Similarities and Differences Among Factors Affecting Complex Declines of Quercus spp., Olea europea, and Actinidia chinensis
Abstract
1. Introduction
2. Oak Declines
2.1. Chronic Oak Decline

2.2. Sudden Oak Decline
2.3. Acute Oak Decline
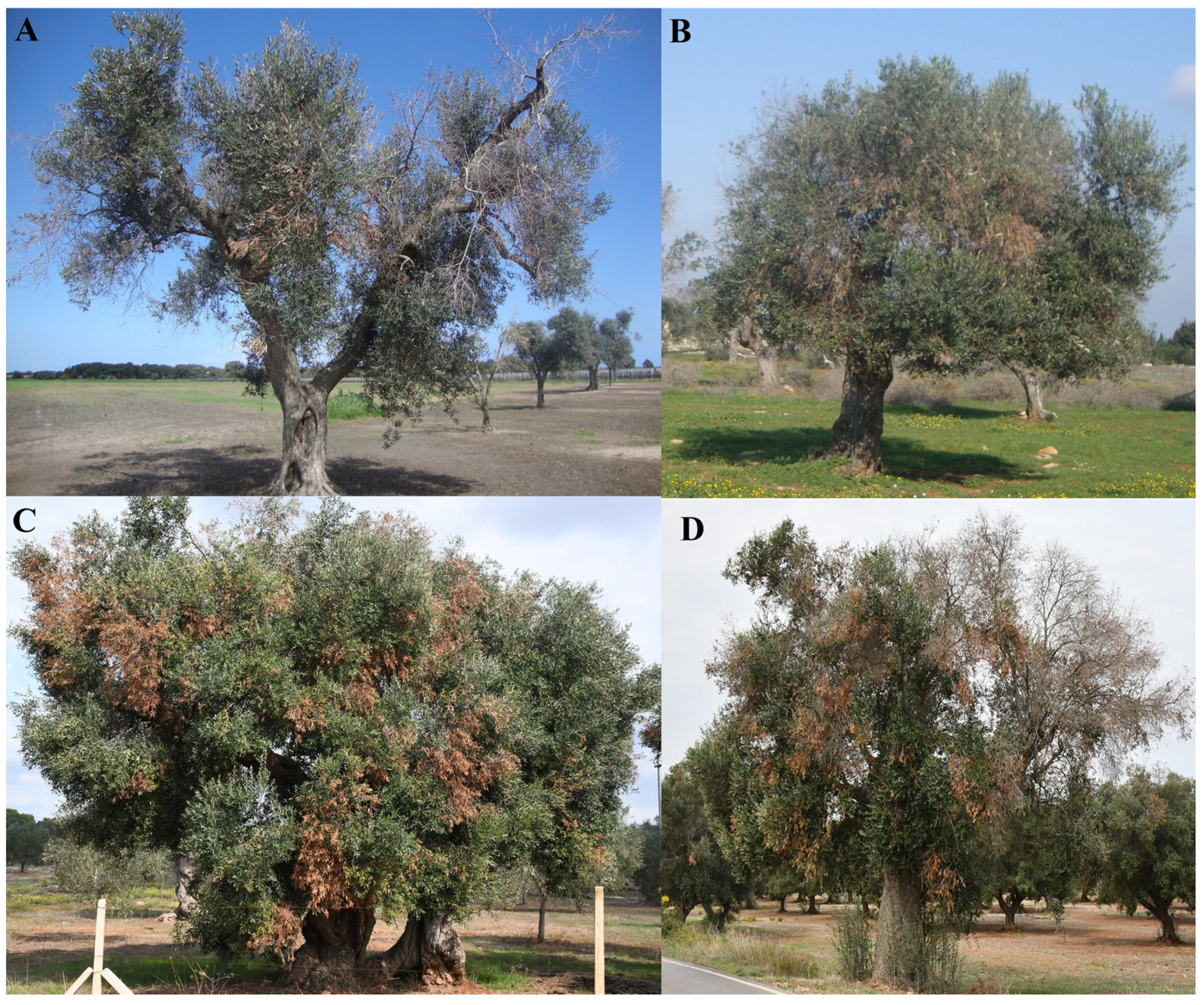
3. Olive Decline
4. Kiwifruit Vine Decline Syndrome
5. Declines and Dysbiosis
6. Similarities and Differences Between Oak, Olive, and Kiwifruit Declines
7. Perspectives
8. Conclusions
Funding
Conflicts of Interest
References
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global decline in large old trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef] [PubMed]
- Wing, I.S.; De Cian, E.; Mistry, M.N. Global vulnerability of crop yields to climate change. J. Environ. Econom. Manag. 2021, 109, 102462. [Google Scholar] [CrossRef]
- Chloupek, O.; Hrstkova, P. Adaptation of crops to environment. Theor. Appl. Genet. 2005, 111, 1316–1321. [Google Scholar] [CrossRef]
- Manion, P.D. Tree Disease Concept; Prentice Hall: Englewood Cliffs, NJ, USA, 1981; 399p. [Google Scholar]
- Manion, P.D. Tree Disease Concept, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1991; 402p. [Google Scholar]
- Sinclair, W.A.; Hudler, G.W. Tree declines: Four concepts of causality. Arboric. Urban For. 1988, 14, 29–35. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Sena, K.; Crocker, E.; Vincelli, P.; Barton, C. Phytophthora cinnamomi as a driver of forest change: Implications for conservation and management. For. Ecol. Manag. 2018, 409, 799–807. [Google Scholar] [CrossRef]
- Gómez-Sanz, V.; Gaston, A.; García-Viñas, J.I.; Serrada-Hierro, R. Site-scale soil conditions influencing the decline of Aleppo pine stands in Mediterranean Spanish woodlands. Plant Soil 2024, 504, 493–504. [Google Scholar] [CrossRef]
- Morris, H. Tree pruning: A modern approach. Int. Dendrol. Soc. 2013, 209. [Google Scholar]
- UN-ECE; FAO. Forest Resources of Europe, CIS, North America, Australia, Japan and New Zealand (Industrialized Temperate/Boreal Countries): UN-ECE/FAO Contribution to the Global Forest Resources Assessment 2000: Main Report; United Nations: Geneva, Switzerland, 2000; ISBN 9211167353. [Google Scholar]
- International Olive Council. Available online: https://www.internationaloliveoil.org/world-market-of-olive-oil-and-table-olives-data-from-december-2024 (accessed on 22 February 2025).
- Worldostats. Available online: https://worldostats.com/kiwi-fruit-production-by-country-2025 (accessed on 22 February 2025).
- Kowsari, M.; Karimi, E. A review on oak decline: The global situation, causative factors, and new research approaches. For. Syst. 2023, 32, eR01. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Petritan, A.M.; Petritan, I.C.; Hevia, A.; Walentowski, H.; Bouriaud, O.; Sanchez-Salguero, R. Climate warming predispose sessile oak forests to drought-induced tree mortality regardless of management legacies. For. Ecol. Manag. 2021, 491, 119097. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. Phytophthora quercina: A new species found on declining oaks. EPPO Rep. Serv. 2000, 066. [Google Scholar]
- Oak, S.W.; Spetich, M.A.; Morin, R.S. Oak decline in central hardwood forests: Frequency, spatial extent and scale. In Managing Forest Ecosystems. Natural Disturbances and Historic Range of Variation; Greenberg, C.H., Collins, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 49–71. [Google Scholar]
- Choi, W.I.; Lee, D.-H.; Jung, J.B.; Park, Y.-S. Oak decline syndrome in Korean forests: History, biology, and prospects for Korean oak wilt. Forests 2022, 13, 964. [Google Scholar] [CrossRef]
- Sicoli, G.; De Gioia, T.; Luisi, N.; Lerario, P. Multiple factors associated with oak decline in southern Italy. Phytopath. Medit. 1998, 37, 1–8. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Kabrik, J.M.; Dey, D.C.; Jensen, R.G.; Wallendorf, M. The role of environmental factors in oak decline and mortality in the Ozark Highlands. For. Ecol. Manag. 2008, 255, 1409–1417. [Google Scholar] [CrossRef]
- Acacio, V.; Dias, F.S.; Catry, F.X.; Bugalho, M.N.; Moreira, F. Canopy cover loss of Mediterranean oak woodlands: Long-term effects of management and climate. Ecosystems 2021, 24, 1775–1791. [Google Scholar] [CrossRef]
- Gosling, R.H.; Jackson, R.W.; Elliot, M.; Nichols, C.P. Oak Declines: Reviewing the evidence for causes, management implications and research gaps. Ecol. Sol. Evid. 2024, 5, e12395. [Google Scholar] [CrossRef]
- Gaertic, D.; Schack-Kirkner, H.; Hildebrand, E.E.; Wilpert, K. The impact of soil aeration on oak decline in southwestern Germany. For. Ecol. Manag. 2002, 159, 15–25. [Google Scholar] [CrossRef]
- Encinas-Valero, M.; Esteban, R.; Heres, A.-M.; Vivas, M.; Fakhet, D.; Aranjuelo, I.; Solla, A.; Moreno, G.; Curiel Juste, J. Holm oak decline is determined by shifts in fine root phenotypic plasticity in response to belowground stress. New. Phytol. 2022, 235, 2237–2251. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Senf, C.; Buras, A.; Zang, C.S.; Ramming, A.; Seidl, R. Excess Forest mortality is consistently linked to drought across Europe. Nat. Comm. 2020, 11, 6200. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.-M.-C. The impact of climate change on forest development: A sustainable approach to management models applied to Mediterranean-type regions. Plants 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Dolezal, J.; Scherrer, D.; Altman, J.; Ziche, D.; Martinez-Sancho, E.; Bigler, C.; Bolte, A.; Colangelo, M.; Dorado-Linan, I.; et al. Revealing legacy effects of extreme droughts on tree growth of oaks across Northern Hemisphere. Sci. Tot. Environ. 2024, 926, 172049. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.; Trenberth, K.E. Climate change and drought: A perspective on drought indices. Curr. Clim. Chang. Rep. 2018, 4, 145–163. [Google Scholar] [CrossRef]
- Siwecki, R.; Ufnalski, K. Review of oak stand decline with special reference to the role of drought in Poland. Eur. J. For. Pathol. 2007, 28, 99–112. [Google Scholar] [CrossRef]
- Bevacqua, E.; Rakovec, O.; Schumacher, D.L.; Kumar, H.; Thober, S.; Samaniego, L.; Seneviratne, S.I.; Zscheischler, J. Direct and lagged climate change effects intensified the 2022 European drought. Nat. Geosci. 2024, 17, 1100–1107. [Google Scholar] [CrossRef]
- Deprez-Loustau, M.-L.; Marcais, B.; Nageleisen, M.-L.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Sanchez-Salguero, R.; Colangelo, M.; Matias, L.; Ripullone, F.; Camarero, J.J. Shifts in growth responses to climate change and exceeded drought-vulnerability thresholds characterize dieback in two Mediterranean deciduous oak. Forests 2020, 11, 714. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-induced oak decline in the western Mediterranean region: An overview of current evidence, mechanisms and management options to improve forest resilience. IForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.-L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Change Biol. 2011, 18, 267–276. [Google Scholar]
- Hilmers, T.; Leroy, B.M.L.; Bae, S.; Hahn, W.A.; Hochrein, S.; Jacobs, M.; Lemme, H.; Muller, J.; Schmied, G.; Weisser, W.W.; et al. Growth response of oak to insect defoliation: Immediate and intermediate perspectives. For. Ecol. Manag. 2023, 549, 121465. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: Drought-affected Italian oaks are smaller and show lower growth prior to tree death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.M. Recent advances in cause-effect research on oak decline in Europe. CABI Rev. 2008, 037, 12. [Google Scholar] [CrossRef]
- Reinmann, A.B.; Bowers, J.T.; Kaur, P.; Kohler, C. Compensatory responses of leaf physiology reduce effects of spring frost defoliation on temperate tree carbon uptake. Front. For. Glob. Chang. 2023, 6, 988233. [Google Scholar] [CrossRef]
- Sakai, A.; Larcher, W. Frost Survival of Plants: Responses and Adaptation to Freezing Stress; Springer Science & Business Media: Heidelberg, Germany, 2012; Volume 62. [Google Scholar]
- Corcobado, T.; Cubera, E.; Moreno, G.; Solla, A. Quercus ilex forests are influenced by annual variation in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi. Agric. For. Meteorol. 2013, 169, 92–99. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 27, 2245–2259. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The many facets of hypoxia in plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Liu, Y.; Miao, P.; Liu, J.; Wang, Z. Updates and prospects: Morphological, physiological, and molecular regulation in crop response to waterlogging stress. Agronomy 2023, 13, 2599. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Sexstone, A.J.; Parkin, T.B.; Revsbech, R.B.; Shelton, D.R. Anaerobic processes in soil. Plant Soil 1984, 76, 197–212. [Google Scholar] [CrossRef]
- Pan, B.; Xia, L.; Lam, S.K.; Wang, E.; Zhang, Y.; Mosier, A.; Chen, D. A global synthesis of soil denitrification: Driving factors and mitigation strategies. Agric. Ecosyst. Environm. 2022, 327, 107850. [Google Scholar] [CrossRef]
- Nagel, A.M.; Long, R.P.; Madden, L.V.; Bonello, P. Association of Phytophthora cinnamomi with white oak decline in Southern Ohio. Plant Dis. 2010, 94, 1026–1034. [Google Scholar] [CrossRef]
- McConnel, M.E.; Balci, Y. Phytophthora cinnamomi as a contributor to white oak decline in the Mid-Atlantic United States forests. Plant Dis. 2014, 98, 319–327. [Google Scholar] [CrossRef]
- Linaldeddu, B.D.; Scanu, B.; Maddau, L.; Franceschini, A. Diplodia corticola and Phytophthora cinnamoni: The main pathogens involved in holm oak decline in Caprera island (Italy). For. Pathol. 2014, 44, 191–200. [Google Scholar] [CrossRef]
- Frisullo, D.; Lima, G.; Magnano di San Lio, G.; Camele, I.; Melissano, L.; Puglisi, I.; Pane, A.; Agosteo, G.E.; Prudente, L.; Cacciola, S.A. Phytophthora cinnamomi involved in the decline of holm oak (Quercus ilex) stands in Southern Italy. For. Sci. 2018, 64, 290–298. [Google Scholar] [CrossRef]
- Seddau, S.; Brandano, A.; Ruiu, P.A.; Sechi, C.; Scanu, B. An overview of Phytophthora species inhabiting declining Quercus suber stands in Sardinia (Italy). Forests 2020, 11, 971. [Google Scholar] [CrossRef]
- Jung, T.; Cooke, D.E.L.; Blaschke, H.; Duncan, J.M.; Osswald, W. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol. Res. 1999, 103, 785–798. [Google Scholar] [CrossRef]
- Linaldeddu, B.D.; Luque, J.; Franceschini, A. Occurrence of Botryosphearia obtusa in declining cork oak trees in Italy. J. Plant Pathol. 2006, 88, S66. [Google Scholar]
- Linaldeddu, B.D.; Franceschini, A.; Luque, J.; Phillips, A.J.L. First report of canker disease caused by Botryosphaeria parva on cork oak trees in Italy. Plant Dis. 2007, 91, 324. [Google Scholar] [CrossRef]
- Linaldeddu, B.D.; Scanu, B.; Schiaffino, A.; Zanda, A.; Franceschini, A. First report of Botryosphaeria dothidea causing canker and branch dieback on Quercus suber in Italy. J. Plant Pathol. 2009, 91, S104. [Google Scholar]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and emerging pathogens threatening cork oak trees: Management options for conserving a unique forest ecosystem. Plant Dis. 2016, 100, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, S.; Abdollahzadeh, J. Taxonomy and pathogenicity of fungi associated with oak decline in northern and central Zagros forests of Iran with emphasis on coelomycetous species. Front. Plant Sci. 2024, 15, 1377441. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Delatour, C. Phytophthora species in oak forests of north-east France. Ann. For. Sci. 1999, 56, 539–547. [Google Scholar] [CrossRef]
- Serrano, M.S.; Romero, M.A.; Homet, P.; Gomez-Aparicio, L. Climate change impact on the population dynamics of exotic pathogens: The case of the worldwide pathogen Phytophthora cinnamomi. Agric. For. Meteorol. 2022, 322, 109002. [Google Scholar] [CrossRef]
- Sallé, A.; Nageleisen, L.-M.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Managem. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect pest and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- Nechita, C.; Iordache, A.M.; Lemr, K.; Levanic, T.; Pluhacek, T. Revidence of declining trees resilience under long term heavy metal stress combined with climate change heating. J. Clean. Prod. 2021, 317, 128428. [Google Scholar] [CrossRef]
- Geo-Izquierdo, G.; Natalini, F.; Cardillo, E. Holm oak dearth is accelerated but not sudden and expresses drought legacies. Sci. Tot. Environ. 2021, 754, e141793. [Google Scholar] [CrossRef]
- Balci, Y.; Halmschlager, E. Incidence of Phytophthora species in oak forest in Austria and their possible involvement in oak decline. For. Pathol. 2003, 33, 157–174. [Google Scholar] [CrossRef]
- Thomas, F.M.; Schafellner, C. Effects of excess nitrogen and drought on the foliar concentration of allelochemicals in young oaks. Angew. Bot. 1999, 73, 222–227. [Google Scholar]
- Losseau, J.; Jonard, M.; Vincke, C. Peduncolate oak decline in southern Belgium: A long-term process highlighting the complex interplay among drought, winter frost, biotic attacks and masting. Can. J. For. Res. 2020, 50, 380–389. [Google Scholar] [CrossRef]
- Garbelotto, M.; Svihra, P.; Rizzo, D.M. Sudden oak death syndrome fells 3 oak species. Cal Agric. 2001, 22, 9–19. [Google Scholar] [CrossRef]
- Kliejunas, J.T. Sudden Oak Death and Phytophthora ramorum: A Summary of the Literature, 2010th ed.; Gen. Tech. Rep. PSW-GTR-234; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2010; 181p. [Google Scholar]
- Grünwald, N.J.; LeBoldus, J.M.; Hamelin, R.C. Ecology and evolution of the sudden oak death pathogen Phytophthora ramorum. Annu. Rev. Phytopath. 2019, 57, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Kozanitas, M.; Metz, M.R.; Osmundson, T.W.; Socorro Serrano, M.; Garbelotto, M. The epidemiology of sudden oak death disease caused by Phytophthora ramorum in a mixed bay laurel-oak woodland provides important clues for disease management. Pathogens 2022, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- McPherson, B.A.; Mori, S.R.; Wood, D.L.; Storer, A.J.; Svihra, P.; Maggi Kelly, N.; Standiford, R.B. Sudden oak death in California: Disease progression in oaks and tanoaks. For. Ecol. Manag. 2005, 213, 71–89. [Google Scholar] [CrossRef]
- Garbelotto, M.; Schmidt, D.; Popenuck, T. Pathogenicity and infectivity of Phytophthora ramorum vary depending on host species, infected plant part, inoculum potential, pathogen genotype, and temperature. Plant Pathol. 2021, 70, 287–304. [Google Scholar] [CrossRef]
- Simler, A.B.; Metz, M.R.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Novel disturbance interactions between fire and an emerging disease impact survival and growth of resprouting trees. Ecology 2018, 99, 2217–2229. [Google Scholar] [CrossRef]
- Cobb, R.C.; Meentmeyer, R.; Rizzo, D.M. Wildfire and forest disease interaction lead to greater loss of soil nutrients and carbon. Oecologia 2016, 182, 265–276. [Google Scholar] [CrossRef]
- Swiecki, T.J.; Bernhardt, E.A. A Reference Manual for Managing Sudden Oak Death in California; Gen. Tech. Rep. PSW-GTR-242; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2013; 129p. [Google Scholar]
- Denman, S.; Brown, N.; Kirk, S.; Jeger, M.; Webber, J. A description of the symptoms of acute oak decline in Britain and a comparative review on causes of similar disorders on oak in Europe. Forestry 2014, 87, 535–551. [Google Scholar] [CrossRef]
- Carluccio, G.; Sabella, E.; Greco, D.; Vergine, M.; Delle Donne, A.G.; Nutricati, E.; Aprile, A.; De Bellis, L.; Luvisi, A. Acute and chronic oak decline in urban and forest ecosystems in Southern Italy. Forestry 2024, 97, 739–749. [Google Scholar] [CrossRef]
- Eichenlaub, L.; Denman, S.; Brady, C.; Maddock, D.; Robledo-Garcia, F.; Aubert, A.; Husson, C.; Robin, C. First report of Brenneria goodwinii, Gibbsiella quercinecans and Rahnella victoriana in declining oaks in France. New Dis. Rep. 2024, 49, e12264. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K. The role of bacteria in acute oak decline in South Poland. Microorganisms 2024, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Amirabad, Y.; Rahimian, H.; Babaeizad, V.; Denman, S. Brenneria spp. and Rahnella victoriana associated with acute oak decline symptoms on oak and hornbeam in Iran. For. Pathol. 2019, 49, e12535. [Google Scholar] [CrossRef]
- Brown, N.; Vanguelova, E.; Parnell, S.; Broadmeadow, S.; Denman, S. Predisposition of forests to biotic disturbance: Predicting the distribution of acute oak decline using environmental factors. For. Ecol. Manag. 2018, 407, 145–154. [Google Scholar] [CrossRef]
- Reed, K.; Forster, J.; Denman, S.; Brown, N.; Leather, S.R.; Inward, D.J. Novel dendrochronological modelling demonstrates that decades of reduced stem growth predispose trees to acute oak decline. For. Ecol. Manag. 2020, 476, 118441. [Google Scholar] [CrossRef]
- Barsoum, N.; A’Hara, S.W.; Cottrell, J.E.; Forster, J.; Garcia, M.S.J.; Schonrogge, K.; Shaw, L. Root ectomycorrhizal status of oak trees symptomatic and asymptomatic for acute oak decline in southern Britain. For. Ecol. Manag. 2021, 482, 118800. [Google Scholar] [CrossRef]
- Denman, S.; Brown, N.; Vanguelova, E.; Crampton, B. Temperate Oak Declines: Biotic and Abiotic Predisposition Drivers; Asiegbu, F.O., Kovalchuck, A., Eds.; Forest microbiology, Forest Tree Health; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 2, pp. 239–263. [Google Scholar]
- Meaden, S.; Metcalf, C.J.E.; Koskella, B. The effects of host age and spatial location on bacterial community composition in the English oak tree (Quercus robur). Environ. Microbiol. Rep. 2016, 8, 649–658. [Google Scholar] [CrossRef]
- Doonan, J.M.; Broberg, M.; Denman, S.; McDonald, J.E. Host-microbiota-insect interactions drive emergent virulence in a complex tree disease. Proc. Royal Soc. B Biol. Sci. 2020, 287, 20200956. [Google Scholar] [CrossRef]
- Denman, S.; Doonan, J.; Ransom-Jones, E.; Broberg, M.; Plummer, S.; Kirk, S.; Scarlett, K.; Griffiths, A.R.; Kaczmarek, M.; Forster, J.; et al. Microbiome and infectivity studies reveal complex polyspecies tree disease in acute oak decline. ISME J. 2018, 12, 386–399. [Google Scholar] [CrossRef]
- Doonan, J.; Denman, S.; Pachebat, J.A.; McDonald, J.E. Genomic analysis of bacteria in the acute oak decline pathobiome. Microb. Genom 2019, 5, e000240. [Google Scholar] [CrossRef]
- Pettifor, B.J.; Doonan, J.; Denman, S.; McDonald, J.E. Survival of Brenneria goodwinii and Gibbsiella quercinecans, associated with acute oak decline, in rainwater and forest soil. Syst. Appl. Microbiol. 2020, 43, 126052. [Google Scholar] [CrossRef] [PubMed]
- Gathercole, L.A.P.; Nocchi, G.; Brown, N.; Coker, T.L.R.; Plumb, W.J.; Stocks, J.J.; Nichols, R.A.; Denman, S.; Buggs, R.J.A. Evidence for the widespread occurrence of bacteria implicated in acute oak decline from incidental genetic sampling. Forests 2021, 12, 1683. [Google Scholar] [CrossRef]
- Brown, N.; Jeger, M.; Kirk, S.; Xu, X.; Denman, S. Spatial and temporal patterns in symptom expression within eight woodlands affected by acute oak decline. For. Ecol. Manag. 2016, 360, 97–109. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Boscia, D.; Saponari, A.; Nigro, F.; Martelli, G.P. Il caso del disseccamento rapido dell’olivo: Sintomatologia ed eziologia. In Batteri Vascolari Fitopatogeni Trasmessi da Insetti; Quaderni Georgofili; Accademia dei Georgofili: Firenze, Italy, 2014; pp. 41–50. [Google Scholar]
- Scortichini, M.; Manetti, G.; Brunetti, A.; Lumia, V.; Sciarroni, S.; Pilotti, M. Xylella fastidiosa subsp. pauca, Neofusicoccum spp., and the decline of olive trees in Salento (Apulia, Italy): Comparison of symptoms, possible interactions, certainties and doubts. Plants 2023, 12, 3593. [Google Scholar]
- Manetti, G.; Brunetti, A.; Sciarroni, L.; Lumia, V.; Bechini, S.; Marangi, P.; Reverberi, M.; Scortichini, M.; Pilotti, M. Diplodia seriata isolated from declining olive trees in Salento (Apulia, Italy): Pathogenicity trials give a glimpse that it is more virulent to drought-stressed olive trees and in warmth-conditioned environment. Plants 2024, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- McElrone, A.J.; Sherald, J.L.; Forseth, I.N. Effects of water stress on symptomatology and growth of Partenocissuc quinquefoliae infected by Xylella fastidiosa. Plant Dis. 2001, 85, 1160–1164. [Google Scholar] [CrossRef]
- Wallis, C.M.; Gorman, Z. Pre-inoculations water deficit effects on grapevine physiology, Xylella fastidiosa titers, and Pierce’s disease progression. BMC Res. Notes 2024, 17, 119. [Google Scholar] [CrossRef]
- Brunetti, A.; Matere, A.; Lumia, V.; Pasciuta, V.; Fusco, V.; Sansone, D.; Marangi, P.; Cristella, N.; Faggioli, F.; Scortichini, M.; et al. Neofusicoccum mediterraneum is involved in a twig and branch dieback of olive trees observed in Salento (Apulia, Italy). Pathogens 2022, 11, 53. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D.; et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017, 7, 17723. [Google Scholar] [CrossRef]
- Manetti, G.; Brunetti, A.; Lumia, V.; Sciarroni, L.; Marangi, P.; Cristella, N.; Faggioli, F.; Reverberi, M.; Scortichini, M.; Pilotti, M. Identification and characterization of Neofusicoccum stellenboschiana in branch and twig dieback-affected olive trees in Italy and comparative pathogenicity with N. Mediterraneum. J. Fungi 2023, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Alfio, M.R.; Balacco, G.; Parisi, A.; Totaro, V.; Fidelibus, M.D. Drought index as indicator of salinization of the Salento aquifer (Southern Italy). Water 2020, 12, 1927. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; Nigro, F.; Sergeeva, V.; Ippolito, A.; Salerno, M.G. Abiotic diseases of olive. J. Plant Pathol. 2012, 94, 469–491. [Google Scholar]
- Ciervo, M. The olive quick decline syndrome (OQDS) diffusion in Apulia region: An apparent contradiction according to the agricultural model. Belgeo 2016, 4. [Google Scholar] [CrossRef]
- Scortichini, M.; Ragno, D. Survey on resilient olive groves previously severely damaged by Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy). Agronomy 2024, 14, 2003. [Google Scholar]
- Bardi, L. Early kiwifruit decline: A soil-borne disease syndrome or a climate change effect on plant-soil relations? Front. Agron. 2020, 7, 3. [Google Scholar] [CrossRef]
- Ferguson, A.R. Kiwifruit: A botanical review. Hort. Rev. 1984, 6, 1–64. [Google Scholar]
- Tacconi, G.; Paltrinieri, S.; Mejia, J.F.; Fuentealba, S.P.; Bertaccini, A.; Tosi, L.; Giacopini, A.; Mazzucchi, U.; Favaron, F.; Sella, L.; et al. Vine decline in kiwifruit: Climate change and effect on waterlogging and Phytophthora in north Italy. Acta Hortic. 2015, 1096, 93–97. [Google Scholar] [CrossRef]
- Savian, F.; Ginaldi, F.; Musetti, R.; Sandrin, N.; Tarquini, G.; Pagliari, L.; Firrao, G.; Martini, M.; Ermacora, P. Studies on the aetiology of kiwifruit decline: Interactions between soil-borne pathogens and waterlogging. Plant Soil 2020, 456, 113–128. [Google Scholar] [CrossRef]
- Savian, F.; Prencipe, S.; Filippini, N.; Nari, L.; Martini, M.; Ermacora, P.; Spadaro, D. Pathogenicity of Phytopythium chamaehyphon: A new player in kiwifruit vine decline syndrome of Actinidia chinensis var. deliciosa “Hayward” in Italy. Plant Dis. 2021, 105, 2781–2784. [Google Scholar] [CrossRef]
- Bardi, L.; Nari, L.; Morone, C.; Faga, M.G.; Malusà, E. Possible role of high temperature and soil biological fertility on kiwifruit early decline syndrome. Front. Agron. 2020, 2, 580659. [Google Scholar] [CrossRef]
- Cardacino, A.; Turco, S.; Balestra, G.M. Seasonal dynamic of kiwifruit microbiome: A case study in a KVDS-affected orchard. Microbiol. Res. 2025, 292, 128044. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, C.; Monaco, S.; Nari, L.; Morone, C.; Palazzi, F.; Bencresciuto, G.F.; Bardi, L. Xylem hydraulic conductance role in kiwifruit decline syndrome occurrence. Horticulturae 2024, 10, 392. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Caldera, E.; Sorrenti, G.; Spinelli, F. Pathogens associated with kiwifruit vine decline in Italy. Agriculture 2020, 10, 119. [Google Scholar] [CrossRef]
- Prencipe, S.; Schiavon, G.; Rosati, M.; Nari, L.; Schena, L.; Spadaro, D. Characterization of Phytopythium species involved in the establishment and development of kiwifruit vine decline syndrome. Microorganisms 2023, 11, 216. [Google Scholar] [CrossRef]
- Guaschino, M.; Garello, M.; Nari, L.; Zhimo, Y.V.; Droby, S.; Spadaro, D. Soil, rhizosphere and root microbiome in kiwifruit vine decline, an emerging multifactorial disease. Front. Microbiol. 2024, 15, 1330865. [Google Scholar] [CrossRef]
- Spigaglia, P.; Barbanti, F.; Marocchi, F.; Mastroleo, M.; Baretta, M.; Ferrante, P.; Caboni, E.; Lucioli, S.; Scortichini, M. Clostridium bifermentans and C. subterminale are associated with kiwifruit vine decline, known as moria, in Italy. Plant Pathol. 2020, 69, 765–774. [Google Scholar] [CrossRef]
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-microbiota interaction: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef]
- Arnault, G.; Mony, C.; Vandenkoornhuyse, P. Plant microbiota dysbiosis and the Anna Karenina principle. Trends Plant Sci. 2023, 28, 18–30. [Google Scholar] [CrossRef]
- Paasch, B.C.; He, S.H. Towards understanding microbiota homeostasis in the plant kingdom. PLoS Pathog. 2021, 17, e1009472. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Gallardo, A.; Gómez-Aparicio, L. Pathogen-induced tree mortality interacts with predicted climate change to alter soil respiration and nutrient availability in Mediterranean systems. Biogeochem. 2019, 3, 53–71. [Google Scholar] [CrossRef]
- Encinas-Valero, M.; Esteban, R.; Heres, A.-M.; Vivas, M.; Solla, A.; Moreno, G.; Corcobado, T.; Odriozola, I.; Garbisu, C.; Epelde, L.; et al. Alteration of the tree-soil microbial system triggers a feedback loop that boosts holm oak decline. Funct. Ecol. 2024, 38, 374–390. [Google Scholar] [CrossRef]
- Pinho, D.; Barroso, C.; Froufe, H.; Brown, N.; Vanguelova, E.; Egas, C.; Denman, S. Linking tree health, rhizosphere physicochemical properties, and microbiome in acute oak decline. Forests 2020, 11, 1153. [Google Scholar] [CrossRef]
- Vergine, M.; Vita, F.; Casati, P.; Passera, A.; Ricciardi, L.; Pavan, S.; Aprile, A.; Sabella, E.; De Bellis, L.; Luvisi, A. Characterization of the olive endophytic community in genotypes displaying a contrasting response to Xylella fastidiosa. BMC Plant Biol. 2024, 24, 337. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Saccà, M.L.; Scotti, C.; Caputo, F. Quantitative reduction of soil bacteria and qualitative microbial changes: Biotic components associated to kiwifruit decline. Plant Soil 2022, 477, 613–628. [Google Scholar] [CrossRef]
- Bergamaschi, V.; Vera, A.; Pirone, L.; Siles, J.A.; Lopez-Mondècar, R.; Luongo, L.; Vitale, S.; Reverberi, M.; Infantino, A.; Bastida, F. Kiwifruit vine decline syndrome (KVDS) alter soil enzyme activity and microbial community. Microorganisms 2024, 12, 2347. [Google Scholar] [CrossRef]
- Manici, M.L.; Maisto, G.; Abbate, C.; Caputo, F.; Memoli, V.; Santorufo, L.; Morello, B.; Zizolfi, M.; Santini, G.; Vitale, S.; et al. Quantitative soil indicators for identifying primary stressors in fruit tree decline: A case study on kiwifruit vine decline syndrome. Appl. Soil Ecol. 2025, 206, 105887. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Penuelas, J. Widespread condition decline, food web destruction, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Bolte, A.; Dorado-Linan, I.; Etzold, S.; Fonti, P. Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef]
- Bendixsen, D.P.; Hallgren, S.W.; Frazier, A.F. Stress factors associated with forest decline in xeric oak forests of south-central United States. For. Ecol. Manag. 2015, 347, 40–48. [Google Scholar] [CrossRef]
- Gieger, T.; Thomas, F.M. Differential response of two Central-European oak species to single and combined stress factors. Trees 2005, 19, 607–618. [Google Scholar] [CrossRef]
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicoli, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular effects of Xylella fastidiosa and drought combined stress in olive trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europea: Revisiting a tale of plant-pathogen interaction. AoB Plants 2021, 13, plab027. [Google Scholar] [CrossRef]
- Niinemets, U.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Chen, S.; Ten Tusscher, K.H.W.J.; Sisidharan, R.; Dekker, S.C.; De Boer, H.J. Parallels between drought and flooding: An integrated framework for plant eco-physiological responses to water stress. Plant-Environ. Interact. 2023, 4, 175–187. [Google Scholar] [CrossRef]
- Mexia, T.; Caldeira, M.C.; Lecomte, X.; Dias, F.S.; Tomé, M.; Nunes, L.; Bugalho, M.N. Is forest certification mitigating oak decline in Mediterranean open woodlands? For. Ecol. Manag. 2024, 568, 122105. [Google Scholar] [CrossRef]
- Corcobado, T.; Solla, A.; Madeira, M.A.; Moreno, G. Combined effects of soil properties and Phytophthora cinnamomi infections on Quercus ilex decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Maddock, D.; Brady, C.; Denman, S.; Arnold, D. Bacteria associated with acute oak decline: Where did they come from? We know where they go. Microorganisms 2023, 17, 2789. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with ink disease and crown decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Chadfield, V.; Pautasso, M. Phytophthora ramorum in England and Wales: Which environmental variables predict county disease incidence? For. Pathol. 2012, 42, 150–159. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; Lopez, R.; Saenz-Romero, C.; Hartmann, H.; Bresheras, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprints for Earth’s forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2001: The Scientific Basis, IPCC Contribution of Working Group to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Metze, D.; Schnecker, J.; Canarini, A.; Fuchslueger, L.; Koch, B.J.; Stone, B.W.; Hungate, B.A.; Hausmann, B.; Schimdt, H.; Schaumberger, A.; et al. Microbial growth under drought is confined to distinct taxa and modified by potential future climate conditions. Nat. Commun. 2023, 14, 5895. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; Imbriani, G.; Girelli, C.R.; Miglietta, P.R.; Scortichini, M.; Fanizzi, F.P. A decade after the outbreak of Xylella fastidiosa subsp. pauca in Apulia (Southern Italy): Methodical literature analysis of research strategies. Plants 2024, 13, 1433. [Google Scholar] [PubMed]
- Scortichini, M. Predisposing factors for “olive quick decline syndrome” in Salento (Apulia, Italy). Agronomy 2020, 10, 1445. [Google Scholar] [CrossRef]
- Scortichini, M.; Loreti, S.; Scala, V.; Pucci, N.; Pilotti, M.; Tatulli, G.; Cesari, E.; L’Aurora, A.; Reverberi, M.; Cristella, N.; et al. Management of the olive decline disease complex caused by Xylella fastidiosa subsp. pauca and Neofusicoccum spp. in Apulia, Italy. Crop Prot. 2024, 184, 106782. [Google Scholar]
- Ruiz-Gomez, F.J.; Navarro-Cerrillo, R.M.; Pérez-de-Luque, A.; Oβwald, W.; Vannini, A.; Morales-Rodriguez, C. Assessment of functional and structural change of soil fungal and oomycete communities in holm oak declined dehesa through metabarcoding analysis. Sci. Rep. 2019, 9, 5315. [Google Scholar] [CrossRef] [PubMed]
- Lyubenova, A.; Baranowska, M.; Menkis, A.; Davydenko, K.; Nowakowska, J.; Borowik, P.; Oszako, T. Prospects for oak cultivation in Europe under changing environmental conditions and increasing pressure from harmful organisms. Forests 2014, 15, 2164. [Google Scholar] [CrossRef]
- Morales-Rodriguez, C.; Vannini, A.; Scanu, B.; Gonzalez-Moreno, P.; Turco, S.; Drais, M.I.; Brandano, A.; Varo Martinez, M.A.; Mazzaglia, A.; Deidda, A.; et al. Challenges to Mediterranean Fagaceae ecosystems affected by Phytophthora cinnamomi and climate change: Integrated pest management perspectives. Curr. For. Rep. 2025, 11, 9. [Google Scholar] [CrossRef]
- Laine, A.-L. Plant disease risk is modified by multiple global change drivers. Curr. Biol. 2023, 33, R574–R583. [Google Scholar] [CrossRef]
- Choudhary, A.; Senthil-Kumar, M. Drought: A context-dependent damper and aggravator of plant diseases. Plant Cell Environ. 2024, 47, 2109–2126. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.; Mendes, R.; Costa, L.A.S.; Bueno, G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Front. Microbiol. 2021, 12, 743512. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigations and the role of plant-growth promoting rhizobacteria. Res. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. recent advances in bacterial amelioration of plant drought and salt stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Holling, C.S.; Carpenter, S.R.; Kinzig, A. Resilience, adaptability, and transformability in social-ecological systems. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Knoot, T.G.; Schulte, L.A.; Tyndall, J.C.; Palik, B.J. The state of the system and steps toward resilience of disturbance-dependent oak forests. Ecol. Soc. 2010, 15, 5. [Google Scholar] [CrossRef]
- Tonelli, E.; Vitali, A.; Brega, F.; Gazol, A.; Colangelo, M.; Urbinati, C.; Camarero, J.J. Thinning improves growth and resilience after severe droughts in Quercus subpyrenaica coppice forests in the Spanish pre-Pyrenees. Dendrochronologia 2023, 77, 126042. [Google Scholar] [CrossRef]
- Kostic, S.; Orlovic, S.; Karaklic, V.; Kesic, L.; Zoric, M.; Stojanovic, D.B. Allometry and post-drought growth resilience of pedunculate oak (Quercus robur L). varieties. Sustainability 2021, 12, 930. [Google Scholar] [CrossRef]
- Scala, V.; Scortichini, M.; Marini, F.; La Montagna, D.; Beccaccioli, M.; Micalizzi, K.; Cacciotti, A.; Pucci, N.; Tatulli, G.; Fiorani, R.; et al. Assessment of fatty acid and oxylipin profile in resprouting olive trees positive to Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy). Plants 2024, 13, 2186. [Google Scholar]
- Mi, Y.; Ma, X.; Chen, S. Resistant evaluation of kiwifruit rootstocks to root zone hypoxia stress. Am. J. Plant Sci. 2013, 4, 945–954. [Google Scholar] [CrossRef]
- Mian, G.; Cipriani, G.; Saro, S.; Martini, M.; Ermacora, P. Potential of different Actinidia genotypes as resistant rootstocks for preventing kiwifruit vine decline syndrome. Horticulturae 2022, 8, 627. [Google Scholar] [CrossRef]
- Kim, G.H.; Choi, E.D. Occurrence of kiwifruit vine decline syndrome and its prevention using rootstocks tolerant to waterlogging. Res. Plant Dis. 2023, 29, 425–432. [Google Scholar] [CrossRef]
- Mian, G.; Cipriani, G.; Firrao, G.; Martini, M.; Ermacora, P. Genetic diversity of Actinidia spp. shapes the oomycetes pattern associated with kiwifruit vine decline syndrome (KVDS). Sci. Rep. 2023, 13, 16449. [Google Scholar] [CrossRef] [PubMed]
- Calabritto, M.; Mininni, A.N.; Di Biase, R.; Petrozza, A.; Summerer, S.; Cellini, F.; Dichio, B. Physiological and image-based phenotyping assessment of waterlogging responses of three kiwifruit rootstocks and grafting combinations. Front. Plant Sci. 2025, 16, 1499432. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gomez, F.J.; Miguel-Rojas, C. Antagonistic potential of native Trichoderma spp. against Phytophthora cinnamomi in the control of holm oak decline in dehesas ecosystems. Forests 2021, 17, 945. [Google Scholar] [CrossRef]
- Costa, D.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Cork oak endophytic fungi as potential biocontrol agents against Biscognauxia mediterranea and Diplodia corticola. J. Fungi 2020, 14, 287. [Google Scholar] [CrossRef]
- Tatulli, G.; Modesti, V.; Pucci, N.; Scala, V.; L’Aurora, A.; Lucchesi, S.; Salustri, M.; Scortichini, M.; Loreti, S. Further in vitro assessment and mid-term evaluation of control strategy of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 85. [Google Scholar]
- Blonda, P.; Tarantino, C.; Scortichini, M.; Maggi, S.; Tarantino, M.; Adamo, M. Satellite monitoring of bio-fertilizer restoration in olive groves affected by Xylella fastidiosa subsp. pauca. Sci. Rep. 2023, 13, 5695. [Google Scholar] [CrossRef]
- Crocker, E.; Gurung, K.; Calvert, J.; Nelson, C.D.; Yang, J. Integrating GIS, remote sensing, and citizen science to map oak decline risk across the Daniel Boone national forest. Remote Sens. 2023, 15, 2250. [Google Scholar] [CrossRef]
- Imanyfar, S.; Hasanlou, M.; Mirzaei Zadeh, V. Mapping oak decline through long-term analysis of time series of satellite images in the forests of Malekshahi, Iran. Int. J. Remote Sens. 2019, 40, 8705–8726. [Google Scholar] [CrossRef]
- Kim, J.H.; Castroverde, C.D.M.; Huang, S.; Li, C.; Hilleary, L.; Seroka, A.; Sohrabi, R.; Medina-Yereda, D.; Hout, B.; Wang, J.; et al. Increasing the resilience of plant immunity to a warmer climate. Nature 2022, 607, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Pelaez-Vico, M.A.; Sinha, R.; Pascual, L.S.; Mittler, R. The impact of multifactorial stress combinations on plants, crops, and ecosystems: How should we prepare for what comes next? Plant J. 2024, 117, 1800–1814. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scortichini, M. Similarities and Differences Among Factors Affecting Complex Declines of Quercus spp., Olea europea, and Actinidia chinensis. Horticulturae 2025, 11, 325. https://doi.org/10.3390/horticulturae11030325
Scortichini M. Similarities and Differences Among Factors Affecting Complex Declines of Quercus spp., Olea europea, and Actinidia chinensis. Horticulturae. 2025; 11(3):325. https://doi.org/10.3390/horticulturae11030325
Chicago/Turabian StyleScortichini, Marco. 2025. "Similarities and Differences Among Factors Affecting Complex Declines of Quercus spp., Olea europea, and Actinidia chinensis" Horticulturae 11, no. 3: 325. https://doi.org/10.3390/horticulturae11030325
APA StyleScortichini, M. (2025). Similarities and Differences Among Factors Affecting Complex Declines of Quercus spp., Olea europea, and Actinidia chinensis. Horticulturae, 11(3), 325. https://doi.org/10.3390/horticulturae11030325




