genes for classical strain improvements of ascomycetes. Appl. Microbiol. Biot. 2001, 56, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Pöggeler, S. Phylogenetic relationships between mating-type sequences from homothallic and heterothallic ascomycetes. Curr. Genet. 1999, 36, 222–231. [Google Scholar] [CrossRef]
- Vreeburg, S.; Nygren, K.; Aanen, D.K. Unholy marriages and eternal triangles: How competition in the mushroom life cycle can lead to genomic conflict. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150533. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.J.; Leonard, T.J. Cytology of the life-cycle of Morchella. Mycol. Res. 1990, 94, 399–406. [Google Scholar] [CrossRef]
- Shen, Q.; Li, C.; Xie, M.; Li, W.; Qian, Z.; Zhang, J. A novel PCR-based approach for rapid identification of Morchella sextelata using species-specific primers. Curr. Microbiol. 2020, 77, 232–237. [Google Scholar] [CrossRef]
- Sonnenberg, A.S.; Baars, J.J.; Gao, W.; Visser, R.G. Developments in breeding of Agaricus bisporus var. bisporus: Progress. made and technical and legal hurdles to take. Appl. Microbiol. Biot. 2017, 101, 1819–1829. [Google Scholar] [CrossRef]
- Xu, L.; Guo, L.; Yu, H. Label-free comparative proteomics analysis revealed heat stress responsive mechanism in Hypsizygus marmoreus. Front. Microbiol. 2020, 11, 3359. [Google Scholar] [CrossRef]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. evaluation of biomass and chitin production of Morchella mushrooms grown on starch-based substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Liu, X.; Cui, B.; Miao, W.; Cheng, W.; Zhao, F. Characteristics analysis reveals the progress of Volvariella volvacea mycelium subculture degeneration. Front. Microbiol. 2019, 10, 2045. [Google Scholar] [CrossRef]
- Horgen, P.A.; Carvalho, D.; Sonnenberg, A.; Li, A.; Van Griensven, L.J.L.D. Chromosomal abnormalities associated with strain degeneration in the cultivated mushroom, Agaricus bisporus. Fungal Genet. Biol. 1996, 20, 229–241. [Google Scholar] [CrossRef]
- Yin, J.; Xin, X.; Weng, Y.; Gui, Z. Transcriptome-wide analysis reveals the progress of Cordyceps militaris subculture degeneration. PLoS ONE 2017, 12, e0186279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hu, J.; Chi, J.; Li, Y.; Yang, B.; Hu, W.; Chen, F.; Xu, C.; Chai, L.; Bao, Y. Label-free proteomics reveals the molecular mechanism of subculture induced strain degeneration and discovery of indicative index for degeneration in Pleurotus ostreatus. Molecules 2020, 25, 4920. [Google Scholar] [CrossRef]
- Yin, J.; Xin, X.D.; Weng, Y.J.; Li, S.H.; Jia, J.Q.; Gui, Z.Z. Genotypic analysis of degenerative Cordyceps militaris cultured in the pupa of Bombyx mori. Entomol. Res. 2018, 48, 137–144. [Google Scholar] [CrossRef]
- He, P.; Wang, K.; Cai, Y.; Liu, W. Live cell confocal laser imaging studies on the nuclear behavior during meiosis and ascosporogenesis in Morchella importuna under artificial cultivation. Micron 2017, 101, 108–113. [Google Scholar] [CrossRef]
- Yuan, B.H.; Li, H.; Liu, L.; Du, X.H. Successful induction and recognition of conidiation, conidial germination and chlamydospore formation in pure culture of Morchella. Fungal Biol. 2021, 125, 285–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).