Abstract
The marketability of fresh granadilla (Passiflora edulis) fruit is significantly reduced by oxidation reactions occurring in its exocarp, which is not directly linked to the organoleptic quality of its arils. However, organic means of mitigating this are not topical in research. This study investigated the potency of moringa (Moringa oleifera)-based coating to preserve antioxidant compounds in granadilla. Physiologically mature fruit of equal size were assigned to a completely randomized design experiment at the North-West University Farm Laboratory. The fruit samples were coated with 2% xanthan gum (commercial coating), 2% moringa, or 2% rosemary coating and kept at shelf-life conditions (25 ± 2 °C; 40 ± 5%RH) for 5 weeks while sampling at weekly intervals. Correlations between the measured parameters were confirmed prior to regression analysis. Significant (p < 0.05) differences were observed in weekly changes in the exocarp pH, total antioxidant compounds (TAO), tartaric acid (TA), malic acid (MA), and citric acid (CA). At the end of storage, the moringa- and xanthan-coated fruit had the highest exocarp pH (7.8) and TAO (0.87 mg/g). Moringa-coated fruit had higher TA and MA (6.0 and 5.36 µg/g, respectively) as well as a significantly higher CA (0.51 µg/g) preserved than the other coatings. Strong correlations between MA and TAO (r > 0.82), as well as TA and TAO (r > 0.86), indicated the potency of developing TAO estimation models using multivariate data from the organic acids. Pre-processed data regression models were developed but were limited by the amount of data collected. Models developed similarly can be used for sustainable TAO assessment as a latent variable to minimize toxic waste that results from wet chemistry analyses.
1. Introduction
An annual yield loss of up to 30% during postharvest storage is a general estimation across the South African fresh fruit industry. Concurrently, a growing consumer demand for organic food products is experienced [1]. These challenges have resulted in a growing interest in postharvest technology like cold storage and controlled atmosphere packaging during exports. Packaging is used to regulate atmospheric gases and branding ability, protect produce from light/moisture, and offer necessary chilling injury buffer during refrigeration [2]. However, a significant portion of seasonal crops are destined for local markets where such technologies are not feasible, as the sector permits profitability if minor investments are made along the value chain.
Previous research has evidenced that aqueous Rosmarinus officinalis L. and Moringa oleifera leaf extracts are amongst the most effective and sustainable bioactive plant-derived extracts for ameliorating physiological and pathological degradation of fresh fruits [3,4]. The high antioxidant and antimicrobial properties of moringa are easily extractable through aqueous extraction methods [5,6]. Fruit coatings incorporated with moringa extracts are widely recommended amongst potential alternatives to cold storage in commercial lines where the fruit remains in ambient temperatures from the orchard to the consumer [7,8]. On the other hand, the effectiveness of rosemary-enriched films for food coating has been associated with their rosmarinic acid release [9]. Plant-based coatings elongate fruits’ shelf life by altering physical and physiological activities and preventing potential infections. Studies of edible organic coatings on various fruits have shown the effectiveness of coatings in maintaining organic compounds including minerals, vitamins, antioxidants, pigments, enzymes, and organic acids during postharvest storage [10,11].
Climacteric fruits experience high respiration at postharvest storage. The process includes oxidation of sugars into carbon dioxide and water. The absorption of sugar during fruit ripening may influence postharvest water loss by disrupting cuticle formation. As such, the inhibition of cell wall invertase LIN5 that results in soluble solutes content has been associated with a reduction in water loss rate and wrinkling in tomato fruits stored at ambient conditions for 12 days [12]. It was indicated that the sink of sugars during fruit growth influences the structure of its exocarp cell wall and cuticle, leading to a significant effect on tomato senescence. Soluble sugars are crucial metabolites in reactive oxygen species (ROS) metabolism, serving as the principal source of carbon and energy while facilitating the production of reducing power through the oxidative pentose phosphate pathway [13,14]. Their role in oxidation results in a significant role in osmoprotection and exocarp cell membrane stabilization [15,16]. An intensive breakdown of polysaccharides such as starch and cellulose into monosaccharides from ROS activities results in a short shelf life. The metabolism of various monosaccharides, including xylose, galactose, and arabinose, as well as mannonic acid and glucuronic acid, which originate from cell wall disassembly, have been reported in pitaya fruit [17]. The metabolism of free mannose from cell wall disassembly and hemicellulose breakdown during fruit senescence has been found in fruits like tomato, apple, and pear [18,19,20]. Organic acids, particularly citric acid, accumulate to high concentrations in citrus fruits [21]. Its decline at postharvest storage due to ROS activities is associated with a short shelf life.
Physiological metabolism associated with postharvest external quality losses of the fresh granadilla fruit is not well documented. However, plant extracts can act as carriers of biocontrol agents and a wide variety of bioactive and/or functional compounds, such as antimicrobials, antioxidants, anti-browning agents, volatile precursors, nutrients, flavoring, and coloring compounds. The addition of these compounds promotes the functional performance of the coatings and enhances stability and quality, thus reducing the biochemical deterioration, enzymatic browning, and the development of off-flavors, as well as the safety of the coated produce [22].
Issues pertaining to the application of plant extracts and essential oils on fresh produce include gauging their effective concentration against oxidative species or pathogens, non-interruption with taste or flavor characteristics, and the maintenance of fresh appearance [23,24,25]. Research indicating the benefits of organic coatings on appearance and internal consumable fruit parts is vast [26]. However, the focus of most postharvest metabolism research has been directed toward preserving the edible fruit parts (meso- and endocarp) while the effect of coatings on exocarps is assessed as a secondary factor or neglected on fruit with unconsumable peels. Coatings are applied as transparent thin layers on the fruit surface, which is the most visual part that attracts or repels consumers at a retailer’s shelf.
An assessment of the coating effect on the metabolic reactions in the exocarp during postharvest storage is necessary. The effectiveness is based on analyses that include laboratory tests to determine concentrations of beneficial compounds such as antioxidants. The waste from phytochemical analyses that use wet chemistry procedures is considered detrimental to the environment. Yet, conducting sustainable and environmental laboratory analyses of certain fruit compounds that usually require toxic chemicals is impossible [27]. It is thus necessary that indirect estimation models are developed from concentrations of associated parameters that are easier and have a higher degree of eco-friendliness during their wet chemistry analyses.
In this study, the ability of plant-based coatings to maintain exocarp total antioxidant compounds was investigated. The study’s objective was to assess the effectiveness of organic coatings, containing moringa or rosemary leaf extracts, on the ‘Ester’ granadilla fruit exocarp organic acids and antioxidant compounds. Moringa-based coating showed higher efficiency, and further studies are recommended. Moreover, models for an indirect statistical estimation of TAO were developed using correlated organic acids.
2. Materials and Methods
2.1. Sample Collection
The ‘Ester’ granadilla (Passiflora edulis) was collected from a vineyard based at Motsu Farm in Mahikeng (−25.81175, 25.46483), North-West province, South Africa. The fruit samples were harvested in June 2024, the harvesting season. The selection of fruits was based on equal sizes with no deformation, defects, or disease symptoms. The fruit samples were harvested at a turning stage (E7: 10–30%), during a cloudy day using pruning secateurs to cut at approximately 2 cm of the pedicels. The samples were packaged in ventilated fruit boxes (K-Pack Supplies, Alberton, Johannesburg, South Africa) with a 2 cm-thick packaging polystyrene to simulate commercial conditions. The samples were one layer per box to minimize the possibility of bruise during transportation and storage. They were taken to the North-West University farm laboratory using a semi-closed pickup truck with a canopy. Upon arrival at the laboratory, they were washed with distilled water to remove any surface contaminants and allowed to dry at room temperature (25 ± 2 °C; 40 ± 5%RH) where they were stored for the entire experiment. The samples were only exposed to artificial laboratory lights during the day (08h00–18h00). The farm laboratory is located along the Nelson Mandela main road to Botswana (−25.791037, 25.620998), 8 km away from Mafikeng City.
2.2. Coating Preparation
The organic extracts from moringa and rosemary were obtained from crushed leaf powders purchased from the market. The moringa powder (Moringa5000 leaf powder, Saint Gilles Packagers, Covent Garden, London, UK) and rosemary leaf powder (Lifestyle Food Dried Herbs, Midrand, South Africa) were used because of their easy accessibility in the region. The extracts were prepared by dissolving 2 g powder in 50 mL of warm distilled water and left in the dark for 12 h to allow the solutions to dissolve thoroughly. Xanthan gum (Health Connections Wholefoods, Diep River, Western Cape, South Africa) is the commercial wax applied on granadilla as a coating. It normally comes as a powder and is recommended for dilution in warm water to a 2% concentration. As such, a 2 g xanthan powder was dissolved in 100 mL of warm water. Thereafter, the moringa or rosemary extract solution was added in a 1:1 ratio to the warm xanthan solution and consistently swirled using a laboratory rod electric stirrer (IKA® RW 20 digital overhead stirrer; MERC Universal lab mixer, 2000 rpm, 230 V, 1/cs; Darmstadt, Germany) to create a sticky paste.
2.3. Coating Application
A total of 150 fruit samples were randomly divided into groups of 50 pieces before the respective coatings were applied. This was a completely randomized design layout where 50 fruits were coated with 2% xanthan (commercial coating), 50 fruits were coated with 2% moringa coating, and 50 fruits were coated with 2% rosemary coating. There was no uncoated control because it would not align with normal practices, and a washed fruit would have no original wax to protect it during storage. The fruit samples were coated manually using a new towel (wetting technique) that was consistently kept wet by re-dipping into the coating after coating each fruit. The fruit samples were then placed in open boxes labeled according to the treatment they received. The coated fruits were allowed to dry and left for seven days before the start of data collection. Data were collected weekly for 5 weeks.
2.4. The pH and Organic Acid Data Collection
The data were collected weekly until the control fruit samples were rotten. The analysis of organic acids followed a procedure by Ncama et al. [28] with slight modification. During the sampling day, each fruit exocarp was collected by removing the internal arils together with the mesocarp. The exocarps were sun-dried until a constant mass was measured in three 1 h intervals. They were then ground into fine particles using a mortar and pestle and were kept in zip-lock plastic bags for other analyses. During the analysis day, 2 g of the ground samples were prepared separately according to their sampling week and the treatments received. The 2 g of the samples were dissolved in 50 mL of distilled water and stirred for 2 min to ensure that the solutions were homogenized. The solution pH was measured using a digital pH meter (PH Meter PH300F; Inspection Tools, Johannesburg, South Africa). Titratable acids were analyzed by mixing 50 mL of the sample solution with 100 mL distilled water and titrating with 0.1 M sodium hydroxide (NaOH) to the end point (pH 8.1). The amount of NaOH used was recorded, and the acidity formula was applied to calculate TA, expressed as mass equivalents (percentage) of the exocarp powder. The organic acids were calculated using the following formulae [18]:
TA (% citric acid) = ((0.0064 × titre (NaOH) mL)/50 mL juice) × 100
TA (% malic acid) = ((0.0067 × titre (NaOH) mL)/50 mL juice) × 100
TA (% tartaric acid) = ((0.0075 × titre (NaOH) mL)/50 mL juice) × 100
2.5. The Analysis of Total Antioxidant Compound Data Collection
The total antioxidant capacity of the samples was evaluated by the phosphor-molybdenum method by Aliyu et al. [29], with slight modification. Briefly, 80% v/v methanol (5 mL) was used for extracting an exocarp powder sample (0.5 g). The aqueous extract was obtained by filtering the liquid mixture through a 0.25 μm syringe filter before analysis. The methanolic extracts (0.3 mL) were combined with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 65 °C for 90 min. The solution was allowed to cool to room temperature before measuring the absorbance at 695 nm using a UV-1800 Spectrophotometer (Shimadzu Scientific Instruments Inc., Columbia, SC, USA). The total antioxidant compounds were expressed as a milligram equivalent of ascorbic acid per gram of exocarp powder in dry matter based on the generated standard curve.
2.6. Data Analysis
The collected raw data of fruit properties were statistically analyzed for differences using the least significant difference (LSD) at a 5% level of significance via Genstat® statistical software (GenStat, 17th edition). The correlations and regression models were developed using the multivariate chemometric data analysis package, The Unscrambler® X software (The Unscrambler® X version 10.5, Camo Software AS, Oslo Science Park, Oslo, Norway).
3. Results
3.1. The Effect of Moringa, Xanthan, and Rosemary Coatings on the pH of Granadilla Exocarp
The pH levels of all coatings increased over time (Figure 1), and the increase fluctuated. After a week in storage, moringa-coated fruit had a higher pH than the other coatings which both had the same exocarp pH level of 5.3 ± 0.2. Thereafter, for the two following weeks, the rosemary coating had the highest pH level while the moringa and xanthan had the same pH levels. A decline in the pH level of rosemary and xanthan was observed at Week 4 with xanthan having the lowest level while moringa had the highest level. The pH of granadillas coated with moringa (7.7 ± 0.4) and xanthan (7.6 ± 0.4) was not significantly different at the last sampling week (Week 5). The rosemary coating resulted in the lowest pH level (7.3 ± 0.4) of granadillas compared to the other coatings. There were no significant differences between all treatments.
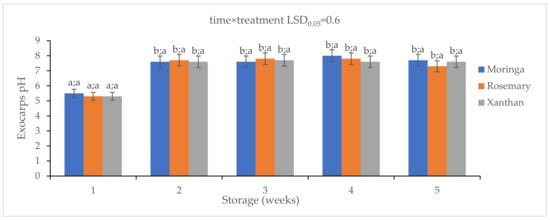
Figure 1.
The effect of 2% moringa, 2% rosemary, and 2% xanthan edible coatings on the pH levels of ‘Ester’ granadilla exocarps during storage (5 weeks; 25 ± 2 °C; 40 ± 5%RH; 8 h light exposure); data are presented as mean ± standard error. The first letters present the significance of the difference caused by time; the second letters present the significance of the difference caused by treatments. The same letters mean a non-significant difference.
3.2. The Effect of Moringa, Xanthan, and Rosemary Coatings on the Citric Acid Concentration from Granadilla Exocarp
There were significant (p < 0.05) differences in the citric acid concentration (CA) amongst the coated fruit exocarps (Figure 2). Moringa had the highest concentration of CA in Week 1. It was followed by rosemary while xanthan had the lowest concentration. A similar trend of differences also happened in Week 2, where moringa had higher CA (1.92 ± 0.1 µg/g) than rosemary and xanthan which were also different from each other with the xanthan having the lowest CA. There was a significant difference between Week 2 and Week 3 in all treatments. However, a non-significant difference between moringa and xanthan was observed in Week 3 and Week 4 whilst the rosemary consistently showed the lowest concentration. This means the effectiveness of moringa will start after two weeks of shelf life on fruit in the market. The moringa coating had a significantly high concentration of CA (0.51 ± 0.01 µg/g) compared to rosemary (0.26 ± 0.01 µg/g) and a higher CA than xanthan (0.38 ± 0.004 µg/g) on the last sampling day.
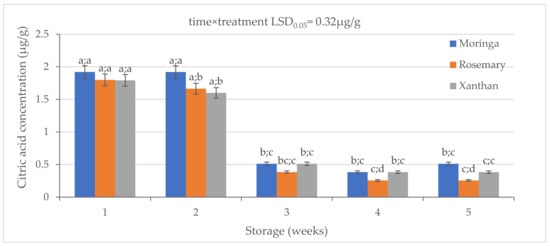
Figure 2.
The effect of 2% moringa, 2% rosemary, and 2% xanthan coatings on the citric acid concentration of ‘Ester’ granadilla exocarps during storage (5 weeks; 25 ± 2 °C; 40 ± 5%RH; 8 h light exposure); data are presented as mean ± standard error. The first letters present the significance of the difference caused by time; the second letters present the significance of the difference caused by treatments. The same letters mean a non-significant difference.
3.3. The Effect of Moringa, Xanthan, and Rosemary Coatings on the Malic Acid Concentration from Granadilla Exocarp
There were significant (p < 0.05) differences observed in the concentration of malic acids (MA) in the coated granadillas (Figure 3). The rosemary coating resulted in a low concentration of malic acid on granadillas throughout the storage. Moringa had the highest MA after one week while the MA of both rosemary and xanthan was the same. The malic acid was highest (2.68 ± 0.3 µg/g) on the xanthan coating in the second week. Moringa followed with a 2.01 ± 0.3 µg/g MA while the xanthan had the lowest MA (1.74 ± 0.2 µg/g). There was no significant difference between moringa and xanthan in Week 3 while rosemary had a significantly lower MA. There was a general decrease in the MA from Week 3 to Week 4 in all treatments, with moringa and xanthan having higher concentrations than rosemary. There were no significant differences between moringa- and xanthan-coated granadillas on malic acid on the last sampling day (5.35 ± 0.2 µg/g), and that concentration was significantly higher than the rosemary coating (2.68 ± 0.1 µg/g). Overall, there was an increase in the malic acid concentration on the exocarp of granadillas.
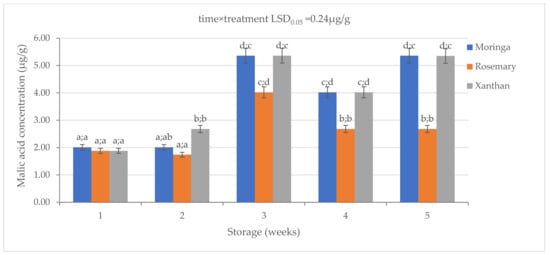
Figure 3.
The effect of 2% moringa, 2% rosemary, and 2% xanthan coatings on the concentration of malic acid in ‘Ester’ granadilla exocarps during storage (5 weeks; 25 ± 2 °C; 40 ± 5%RH; 8 h light exposure); data are presented as mean ± standard error. The first letters present the significance of the difference caused by time; the second letters present the significance of the difference caused by treatments. The same letters mean a non-significant difference.
3.4. The Effect of Moringa, Xanthan, and Rosemary Coatings on the Tartaric Acid Concentration from Granadilla Exocarp
There was a general decrease in the concentration of tartaric acids (TA) in the treated granadilla exocarp (Figure 4). A significant difference was observed in Week 2 when the xanthan coating resulted in the lowest (13.0 ± 0.1 µg/g) concentration of tartaric acid on treated granadilla fruit compared to rosemary (19.5 ± 0.2 µg/g) and moringa (22.5 ± 0.2 µg/g) coatings. There was a further decrease from Week 2 to Week 3 where moringa had a non-significant difference with xanthan (6.0 ± 0.01 µg/g) while rosemary had the lowest TA of 4.5 ± 0.005 µg/g. There was a significant change in TA from Week 3 to Week 4 in moringa and xanthan, and a significant difference was observed in rosemary. There was a non-significant difference between moringa and xanthan, and the rosemary had the lowest TA concentration. An increase in moringa and xanthan was observed from Week 4 to Week 5 whilst there was no change in the rosemary coating. Rosemary coating ended with the lowest tartaric acid (3.0 ± 0.005 µg/g) on the last day of sampling compared with moringa (6.0 ± 0.005 µg/g) and xanthan (6.0 ± 0.003 µg/g).
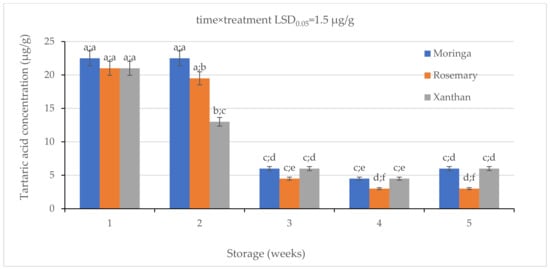
Figure 4.
The effect of 2% moringa, 2% rosemary, and 2% xanthan coatings on the concentration of tartaric acid in ‘Ester’ granadilla exocarps during storage (5 Weeks; 25 ± 2 °C; 40 ± 5%RH; 8 h light exposure); data are presented as mean ± standard error. The first letters present the significance of the difference caused by time; the second letters present the significance of the difference caused by treatments. The same letters mean a non-significant difference.
3.5. The Effect of Moringa, Xanthan, and Rosemary Coatings on the Antioxidant Compounds in Granadilla Fruit Exocarp
The ability of moringa coating to maintain the total antioxidant compounds (TAO) was demonstrated in the present study (Figure 5). There was a significant drop in the antioxidant compounds from Week 1 to Week 2, which was attributed to the fruit’s adaptation to the shelf-life conditions. From Week 2, the fruit samples began defending themselves against oxidation resulting in an increase in TAO concentration. Notably, moringa coating had the lowest concentration of 0.27 mg/g followed by the rosemary coating (0.29 µg/g) and the xanthan coating (0.36 µg/g) in Week 2. There was a significant increase in the TAO from Week 2 to Week 3. The highest concentration of TAO was observed in moringa (0.81 mg/g) followed by xanthan (0.68 mg/g) and rosemary (0.58 mg/g). No significant changes were observed from Week 3 to Week 4. Moringa had the highest TAO (0.83 mg/g) followed by rosemary (0.71 mg/g) and xanthan (0.67 mg/g). No significant change was observed in moringa from Week 4 to Week 5 (0.88 mg/g) while a significant change was observed in rosemary (0.78 mg/g) and xanthan (0.87 mg/g).
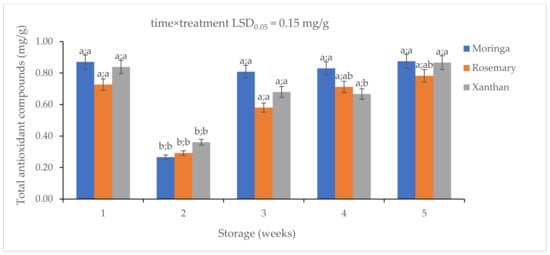
Figure 5.
The effect of 2% moringa, 2% rosemary, and 2% xanthan coatings on the concentration of total antioxidant compounds in ‘Ester’ granadilla exocarps during storage (5 weeks; 25 ± 2 °C; 40 ± 5%RH; 8 h light exposure); data are presented as mean ± standard error. The first letters present the significance of the difference caused by time; the second letters present the significance of the difference caused by treatments. The same letters mean a non-significant difference.
3.6. Correlations of Total Antioxidant Compounds and Organic Acidity
There were high correlations between the measured organic acids and the total antioxidant compounds (Table 1). A strong positive correlation of total antioxidant compounds (TAO) to citric acid (r = 0.77), malic acid (r = 0.82), and tartaric acid (r = 0.86) was observed.

Table 1.
The correlation (Pearson’s r) coefficient of ‘Ester’ granadilla exocarp parameters.
3.7. Regression Modelling
Regression models of the acidity parameters and total antioxidant compounds were developed. The F-values indicated a high association between all three organic acids when the models were calibrated without pre-processing the data (Table 2). A small p-value (p < 0.05) indicates a significant relationship between the variables used for model calibration. All the acids were found to have a significant relationship with TAO.

Table 2.
The parameters of the generated models for indirect assessment of TAO from the granadilla exocarp organic acid concentrations.
4. Discussion
The pH levels of all treatments increased over time, and the increase fluctuated. The same trend was previously reported on passion fruit [30]. However, it is a common physiological change that fruits experience an increase in total soluble solute concentration and a decrease in acidity during storage [31]. There were no significant differences. Thus, it was associated with a low rate of change from the organic acids to the soluble solutes [31]. However, the change was not associated with rosemary-coated fruit having an elongated shelf life since only the pH exhibited the conservational feature in this treatment group.
The CA of all coated fruit gradually decreased over time which can be associated with the storage condition when fruit were not getting anything from the plant to generate more compounds. This also includes the loss of water from granadilla fruit at storage [32], which is highly affected by the cuticular wax components of the fruit [33]. The removal of natural wax that happened during the coating process of the granadilla fruit can be associated with changes in the cuticle components that led to low moisture and CA extractability. Plant tissues with high moisture contents release high concentrations of organic acids during the wet chemistry analyses [34]. This is aligned with the high CA in the first two weeks and the decrease over the shelf-life duration as the fruits continued to transpire and respire after being harvested from the mother plant.
There was an increase in the malic acid concentration on the exocarp of granadillas. It is hypothetical that there should be an increase in the concentration of soluble compounds of fresh fruits during their shelf life. This is because the moisture content decreases over time and the cellular concentrations in plant tissues increase under water deficit conditions [35]. This is beneficial in fruit pieces that have higher concentrations of organic acids than sugars because they will be effective for defense against oxidative compounds and elongate the storage quality.
Moringa coating had a similar tartaric acid concentration to the xanthan coating. Generally, the moringa leaf extract is known to contain tartaric acids [36], which cannot be aligned to a similar concentration to xanthan. The hypothesis is that there are other factors that need consideration before tartaric acid concentration is discussed. Those would include its relationship to various phytochemicals that are heavily available in moringa leaf extract. Compounds found in moringa leaf extracts are very diverse [37]. Specifically, moringa is a rich source of various antioxidants such as phenolics, flavonoids, and ascorbic acid; these antioxidants reduce oxidation in fruit, thereby reducing the rate of tartaric decrease. On the other side, when moringa is used as a coating, it lowers metabolic processes such as respiration, leading to the reduced consumption of tartaric acid.
There was an abrupt decrease from Week 1 to Week 2 and then an increase from Week 2 to Week 3 in all treatments. Rosemary coating had the lowest TAO concentration from Week 2 to Week 5. Rosemary extracts, such as rosmarinic and carnosic acids [9], are characterized by containing colorless antioxidant compounds [38], which are not appropriately assayed by using spectrophotometer absorptions such as the method of Aliyu et al. [29]. It is possible that the TAO of the rosemary coatings was high but did not contribute significantly to the colored TAOs of the granadilla exocarps. Moreover, the TAO concentration of rosemary-treated fruit was low from Week 1. Although it was higher than that of xanthan-coated fruit on Week 4.
The strong positive correlations of total antioxidant compounds (TAO) to citric acid, malic acid, and tartaric acid indicated that these organic compounds play a major role as part of the antioxidant complex found in the granadilla exocarp. Citric acid is known to be associated with vitamin C, which is generally used as the equivalent compound during the estimation of TAO [39]. The high TAO correlation to these acids can be used to improve the longevity of fresh granadilla fruit in shelf life. This can be attained by using plant extracts from plants with tissues known to have high concentrations of bioactive acids such as citrus for citric acid [40], pomes for malic acid [41], and grapes for tartaric acid [42]. In this study, the use of moringa and rosemary extracts improved the correlation of TAO to all the analyzed acids indicating the broad-based nature of the content of TAOs found in these non-consumed plants.
The F-values indicated a high association between all three organic acids when the models were calibrated without pre-processing the data. Briefly, F-values greater than 1 indicate that the variance between groups is greater than the variance within the groups, supporting the robustness of the models. Therefore, the models developed did not have all possible extremes of the acids and TAO concentrations. Notably, only the citric acid had a successful model with TAO after data were subjected to exponential smoothing prior to calibrations. This suggests that data pre-processing is acid specific and requires various treatments for reducing noise and obtaining meaningful results. During laboratory analyses, an ascorbic acid equivalent has been used to estimate TAO [43]. Structurally, citric acid is an oxidized ascorbic acid (L–ascorbic acid) with one more oxygen atom [44]. This structural similarity contributed to the strong association between citric acid concentrations and TAO. The use of ascorbic acid as the equivalent of TAO resulted in the high association of citric acid concentrations even when the data were pre-processed.
Although all the acids were found to have a significant relationship with TAO. The efficiency of the models was not significant as indicated by the values of F—significance above 0.05. In spectroscopy models, this occurs if the data used for calibrations are not sufficient to contain samples adequate for both internal cross-validation and external validation [45]. The models need sourcing of additional data that will reduce possible outliers and model noise during validations. Reliable multivariate models rely on broad variations to improve robustness during external validations [46]. Hereon, the assessment of TAO as a latent variable will be improved by adding more data from granadilla fruit at various maturity levels with broader variance in acidity and TAO concentrations. This will reduce the root mean square error of calibration and root mean square error of prediction to form a high residual prediction deviation.
5. Conclusions
The efficacy and formulation of bioactive plant-based coating incorporating Moringa oleifera were demonstrated. Given the limited research on plant-based coatings for granadilla preservation, this study contributes valuable knowledge toward sustainable postharvest management strategies. Future studies are needed to investigate the development of plant-based adhesives that can contribute to the stickiness of the bioactive extracts because the manufacture of Xanthan, although naturally occurring, needs the presence of Xanthomonas campestris which does not ensure sustainability. The models developed in this study were limited by the span of the data collected. Multivariate data will be incorporated using the ‘fit in’ preprocessing of the models for a broader calibration set and cross-validation trials.
Author Contributions
Y.C.M., concept, experiments, original draft writing; N.J.S., S.M. and B.L.N., review and editing; K.N., supervision, review, editing, and submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Acknowledgements to The Subtrop Lindsey Milne Bursary; the North-West University for providing access to the internet to various platforms to access articles; Sibongile Xaba for providing all the resources required for all the experiments and her technical support; and MS Mabuya for his assistance during experimental activities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marokhu, M.K.; Fatoki, O.O. Young customers’ intention to purchase organic food in South Africa: Extending the theory of planned behaviour. Food Res. 2024, 3, 351–364. [Google Scholar] [CrossRef]
- Viacava, G.E.; Ansorena, M.R.; Marcovich, N.E. Multilayered films for food packaging. In Nanostructured Materials for Food Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 447–475. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Kotsou, K.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds. Int. J. Mol. Sci. 2024, 25, 7708. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, P.; Alhassan, A.R.; Ayamgama, A.A.; Adzaworlu, E.G.; Afoakwah, N.A.; Mahunu, G.K.; Amagloh, F.K. Phytochemical analysis, enumeration, isolation, and antimicrobial activity of lemongrass and moringa leaves extracts. J. Agric. Food Res. 2023, 12, 100579. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z.; Mbili, N.C. The solvent-dependent efficacy of Moringa leaf extract as an antifungal ingredient of organic coatings for citrus fruit. In Acta Horticulturae, Proceedings of the II International Symposium on Moringa 1306, Pretoria, South Africa, 10–13 November 2019; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2021; pp. 317–322. [Google Scholar] [CrossRef]
- Ngcobo, B.L.; Mbuyisa, S. November. Recent developments in environmentally friendly techniques for extracting bioactive compounds from moringa plant parts. In Acta Horticulturae, Proceedings of the III International Symposium on Moringa 1394, Aracaju, Brazil, 8–10 November 2023; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2023; pp. 93–98. [Google Scholar] [CrossRef]
- Chan-Matú, D.I.; Toledo-López, V.M.; Vargas, M.D.L.V.Y.; Rincon-Arriaga, S.; Rodríguez-Félix, A.; Madera-Santana, T.J. Preparation and characterization of chitosan-based bioactive films incorporating Moringa oleifera leaves extract. J. Food Meas. Charact. 2021, 15, 4813–4824. [Google Scholar] [CrossRef]
- Aghaei Dargiri, S.; Rastegar, S.; Mohammadi, M. Chitosan based coating enriched with Spirulina platensis and moringa leaf extracts preserved the postharvest quality of Mexican Lime (Citrus aurantifolia). J. Hortic. Postharvest Res. 2024, 8, 105–124. [Google Scholar] [CrossRef]
- Kahya, N.; Kestir, S.M.; Öztürk, S.; Yolaç, A.; Torlak, E.; Kalaycıoğlu, Z.; Akın-Evingür, G.; Erim, F.B. Antioxidant and antimicrobial chitosan films enriched with aqueous sage and rosemary extracts as food coating materials: Characterization of the films and detection of rosmarinic acid release. Int. J. Biol. Macromol. 2022, 217, 470–480. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. Extending the Shelf Life of Apples After Harvest Using Edible Coatings as Active Packaging—A Review. Appl. Sci. 2025, 15, 767. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, A.M. Edible coatings and films for shelf-life extension of fruit and vegetables. Biomater. Adv. 2023, 154, 213632. [Google Scholar] [CrossRef]
- Vallarino, J.G.; Trevor, H.Y.; Eugenia, M.; Jocelyn, K.R.; Alisdair, R.F.; Sonia, O. Postharvest changes in LIN5-down-regulated plants suggest a role for sugar deficiency in cuticle metabolism during ripening. Phytochemistry 2017, 142, 11–20. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front. Plant Sci. 2013, 4, 282. [Google Scholar] [CrossRef]
- Keunen, E.L.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.I.; Cuypers, A.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Davik, J.; Koehler, G.; From, B.; Torp, T.; Rohloff, J.; Eidem, P.; Wilson, R.C.; Sønsteby, A.; Randall, S.K.; Alsheikh, M.D. alcohol dehydrogenase, and central metabolite levels are associated with cold tolerance in diploid strawberry (Fragaria spp.). Planta 2013, 237, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.; Gärtner, T.; Erban, A.; Kopka, J.; Selbig, J.; Hincha, D.K. Predicting Arabidopsis freezing tolerance and heterosis in freezing tolerance from metabolite composition. Mol. Plant 2010, 3, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, Y.; Zhang, Z.; Li, T.; Jiang, Y.; Gao, H.; Yun, Z. Effect of blue light on primary metabolite and volatile compound profiling in the peel of red pitaya. Postharvest Biol. Technol. 2020, 160, 111059. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Hertog, M.L.; Van de Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.H.; Nicolai, B.M. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Pedreschi, R.; Franck, C.; Lammertyn, J.; Erban, A.; Kopka, J.; Hertog, M.; Verlinden, B.; Nicolaï, B. Metabolic profiling of ‘Conference’ pears under low oxygen stress. Postharvest Biol. Technol. 2009, 51, 123–130. [Google Scholar] [CrossRef]
- Hatoum, D.; Annaratone, C.; Hertog, M.L.A.T.M.; Geeraerd, A.H.; Nicolai, B.M. Targeted metabolomics study of “Braeburn” apples during long-term storage. Postharvest Biol. Technol. 2014, 96, 33–41. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, A.; Liu, S.; Sheng, L.; Ma, Q.; Zhang, L.; Nishawy, E.M.E.; Zeng, Y.; Xu, J.; Ma, Z.; et al. Integration of Metabolomics and Subcellular Organelle Expression Microarray to Increase Understanding the Organic Acid Changes in Post-harvest Citrus Fruit. J. Integr. Plant Biol. 2013, 55, 1038–1053. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT 2021, 152, 112346. [Google Scholar] [CrossRef]
- Ncama, K.; Mditshwa, A.; Tesfay, S.Z.; Mbili, N.C.; Magwaza, L.S. Topical procedures adopted in testing and application of plant-based extracts as bio-fungicides in controlling postharvest decay of fresh produce. Crop Prot. 2019, 115, 142–151. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Chen, D.; Chen, T.; Chen, Y.; Zhang, Z.; Li, B.; Tian, S. Bio-source substances against postharvest diseases of fruits: Mechanisms, applications and perspectives. Postharvest Biol. Technol. 2023, 198, 112240. [Google Scholar] [CrossRef]
- Sharma, C.; Pathak, P.; Yadav, S.P.; Gautam, S. Potential of emerging “all-natural” edible coatings to prevent post-harvest losses of vegetables and fruits for sustainable agriculture. Prog. Org. Coat. 2024, 193, 108537. [Google Scholar] [CrossRef]
- Nandy, S.; Fortunato, E.; Martins, R. Green economy and waste management: An inevitable plan for materials science. Prog. Nat. Sci. Mater. Int. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Ncama, K.; Sithole, N.J. The effect of nitrogen fertilizer and water supply levels on the growth, antioxidant compounds, and organic acids of baby lettuce. Agronomy 2022, 12, 614. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Ibrahim, M.A.; Musa, A.M.; Musa, A.O.; Kiplimo, J.J.; Oyewale, A.O. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes difformis Engl. (Araceae). Acta Pol. Pharm. 2013, 70, 115–121. [Google Scholar]
- Silva, F.C.; Fagundes, M.C.P.; Rufini, J.C.M.; da Silva Lima, N.L.; Alves, G.S.; Souza, W.G. Sustainable innovations in postharvest conservation of passion fruit. Contrib. Las Cienc. Soc. 2024, 17, e7357. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Pan, Y.; Shi, L.; Li, X. Effects of harvest maturity stage on postharvest quality of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage. Sci. Hortic. 2021, 277, 109778. [Google Scholar] [CrossRef]
- Li, X.; Pei, Z.; Meng, L.; Jiang, Y.; Liu, H.; Pan, Y. Investigation on epidermal structure and water migration of postharvest passion fruit during storage. J. Food Sci. 2023, 88, 4046–4058. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Tan, Y.; Pan, Y. Revealing the crucial role of cuticular wax components in postharvest water loss in passion fruit (Passiflora edulis Sims.). Postharvest Biol. Technol. 2024, 213, 112974. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in lipid extraction methods—A review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Ncama, K.; Aremu, O.A.; Sithole, N.J. Plant Adaptation to environmental stress: Drought, chilling, heat, and salinity. In Environment and Climate-Smart Food Production; Springer: Berlin/Heidelberg, Germany, 2022; pp. 151–179. [Google Scholar] [CrossRef]
- Alhaj Alali, F.; Askari Sarcheshmeh, M.A.; Bababalar, M. Evaluating the effects of citric acid application on reducing decay, maintaining edibility and shelf life of peach fruits in cold storage. Int. J. Hortic. Sci. Technol. 2023, 10, 149–160. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; El-Otmani, M.; Agustí, M. Examining the impact of dry climates temperature on citrus fruit internal ripening. Sci. Hortic. 2024, 337, 113501. [Google Scholar] [CrossRef]
- Balık, S.; Kaya, T.; Aslantaş, R. Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”. Horticulturae 2023, 9, 1171. [Google Scholar] [CrossRef]
- Li, M.; Su, J.; Yang, H.; Feng, L.; Wang, M.; Xu, G.; Shao, J.; Ma, C. Grape Tartaric Acid: Chemistry, Function, Metabolism, and Regulation. Horticulturae 2023, 9, 1173. [Google Scholar] [CrossRef]
- Muangthai, P.; Nookaew, P. Monitoring on some organic acids in fresh and processed rural plant leaves in Thailand. Asian J. Nat. Appl. Sci. 2015, 4, 82–89. [Google Scholar]
- Segwatibe, M.K.; Cosa, S.; Bassey, K. Antioxidant and antimicrobial evaluations of Moringa oleifera Lam leaves extract and isolated compounds. Molecules 2023, 28, 899. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, A.; Shah, S.S.; Bamezai, R.K. Analysis of interactions between streptomycin sulphate and aqueous food acids (L-ascorbic acid and citric acid): Physico-chemical and spectroscopic insights. J. Chem. Thermodyn. 2020, 151, 106207. [Google Scholar] [CrossRef]
- Dardenne, P.; Sinnaeve, G.; Baeten, V. Multivariate calibration and chemometrics for near infrared spectroscopy: Which method? J. Near Infrared Spectrosc. 2000, 8, 229–237. [Google Scholar]
- Zhang, X.; Yang, J. Advanced chemometrics toward robust spectral analysis for fruit quality evaluation. Trends Food Sci. Technol. 2024, 150, 104612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).