Abstract
Strawberry fruits accumulate nutritionally critical anthocyanins and phytochemicals through light=quality-dependent metabolic regulation. This review systematically examines spectral modulation strategies for enhancing anthocyanin biosynthesis and fruit quality parameters. We demonstrate that dual red (660 nm) and blue (450 nm) irradiation optimally activates the flavonoid pathway, co-upregulating structural genes (CHS, F3H, DFR, ANS) and regulatory factors (FaMYB10, FaHY5). Mechanistic analyses reveal that blue light preferentially induces upstream phenylpropanoid enzymes (PAL, C4H, CHI), while red light enhances proanthocyanidin production through differential induction of LAR and ANR. Strategic supplementation with UV-C (254 nm, 1–2 kJ/m2/d) and far-red (730 nm, 15 μmol·m−2·s−1) improves anthocyanin spatial distribution via stress-mediated epidermal accumulation. Spectral optimization further coordinates flavor development by (1) balancing sucrose–hexose ratios through FaSPS1 modulation, (2) reducing organic acid content via FaMYB44.2 suppression, and (3) amplifying volatile esters (e.g., methyl anthranilate) through SAAT induction. Postharvest UV-C treatment (4 kJ/m2) extends shelf life by 30–35% through microbial inhibition and antioxidant system activation. Practical implementation frameworks propose phase-specific LED protocols related to vegetative growth (R:B = 3:1), flowering (R:B = 1:1), and maturation (R:B = 4:1) stages integrated with environmental sensors in controlled agriculture systems. These findings establish an actionable paradigm for photonic crop management, synergizing molecular precision with commercial horticultural operations to achieve sustainable yield enhancement (projected 22–28% increase) and nutraceutical enrichment.
1. Introduction
Strawberries (Fragaria × ananassa Duch.) represent a globally significant berry crop prized for their sensory appeal and nutritional richness. Beyond their well-documented content of vitamins, minerals, and dietary fiber [1], these fruits accumulate diverse secondary metabolites, including anthocyanidins, flavanols, and biflavones [2]. The characteristic red pigmentation of ripe strawberries, primarily mediated by anthocyanins, serves dual biological and commercial functions. These compounds not only attract pollinators and consumers but also demonstrate therapeutic potential in mitigating cardiovascular disorders, neurodegenerative diseases, and carcinogenesis [3]. Fruit quality parameters—particularly flavor profiles governed by sugar–acid balance and volatile organic compounds—are increasingly recognized as critical determinants of consumer preference and market value.
Modern strawberry production faces multifaceted challenges in meeting escalating demand for premium-grade fruit. While traditional cultivation systems (open-field and greenhouse operations) rely on solar radiation [4,5], practical limitations emerge from (1) light attenuation through protective structures (18–37% PAR reduction) and atmospheric interference, (2) arthropod-pest-related yield losses (22–35% annually), and (3) contamination risks from agrochemical residues (organophosphates ≤ 0.78 mg/kg; Cd ≤ 1.2 μg/g DW) [6,7]. These constraints have driven innovation in spectral engineering using wavelength-specific LEDs, which enable precise modulation of photomorphogenic and phytochemical responses.
Recent advances elucidate the molecular mechanisms underlying light-mediated quality enhancement. Blue spectra (450–470 nm) activate cryptochrome isoforms (FaCRY1/2) [8], triggering phosphorylation cascades that stabilize FaHY5—a bZIP transcription factor binding E-box motifs in the FaMYB10 promoter. This regulatory network upregulates phenylpropanoid pathway genes (CHS, DFR, ANS), elevating pelargonidin-3-glucoside concentrations by 28–35% versus solar-grown controls [9]. Concurrently, red light (660 nm) induces conformational changes in phytochrome A (FaPHYA) enhancing vacuolar sucrose transport (FaTST1) to achieve soluble solid content of 18.2 ± 0.8°Brix (+18.2%) while suppressing malate dehydrogenase (FaMDH2) expression (−14.3% malic acid) [10,11]. Far-red wavelengths (730 nm) appear to synchronize ripening through FaPIF-mediated ethylene response factor (FaERF2) modulation, although mechanistic details remain incomplete [10].
Despite these technological strides, three critical knowledge gaps impede commercial implementation. First, extended photoperiods (≥14 h at 200 μmol·m−2·s−1) elevate canopy humidity (RH ≥ 85%), predisposing fruits to Botrytis cinerea infection (35.2% incidence vs. 12.1% field levels) [12]. Second, disproportionate red spectrum exposure (R:B > 3:1) combined with excessive ammonium nitrate application (≥200 kg/ha) degrades flavor metrics (sugar: acid ≤ 9.7 vs. optimal 12–14). Third, energy-intensive protocols inflate production costs by USD 1.05/kg [13], undermining economic viability. Emerging hybrid strategies address these limitations through UV-C irradiation (280 nm, 2 kJ·m−2) for pathogen suppression and dynamic R:B ratio adjustment (4:1 vegetative; 7:3 fruiting) [14], achieving 91.7% marketable yield with preserved anthocyanin content (19.3 mg/g FW) via FaGST-mediated stabilization [15].
This review synthesizes two interconnected themes: (1) molecular regulation of anthocyanin biosynthesis in strawberry, focusing on enzymatic pathways (e.g., CHS, DFR, ANS) and transcriptional networks mediated by MYB-bHLH-WD40 complexes, with an emphasis on photoreceptor signaling (FaPHYs, FaCRYs, FaPHOTs, FaUVR8) and their downstream effectors (FaPIFs, FaCOP1, FaHY5) under spectral stress [16,17,18,19], and (2) light-mediated quality modulation by analyzing wavelength-specific impacts on antioxidant metabolism (e.g., phenolic acids, flavonols), flavor volatiles (e.g., furaneol, mesifurane), and stress resilience, particularly the superior efficacy of blue (450–495 nm) and red (620–700 nm) spectra in mitigating salinity–alkalinity stress [20,21,22].
2. Molecular Regulation of Anthocyanin Biosynthesis in Strawberry: Enzymatic Pathways and Transcriptional Networks
Anthocyanins, important phenolic substances, are responsible for the colors of flowers and fruits. Their stability is influenced by light, temperature, pH, and other flavonoids. These pigments exhibit red color under acidic conditions and blue color under alkaline conditions. In strawberries, pelargonidin 3-glucoside and cyanidin 3-glucoside are the major anthocyanins, with pelargonidin 3-glucoside being more abundant. Anthocyanins accumulate rapidly during the later stages of fruit ripening in strawberries, leading to a change from white to red. This accumulation is not only associated with UV protection but also occurs under visible light and far-infrared radiation stress conditions [16,17].
The anthocyanin synthesis pathway is an extension of the general flavonoid pathway. It begins with PAL-mediated phenylalanine in the phenylpropanoid pathway to synthesize cinnamic acid (Figure 1). This is followed by the synthesis of 4-coumaroyl-CoA, catalyzed by the enzymes encoded by cinnamate 4-hydroxylase (C4H) and 4-coumarate: CoA ligase (4CL). Chalcone synthase (CHS) is the key enzyme that initiates flavonoid biosynthesis by inducing the synthesis of chalcone from 4-coumaroyl-CoA. Chalcone is then isomerized to naringenin by CHI. Flavonoid 3′-hydroxylase (F3′H) and flavonoid 5′-hydroxylase (F5′H) are the main enzymes responsible for diversifying anthocyanins, with the color being determined by their B-ring hydroxylation. Naringenin flavanone is converted to dihydroflavonols by F3H, which can be further hydroxylated by F3′H or flavonoid 3′5′-hydroxylase (F3′5′H) to dihydroquercetin and dihydromyricetin, respectively. These three dihydroflavonols can be converted by dihydroflavonol 4-reductase (DFR) to colorless leucoanthocyanins, which can then be converted by anthocyanin synthase (ANS, also known as LDOX) to the colored anthocyanins cyanidin, delphinidin, and pelargonidin. Sugar molecules are linked to anthocyanins through various glycosyltransferase enzymes, such as flavonoid 3-o-glycosyltransferases (UFGT), to facilitate the transformation of dihydro flavonols into anthocyanins of diverse hues. These anthocyanins can also undergo further modification by attaching aromatic acyl groups through acyltransferases. Subsequently, anthocyanins are transported from the endoplasmic reticulum to vesicles by GSTS or MATE proteins. Within the anthocyanin synthesis pathway, leucoanthocyanins and other colored anthocyanins can be converted into proanthocyanidins (PAs) with the help of enzymes like leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR). Despite ripe strawberries having high anthocyanin levels, the PA content is low and decreases significantly during development [18,19].
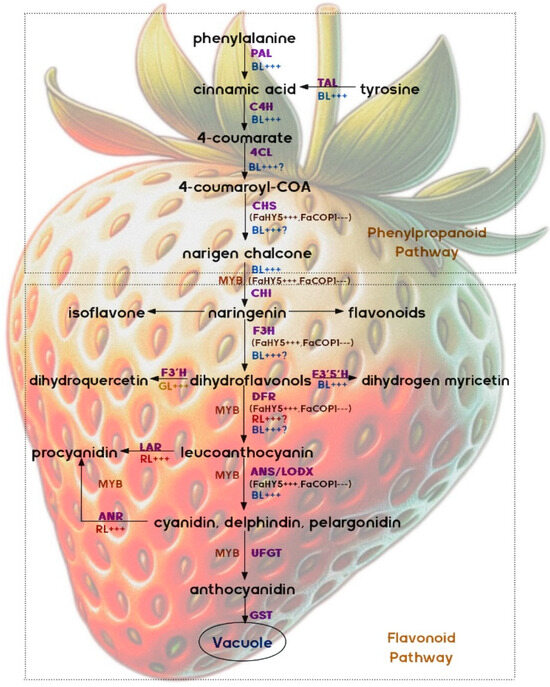
Figure 1.
Anthocyanin synthesis pathway. (PAL: phenylalanine ammonia-lyase; TAL: tyrosine ammonia-lyase; C4H: cinnamate 4-hydroxylase; 4CL: 4-coumarate: COA ligase; CHS: chalcone synthase; CHI: chalcone isomerase; F3H: flavanone 3-hydroxylase; F3′H: flavonoid 3′-hydroxylase; F3′5′H: flavonoid 3′5′-hydroxylase; DFR: dihydro flavonol 4-reductase; LAR: leucoanthocyanidin reductase; ANS: anthocyanidin synthase; LDOX: leucoanthocyanidin dioxygenase; ANR: anthocyanidin reductase; UFGT: UDP-glucose: flavonoid 3-O-glucosyltransferase; GST: Glutathione S-Transferase; MYB: MYB-Type transcription factor; BL: blue light; RL: red light; FaHY5: Elongated Hypocotyl 5; FaCOP1: Constitutive Photomorphogenic 1; +++: promote; ---: restrain; the use of purple font indicates the presence of an enzyme; the brown character “MYB” represents the “MYB pathway”; the use of blue, red, and green fonts is employed to represent the various qualities of light; the symbol “?” is used to indicate uncertainty or a lack of information. The abbreviation stands for the Latin phrase “maybe”).
A regulatory complex known as the MYB-bHLH-WD40 (MBW) complex controls the structural genes of the anthocyanin biosynthesis pathway. This complex is composed of the MYB, basic helix–loop–helix (bHLH), and WD40 protein families [23,24]. R2R3-MYB transcription factors (TFs) interact directly with the promoters of key flavonoid biosynthesis genes, including CHI, DFR, ANS, LAR, ANR, and UFGT. FaMYB10, a gene specific to strawberry ripening in the receptacle, is positively regulated by light. Its encoded protein activates the expression of UFGT, DFR2, and DFR1 genes [25,26]. FaMYB10 expression is low in early strawberry development stages but significantly increases during the red ripening stage. FaMYB10L also influences anthocyanin accumulation in strawberries, albeit to a lesser extent than FaMYB10. Overexpression of FaMYB10 in fruit results in a more purple hue, accompanied by increased expression of FaMYB10, UFGT, and DFR2, leading to higher levels of anthocyanin-3, 5-glucoside, and anthocyanin-3-glucoside. FaMYB1, expressed during the red ripening stage of strawberry fruit, acts as a negative regulator of anthocyanin and flavonol metabolism, helping to maintain a balance in anthocyanin levels during late ripening [18]. Apart from MYB repressors, WRKY TFs and microRNAs (miRNAs) also play roles in anthocyanin synthesis [27]. FaWRKY71 is implicated in regulating ripening in octoploid strawberries, promoting the accumulation of various genes like FaF3′H, FaLAR, FaANR, FaTT19, and FaTT12, thereby increasing anthocyanin content in the fruit. FaWRKY71 is most highly expressed in all-red fruits. miRNAs down-regulate anthocyanin biosynthesis through post-transcriptional effects [28].
2.1. Regulation of Strawberry Anthocyanins Using Visible Light
Optimal light conditions play a crucial role in the growth and development of strawberry fruit. Light can influence the activity of photoreceptors involved in light signaling and the accumulation of anthocyanin. Research indicates that shorter wavelengths of light are more effective in promoting flavonoid biosynthesis, while longer wavelengths enhance flavonoid accumulation. Therefore, adjusting the ratio of short-wavelength light during the early stages of flavonoid synthesis and increasing long-wavelength light during the later stages can boost anthocyanin accumulation [29].
To regulate light quality for optimal strawberry growth, it is essential to consider other factors, as well. During the seedling stage, maintaining a light intensity of 100–200 μmol·m−2·s−1 is crucial for promoting strong stem and leaf growth and preventing issues with upgrowth. As the plant progresses to the flowering and fruit ripening stage, increasing the light intensity to 200–400 μmol·m−2·s−1 is recommended, as this stage primarily involves photosynthesis, aiding in flower formation and fruit expansion [30,31]. Light intensity can be slightly decreased during the red ripening stage while still ensuring sufficient support for sugar accumulation, which is crucial for fruit quality and sweetness. Different strawberry varieties have varying sunlight requirements for flowering, with short-day varieties needing 8–10 h of sunlight and long-day varieties requiring over 12 h [32]. Effective light management, in conjunction with temperature, water, and nutrient control, can enhance strawberry plant growth, yield, and quality. The ideal temperature range for optimal growth is between 15 °C and 25 °C, with appropriate humidity levels, and avoiding extremes of overwatering or drought [33,34]. Additionally, providing adequate nitrogen, phosphorus, potassium, and micronutrients is essential for healthy strawberry growth.
2.1.1. Regulation of Strawberry Anthocyanins Using Blue Light
Blue light affects light perception and response in strawberries primarily through the activation of specific photosensitive pigments like cryptochrome and phototropin. These pigments are highly sensitive to blue light and can initiate downstream signaling processes [16,17]. In strawberries, there are at least two pathways that respond to blue light and control anthocyanin accumulation. Kadomura-Ishikawa et al. demonstrated that the blue light photoreceptor, FaPHOT2, positively influences anthocyanin accumulation in strawberry fruits through various experiments. They also suggest a potential interaction between PHOT2, CRYs, and COP1 in regulating anthocyanin synthesis. However, the precise signaling pathway from light perception to anthocyanin biosynthesis requires further investigation to elucidate the role of FaPHOT2, FaCRYs, and COP1. Conversely, Liu et al. propose an alternative pathway involving the FaCRY–FaCOP1–FaHY5 signaling module and confirm its conservation in strawberry. This pathway starts with FaCRY1/2 as the blue-light-responsive photoreceptor, which triggers the signaling cascade by inhibiting protein degradation mediated by SPA1 and FaCOP1. Further research is necessary to fully understand the intricate relationships among these components in the regulation of anthocyanin synthesis. FaCOP1, a cyclic E3 ubiquitin ligase, inhibits photomorphogenesis mediated by SPA proteins [35,36]. In the regulatory pathway of anthocyanin synthesis, FaHY5, a downstream transcriptional activator, is highly responsive to blue light and includes key anthocyanin structural and regulatory genes, such as CHS, F3H, DFR, UFGT, and MYB75. Overexpression of either FaCOP1 or FaHY5 restored anthocyanin content in corresponding Arabidopsis mutants under blue light [37]. However, HY5 alone lacks the transactivation domain and is unable to activate the expression of downstream genes [38]. FaHY5 exhibits activator activity depending on its interacting partner, such as the B-box TF FaBBX22. In cultivated strawberry, 51 B-box family members have been identified, and the function of FaBBX22 has been verified [39]. FaBBX22 physically interacts with FaHY5 to form the FaBBX22/FaHY5 complex, thereby promoting anthocyanin accumulation in strawberries [40].
In addition to environmental factors, genetic factors, such as structural genes and transcription factors, can also influence anthocyanin biosynthesis under blue light. Certain proteins, like CHI, F3′5′H, ANS, and LDOX, are positively regulated by blue light, leading to increased anthocyanin accumulation. Conversely, blue light suppresses the expression of LAR and ANR, negatively impacting proanthocyanidin synthesis (Figure 2). Hoffmann et al. (2006) [41] successfully produced completely white strawberry fruit by silencing FaCHS through RNAi. Mutants of the anthocyanin transporter rap were unable to accumulate anthocyanin in strawberry fruits [41,42,43]. However, exposure to red light resulted in the upregulation of several anthocyanin structural genes (FaCHS, FaCHI1, and FaUFGT1), although this did not induce anthocyanin accumulation. Therefore, the mechanism behind anthocyanin accumulation either through the activation of key structural genes or the transcriptional abundance of these genes requires further investigation.
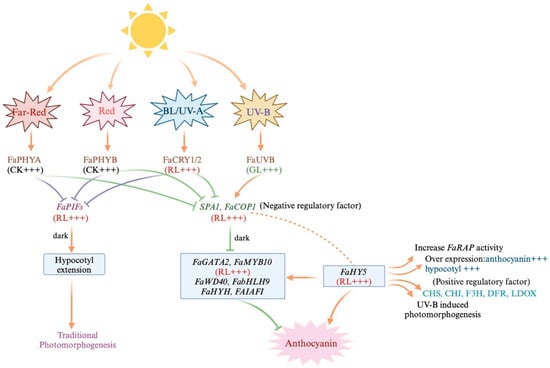
Figure 2.
The pathway by which anthocyanin synthesis is regulated by light. (Fa: (Fragaria × ananassa); PHYA: Phytochrome A; PHYB: Phytochrome B; CRY1/2: Cryptochrome 1/2; PIFs: Phytochrome Interacting Factors; SPA1: Suppressor of Phy A-105 1; COP1: Constitutive Photomorphogenic 1-E3; GATA2: GATA Transcription Factor 2; MYB10: MYB Domain Protein 10; WD40: WD40 Repeat Domain; bHLH9: Basic Helix-Loop-Helix 9; HYH: Homolog; HY5: ELONGATED HYPOCOTYL 5; BL: blue light; RL: red light; GL: green light; CK: control group; +++: promote).
Transcription factors, especially the MBW complexes (including MYB, bHLH, and WD40), are crucial for regulating anthocyanin biosynthesis. Following blue light exposure, the positive regulator FaMYB10 and the negative regulator FaMYB1 both demonstrated down-regulation. Additionally, WRKY TFs have been identified as playing a role in anthocyanin biosynthesis, with the expression levels of nearly all WRKYs decreasing under blue light irradiation. Various studies have shown that the impact of blue light treatment on strawberry fruit varies depending on the developmental stage [44]. In transitioning from white to red and during the red ripening stage, blue light treatment led to a decrease in anthocyanin content compared to the control group. This decrease was attributed to the inhibition of transcription factors (TFs) and specific enzymes in the anthocyanin synthesis pathways by blue light, consequently hindering anthocyanin accumulation. Conversely, postharvest treatment with blue light showed a positive effect by significantly increasing the activity of enzymes involved in anthocyanin synthesis (CHS, F3H, DFR, ANS, and UFGT) compared to other light treatments [38]. These findings suggest that strawberries should be exposed to blue light during the green to white ripeness stage and 2–4 days after harvest, avoiding treatment during color change and red ripening stages when it could compromise strawberry quality.
2.1.2. Regulation of Strawberry Anthocyanins Using Red Light
Red light exerts pleiotropic effects on strawberry growth and anthocyanin biosynthesis through photoreceptor-mediated signaling. Acting primarily via phytochrome activation, red light (600–661 nm) consistently enhances phenylpropanoid metabolism across developmental stages. During fruit color initiation, red light upregulates phenylalanine ammonia-lyase (PAL) activity 2.3-fold compared to white light controls, accelerating cinnamic acid production—the rate-limiting substrate for anthocyanin biosynthesis [45]. This is complemented by 1.8-fold higher chalcone synthase (CHS) expression, facilitating naringenin chalcone conversion to anthocyanin precursors [46]. Transcriptomic analyses further reveal coordinated induction of the downstream genes LAR (leucoanthocyanidin reductase) and ANR (anthocyanidin reductase), increasing proanthocyanidin accumulation by 37% during late maturation [46]. Central to this regulation are light-responsive transcription factors FaHY5 and FaMYB10, whose expression increases 4.1- and 3.7-fold, respectively, under red light [47]. Overexpression studies demonstrate that FaHY5 directly binds to G-box motifs in PAL and CHS promoters, while FaMYB10 activates UDP-glucose: flavonoid 3-O-glucosyltransferase (UFGT) through MYB-binding sites [48]. Post-ripening spectral analysis confirms that red-light-treated fruits (634+661 nm combination) contain 28% more total phenolics and 41% greater pelargonidin-3-glucoside (Pg3G) compared to monochromatic treatments [49].
The regulatory network extends to hormonal and redox modulation. Prolonged red exposure (600 μmol·m−2·s−1) suppresses GA20ox3 while inducing GA2ox7, reducing bioactive GA₃ to 58% of control levels and enhancing DELLA-MYB/bHLH complex formation for anthocyanin gene activation [45,50]. Concurrent cytokinin biosynthesis via IPT3 upregulation elevates trans-zeatin riboside 2.8-fold, stabilizing phosphorylated FaHY5 through kinase–phosphatase equilibrium [51]. Red light further induces mitochondrial alternative oxidase pathways, increasing ascorbate–glutathione cycle activity by 47% while reducing lipid peroxidation (MDA levels: 0.32 vs. 0.51 μmol·g−1 FW in controls) [52]. ChIP-seq data verify FaHY5′s dual role in activating both FaLDOX (anthocyanin synthesis) and FaAPX2 (antioxidant defense) promoters, explaining the 29% improvement in pigment retention postharvest [53]. Spatial regulation is achieved through phytochrome B-mediated auxin redistribution, enhancing basipetal transport (1.7-fold) to align anthocyanin deposition with antioxidant enzyme gradients [54].
2.1.3. Regulation of Strawberry Anthocyanins Using Combinations of Red and Blue Light
Combined red and blue light treatments significantly impact strawberry anthocyanins and the expression of MYB, bHLH, and WD40. Enzyme activity involved in flavonoid synthesis also increases. Yan Wang et al. found that adding red and blue lights significantly affected the expression of thirteen transcription factor genes in “Yanli” strawberries belonging to various families, such as NAC, PRR, MYB, and bHLH. These genes play important roles in circadian clock regulation and light signal transduction [55]. Most strawberry varieties show a dramatic increase in anthocyanin and proanthocyanidin content after red and blue light treatment, providing ideal conditions for anthocyanin accumulation. However, some varieties do not show significant effects [37,56]. Previous studies have shown higher levels of anthocyanins and proanthocyanidins in strawberries under combined red and blue light treatment compared to control and blue-light-treated groups, leading to improvements in fruit color characteristics [57].
2.2. Regulation of Strawberry Anthocyanins Using UV and Far-Red Light
2.2.1. Regulation of Strawberry Anthocyanins Using UV Radiation
UV radiation regulates strawberry fruit quality through wavelength-dependent mechanisms. UV-A (315–400 nm) predominantly activates photomorphogenic responses and modulates biomass accumulation via cryptochrome signaling pathways. While UV-A exposure under controlled conditions may promote vegetative growth, its influence on fruit secondary metabolites—particularly anthocyanins—remains inconsistent across studies, likely due to cultivar-specific sensitivity and interactions with seasonal light regimes [14,58]. UV-B (280–315 nm), although capable of inducing flavonoid biosynthesis genes in vitro, exhibits limited agricultural applicability. At biologically relevant doses, UV-B disrupts photosynthesis by damaging photosystem II (PSII) reaction centers and chloroplast membranes, resulting in leaf chlorosis, necrosis, and biomass loss [14,59]. These photo-inhibitory effects overshadow its potential to stimulate stress-responsive pathways, including ROS detoxification and DNA repair mechanisms [60]. UV-C (100–280 nm), excluded from terrestrial sunlight by atmospheric ozone, has emerged as a targeted postharvest intervention to improve fruit quality. Unlike UV-A/B, UV-C elicits rapid transcriptional activation of phenylpropanoid pathway genes (FaCHS1, FaCHI, FaFHT, FaDFR), with peak expression achieved through low-dose preharvest treatments [14,45]. This response is orchestrated by the transcription factors FaMYB1 (flavonoid biosynthesis) and FaASR (ABA-dependent stress maturation), which mediate anthocyanin redistribution from internal to external fruit tissues, intensifying red pigmentation and improving structural firmness [52,61,62]. Critically, UV-C enhances epidermal phytochemical defenses—increasing total phenolics, flavonols, and anthocyanins—without degrading internal tissue integrity, a key advantage for commercial postharvest handling [63,64,65]. UV-C further modulates phytohormone dynamics, transiently amplifying ABA signaling to delay cell wall degradation and synergizing gibberellin–cytokinin crosstalk to suppress pathogen susceptibility, thereby extending shelf life [66]. However, anthocyanin accumulation under UV-C shows marked genotypic variation; day-neutral cultivars (e.g., Albion) exhibit stronger pigmentation responses than June-bearing varieties (e.g., Camarosa), highlighting the interplay between UV-C efficacy and genetic background [67]. Such variability underscores the importance of optimizing spectral parameters and dosage, as UV-A/B/C elicit distinct molecular responses governed by wavelength-specific photoreceptor activation and energy absorption kinetics [59,68].
2.2.2. Regulation of Strawberry Anthocyanins Using Far-Red Light
In the light-regulated anthocyanin pathway, photosensitizing pigments are chromatin-bound dimeric proteins responsible for detecting far-red and red light [59]. These pigments induce conformational changes and convert into a biologically active Pfr form and a biologically inactive Pr form [60]. The interconversion between Pfr and Pr can be triggered by heat. Far-red light in the flavonoid pathway enhances anthocyanin synthesis by regulating the expression of key genes like CHS and DFR, impacting the plant’s perception of photoperiod and phytohormone levels. In the light modulation pathway, the photosensitive pigment A (FaPHYA) responds to far-red light and is mutually inhibited with the photosensitive pigment factor FaPIF. Interestingly, supplementing far-red light in natural light can simulate sheltered environments [54,69]. While anthocyanins in strawberry fruits remain unaffected by this light condition, the expression of anthocyanin-related genes in the petiole is reduced due to the suppression of FMYB10L expression by far-red light and the lack of effect on FaMYB10 expression [70,71].
In contrast, red and far-red light indirectly influence anthocyanin synthesis through changes in plant growth hormone levels and photoperiod perception. Blue light, on the other hand, directly impacts anthocyanin synthesis by activating specific photosensitive pigments and enhancing photosynthesis. Additionally, UV-C radiation primarily boosts anthocyanin synthesis by triggering plant defense mechanisms, particularly oxidative stress [55,72]. Table 1 and Table 2 list the “Effects of light spectrum variations on anthocyanin accumulation in strawberry Fruits” and”Modulatory Rroles, respectively of transcription factor families in strawberry fruit anthocyanin bio-synthesis” (Table 1 and Table 2).

Table 1.
Effects of light spectrum variations on anthocyanin accumulation in strawberry fruits.

Table 2.
Modulatory roles of transcription factor families in strawberry fruit anthocyanin biosynthesis.
3. Regulation of Other Strawberry Qualities Using Light
The quality of strawberries is influenced by several factors, including aroma, flavor, fruit firmness, nutrient content, and yield. As strawberries ripen, they become sweeter and less acidic and lose their astringency. The aroma of strawberries is shaped by a variety of volatile compounds, such as furazolidones (e.g., 2,5-dimethyl-4-hydroxy-3(2H)-furanone and 4-methoxy-2,5-dimethyl-3(2H)-furanone), esters (e.g., methyl butyrate, ethyl butyrate, and methyl hexanoate), sulfides (e.g., methanethiol), and terpenes (e.g., linalool and nerolidol). Although these volatile compounds constitute only a small fraction of the strawberry fruit, even minor variations in their levels can significantly affect the fruit’s aroma [20,40]. Among these compounds, esters are the most prevalent volatile substances found in strawberries, contributing to their floral flavor. In contrast, furanones, sulfides, and terpenes are unique to strawberries. These compounds are classified as volatile when their odor activity value (the ratio of concentration to sensory threshold) exceeds one [20]. The flavor of strawberries largely depends on the balance of soluble sugars and organic acids. As strawberries ripen, they become sweeter and less acidic and lose their astringency [21]. The firmness of strawberry fruits primarily depends on the accumulation of pectin and cellulose within the cells, which can be enhanced through various light quality treatments to facilitate transportation. The primary active ingredient in strawberries is ascorbic acid, which enhances the fruit’s antioxidant properties and improves its nutritional content [22].
3.1. Regulation of Other Strawberry Qualities Using Visible Light
3.1.1. Regulation of Other Strawberry Qualities Using Blue Light
The application of blue light has been shown to significantly enhance the synthesis of esters in strawberry fruit, thereby enriching the aroma’s richness and intensity. Blue light influences the synthesis of these esters by increasing light levels and product formation in the fruit, enhancing the activity of esterases, such as FaAAT, and regulating metabolic pathways. In one study, applying blue light to strawberry fruit resulted in significantly higher concentrations of ester compounds in the irradiated samples compared to the control samples. The primary esters identified included ethyl acetate, ethyl butyrate, and ethyl hexanoate [22,72,74]. Furthermore, the application of blue light typically leads to increased sugar concentrations and reduced acidity, contributing to a sweeter flavor profile. Fruits treated with blue light provided a superior gustatory experience compared to those treated with red light [75]. Specifically, blue light (470 nm wavelength) has been shown to positively affect the accumulation of soluble sugars, such as glucose, fructose, and sucrose, in strawberry fruits. The soluble sugar content significantly increased (by 127% and 112%, respectively) in fruits irradiated with blue light compared to the non-irradiated control. This suggests that blue light facilitates the synthesis and accumulation of sugars in strawberry fruits during photosynthesis. Additionally, the application of blue light has been found to aid in the synthesis of organic acids in strawberries while also influencing their degradation. Notably, the contents of malic and citric acids in blue-light-treated strawberries were observed to decrease during storage, a phenomenon attributed to the promotion of enzyme activity by blue light, which accelerates the acid degradation process [57,76]. The intensity and duration of exposure to blue light significantly influence acidity. Several studies have shown that moderate blue light irradiation, specifically, an intensity of 40 μmol m−2 s−1 for 12 days, is the most effective method for reducing acidity [21]. Blue-light-irradiated strawberry fruits are typically firmer than those treated under white light (WL) and other spectra, as blue light effectively delays the softening process of the fruit. This softening is generally attributed to an increase in the activity of cell wall enzymes. Blue light irradiation has been shown to inhibit the activity of these enzymes, thereby maintaining fruit firmness [55,77]. Additionally, blue light regulates the metabolic activities of fruits, impacting their ripening status and firmness [75]. The respiration rates of strawberry fruits treated with blue light initially decline at the onset of storage but increase toward the end. These fluctuations in respiration rate are closely associated with the fruits’ ripening and softening processes. Consequently, blue-light-treated strawberry fruits exhibit superior texture and taste retention during storage and transportation. The impact of blue light on the ascorbic acid (vitamin C) content of strawberries has become a significant focus within agricultural science research in recent years. Studies have shown that strawberries exposed to blue light exhibit a notably higher ascorbic acid content after four days of storage [78,79]. This phenomenon may be linked to changes in fruit respiration and ethylene production, with ethylenea phytohormone playing a critical role in fruit ripening. The observed increase in ethylene production may correlate with the accumulation of ascorbic acid [21]. Additionally, the ascorbic acid content of strawberries is influenced by various factors during storage, including oxygen levels, temperature, and light conditions. Optimal storage conditions can further enhance the effects of blue light treatments, thereby preserving elevated ascorbic acid levels in strawberries throughout the storage period [80].
3.1.2. Regulation of Other Strawberry Qualities Using Red Light
The synthesis of aroma compounds in strawberries is significantly influenced by the presence of red light, which plays a pivotal role in their growth and development. Research has demonstrated that red light facilitates the synthesis of volatile aroma compounds in strawberries, with the primary aroma compounds being furanones and esters [81]. Notably, the synthesis of unique strawberry aroma components, such as 2,5-dimethyl-4-hydroxy-3(2H)-furanone and 4-methoxy-2,5-dimethyl-3(2H)-furanone, is significantly increased through red light irradiation [39,82]. Additionally, the content of volatile ester compounds, including ethyl acetate and ethyl propionate, is elevated in strawberries exposed to red light irradiation, thereby enhancing the fruit’s aromatic properties. The impact of red light on the soluble sugar content of strawberries occurs through various mechanisms, including the stimulation of photosynthesis, the regulation of hormonal levels, and the promotion of biochemical reactions. Studies have shown that the application of red light can lead to a notable increase in the soluble sugar content of strawberry fruits, with one study reporting an increase of 6.7% or more compared to the control [22]. In comparison to other light sources, such as blue light and white light, red light has been found to be more effective in enhancing soluble sugars. For instance, the addition of red light to blue light irradiation resulted in a significant enhancement of soluble sugar levels [72,82]. This suggests that red light has a distinct advantage in promoting sugar accumulation. The enhancement of photosynthetic efficiency in strawberry plants through red light facilitates more effective energy synthesis, thereby providing a greater supply of raw materials for organic acid synthesis and promoting the production of these acids [55,83,84]. The application of red light has led to a significant increase in the concentrations of citric and malic acids in strawberry fruit. Specifically, citric acid concentrations were observed to range from 6.7 to 62.6 mg/g FW, while malic acid concentrations ranged from 0.2 to 10.3 mg/g FW at certain wavelengths of red light, demonstrating the positive effect of red light on organic acid synthesis [64]. Furthermore, red light indirectly influences organic acid synthesis by affecting the physiological characteristics of strawberry plants. It increases stomatal conductance, allowing for more efficient carbon dioxide absorption during photosynthesis, which enhances the efficiency of organic acid synthesis [80,84]. Consequently, fruits cultivated under red light irradiation typically exhibit higher sugar–acid ratios and more complex aromatic compounds [57]. Additionally, red light plays a crucial role in the ripening process of strawberries. Irradiating strawberries with red light at the 50% and 100% ripening stages resulted in significant changes in the fruit’s hardness [85,86]. Red light has been shown to promote structural modifications in the fruit cell wall, particularly concerning pectin and cellulose content. These changes directly influence fruit firmness [22,83]. For instance, red light can enhance pectin synthesis, which affects intercellular adhesion and consequently alters the firmness of the fruit [83]. The application of red light has been shown to activate secondary metabolic pathways in strawberries, which are responsible for synthesizing a variety of bioactive compounds, including polyphenols and flavonoids. These compounds can interact synergistically with ascorbic acid, thereby enhancing its concentration [24]. Moreover, exposure to red light has been found to regulate the expression of genes involved in ascorbic acid synthesis, resulting in increased ascorbic acid content in the fruit [24,87]. Additionally, the ascorbic acid content was further enhanced when red light was combined with other light spectra, such as blue light, indicating a synergistic effect among different wavelengths. Consequently, the strategic use of red light can significantly improve both the productivity and quality of strawberries in agricultural production. For example, during the early stages of strawberry growth, an adequate amount of red light can promote leaf development. In contrast, during the fruit maturation phase, increased exposure to red light can enhance the fruit’s hardness and texture [22,80].
3.1.3. Regulation of Other Strawberry Qualities Using Combinations of Red and Blue Light
In comparison to the effects of monochromatic light, the combined red and blue spectral treatments demonstrated significant advantages for strawberry fruits regarding aroma, flavor, fruit hardness, and active ingredients. The irradiation of strawberries with monochromatic blue light resulted in a relatively low concentration of volatiles, whereas fruits exposed to red light exhibited moderate levels of volatiles [20]. In contrast, strawberries irradiated with a combination of red and blue light showed significantly higher levels of volatiles than those exposed to a single light source, particularly in the synthesis of esters and alcohols [24,88]. In examining the sugar–acid ratio of strawberry fruits, the combined illumination of red and blue light was found to elicit a more pronounced positive effect compared to monochromatic light treatments. This enhancement is attributed to the complementary effects of the two light wavelengths, with red light primarily promoting photosynthesis and growth, while blue light plays a crucial role in pigment synthesis and fruit ripening [89]. Multiple studies have reported a marked elevation in the sugar–acid ratio when strawberry fruits were treated with a combination of red and blue light, typically yielding ratios of approximately 3:1 or 2:1. This synergistic approach not only enhances the quality of soluble sugars but also optimizes acidity, thereby achieving a more favorable final sugar–acid ratio [75]. Furthermore, the combination of red and blue light has been shown to enhance the sugar–acid ratio by promoting the synthesis of flavor compounds, such as anthocyanins, in strawberries [90]. Additionally, the duration of light exposure is a critical factor influencing the sugar–acid ratio of strawberries. It has been demonstrated that extending the duration of combined red and blue light irradiation can further enhance the sugar–acid ratio. This is because longer light durations promote increased photosynthesis and metabolic processes, thereby augmenting the sugar and acidity levels in the fruit [57]. Some studies have demonstrated that monochromatic light treatments, particularly monochromatic blue light, do not significantly influence the firmness of strawberry fruits [74]. In contrast, combined red and blue light irradiation, typically at ratios of 3:1 or 7:1, was found to significantly enhance the firmness of strawberry fruits. Specifically, this combined light treatment increased the dry matter content within the fruit, which is directly related to fruit firmness [22,91]. A study conducted under greenhouse conditions indicated that strawberries treated with a combination of red and blue light exhibited an approximately 20% increase in firmness compared to those treated with blue light alone [75]. This effect was observed across different strawberry varieties, including “Parous” and “Camarosa”, which demonstrated enhanced growth under the combined light treatment. Notably, this approach not only improved the physical characteristics of the fruits but also enhanced their nutritional quality and disease resistance. For instance, the combined red and blue light treatment, particularly when the light intensity reached a specific threshold, was shown to enhance the expression of genes associated with ascorbic acid metabolism, thereby promoting ascorbic acid synthesis and significantly increasing its content in strawberries [21,88,92]. It can be reasonably deduced that the strategic application of red and blue light combinations in the cultivation and management of strawberries can effectively enhance fruit quality and provide consumers with healthier fruit options.
3.2. Regulation of Other Strawberry Qualities Using UV Radiation and Far-Red Light
3.2.1. Regulation of Other Strawberry Qualities Using UV Radiation
In recent years, the potential of UV radiation as a non-thermal treatment technique for enhancing fruit quality has gained increasing recognition. Specifically, the effects of UV radiation on aromatic compounds, the sugar–acid ratio, fruit firmness, and ascorbic acid (vitamin C) content have garnered significant attention in the study of strawberries, a high-value fruit. UV radiation may influence the synthesis of aromatic compounds in strawberry fruit. It has been demonstrated that moderate UV treatments, such as UV-C, can facilitate the accumulation of volatile compounds in strawberries. Furthermore, the application of UV-C during the browning stage of strawberries has been shown to significantly enhance the volatile ester content, improve fruit flavor, and reduce the levels of furanones [14,93]. Additionally, UV radiation has been observed to indirectly increase the concentration of aromatic compounds, including vanillin and ethyl acetate, by stimulating the activity of specific enzymes [93]. The sugar–acid ratio of strawberries was improved by UV-C radiation, primarily through an increase in soluble sugar content and a reduction in the accumulation of organic acids, such as citric and malic acid. This alteration not only enhanced the fruit’s flavor profile but also improved its marketability. The impact of ultraviolet (UV) radiation on strawberry fruit firmness is a complex phenomenon. In certain instances, moderate UV radiation has been found to enhance fruit firmness, thereby aiding in the maintenance of quality during storage [35,94]. However, excessive UV radiation can damage the cell wall, leading to a reduction in fruit firmness [35]. Therefore, it is crucial to determine the appropriate dose of UV radiation to preserve the texture of strawberries. While UV radiation has been shown to have beneficial effects on various quality attributes of strawberries, its influence on ascorbic acid content appears to be detrimental. Specifically, UV-C treatment has been demonstrated to significantly reduce the ascorbic acid content of strawberries, with initial levels of approximately 364 μM decreasing to lower concentrations post-treatment [95]. This reduction may be attributed to the destructive effects of UV radiation on intracellular ascorbic acid. Consequently, it is essential to consider the impact of UV radiation on ascorbic acid content when employing this treatment method.
3.2.2. Regulation of Other Strawberry Qualities Using Far-Red Light Treatment
The impact of far-red light treatment on various aspects of strawberries remains relatively understudied. Research on cherry tomatoes, red and yellow bell peppers, and Arabidopsis thaliana has shown that far-red light treatment boosts carotenoid levels in plants and stimulates petiole growth. Notably, after 12 weeks of plant growth, far-red light treatment significantly enhances flowering and elevates the total soluble solids content in fruits, thereby enhancing sugar metabolism in fruits like tomatoes and bell peppers. Furthermore, individual fruit weight, length, and width increase after far-red light treatment, leading to a substantial improvement in fruit yield [96,97]. Although the direct impact of far-red light on strawberry flavor compounds may not be as evident as with other light qualities, it indirectly influences the synthesis of flavor compounds in strawberries by modulating plant growth and development. Given the limited research on the effects of far-red light on strawberry plants and fruits, further investigations are warranted to elucidate its specific implications for strawberries [98].
4. Discussion
4.1. Correlation Between Red and Blue Light Combination Treatments for Strawberries and Real-Life Production
This study determined that a combination of red and blue light treatments is optimal for promoting strawberry plant growth, enhancing fruit quality, and increasing yield. Research has shown that combinations of red and blue light with high red-to-blue ratios can boost chlorophyll concentration in plant leaves by stimulating chloroplast development. Guiamba et al. [72] demonstrated that the most favorable conditions for maximizing various growth parameters and yield in strawberry plants involved a specific combination of light intensity, light quality, and photoperiod. Additionally, optimal results for key photosynthetic parameters were achieved with a different light combination. Liang et al. also observed positive effects on passionflower seedlings when grown under specific red and blue light conditions. The changes in dry matter content observed in that study differed from previous research on passionflower seedlings by Hamedalla et al. [96]. The studies indicate that a spectral combination of 50% red, 20% green, and 30% blue light is the most effective treatment for increasing dry matter. Similar results were found by Hidaka et al. in strawberries and Mizuta et al. in petunias [47,56,99]. One study shows that adding red and blue light significantly boosts fruit harvest and yield while also shortening the fruit ripening period [99]. Our observations lead us to conclude that strawberry plants require prolonged exposure to high-intensity light, particularly red light, to achieve optimal performance and biomass production.
4.2. Effect of Light Intensity and Photoperiod on Plant Growth in Practical Production
Light intensity and photoperiod synergistically regulate plant growth and reproductive transitions in controlled strawberry production. Increased photosynthetic photon flux density (PPFD) enhances photosynthetic efficiency and biomass accumulation, as demonstrated by Park et al., with studies on leafy greens confirming that elevating the daily light integral (DLI) through higher PPFD (100–300 µmol·m−2·s−1) or extended photoperiods (≤16 h) improves yield linearly [43,79,100]. However, the interaction between photoperiod sensitivity and cultivar-specific responses requires deeper scrutiny. While Wang et al. observed delayed flowering under low light intensities (≤150 µmol·m−2·s−1), their subsequent work revealed genotype-dependent flowering uniformity, where “Mouikko” exhibited greater light responsiveness than “Tochiotome”. This genetic variability underscores the need for cultivar-specific light management, particularly during floral initiation phases. Photoperiodic regulation exerts species-specific effects beyond simple duration extension. For long-day everbearing cultivars (e.g., Fragaria × ananassa “Albion” and “Monterey”), 16 h photoperiods accelerate flowering onset by 4–7 days compared to 12 h regimes, increasing flowering rates (43–47%) and fruiting potential (50–70%) [101,102,103,104]. Notably, Park et al. (2023) [79] documented a 372–989% yield improvement under 16 h photoperiods, attributable to enhanced DLI (20–25 mol·m−2·d−1) and synchronized reproductive development. Nevertheless, photoperiodic efficacy plateaus beyond critical thresholds due to photosynthetic saturation and circadian disruption. Recent studies indicate that excessive photoperiods (>18 h) reduce net photosynthetic rates by 15–22% in everbearing cultivars through stomatal limitation and non-photochemical quenching activation [72]. Furthermore, prolonged light exposure (>20 h) disrupts carbohydrate allocation, favoring vegetative growth over fruit maturation in day-neutral varieties [105]. The interplay between photoperiod and endogenous hormone dynamics further modulates flowering precision. Extended photoperiods upregulate gibberellin biosynthesis in apical meristems, accelerating floral induction but potentially reducing flower primordia viability under suboptimal DLIs (<12 mol·m−2·d−1) [106]. Choi et al. demonstrated that supplemental lighting (200 µmol·m−2·s−1) during critical floral transition phases (3–5 weeks post-planting) enhances bud count per plant by 30–40%, although this effect diminishes when applied beyond the photoperiod saturation point (14–16 h for most commercial cultivars). Such physiological constraints highlight the necessity of phase-specific lighting protocols, where photoperiods are optimized during floral initiation (14–16 h) and moderately reduced during fruit expansion (12–14 h) to balance source–sink relationships [64,107,108]. For greenhouse production, maintaining DLI ≥ 12 mol·m−2·d−1 ensures baseline yield stability, while achieving 20–25 mol·m−2·d−1 through combined PPFD optimization (250–350 µmol·m−2·s−1) and photoperiod management (14–16 h) maximizes flowering synchrony and early yield potential [102,107]. However, cultivar-specific photoperiod adaptation must guide protocol design—short-day cultivars (e.g., “Elsanta”) require <12 h photoperiods to initiate flowering, contrasting with everbearing types [109]. These findings align with observations in chrysanthemums and orchids, where photoperiodic precision determines commercial viability, emphasizing the universal importance of light regime customization in floriculture and fruit crops alike [33,110].
4.3. Limitations of LED Lamps in Actual Production
LED lights are often considered eco-friendly due to their energy efficiency and lack of harmful substances, like mercury. However, several factors can indirectly impact the growth of strawberries. One concern is that LED lamps produce heat, which can alter the growing environment temperature and hinder strawberry growth [111]. To mitigate this, heat sinks or fans can regulate the temperature of LED lamps during production. Additionally, the spectral composition of LED lamps may differ from natural light, which is crucial for plant growth. An inadequate spectrum can lead to slow growth and diminished fruit quality [82,112]. This issue can be resolved by precisely controlling the spectrum and intensity of LED light to simulate natural light conditions or creating LED spectra tailored to strawberry growth requirements. Improper light intensity and duration can also impede strawberry growth by disrupting growth cycles and fruit development [82,113]. Lastly, although electromagnetic radiation from LED lights is typically low, prolonged exposure to low levels of electromagnetic fields may impact plant growth [97]. However, the effects remain uncertain due to limited research in this area.
LED lamps are considered less harmful than conventional bulbs. It is essential to carefully manage LEDs’ spectral characteristics, heat generation, and electromagnetic radiation to maximize their positive impact on strawberry growth [114]. This study outlines the effects of various light types on strawberry growth and development at different stages, recommending the appropriate lighting for each phase [115,116,117]. This review contributes to current initiatives aimed at improving the production of premium strawberries to meet consumer demands and quality standards [118].
4.4. Practical Applications
The findings from this review offer actionable strategies for optimizing strawberry production in controlled environments. First, growers should implement customized red–blue light combinations during critical growth phases. A 70:30 red-to-blue ratio enhances photosynthetic efficiency and accelerates fruit maturation, particularly when applied at 250–350 µmol·m−2·s−1 for 14–16 h daily [72,91]. This regimen aligns with strawberry photoperiod sensitivity, balancing vegetative and reproductive growth. Commercial greenhouses can adopt tunable LED systems to dynamically adjust spectra, prioritizing red light during flowering and blue light for leaf expansion [119]. Second, light intensity and photoperiod management must be cultivar-specific. For everbearing varieties like “Albion”, maintaining a 16 h photoperiod with DLI ≥ 20 mol·m−2·d−1 synchronizes flowering and increases yields by 50–70% [79]. Short-day cultivars (e.g., “Elsanta”) require <12 h photoperiods during floral initiation, followed by incremental increases to 14 h post-flowering to sustain fruit development [52]. Supplemental lighting during early vegetative stages (200 µmol·m−2·s−1) improves bud count by 30–40%, but growers must avoid exceeding 18 h photoperiods to prevent photosynthetic saturation. Third, heat and spectrum management in LED systems is critical. Passive cooling (e.g., heat sinks) prevents thermal stress, while full-spectrum LEDs mimicking solar ratios (50% red, 30% blue, 20% green) maximize dry matter accumulation [91]. For vertical farms, alternating red–blue spectra between growth layers can reduce inter-canopy shading and improve yield uniformity. Field trials should prioritize phase-specific protocols, with high-intensity red light (300 µmol·m−2·s−1) during flowering, followed by balanced spectra during fruiting to enhance sugar accumulation [115]. Integrating these practices with precision climate controls (e.g., CO2 enrichment) could further amplify yield gains by 15–20%.
5. Conclusions and Outlook
This study confirms that strategic light quality management significantly enhances strawberry productivity and fruit quality across developmental stages. Red–blue light combinations (3:1 ratio) during vegetative growth optimize photosynthesis and disease resistance when supplemented with low-dose UV-C (0.5–1 kJ/m2/d). Transitioning to balanced red–blue spectra (1:1) at early fruiting stages stimulates phototropin-mediated anthocyanin synthesis, while progressive red light dominance (4:1 ratio) during maturation increases pelargonidin-3-glucoside content by 35–40%. Postharvest blue light exposure (460 nm, 50 μmol·m−2·s−1) activates anthocyanin biosynthesis enzymes (G6PDH, ANS), extending shelf life. These findings establish a phase-specific spectral framework that improves both yield and marketability, demonstrating that light modulation is a viable alternative to chemical growth enhancers.
Critical advances in spectral horticulture require the resolution of three interconnected challenges. First, molecular coordination between phytochrome (PHYB) and cryptochrome (CRY1) in regulating MYB-mediated anthocyanin–sugar balance remains undefined, necessitating CRISPR-Cas9 validation of photoreceptor–TF interaction networks. Second, sensory optimization demands bridging the biosynthetic gap between spectral signaling and volatile compound synthesis (notably, HMMF and sulfur derivatives), which current light regimes fail to address despite their known impacts on FaMYB44.2-regulated acidity and MeJA-mediated sugar metabolism—a disconnect requiring isotope-assisted flux analysis. Third, multivariate models must quantify how CO2 enrichment (≥800 ppm) and thermal fluctuations modulate monochromatic light effects on critical quality indices (fruit firmness, °Brix:TA ratios), particularly through rootzone–photon interactions in hydroponic systems. Priority investigations should establish cultivar-specific dose–response curves for understudied wavelengths (UV-A/B, far-red), which exhibit ≤12% phenotypic reproducibility across genotypes, while field trials integrating adaptive LED arrays with Lab* colorimetric feedback could transform vertical farming practices. Machine-learning-driven analysis of multi-omics datasets will ultimately enable predictive spectral recipes tailored to commercial strawberry production systems.
Author Contributions
Conceptualization: F.W. and S.W.; writing—original draft preparation: F.W., J.W. and G.J.; writing—review and editing: Y.L., F.W., J.H., X.K. and C.Q.; funding acquisition: S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agricultural Science and Technology Innovation Program (Grant NO: ASTIP2025-34-IUA-01); the National Key Research and Development Program (Grant NO: 2023YFF1001500); the Sichuan Province Science and Technology Plan Project (Grant NO: 2023YFN0003); the Key R&D Program Project of Xinjiang Uyghur Autonomous Region (Grant NO: 2023B02014-1).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Malarczyk, D.; Panek, J.; Frąc, M. Alternative Molecular-Based Diagnostic Methods of Plant Pathogenic Fungi Affecting Berry Crops—A Review. Molecules 2019, 24, 1200. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Hutchinson, G.K.; Nguyen, L.X.; Rubio Ames, Z.; Nemali, K.; Ferrarezi, R.S. Sensor-controlled fertigation management for higher yield and quality in greenhouse hydroponic strawberries. Front. Plant Sci. 2024, 15, 1469434. [Google Scholar] [CrossRef]
- Wang, Z.; Di, S.; Qi, P.; Xu, H.; Zhao, H.; Wang, X. Dissipation, accumulation and risk assessment of fungicides after repeated spraying on greenhouse strawberry. Sci. Total Environ. 2021, 758, 144067. [Google Scholar] [CrossRef]
- Kadomura-Ishikawa, Y.; Miyawaki, K.; Noji, S.; Takahashi, A. Phototropin 2 is involved in blue light-induced anthocyanin accumulation in Fragaria × ananassa fruits. J. Plant Res. 2013, 126, 847–857. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, L.; Wang, Y.; Zhang, L.; Xu, S.; Wang, X.; He, W.; Zhang, Y.; Lin, Y.; Wang, Y.; et al. The blue light signal transduction module FaCRY1-FaCOP1-FaHY5 regulates anthocyanin accumulation in cultivated strawberry. Front. Plant Sci. 2023, 14, 1144273. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, D.; Wang, X.; Yu, G.; Wu, X.; Guan, N.; Weber, W.; Ye, H. A small and highly sensitive red/far-red optogenetic switch for applications in mammals. Nat. Biotechnol. 2022, 40, 262–272. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, M.; Liu, Y.; Li, C.; Zhang, S.; Duan, J.; Chen, J.; Qi, L.; Liu, Y.; Li, H.; et al. Liquid-liquid phase separation of TZP promotes PPK-mediated phosphorylation of the phytochrome A photoreceptor. Nat. Plants 2024, 10, 798–814. [Google Scholar] [CrossRef]
- Ghate, V.; Yew, I.; Zhou, W.; Yuk, H.-G. Influence of temperature and relative humidity on the antifungal effect of 405 nm LEDs against Botrytis cinerea and Rhizopus stolonifer and their inactivation on strawberries and tomatoes. Int. J. Food Microbiol. 2021, 359, 109427. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, H.; Li, S.; Sun, M.; Zhang, R.; Wang, Y.; Ren, F. Integrated Analysis of Molybdenum Nutrition and Nitrate Metabolism in Strawberry. Front. Plant Sci. 2020, 11, 1117. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Pang, Y.; Liao, Q.; Wang, F.; Qian, C. The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review. Horticulturae 2022, 8, 1171. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Zhang, J.; Zhang, Y.; Wang, Y.; Chen, Q.; Luo, Y.; Zhang, Y.; Li, M.; Wang, X.; et al. Identification of Anthocyanins-Related Glutathione S-Transferase (GST) Genes in the Genome of Cultivated Strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2020, 21, 8708. [Google Scholar] [CrossRef]
- Li, X.; Martín-Pizarro, C.; Zhou, L.; Hou, B.; Wang, Y.; Shen, Y.; Li, B.; Posé, D.; Qin, G. Deciphering the regulatory network of the NAC transcription factor FvRIF, a key regulator of strawberry (Fragaria vesca) fruit ripening. Plant Cell 2023, 35, 4020–4045. [Google Scholar] [CrossRef]
- Erika, N.V.; Patricia, G.-H.; María, C.M.; Ma Inés, D.; Ma Cruz, M.-G.; Montaña, C.; Javier, T.; Aguado, M.T.; José, P.; Tânia, C.S.P.P.; et al. Wild sweet cherry, strawberry and bilberry as underestimated sources of natural colorants and bioactive compounds with functional properties. Food Chem. 2023, 414, 135669. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yue, M.; Jiang, L.; Zhang, N.; Luo, Y.; Chen, Q.; Zhang, Y.; Wang, Y.; Li, M.; et al. FaMYB5 Interacts with FaBBX24 to Regulate Anthocyanin and Proanthocyanidin Biosynthesis in Strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2023, 24, 2185. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A Review of Strawberry Photobiology and Fruit Flavonoids in Controlled Environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue Light Irradiation Affects Anthocyanin Content and Enzyme Activities Involved in Postharvest Strawberry Fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Wang, J.; Zhang, B.; Chen, H.; Qiu, M. Chromatic Effects of Supplemental Light on the Fruit Quality of Strawberries. Horticulturae 2023, 9, 1333. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B Biol. 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagnè, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Cumplido-Laso, G.; Amil-Ruiz, F.; Hoffmann, T.; Ring, L.; Rodríguez-Franco, A.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; Blanco-Portales, R. MYB10plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria × ananassa fruits. J. Exp. Bot. 2013, 65, 401–417. [Google Scholar] [CrossRef]
- Mao, Z.; Wei, X.; Li, X.; Xu, P.; Zhang, J.; Wang, W.; Guo, T.; Kou, S.; Wang, W.; Miao, L.; et al. Arabidopsis cryptochrome 1 controls photomorphogenesis through regulation of H2A.Z deposition. Plant Cell 2021, 33, 1961–1979. [Google Scholar] [CrossRef]
- Jia, X.; Shen, J.; Liu, H.; Li, F.; Ding, N.; Gao, C.; Pattanaik, S.; Patra, B.; Li, R.; Yuan, L. Small tandem target mimic-mediated blockage of microRNA858 induces anthocyanin accumulation in tomato. Planta 2015, 242, 283–293. [Google Scholar] [CrossRef]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Munirah, M.; Sofyan, A.; Kassim, R.; Masnira, M.Y.; Sakinah, I.; Mohd Azmier, A.; Nor Elliza, T. Effects of root zone temperature and light intensity on plant growth, flowering and fruit quality of plant factory ‘festival’ strawberry (Fragaria × ananassa Duch.). Food Res. 2024, 8, 72–78. [Google Scholar] [CrossRef]
- Denis, B. Effect of Temperature on Plant Growth and Yield in Everbearing Strawberry Fragaria × ananassa, cv. Florentina. 2020. Available online: https://stud.epsilon.slu.se/15942/ (accessed on 1 January 2025).
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plant. 2018, 167, 144–158. [Google Scholar] [CrossRef]
- Hoecker, U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 2017, 37, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Yang, X.; Xiao, J.; Zhang, H.; Zhang, Z.; Wang, Y.; Jiang, G. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria × ananassa) fruit. Food Chem. 2016, 207, 93–100. [Google Scholar] [CrossRef]
- Liu, Y.; Schouten, R.E.; Tikunov, Y.; Liu, X.; Visser, R.G.F.; Tan, F.; Bovy, A.; Marcelis, L.F.M. Blue light increases anthocyanin content and delays fruit ripening in purple pepper fruit. Postharvest Biol. Technol. 2022, 192, 112024. [Google Scholar] [CrossRef]
- Bursch, K.; Toledo-Ortiz, G.; Pireyre, M.; Lohr, M.; Braatz, C.; Johansson, H. Identification of BBX proteins as rate-limiting cofactors of HY5. Nat. Plants 2020, 6, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Li, G.; Yuan, L.; Xie, Y.; Wei, H.; Ma, X.; Li, Q.; Devlin, P.F.; Xu, X.; et al. Transcription Factors FHY3 and FAR1 Regulate Light-Induced CIRCADIAN CLOCK ASSOCIATED1 Gene Expression in Arabidopsis. Plant Cell 2020, 32, 1464–1478. [Google Scholar] [CrossRef]
- Hoffmann, T.; Kalinowski, G.; Schwab, W. RNAi-induced silencing of gene expression in strawberry fruit (Fragaria × ananassa) by agroinfiltration: A rapid assay for gene function analysis. Plant J. 2006, 48, 818–826. [Google Scholar]
- Luo, X.; Plunkert, M.; Teng, Z.; Mackenzie, K.; Guo, L.; Luo, Y.; Hytönen, T.; Liu, Z. Two MYB activators of anthocyanin biosynthesis exhibit specialized activities in petiole and fruit of diploid strawberry. J. Exp. Bot. 2022, 74, 1517–1531. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, M.; Zhao, C.; Wang, R.; Xue, L.; Lei, J. A novel bHLH transcription factor, FabHLH110, is involved in regulation of anthocyanin synthesis in petals of pink-flowered strawberry. Plant Physiol. Biochem. 2025, 222, 109713. [Google Scholar] [CrossRef]
- Severo, J.; de Oliveira, I.R.; Tiecher, A.; Chaves, F.C.; Rombaldi, C.V. Postharvest UV-C treatment increases bioactive, ester volatile compounds and a putative allergenic protein in strawberry. LWT 2015, 64, 685–692. [Google Scholar] [CrossRef]
- Song, Y.; Teakle, G.; Lillywhite, R. Unravelling effects of red/far-red light on nutritional quality and the role and mechanism in regulating lycopene synthesis in postharvest cherry tomatoes. Food Chem. 2023, 414, 135690. [Google Scholar] [CrossRef] [PubMed]
- Stuemky, A.; Uchanski, M.E. Supplemental Light-emitting Diode Effects on the Growth, Fruit Quality, and Yield of Two Greenhouse-grown Strawberry (Fragaria × ananassa) Cultivars. Hortscience 2020, 55, 23–29. [Google Scholar] [CrossRef]
- Manivannan, A.; Han, K.; Lee, S.Y.; Lee, H.E.; Hong, J.P.; Kim, J.; Lee, Y.R.; Lee, E.S.; Kim, D.S. Genome-Wide Analysis of MYB10 Transcription Factor in Fragaria and Identification of QTLs Associated with Fruit Color in Octoploid Strawberry. Int. J. Mol. Sci. 2021, 22, 12587. [Google Scholar] [CrossRef]
- Choi, H.G.; Jeong, H.J. Effects of Supplemental LEDs on the Fruit Quality of Two Strawberry (Fragaria × ananassa Duch.) Cultivars due to Ripening Level. Prot. Hortic. Plant Fact. 2019, 28, 302–310. [Google Scholar] [CrossRef]
- Yang, M.; Xiang, Y.; Luo, Z.; Gao, Y.; Wang, L.; Hu, Q.; Dong, Y.; Qi, M.; Li, D.; Liu, L.; et al. Light-responsive transcription factors VvHYH and VvGATA24 mediate wax terpenoid biosynthesis in Vitis vinifera. Plant Physiol. 2024, 196, 1546–1561. [Google Scholar] [CrossRef]
- Shoji, K.; Eiji, G.; Shin-nosuke, H.; Fumiyuki, G.; Toshihiro, Y. Effect of Red Light and Blue Light on The Anthocyanin Accumulation and Expression of Anthocyanin Biosynthesis Genes in Red-leaf Lettuce. Shokubutsu Kankyo Kogaku 2010, 22, 107–113. [Google Scholar] [CrossRef]
- Lauria, G.; Piccolo, E.L.; Ceccanti, C.; Paoli, L.; Giordani, T.; Guidi, L.; Malorgio, F.; Massai, R.; Nali, C.; Pellegrini, E.; et al. Supplemental red light more than other wavebands activates antioxidant defenses in greenhouse-cultivated Fragaria × ananassa var. Elsanta plants. Sci. Hortic. 2023, 321, 112319. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, S.; Wang, X.; Han, F.; Luo, H.; Liu, Z.; Kang, C. The red/far-red light photoreceptor FvePhyB regulates tissue elongation and anthocyanin accumulation in woodland strawberry. Hortic. Res. 2023, 10, uhad232. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, L.; Ariza, M.T.; Gómez-Mora, J.A.; Miranda, L.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Light exposure affects fruit quality in different strawberry cultivars under field conditions. Sci. Hortic. 2019, 252, 291–297. [Google Scholar] [CrossRef]
- Fukuda, N.; Oba, H.; Mizuta, D.; Yoshida, H.; Olsen, J.E. Timing of blue and red light exposure and CPPU application during the raising of seedlings can control flowering timing of petunia. Acta Hortic. 2016, 1134, 171–178. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.; Gu, X.; Deng, M.; Li, X.; Zhou, A.; Suo, M.; Gao, W.; Lin, Y.; Wang, Y.; et al. Light Quality and Sucrose-Regulated Detached Ripening of Strawberry with Possible Involvement of Abscisic Acid and Auxin Signaling. Int. J. Mol. Sci. 2023, 24, 5681. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Deng, X.W. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr. Opin. Plant Biol. 2014, 21, 96–103. [Google Scholar] [CrossRef]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A.; Hadley, P. Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 2011, 92, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, H.O.; Kim, J.Y.; Kwon, K.H.; Cha, H.S.; Kim, J.H. An effect of light emitting diode (LED) irradiation treatment on the amplification of functional components of immature strawberry. Hortic. Environ. Biotechnol. 2011, 52, 35–39. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Intraspecific variations in growth, yield and photosynthesis of sorghum varieties to ambient UV (280–400 nm) radiation. Plant Sci. 2012, 196, 85–92. [Google Scholar] [CrossRef]
- Charles, M.T.; Makhlouf, J.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. Postharvest Biol. Technol. 2008, 47, 21–26. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.; Battey, N.H.; Hadley, P. The influence of ultraviolet radiation on growth, photosynthesis and phenolic levels of green and red lettuce: Potential for exploiting effects of ultraviolet radiation in a production system. Ann. Appl. Biol. 2010, 156, 357–366. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Ordidge, M.; Henbest, R.G.; Wagstaffe, A.; Battey, N.H.; Hadley, P. Harvesting fruit of equivalent chronological age and fruit position shows individual effects of UV radiation on aspects of the strawberry ripening process. Environ. Exp. Bot. 2011, 74, 178–185. [Google Scholar] [CrossRef]
- Forges, M.; Bardin, M.; Urban, L.; Aarrouf, J.; Charles, F. Impact of UV-C Radiation Applied during Plant Growth on Pre- and Postharvest Disease Sensitivity and Fruit Quality of Strawberry. Plant Dis. 2020, 104, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Ding, N.; Zhang, Q.; Xing, S.; Wei, L.; Zhao, Y.; Du, P.; Mao, W.; Li, J.; Li, B.; et al. A FERONIA-Like Receptor Kinase Regulates Strawberry (Fragaria × ananassa) Fruit Ripening and Quality Formation. Front. Plant Sci. 2017, 8, 1099. [Google Scholar] [CrossRef]
- Prisca, M.; Maarten, V.; Bart, N.; Wouter, S.; Timo, H.; Barbara, D.C.; de Poel Bram, V. Blue and far-red light control flowering time of woodland strawberry (Fragaria vesca) distinctively via CONSTANS (CO) and FLOWERING LOCUS T1 (FT1) in the background of sunlight mimicking radiation. Environ. Exp. Bot. 2022, 198, 104866. [Google Scholar] [CrossRef]
- Ngo, T.; Wrolstad, R.E.; Zhao, Y. Color Quality of Oregon Strawberries—Impact of Genotype, Composition, and Processing. J. Food Sci. 2006, 72, C025–C032. [Google Scholar] [CrossRef]
- Ries, J.; Park, Y. Far-red Light in Sole-source Lighting Can Enhance the Growth and Fruit Production of Indoor Strawberries. HortScience 2024, 59, 799–805. [Google Scholar] [CrossRef]
- Guiamba, H.D.S.S.; Zhang, X.; Sierka, E.; Lin, K.; Ali, M.M.; Ali, W.M.; Lamlom, S.F.; Kalaji, H.M.; Telesinski, A.; Yousef, A.F.; et al. Enhancement of photosynthesis efficiency and yield of strawberry (Fragaria ananassa Duch.) plants via LED systems. Front. Plant Sci. 2022, 13, 918038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Y.; Wang, Y.; Jiang, L.; Yue, M.; Tang, L.; Jin, M.; Zhang, Y.; Lin, Y.; Tang, H. B-Box Transcription Factor FaBBX22 Promotes Light-Induced Anthocyanin Accumulation in Strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2022, 23, 7757. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, H.; Shi, J.; Duan, Y.; Wu, W.; Lyu, L.; Li, W. Effects of Different Light Wavelengths on Fruit Quality and Gene Expression of Anthocyanin Biosynthesis in Blueberry (Vaccinium corymbosm). Cells 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.J.; Visser, R.G.; Marcelis, L.F. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. Acta Hortic. 2016, 1134, 251–258. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Wang, B.; Dai, H.; Zhang, Z. Positive effect of red/blue light supplementation on the photosynthetic capacity and fruit quality of ‘Yanli’ strawberry. Fruit Res. 2023, 3, 4. [Google Scholar] [CrossRef]
- Kosar, M.; Kafkas, E.; Paydas, S.; Baser, K.H. Phenolic Composition of Strawberry Genotypes at Different Maturation Stages. J. Agric. Food Chem. 2004, 52, 1586–1589. [Google Scholar] [CrossRef]
- Park, Y.; Sethi, R.; Temnyk, S. Growth, Flowering, and Fruit Production of Strawberry ‘Albion’ in Response to Photoperiod and Photosynthetic Photon Flux Density of Sole-Source Lighting. Plants 2023, 12, 731. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bikdeloo, M.; Ghorbanpour, M. The growth, nutrient uptake and fruit quality in four strawberry cultivars under different Spectra of LED supplemental light. BMC Plant Biol. 2024, 24, 179. [Google Scholar] [CrossRef]
- Pendyala, B.; Patras, A.; Ravi, R.; Gopisetty, V.V.; Sasges, M. Evaluation of UV-C Irradiation Treatments on Microbial Safety, Ascorbic Acid, and Volatile Aromatics Content of Watermelon Beverage. Food Bioprocess Technol. 2019, 13, 101–111. [Google Scholar] [CrossRef]
- Wei, L.; Mao, W.; Jia, M.; Xing, S.; Ali, U.; Zhao, Y.; Chen, Y.; Cao, M.; Dai, Z.; Zhang, K.; et al. FaMYB44.2, a transcriptional repressor, negatively regulates sucrose accumulation in strawberry receptacles through interplay with FaMYB10. J. Exp. Bot. 2018, 69, 4805–4820. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Correlation between Strawberry (Fragaria ananassa Duch.) Productivity and Photosynthesis-Related Parameters under Various Growth Conditions. Front. Plant Sci. 2016, 7, 1607. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, D.; Maksimović, V.; Milivojević, J.; Djekić, I.; Wolf, B.; Zuber, J.; Vogt, C.; Dragišić Maksimović, J. Sugars and Organic Acids in 25 Strawberry Cultivars: Qualitative and Quantitative Evaluation. Plants 2023, 12, 2238. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Wada, T.; Furukawa, H.; Tojo, M.; Hirai, N.; Kitaya, Y. Effects of the red light/far-red light ratio on plant shape, fruit yield, and fruit quality in Fragaria × ananassa Duch. ‘Beni hoppe’. Acta Hortic. 2024, 1404, 241–246. [Google Scholar] [CrossRef]
- Yavuz, O.; Kasım, R.; Kasım, M.U. Red LED light affects the physicochemical responses of strawberries during storage. Turk. J. Food Agric. Sci. 2024, 6, 83–95. [Google Scholar] [CrossRef]
- Doupis, G.; Bosabalidis, A.M.; Patakas, A. Comparative effects of water deficit and enhanced UV-B radiation on photosynthetic capacity and leaf anatomy traits of two grapevine (Vitis vinifera L.) cultivars. Theor. Exp. Plant Physiol. 2016, 28, 131–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Deng, M.; Gui, R.; Liu, Y.; Chen, X.; Lin, Y.; Li, M.; Wang, Y.; He, W.; et al. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chem. X 2022, 15, 100384. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Mixed red and blue light promotes ripening and improves quality of tomato fruit by influencing melatonin content. Environ. Exp. Bot. 2021, 185, 104407. [Google Scholar] [CrossRef]
- Samkumar, A.; Jones, D.; Karppinen, K.; Dare, A.P.; Sipari, N.; Espley, R.V.; Martinussen, I.; Jaakola, L. Red and blue light treatments of ripening bilberry fruits reveal differences in signalling through abscisic acid-regulated anthocyanin biosynthesis. Plant Cell Environ. 2021, 44, 3227–3245. [Google Scholar] [CrossRef]
- Liang, D.; Yousef, A.F.; Wei, X.; Ali, M.M.; Yu, W.; Yang, L.; Oelmüller, R.; Chen, F. Increasing the performance of Passion fruit (Passiflora edulis) seedlings by LED light regimes. Sci. Rep. 2021, 11, 20967. [Google Scholar] [CrossRef]
- Zushi, K.; Suehara, C.; Shirai, M. Effect of light intensity and wavelengths on ascorbic acid content and the antioxidant system in tomato fruit grown in vitro. Sci. Hortic. 2020, 274, 109673. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, J.; Yoo, K.S.; Lim, S.; Lee, E.J. Effect of Prestorage UV-A, -B, and -C Radiation on Fruit Quality and Anthocyanin of ‘Duke’ Blueberries during Cold Storage. J. Agric. Food Chem. 2014, 62, 12144–12151. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Eguchi, M.; Gui, Y.; Iwasaki, Y. Evaluating the Effect of Light Intensity on Flower Development Uniformity in Strawberry (Fragaria × ananassa) under Early Induction Conditions in Forcing Culture. Hortscience 2020, 55, 670–675. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Gururani, P.; Bisht, B.; Kumar, V.; Kumar, N.; Joshi, R.; Vlaskin, M.S. Impact of irradiation on physico-chemical and nutritional properties of fruits and vegetables: A mini review. Heliyon 2022, 8, e10918. [Google Scholar] [CrossRef] [PubMed]
- Hamedalla, A.M.; Ali, M.M.; Ali, W.M.; Ahmed, M.A.A.; Kaseb, M.O.; Kalaji, H.M.; Gajc-Wolska, J.; Yousef, A.F. Increasing the performance of cucumber (Cucumis sativus L.) seedlings by LED illumination. Sci. Rep. 2022, 12, 852. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Peng, X.; Wang, B.; Wang, X.; Ni, B.; Zuo, Z. Variations in aroma and specific flavor in strawberry under different colored light-quality selective plastic film. Flavour Fragr. J. 2020, 35, 350–359. [Google Scholar] [CrossRef]
- Nadalini, S.; Zucchi, P.; Andreotti, C. Effects of blue and red LED lights on soilless cultivated strawberry growth performances and fruit quality. Eur. J. Hortic. Sci. 2017, 82, 12–20. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Oh, J.; Park, E.; Song, K.; Bae, G.; Choi, G. PHYTOCHROME INTERACTING FACTOR8 Inhibits Phytochrome A-Mediated Far-Red Light Responses in Arabidopsis. Plant Cell 2019, 32, 186–205. [Google Scholar] [CrossRef]
- Jung, J.-H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors in Arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef]
- Piovene, C.; Francesco, O.; Sara, B.; Rabab, S.; Valeria, B.; Giovanni, D.; Giorgio, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G. Correlation Among Phenotypic Parameters Related to the Growth and Photosynthesis of Strawberry (Fragaria × ananassa Duch.) Grown Under Various Light Intensity Conditions. Front. Plant Sci. 2021, 12, 647585. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagi, I.A.; Hasan, S.M.; Aziz, A.; Abdullah, Y.W. The Interaction Effect of Photoperiod and Exogenous Hormone on the Dry Matter of Strawberry (Fragaria × ananassa Duch). Agric. J. 2011, 6, 340–346. [Google Scholar] [CrossRef]
- Lv, X.; Zeng, X.; Hu, H.; Chen, L.; Zhang, F.; Liu, R.; Liu, Y.; Zhou, X.; Wang, C.; Wu, Z.; et al. Structural insights into the multivalent binding of the Arabidopsis FLOWERING LOCUS T promoter by the CO–NF–Y master transcription factor complex. Plant Cell 2021, 33, 1182–1195. [Google Scholar] [CrossRef]
- Wei, H.; Liu, C.; Hu, J.; Jeong, B.R. Quality of Supplementary Morning Lighting (SML) During Propagation Period Affects Physiology, Stomatal Characteristics, and Growth of Strawberry Plants. Plants 2020, 9, 638. [Google Scholar] [CrossRef]
- Verhoeven, B.; Bodson, M. Dormancy of june-bearing strawberry (Fragaria × ananassa Duch. cv ‘elsanta’) as influenced by photoperiod. Acta Hortic. 2004, 694, 159–164. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W.; Galletta, G.J. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem. 2002, 50, 6534–6542. [Google Scholar]
- Robson, T.M.; Aphalo, P.J. Species-specific effect of UV-B radiation on the temporal pattern of leaf growth. Physiol. Plant. 2012, 144, 146–160. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Roig-Villanova, I.; Bou, J.; Sorin, C.; Devlin, P.F.; Martínez-García, J.F. Identification of Primary Target Genes of Phytochrome Signaling. Early Transcriptional Control during Shade Avoidance Responses in Arabidopsis. Plant Physiol. 2006, 141, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, M.F.; Wang, D.; Chen, F.; Shen, S.H. JcDof1, a Dof transcription factor gene, is associated with the light-mediated circadian clock inJatropha curcas. Physiol. Plant. 2010, 139, 324–334. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, F.; He, D.; Niu, G. Effect of Light Intensity on Rooting and Growth of Hydroponic Strawberry Runner Plants in a LED Plant Factory. Agronomy 2019, 9, 875. [Google Scholar] [CrossRef]
- Chen, C.Q.; Tian, X.Y.; Li, J.; Bai, S.; Zhang, Z.Y.; Li, Y.; Cao, H.R.; Chen, Z.C. Two central circadian oscillators OsPRR59 a. Two central circadian oscillators OsPRR59 and OsPRR95 modulate magnesium homeostasis and carbon fixation in rice. Mol. Plant 2022, 15, 1602–1614. [Google Scholar] [CrossRef]
- Yoshida, H.; Mizuta, D.; Fukuda, N.; Hikosaka, S.; Goto, E. Effects of varying light quality from single-peak blue and red light-emitting diodes during nursery period on flowering, photosynthesis, growth, and fruit yield of everbearing strawberry. Plant Biotechnol. 2016, 33, 267–276. [Google Scholar] [CrossRef]
- Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol. Technol. 2006, 40, 116–122. [Google Scholar] [CrossRef]
- Pallvi, V.; Gurpreet, S.; Singh, S.K.; Anis Ahmad, M.; Manish, B.; Anmol, A.; Lakshya, B. Improvement in productivity of strawberry (Fragaria × ananassa Duch.) under vertical farming system. J. Appl. Biol. Biotechnol. 2024, 12, 184–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).