Abstract
This study examines how various rootstocks affect the pomological, biochemical, and aroma contents of the local ‘Sırrı’ peach genotype grown in the Lapseki region of Türkiye. The research focused on peach trees grafted onto three distinct rootstocks: ‘Seedling’, ‘GF-677’ (P. persica × P. amygdalus), and ‘Rootpac-R’ (P. cerasifera × P. amygdalus). The results showed that peaches from the ‘Seedling’ and ‘GF-677’ rootstocks had larger sizes, greater weights, and brighter colors compared to those from ‘Rootpac-R’. Furthermore, the rootstocks impacted essential quality factors such as soluble solid content, firmness, fruit–flesh ratio, titratable acidity, and total phenolic content levels. The analysis of volatile compounds indicated that aldehydes (which varied from 67.02% to 63.74%), lactones (which changed from 9.14% to 7.99%), and esters (which changed from 12.51% to 11.92%) were the major aroma types in ‘Sırrı’ peaches, with the ‘GF-677’ rootstock exhibiting amplified fruity and sweet aromas due to increased lactone levels. Principal Component Analysis (PCA) revealed the significant effects of rootstocks on both pomological and biochemical characteristics, with ‘Seedling’ showing elevated biophenol levels and ‘GF-677’ contributing to a firmer texture. These findings underscore the importance of rootstock choice in enhancing fruit quality and aroma, indicating that the ‘Sırrı’ genotype is highly suitable for commercial production and future breeding efforts.
1. Introduction
Peaches (Prunus persica L. Batsch) belong to the genus Prunus L., which is part of the subfamily Amygdaloideae in the family Rosaceae, under the order Rosales. The subspecies of this genus is Amygdalus, classified in the section Prunus. It is generally accepted that peach cultivation originated in China around 8000 BC [1], though the precise date of its introduction to present-day Türkiye remains uncertain. Peaches rank globally among the most widely cultivated and diverse temperate fruit species due to their natural self-fertility and adaptability. The species thrive in temperate and subtropical regions between 30° and 45° north and south latitudes [2,3,4,5,6].
According to data from the past decade, global peach and nectarine production reached 27,077,873 tons in 2023, representing an approximate 24% increase. China, Spain, and Türkiye are the world’s leading producers of peaches [7]. In 2023, Türkiye harvested 1,076,852 tons of peaches and nectarines, with the highest production occurring in the provinces of Mersin, Çanakkale, Bursa, Denizli, and Izmir [8].
The climate of Çanakkale creates opportunities for cultivating various fruit species, leading to rich fruit genotypes and variety biodiversity. In the Çanakkale region, especially in the Lapseki district, the quality of peach cultivation was certified with the ‘Lapseki Peach’ geographical indication, granted on 10 May 2022. In the region, cultivation primarily utilizes nearly 40 standard commercial varieties, including ‘Early May Crest’, ‘May Crest’, ‘Dixired’, ‘Redhaven’, ‘Gloheaven’, ‘Cresthaven’, ‘O’Henry’, ‘J.H. Hale’, ‘Blake’, ‘Monroe’, ‘Elegant Lady’, ‘Venus’, ‘Silver King’, ‘Caldesi-2000’, ‘Caldesi-85’, ‘Summer Red’, and ‘Extreme’. Additionally, some local genotypes, such as ‘Abdos’ and ‘Sırrı’ (unregistered local genotypes), which have been cultivated for many years and are well adapted to the region, are grown extensively. Their superior quality sets these local genotypes apart from other standard peach varieties [9,10].
In modern fruit cultivation, as with many other fruit species, one of the most critical factors for successful peach cultivation is the selection of rootstocks. The use of specific rootstocks is driven by various factors, including the requirement to grow peaches under unfavorable climatic and soil conditions, regulating growth, reducing the juvenile sterility period, improving fruit quality and yield, facilitating the management of pests and diseases, and more. In addition to anchoring the plant to the soil, it has been proven that rootstocks significantly influence many characteristics of the grafted scion, such as fruit yield, quality, and post-harvest storage, and that these characteristics are transferred to the grafted cultivar. Considering this information, selecting the right rootstock is one of the most critical aspects of successful peach cultivation [11].
Fruit quality is a critical factor for market success. Consumers are influenced by certain factors, such as the appearance, taste, and aroma of fresh fruits both before and during consumption. These quality parameters are closely related to various chemical and physical characteristics, such as mineral content, sugar profile, color, pH, fruit size, weight, skin thickness, and firmness, all of which are significantly affected by the rootstock. Alongside many quality attributes, fruit aroma plays a vital role in consumer preferences and arises from the combined effects of numerous volatile compounds. Aroma, along with sugar content and acidity, is a highly complex trait that constitutes an important quality attribute of the sensory characteristics of fresh fruits. Peach fruit contains approximately 100 different aroma compounds, including aldehydes, alcohols, esters, terpenoids, ketones, lactones, and C13 norisoprenoids [12,13]. Among these, aldehydes (‘fresh green’, ‘unripe fruit’), alcohols, esters (‘fruity’, ‘green’), lactones (‘peach-like’, ‘sweet’), and terpenoids (‘floral’) are the main components that contribute to the aroma of peaches. Lactones play a vital role in defining the peach scent. Volatile compounds mainly consist of substances that can be detected even in tiny amounts, which makes them crucial for consumer preferences. This critical characteristic significantly influences consumer choice [9,14,15,16,17,18,19].
This study aimed to explore how various rootstocks influence fruit quality (pomological and biochemical characteristics), mainly the volatile compound composition of the local peach genotype ‘Sırrı’.
2. Materials and Methods
2.1. Materials
This study was conducted in the commercial orchards of producers in Umurbey, Lapseki district, Çanakkale province (Figure 1). The material of the study consisted of fruits harvested on 25 September 2023, from orchards located in the Lapseki district of Çanakkale province. In 2023, it was noted that the climate of the Lapseki region in Çanakkale province was roughly comparable to what is shown in the climate data from many other years [20]. These orchards were established with the ‘Sırrı’ peach ecotype grafted onto ‘Seedling’, ‘GF 677’, and ‘Replantpac’ (‘Rootpac-R’) rootstocks, and they were in full-bearing age.
Figure 1.
The commercial orchards where the fruits for the research were harvested.
Peach rootstocks develop uniformly. This is because peaches are primarily self-fertile and yield pits that grow in a uniform structure. Consequently, peach cultivators frequently use peach seedlings as rootstocks. The rootstock ‘GF677’, obtained in 1939 by the INRA Research Organization in France as a natural hybrid of peach and almond (Prunus persica × Prunus amygdalus), is an ideal rootstock for earliness and high yield. While it is resistant to drought, high pH, and high salinity, it is also sensitive to biotic conditions such as nematodes, root rot, and branch cancer. The ‘Replantpac’ (‘Rootpac-R’) rootstock is a hybrid of plum and almond (Prunus cerasifera × Prunus amygdalus). This highly productive and versatile new-generation rootstock is ideal for replanting orchards, tolerates challenging soil conditions, and is compatible with varieties of peach, nectarine, plum, almond, and apricot [21,22].
‘Sırrı’ peach genotype plots in the orchards were established in 2011 at 4.5 × 4 planting density and pruned according to the free goblet shape system. Standard practices such as drip irrigation, fertilization, pest control, and summer pruning were routinely applied. ‘Sırrı’ is a very late-maturing peach genotype (harvest period: mid-September to early October), with the fruit characterized by a yellow ground color overlaid with patches of light red, yellow flesh, and a freestone pit. The fruits of the ‘Sırrı’ peach genotype at the ripening stage were harvested using the tissue integrity and skin ground color as the maturity indices for the ecotype [23,24]. The study involved eighteen trees, with three replicates for each rootstock and two trees per replicate. Pomological measurements and biochemical analyses were carried out on 10 randomly selected fruits collected from various sides of the trees within the replicate.
2.2. Methods
After harvest, the fruit samples were promptly analyzed at the Pomology Laboratory (Department of Horticulture, Faculty of Agriculture, Çanakkale Onsekiz Mart University). The pomological traits were evaluated by measuring the fruit width (mm), fruit length (mm), pit width (mm), pit length (mm), fruit weight (g), pit weight (g), fruit flesh ratio (%), fruit skin and flesh color (L, hue (h°), chroma (C) (using a CR-400 Chroma Meter (Konica Minolta Inc., Tokyo, Japan), firmness (kg/cm2), soluble solid content (SSC) (%), pH, titratable acidity (TA) (Milwaukee® desktop type pH/Orp Meter, Rocky Mount, NC, USA) (g/100 mL Malic acid), and total phenolic compound content (μg/100 mL GAE). Measurements were taken with precision instruments: a digital caliper (±0.01 mm), a Sartorius balance (0.01 g), a digital refractometer (Atago, Tokyo, Japan) for SSC, and a chromameter for color (L, hue, and chroma). Fruit peel was removed from the mid-plane of the fruit in two opposite regions and fruit firmness (kg/cm2) was determined using a Chatillon® penetrometer (5.1 mm tip).
The total phenolic compound content was determined using the method described by Ekinci et al. [25]. For the analysis, 5 g of peach fruit was weighed, and 20 mL of an 80% methanol–water mixture was added. The mixture was homogenized by stirring for 1 min and subsequently filtered using coarse filter paper. A 100 µL aliquot of the filtrate was mixed with 3400 µL of distilled water, followed by the addition of 2000 µL of 10% Folin–Ciocalteu reagent (Merck, Darmstadt, Germany) and homogenization for 15 s. Subsequently, 2000 µL of 10% Na2CO3 (Sigma-Aldrich, St. Louis, MO, USA) solution was added and mixed again for 15 s. The mixture was then incubated in a water bath at 40 °C for 30 min. Absorbance measurements were conducted at 765 nm using a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan), with a blank prepared using distilled water. The total phenolic content was quantified using a calibration curve generated with gallic acid.
The skin and flesh colors of the fruit were measured from two opposite sides in the equatorial region of the fruit. When determining the colors of the fruit, a higher L value indicates increased brightness, while an increase in the chroma value signifies greater color intensity, making the color appear more vibrant. The hue angle, on the other hand, reflects different color tones: as it approaches 0, the color becomes dark burgundy; nearing 45, it shifts to a bright red; closer to 60, it appears orange; and approaching 90, it transitions to yellow.
The qualitative and quantitative analyses of the volatile compounds were conducted using a GC-MS system (QP2010 Plus, Shimadzu Corporation, Kyoto, Japan). For qualitative analysis (identification of compounds), the WILEY and NIST libraries were used in conjunction with a mass spectrometer. To extract volatile compounds, 50 g of fruit purees was treated with 100 mL of diethyl ether in an Erlenmeyer flask. The solvents were then concentrated to 1 mL using centrifugation and a concentrator. Additionally, 4-Nonanol (Sigma Aldrich, St Quentin Fallavier, France) was used as the internal standard for quantitative analysis [26,27].
For compound separation, a DB wax capillary column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies, Santa Clara, CA, USA), featuring polyethylene glycol (PEG) as the stationary phase and helium gas as the mobile phase, was employed. The aroma profiles were determined using a 1:50 split injection mode at an injection temperature of 280 °C, with the ion and interface temperatures set at 250 °C and 230 °C, respectively. Compounds were identified in the detector with a mass range of 40–350 amu (m/z), a scan rate of 666 amu/s, and an ionization energy of 70 eV. The oven temperature program began with a 1 min hold at 40 °C, followed by a ramp up to 60 °C at a rate of 4 °C/min, and then a 1 min hold. The temperature subsequently increased to 200 °C at the same rate of 4 °C/min, followed by a 2 min hold, and finally ramped up to 250 °C at 10 °C/min for 10 min. The total analysis time was 59 min.
2.3. Statistical Analyses
In this research, a randomized complete block design with three replications was utilized. The data collected at the end of the study were analyzed using analysis of variance (ANOVA) with the statistical software package ‘SAS® ver. 9.0’. The mean values for the treatments were assessed at a significance level of p < 0.05 using the Tukey multiple comparison test. Biplot graphs were created using the ‘Minitab ver. 16’ statistical program.
3. Results and Discussion
The fruit quality characteristics of the ‘Sırrı’ peach genotype varied significantly among the rootstocks (Table 1). Overall, it was found that the fruits of the ‘Sırrı’ genotype cultivated on the ‘GF-677’ and ‘Seedling’ rootstocks were larger and heavier, and their skin shone more brightly. When examined pomologically, the fruit width ranged from 79.92 to 74.12 mm, and the length varied from 78.58 to 72.46 mm. The fruits with the highest weight were found on cultivars grafted onto the ‘Seedling’ rootstock (284.40 g), whereas the lightest fruits were noted on those grafted onto the ‘Rootpac-R’ rootstock (221.18 g).

Table 1.
Pomological characteristics of the fruits of the ‘Sırrı’ peach genotype cultivated on various rootstocks.
Gür and Pırlak [28] conducted a study in Egirdir using 16 different peach cultivars grafted onto the peach ‘Seedling’ rootstock. Consequently, they found that the fruit weights of the peach cultivars ranged from 133.40 g to 252.40 g, while the fruit widths varied from 88.00 mm to 63.40 mm. Şeker et al. [29] conducted a study using the ‘Cresthaven’ cultivar, which was grafted onto five different rootstocks: ‘Uzunoglu Seedling’ (Prunus persica), ‘GF677’ (P. persica × P. dulcis), ‘Nemaguard’ (P. persica × P. davidiana), ‘Cadaman’ (P. persica × P. davidiana), and ‘St. Julien GF 655/2’ (P. insititia). They observed the highest fruit weight on the ‘Cadaman’ rootstock (211.61 ± 6.06 g) and the lowest fruit weight on the ‘St. Julien GF 655/2’ rootstock (178.70 ± 3.01 g). The variations in fruit weight observed among different rootstocks in their study align with the findings of the current study, emphasizing the substantial impact of rootstock selection on fruit size and weight.
Fruits grown on the ‘GF-677’ rootstock had the highest width (22.32 mm), length (45.95 mm), and weight (11.44 g) of pits, while those grown on the ‘Rootpac-R’ rootstock had the pits with the smallest width, length, and weight (15.62 mm, 38.00 mm, and 7.30 g, respectively) (Table 1). Çalışkan et al. [30] reported pit weights ranging from 5.0 to 11.9 g in a study on five peach and five nectarine cultivars grafted onto ‘Seedling’ rootstocks. Türkmen [31] reported pit weight values between 3.71 g and 4.21 g in a study with six peach and seven nectarine cultivars. It was observed that the ‘GF-677’ rootstock increased pit size, while the Rootpac-R rootstock decreased it. This variation affected the flesh ratios of fruits. The highest flesh ratio was achieved with the ‘Rootpac-R’ rootstock at 96.68%, while the ‘GF-677’ rootstock had a slightly lower ratio of 95.58%. Gür et al. [23] reported that the fruit–flesh ratios of peach varieties grafted onto the ‘GF-677’ rootstock ranged from 91.92% to 97.12%, with the ‘Sırrı’ genotype exhibiting the highest flesh ratio. When examining the skin colors of the fruits, it was observed that fruits grafted onto the ‘GF-677’ and ‘Seedling’ rootstocks had brighter skin colors (46.49 and 45.35, respectively) and more orange hues (53.67 and 51.54, respectively). In contrast, fruits grafted onto the ‘Rootpac-R’ rootstock displayed lower brightness (32.70) and more red-colored skin (34.15). Regarding chroma, which indicates color intensity, fruits on the ‘GF-677’ rootstock showed the highest value (28.61), whereas those on Rootpac-R had the lowest (26.55) (Table 1).
The rootstock did not significantly affect the fruit flesh’s brightness (L*) or hue values, as all rootstocks showed similar brightness and hue values. However, the fruits grown on the ‘GF-677’ rootstock showed a more intense color tone (chroma), with a value of 33.57. In contrast, the ‘Rootpac-R’ and ‘Seedling’ rootstocks had similar chroma values of 31.88 and 31.02, respectively (Table 1).
Yıldız [32] examined the color characteristics of 11 processing peach cultivars grafted onto the ‘GF-677’ rootstock. The study revealed that the L* values, which indicate fruit brightness, ranged from 81.44 to 72.02 on the fruit skin. The chroma values ranged from 21.71 to 9.85, and the hue angle values varied from 107.61 to 85.51. Similarly, Gür et al. [23] conducted a study that found that the L* values, which represent fruit brightness, ranged from 19.54 to 57.29 on the fruit skin, while the chroma values ranged from 17.42 to 48.93. Furthermore, the L* values in the fruit flesh ranged from 47.78 to 59.22, while the chroma values varied from 26.51 to 49.28.
Fruits grown on the ‘GF-677’ rootstock exhibited firmer flesh (3.87 kg/cm2) compared to those cultivated on the other two rootstocks, potentially enhancing their post-harvest durability. Fruits grown on the ‘Seedling’ rootstock showed the highest SSC (12.57%), which, while indicative of more intense sweetness, also serves as a criterion for more advanced ripeness. Gür et al. [23] reported that the soluble solid content in 16 peach cultivars ranged from 7.53% to 14.50%. Additionally, the ‘Sırrı’ peach genotype grafted onto the ‘GF-677’ rootstock was measured at 13.10% ± 0.27. These findings were comparable to those of the earlier study. The SSC and TA contents of the fruit vary not only according to genotype, but also due to environmental and seasonal conditions, yield, sunlight exposure, and ripeness [22]. Fruit firmness not only relates to mass, but also to cell structure, cell wall components, and the maturity level of the fruit. For instance, the highest soluble solid content (SSC) was noted in fruit grown on the ‘Seedling’ rootstock. Elevated SSC values typically indicate a more advanced level of ripeness, which may result in a decrease in fruit firmness. Kader [33] explains that a higher soluble solid content (SSC) is usually linked to advanced ripening, which subsequently results in decreased fruit firmness due to the breakdown of the cell wall components. This supports the notion that the observed variation in firmness may not solely stem from fruit weight, but could also be influenced by differences in the biochemical composition and ripening stage.
The impact of rootstock on fruit pH was not statistically significant, as all rootstocks displayed similar pH values. Gür [28] reported fruit firmness values of 1.90 kg and 1.43 kg, along with pH values of 3.80 and 3.71 for the ‘Vesuvio’ and ‘Muir’ cultivars, respectively. In terms of malic acid content, fruits grown on the ‘Seedling’ rootstock exhibited the lowest value (0.587), while those on the ‘GF-677’ rootstock showed the highest acidity (0.677). Seker et al. [29] reported that the malic acid content of the ‘Cresthaven’ peach cultivar grown with different rootstocks varied between 0.35 and 0.65 g/100 g. According to Gur et al. [23], the citric acid content of the ‘Sırrı’ peach variety was 0.89 g/100 mL (0.85 g/100 mL malic acid).
The results indicate that rootstocks significantly influence various characteristics of peach fruits. While the ‘GF-677’ and ‘Seedling’ rootstocks generally produce larger and heavier fruits, the ‘Rootpac-R’ rootstock results in higher fruit–flesh ratios. Different rootstocks play a crucial role in determining several quality traits.
This study found a statistically significant difference in the total phenolic compound content of the fruits from the ‘Sırrı’ genotype grown on different rootstocks. The total phenolic content was measured at 65.92 mg/kg GAE for the ‘Seedling’ rootstock, 62.81 mg/kg GAE for the ‘Rootpac-R’ rootstock, and 60.01 mg/kg GAE for the ‘GF-677’ rootstock (Table 1). Significantly, the total phenolic content of the ‘Sırrı’ fruit genotype grafted onto the ‘Seedling’ rootstock was higher than that of the other rootstocks. Crisosto and Valero [34,35] reported that the total phenolic content for California peach cultivars ranged from 28 to 111 mg/100 g FW for white-fleshed peaches and from 21 to 61 mg/100 g FW for yellow-fleshed peaches, while other European cultivars had a content between 38 and 240 mg/100 g FW. Researchers also noted that the phenolic content in peach fruits influences visual appearance (color), taste (astringency), and antioxidant properties related to health [34,36].
In the study examining the effects of rootstocks grafted onto the ‘Sırrı’ genotype on the aroma compounds in fruits, the identified aroma compounds are represented by their relative amounts (%) in the overall aroma profile (Table 2). It was found that they influenced the total proportions of aldehydes, which are the desired group of volatile compounds in peaches (67.02% for ‘GF-677’, 66.56% for ‘Rootpac-R’, and 63.74% for ‘Seedling’). Notably, hexanal (34.33% for ‘Rootpac-R’, 33.01% for ‘GF-677’, and 32.31% for ‘Seedling’) and (E)-2-hexenal (28.59% for ‘GF-677’, 27.78% for ‘Rootpac-R’, and 26.63% for ‘Seedlin’) were statistically significant. Additionally, statistically significant changes were found in hydrocarbons, which are generally undesired aroma compound groups in fruits, as well as in hexane, the only hydrocarbon detected in this group. Regarding other aroma compounds, which were found at a lesser level, the influence of the rootstocks did not show a statistical difference in the fruit genotype.

Table 2.
Identified and determined volatile compounds (%) in the fruits of the ‘Sırrı’ peach genotype cultivated on different rootstocks.
In this study, the effects of rootstocks on aroma compounds were investigated. Four aldehyde aroma compounds were identified: hexanal, (E)-2-hexenal, benzaldehyde, and (E)-2-pentenal; four important lactone compounds for peaches: δ-decalactone, γ-decalactone, δ-octalactone, and γ-hexalactone; three alcohol compounds: (Z)-3-hexenol, 1-hexanol, and (E)-2-hexenol; five ester compounds: ethyl acetate, hexyl acetate, (Z)-3-hexyl acetate, (E)-2-hexyl acetate, and butyric acid hexyl ester; three terpenoid compounds: linalool, D-limonene, and ocimene; and one hydrocarbon compound: hexane (Table 2).
In this study, the effects of rootstocks on the aroma of the ‘Sırrı’ genotype were found to be comparable. The key aroma compounds in peaches are aldehyde and lactone groups. The rootstocks used for the ‘Sırrı’ genotype influenced these two aroma groups. In their exploration of aromatic compounds in peach and nectarine cultivars grown in the ecological conditions of Çanakkale, Şeker et al. [26] noted that aldehyde and lactone groups were important aroma compounds. In another study conducted by Şeker et al. [29], they investigated volatile compounds in the ‘Cresthaven’ peach cultivar grown on five different rootstocks and identified similar aroma compounds. They also reported that different rootstocks affected the aroma compounds of the cultivars.
Aldehydes are among the primary volatile groups contributing to peach aroma, with the total aldehyde content varying from 63.74% in ‘Seedling’ rootstock fruits to 67.02% in those from the ‘GF-677’ rootstock. Among the most common aldehydes, hexanal and (E)-2-hexenal enhance the fruit’s green and fresh characteristic scent. Benzaldehyde, which imparts an almond-like odor profile, had the highest concentration in fruits from the ‘GF-677’ rootstock (3.41%). Similarly, (E)-2-pentenal was found at relatively consistent levels across the rootstocks. These variations indicate that the choice of rootstock may influence the strength of the fruit’s green and fresh aroma [29,37].
Lactones, particularly δ-decalactone and γ-decalactone, play a significant role in creating the distinctive sweet and fruity flavor of peaches. The total lactone content was highest in fruits of the ‘GF-677’ rootstock at 9.14% and lowest in those of the ‘Seedling’ rootstock at 7.99%. δ-decalactone, a key compound in peach aroma, was found in the highest concentration in fruits of the ‘GF-677’ rootstock, while it was lowest in those of the ‘Seedling’ rootstock. This suggests that the choice of rootstock may influence the sweet and fruity flavor of the fruit [29,37].
Alcohols, especially (Z)-3-hexen-1-ol and (E)-2-hexen-1-ol, are associated with the fruit’s green and slightly floral aroma [37]. Total alcohol content did not vary significantly among rootstocks, ranging from 3.88% in ‘GF-677’ to 4.44% in ‘Seedling’.
Esters also play a significant role in the sweet and fruity aroma of the fruit. The total ester content was slightly higher in ‘GF-677’ rootstock fruits at 12.51% compared to ‘Rootpac-R’ fruits at 12.41% and ‘Seedling’ fruits at 11.92%. Among the esters, ethyl acetate and hexyl acetate were the most dominant compounds, significantly enhancing the characteristic fruity flavors of the peach aroma.
Terpenoids and hydrocarbons are volatile compounds that influence the floral and herbal scent of the fruit and show considerable variation among rootstocks [37]. Although the total terpenoid content was relatively similar among the three rootstocks (5.37–5.76%), the total hydrocarbon content (6.53%) was significantly higher in ‘Seedling’ rootstock fruits compared to those from ‘Rootpac-R’ (2.25%) and ‘GF-677’ (1.7%). Fruits from the ‘Seedling’ rootstock were generally richer in hydrocarbons that contain undesirable flavor components.
In conclusion, the data indicate that rootstock selection significantly affects the volatile compound profiles of peach fruits. The ‘GF-677’ rootstock imparts a fresher and fruitier flavor profile, with higher aldehyde and lactone contents, whereas ‘Rootpac-R’ presents a more balanced distribution of volatile compounds. In contrast, the ‘Seedling’ rootstock can yield a more complex flavor profile, particularly due to its elevated hydrocarbon levels. Understanding these differences is crucial for peach cultivation, in order to optimize fruit quality and develop aroma profiles that align with consumer preferences.
Gur [38] reported that rootstocks can significantly influence aroma composition and the ratios of volatile compounds through different carbohydrate and fatty acid metabolism pathways. Therefore, various rootstocks should be utilized to enhance specific aroma compounds in fruits.
The biplot graph was constructed to evaluate the effects of different rootstocks on the pomological and biochemical characteristics of peach fruits and was visualized using Principal Component Analysis (PCA). The graph shows the distribution of variables and rootstocks along two principal component axes (PC-1 and PC-2). PC-1 explains 52.6% of the total variance, and PC-2 explains 47.4%. These high explanatory ratios suggest that the studied characteristics of peach fruits are represented mainly by these two principal component axes (Figure 2).
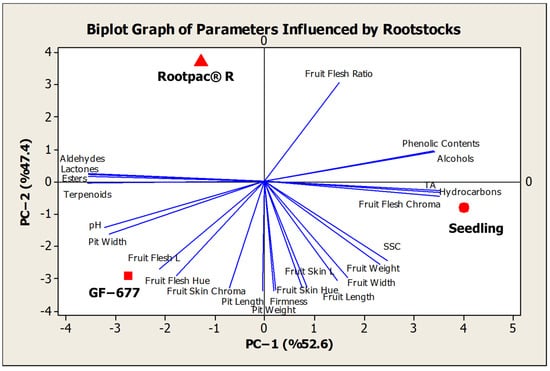
Figure 2.
Biplot graph of parameters affected by rootstocks.
The graph displays three different rootstocks (‘Rootpac-R’, ‘GF-677’, and ‘Seedling’) indicated by red symbols, analyzing their relationships with fruit characteristics. The ‘GF-677’ rootstock shows a negative correlation with variables such as fruit flesh hue, fruit skin chroma, and pit width. The ‘Seedling’ rootstock exhibits a stronger relationship with biochemical components, including total hydrocarbon, total alcohol, and total phenolic compound content. The ‘Rootpac-R’ rootstock demonstrates a powerful relationship with the fruit–flesh ratio, suggesting that this rootstock has a higher fruit–flesh ratio compared to the others.
The directions and lengths of the vectors representing the variables illustrate the contributions of the respective traits to the principal components. Pomological characteristics, such as fruit size, weight, and pit parameters, are more prominent in the PC-1 direction, whereas biochemical components like phenolic compounds, esters, and terpenoids vary in the PC-2 direction. The positive position of the ‘Seedling’ rootstock on the PC-1 axis indicates that it has higher values, particularly regarding total phenolic compound content and hydrocarbons. Conversely, the negative position of the ‘GF-677’ rootstock on the PC-1 axis shows that this rootstock has a strong negative relationship with characteristics such as fruit flesh color, pit width, and fruit skin color.
When the distribution of biochemical characteristics was analyzed, it was observed that traits such as total phenolic compound content, total alcohol, and hydrocarbons varied along the PC-2 axis. The ‘Rootpac-R’ rootstock showed a positive correlation with the fruit–flesh ratio and several volatile compounds. A high fruit–flesh ratio may be an important quality criterion that may directly affect consumer preferences. Additionally, the ‘GF-677’ rootstock was more closely correlated with characteristics like pH and pit width, but exhibited a weaker relationship with volatile compounds.
This analysis demonstrates that selecting the right rootstock in peach cultivation significantly impacts both the pomological and biochemical characteristics of the fruit. Rootstocks appear to play a crucial role in determining fruit size, fruit flesh ratio, biochemical characteristics, and aroma composition. These findings can assist growers and researchers in choosing the most suitable rootstocks by focusing on specific quality attributes. This is especially important in commercial production, where selecting rootstocks that provide taste, aroma, and textural qualities that align with consumer expectations is essential.
4. Conclusions
The results of studying the effects of rootstocks on the pomological, biochemical, and volatile components of the ‘Sırrı’ peach genotype can be summarized as follows:
Grafting onto various rootstocks significantly influenced the fruit characteristics of the ‘Sırrı’ peach genotype. Depending on the grafted rootstock, traits such as fruit size, weight, peel color, pit structure, firmness, SSC, and TA varied notably.
Trees grafted onto ‘GF-677’ and ‘Seedling’ rootstocks produced larger, heavier, and shinier fruits. This can be attributed to the positive effects of rootstocks on fruit development and nutrient delivery. The heaviest fruits were from the ‘Seedling’ rootstock, while the lightest fruits were sourced from ‘Rootpac-R’ rootstock. Likewise, in terms of pit size, ‘GF-677’ produced the largest and heaviest pits, whereas ‘Rootpac-R’ generated the smallest ones. These differences highlight the direct influence of rootstocks on fruit anatomy.
The brightness and color of the fruit skin also varied based on the rootstock. ‘GF-677’ and ‘Seedling’ rootstocks yielded brighter and more orange tones, while ‘Rootpac-R’ produced a duller and more red hue. In terms of color intensity, the highest value was recorded for ‘GF-677’, while the lowest was noted for ‘Rootpac-R’.
Although rootstocks did not significantly impact flesh brightness and hue values, ‘GF-677’ excelled in fruit firmness. This presents a crucial advantage for the post-harvest durability of the fruits. Regarding SSC, the ‘Seedling’ rootstock yielded higher levels, which may influence fruit quality and consumer preferences. Additionally, the ‘GF-677’ rootstock produced larger pits, while ‘Rootpac-R’ offered a greater fruit–flesh ratio.
In conclusion, grafting onto different rootstocks significantly influenced various traits that determine the fruit quality and market value of the ‘Sırrı’ peach genotype, revealing notable differences in factors such as pit size, fruit firmness, skin brightness, and SSC.
At the end of the research, a total of 20 volatile compounds were identified in all rootstocks of the ‘Sırrı’ peach genotype. In a study examining the effects of rootstocks on the aroma volatile compounds of the ‘Sırrı’ peach genotype, aldehydes were identified as the most prevalent among all detected aroma compounds, followed by esters and lactones. Regarding volatile compounds, no significant differences were observed between the rootstocks, excluding hexanal, (E)-2-hexenal, total aldehydes, and hexane compounds. The genotype known as ‘Sırrı’ is highly promising for the region due to its very late ripening period, which coincides with the end of the peach and nectarine harvest season. Additionally, this genotype is often preferred in the region because of its suitability for transportation and post-harvest storage. This genotype attracts attention as promising in terms of many characteristics. More research should be carried out on it, and it should be registered as a variety and popularized in the South Marmara region of Türkiye. This study examined the effects of different rootstocks on the pomological, biochemical, and volatile components of the ‘Sırrı’ peach genotype. Fruits grafted onto the ‘GF-677’ and ‘Seedling’ rootstocks were generally larger and heavier, and had shinier skin, while those on ‘Rootpac-R’ were smaller and duller. Rootstocks influenced pit size and pit weight, with ‘GF-677’ producing the largest pits and ‘Rootpac-R’ the smallest. Fruit flesh firmness was highest in ‘GF-677’ rootstocks, while ‘Seedling’ resulted in the highest soluble solid content. In this study, 20 volatile compounds were identified, with aldehydes being the most dominant. While most aroma compounds were unaffected by rootstocks, some variations were noted for their C-6 compounds such as hexanal, (E)-2-hexenal, and hexane.
Based on previous experiences from production orchards, as well as the results presented in this study, it would be beneficial for the examined genotype to enter the registration and recognition processes as a cultivar.
Author Contributions
The authors confirm the contributions to the paper as follows: conceptualization, E.G.; methodology, software, and validation, M.A.G. and N.Y.; formal analysis, N.Y. and M.A.G.; investigation, E.G.; resources, E.G. and N.Y.; data curation, M.A.G. and N.Y.; writing—original draft preparation, M.A.G. and N.Y.; writing—review and editing, M.S.; visualization, M.A.G.; supervision, M.S.; project administration, E.G.; funding acquisition, E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Çanakkale Onsekiz Mart University Scientific Research Projects (COMU-BAP), under project number FHD-2022-4173.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [M.A. Gundogdu] upon reasonable request.
Acknowledgments
The financial support from Canakkale Onsekiz Mart University Scientific Research Commission (COMU-BAP: FHD-2022-4173) is acknowledged. We would like to thank agricultural engineer Sefer Demir for his contribution to the study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zheng, Y.; Crawford, G.W.; Chen, X. Archaeological Evidence for Peach (Prunus persica) Cultivation and Domestication in China. PLoS ONE 2014, 9, e106595. [Google Scholar] [CrossRef] [PubMed]
- Özçağıran, R.; Ünal, A.; Özeker, E.; İsfendiyaroğlu, M. Ilıman İklim Meyve Türleri: Sert Çekirdekli Meyveler Cilt-I; Ege Üniversitesi Ziraat Fakültesi Yayınları: İzmir, Türkiye, 2011; pp. 1–500. [Google Scholar]
- Byrne, D.H.; Raseira, M.B.; Bassi, D.; Piagnani, M.C.; Gasic, K.; Reighard, G.L.; Monero, M.A.; Pérez, S. Peach. In Fruit Breeding, 2nd ed.; Badenes, M.L., Byrne, D.H., Eds.; Springer: New York, NY, USA, 2012; pp. 505–569. [Google Scholar]
- Bassi, D.; Monet, R. Botany and Taxonomy. In The Peach Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: London, UK, 2008; pp. 1–36. [Google Scholar]
- Yılmaz, N.; Gür, E.; Polatöz, S.; Gündoğdu, M.A.; Şeker, M. The Peach: Brief Description and Growing. In Recent Headways in Pomology; Pakyürek, M., Ed.; Iksad Publishing House: Ankara, Türkiye, 2021; pp. 151–172. [Google Scholar]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of aroma trait of the white-fleshed peach ‘Hu Jing Mi Lu’ and the yellow-fleshed peach ‘Jin Yuan’ based on odor activity value and odor characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- FAOSTAT Production Statistics. Available online: http://www.fao.org/faostat/en/#data/QC/ (accessed on 5 January 2025).
- TUIK Crop Production Statistics. Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr (accessed on 5 January 2025).
- Gür, E. Comparison of AFLP Polymorphism and Aromatic Compounds of White Nectarine Types with Important Prunus Species and Varieties, Creation of Hybrid Populations. Ph.D. Thesis, Çanakkale Onsekiz Mart University, Çanakkale, Türkiye, 2012. [Google Scholar]
- Yıldız, N.; Gür, E.; Kaçan, A. Agricultural Potential of Lapseki District. LJAR 2020, 1, 83–89. [Google Scholar]
- Lesmes-Vesga, R.A.; Cano, L.M.; Ritenour, M.A.; Sarkhosh, A.; Chaparro, J.X.; Rossi, L. Variation in the root system architecture of peach × (peach × almond) backcrosses. Plants 2023, 12, 1874. [Google Scholar] [CrossRef]
- Muto, A.; Müller, C.T.; Bruno, L.; McGregor, L.; Ferrante, A.; Chiappetta, A.A.; Bitonti, M.B.; Rogers, H.J.; Spadafora, N.D. Fruit volatilome profiling through GC × GC-ToF-MS and gene expression analyses reveal differences amongst peach cultivars in their response to cold storage. Sci. Rep. 2020, 10, 18333. [Google Scholar]
- Sirangelo, T.M.; Rogers, H.J.; Spadafora, N.D. Molecular investigations of peach post-harvest ripening processes and VOC biosynthesis pathways: A review focused on integrated genomic, transcriptomic, and metabolomic approaches. Chem. Proc. 2022, 10, 8. [Google Scholar] [CrossRef]
- Loreti, F.; Massai, R. State of the art on peach rootstocks and orchard systems. Acta Hortic. 2006, 713, 253–268. [Google Scholar]
- Wang, Y.J.; Yang, C.X.; Li, S.H.; Yang, L.; Wang, Y.N.; Zhao, J.B.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar]
- Rubiola, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicch, C. Analysis of the plant volatile fraction. In The Chemistry and Biology of Volatiles; Herrmann, A., Ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2010; pp. 49–93. [Google Scholar]
- I Forcada, C.F.; Gogorcena, Y.; Moreno, M.Á. Agronomical and fruit quality traits of two peach cultivars on peach-almond hybrid rootstocks growing on Mediterranean conditions. Sci. Hortic. 2012, 140, 157–163. [Google Scholar]
- Mohammed, J.; Belisle, C.E.; Wang, S.; Itle, R.A.; Adhikari, K.; Chavez, D.J. Volatile profile characterization of commercial peach (Prunus persica) cultivars grown in Georgia, USA. Horticulturae 2021, 7, 516. [Google Scholar] [CrossRef]
- Li, X.; Gao, P.; Zhang, C.; Xiao, X.; Chen, C.; Song, F. Aroma of peach fruit: A review on aroma volatile compounds and underlying regulatory mechanisms. Int. J. Food Sci. Technol. 2023, 58, 4965–4979. [Google Scholar]
- MGM. 2023 Climate Assessment. 2024. Available online: https://www.mgm.gov.tr/FILES/iklim/yillikiklim/2023-iklim-raporu.pdf (accessed on 20 March 2025).
- Ertürk, Ü.; Oran, R.B.; Kosar, D.A. Peach Rootstocks and Characteristics. In Peach and Nectarine Cultivation; Pakyürek, M., Seker, M., Gür, E., Eds.; Iksad Publishing House: Ankara, Türkiye, 2024; pp. 65–78. [Google Scholar]
- Reighard, G.L.; Loreti, F. Rootstock Development. In The Peach Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: London, UK, 2008; pp. 193–220. [Google Scholar]
- Gür, E.; Gündoğdu, M.A.; Şeker, M. Determination of pomological characteristics of peach varieties extensively cultivated in Lapseki ecology. LJAR 2020, 1, 90–100. [Google Scholar]
- Şeker, M.; Kaya, C. Peach Breeding: Breeding Methods, Future Perspectives and Innovative Approaches. In Peach and Nectarine Cultivation; Pakyürek, M., Seker, M., Gür, E., Eds.; Iksad Publishing House: Ankara, Türkiye, 2024; pp. 33–63. [Google Scholar]
- Ekinci, N.; Varli Yunusoglu, S.; Çelik, M. Quality Criteria and Storage Performance of ‘Hayward’ Kiwifruit (Actinidia chinensis var. deliciosa) Cultivated in Gonen Plain. COMU J. Agric. Fac. 2023, 11, 58–65. [Google Scholar]
- Şeker, M.; Kaçan, A.; Gür, E.; Ekinci, N.; Gündoğdu, M.A. Investigation of aromatic compounds of peach and nectarine varieties grown in Canakkale ecological conditions. Res. J. Agric. Sci. 2013, 1, 62–67. [Google Scholar]
- Şeker, M.; Gür, E.; Ekinci, N.; Gündoğdu, M.A. Volatile constituents of different apricot varieties in cool subtropical climate conditions. Hortic. Int. J. 2018, 2, 103–111. [Google Scholar]
- Gür, I.; Pırlak, L. Determination of phenological and pomological characters of some peach cultivars grown in Eğirdir ecological conditions. Derim 2011, 28, 27–41. [Google Scholar]
- Şeker, M.; Ekinci, N.; Gür, E. Effects of different rootstocks on aroma volatile constituents in the fruits of peach (Prunus persica L. Batsch cv. “Cresthaven”). N. Z. J. Crop Hortic. Sci. 2017, 45, 1–13. [Google Scholar]
- Caliskan, O.; Kamiloglu, O.; Polat, A.A. Performance of some peach and nectarine cultivars under East Mediterranean (Hatay/Turkey) conditions. Acta Hortic. 2012, 940, 407–414. [Google Scholar]
- Türkmen, Ö. Investigations on the Performances of Some New Peach and Nectarine Cultivars Grown in Çukurova Conditions. Master’s Thesis, Çukurova University, Adana, Türkiye, 2003. [Google Scholar]
- Yıldız, N. Investigation of the Phenological and Pomological Characteristics of Some (Non-Melting) Peach (Prunus persica var. lonuqinosa) Cultivars. Master’s Thesis, Uludağ University, Bursa, Türkiye, 2018. [Google Scholar]
- Kader, A.A. Fruit Maturity, Ripening, And Quality Relationships. Acta Hortic. 1999, 485, 203–208. [Google Scholar]
- Crisosto, C.H.; Valero, D. Harvesting and Postharvest Handling of Peaches for the Fresh Market. In The Peach Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: London, UK, 2008; pp. 575–596. [Google Scholar]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolics compounds, carotenoids, and vitamin C content of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLCDAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [PubMed]
- Şeker, M.; Gündoğdu, M.A.; Ekinci, N.; Gür, E. Recent developments on aroma biochemistry in fresh fruits. Int. J. Innov. Approaches Sci. Res. 2021, 5, 84–103. [Google Scholar] [CrossRef]
- Gür, E. The effects of different rootstocks on aroma volatile constituents in the fruits of ‘Fuji’ apples (Malus domestica Borkh). Appl. Ecol. Environ. Res. 2019, 17, 11745–11756. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).