Metabolic Response Induced by Methyl Jasmonate and Benzothiadiazole in Vitis vinifera cv. Monastrell Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Treatments
2.3. Extraction of Phenolic Compounds
2.4. Analysis of Phenolic Compounds
2.5. Statistical Analysis
3. Results
3.1. Identification of Phenolic Compounds
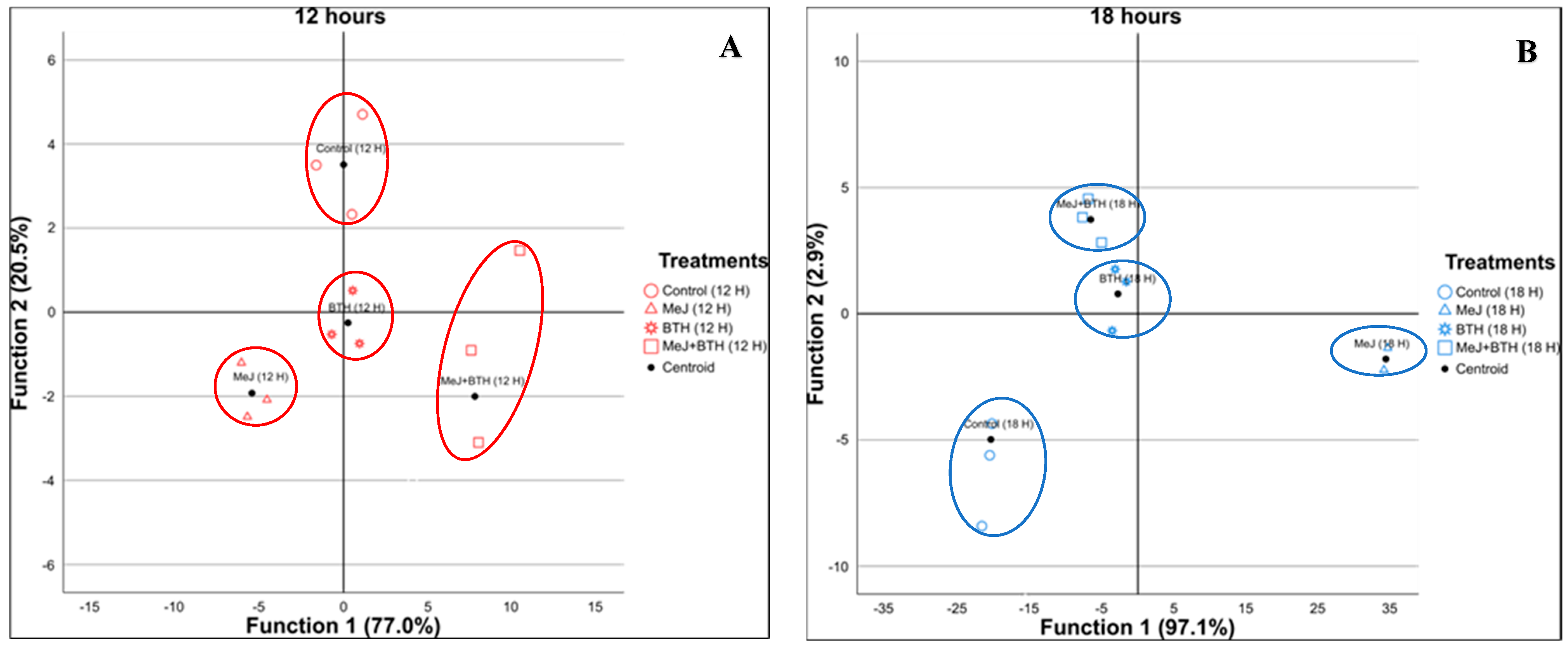
3.2. General Discriminant Analysis
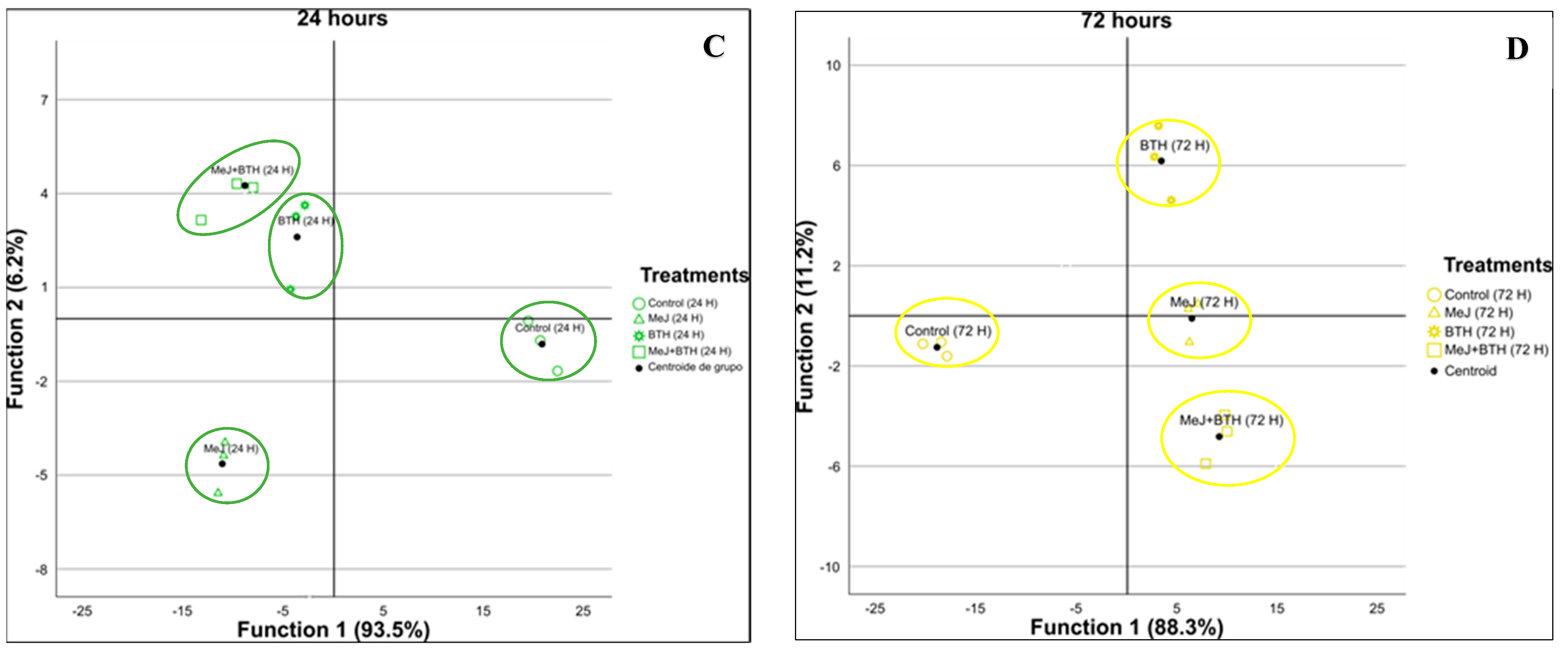
3.3. Discriminant Analyses at Different Time Points
3.4. Effect of Elicitors on the Evolution of Various Phenolic Compounds
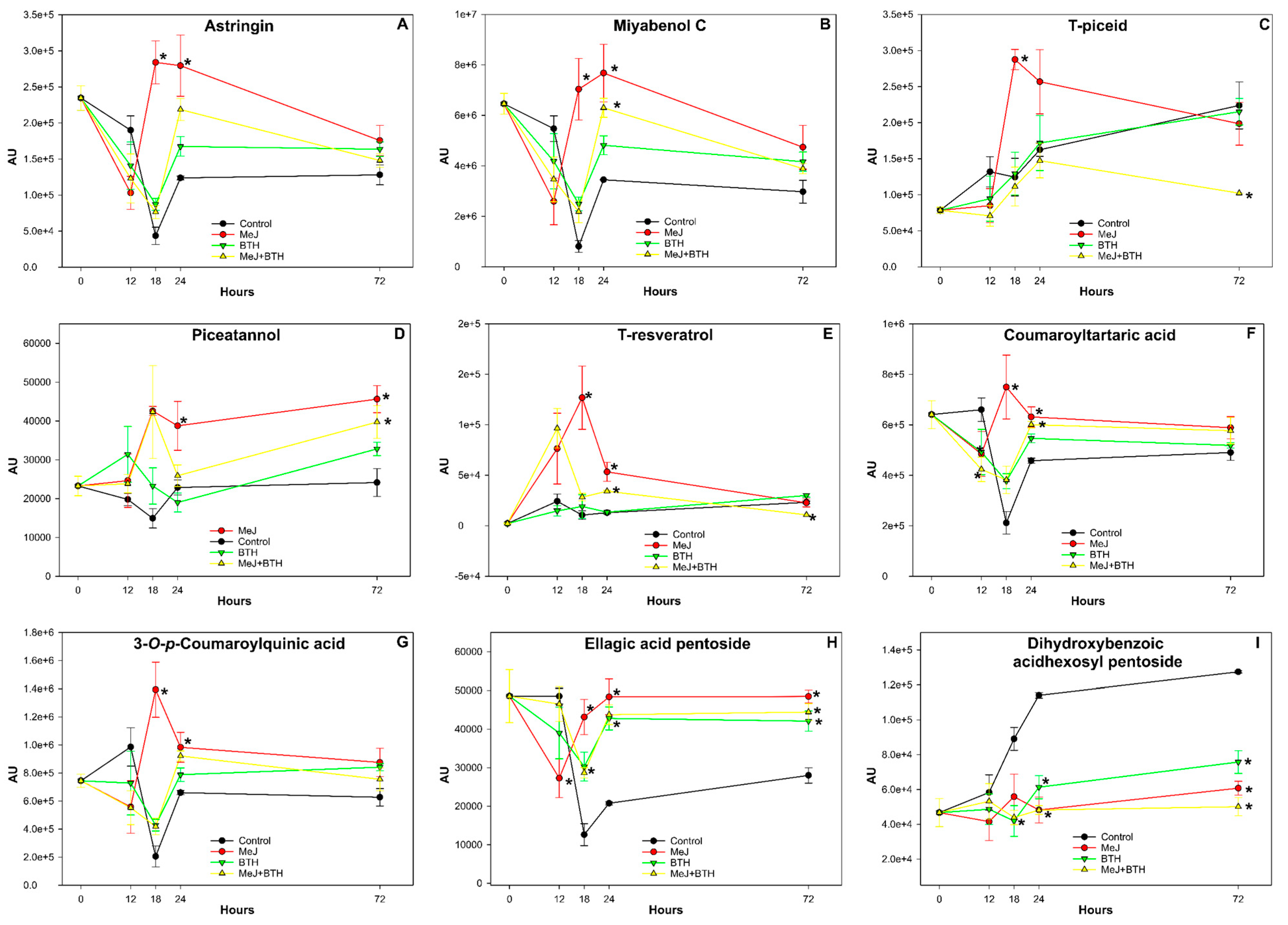
3.4.1. Stilbenes
- Astringin: Figure 3A shows a general decrease in astringin concentration across all analysed samples during the first 12 h of the study. At 18 h, astringin concentration continued to decrease in the Control, BTH, and MeJ + BTH groups, except for the MeJ-treated samples, which reached their maximum concentration at this time. From 18 to 24 h, astringin concentration increased in the control, BTH, and MeJ + BTH samples, while it began to decrease in the MeJ-treated samples. After this point, the concentration of astringin declined in all samples until the end of the study (72 h). It is worth noting that MeJ-treated plants were the only ones showing a significantly higher concentration of astringin at the 18 and 24-h samplings compared to the other treatments.
- Miyabenol C: The general decrease in Miyabenol C concentration was recorded during the first 12 h of treatment (Figure 3B) and observed in all analysed samples. At the 18-h sampling, the Control, BTH, and MeJ + BTH samples continued to decrease, except for the MeJ-treated samples, which experienced a significant increase in Miyabenol C concentration. After 24 h, all samples showed higher levels of Miyabenol C, with MeJ and MeJ + BTH-treated plants showing statistically significant differences from Control plants. From this point, Miyabenol C concentration decreased in all leaves until the end of the study.
- T-piceid: In Figure 3C, an overall increase in T-piceid concentration was observed in all samples during the first 12 h, with the exception of a slight decrease in the MeJ + BTH group. At the 18-h sampling, this upward trend persisted, particularly notable in the MeJ group, which reached its peak concentration at this point. At 24 h after treatment initiation, all samples continued to show an increase in T-piceid concentration, except for the MeJ group, which began to decrease. At the end of the study, the Control and BTH samples continued to increase T-piceid concentration, while the MeJ group decreased to levels similar to the other groups. In contrast, the MeJ + BTH samples showed a decline to the lowest levels compared to the other treatments.
- Piceatannol: During the first 12 h, Piceatannol concentration increased in all treated plants (Figure 3D) except for the control group. At the 18-h sampling, the MeJ and MeJ + BTH groups continued to show an increase in concentration, contrasting with the control group, which continued to decline, while the MeJ + BTH group began to decrease. In the subsequent sampling (24 h), the MeJ, BTH, and MeJ + BTH samples continued to decrease in Piceatannol concentration, except for the control group, which showed a slight increase. At the end of the study, a general increase in Piceatannol concentration was observed in all samples, being more pronounced in the treated plants. Specifically, the MeJ and MeJ + BTH treatments were statistically distinguishable from the control samples.
- T-Resveratrol: In the first 12 h, an increase in T-resveratrol concentration was observed in all treatments (Figure 3E), with this increase being especially notable in the MeJ and MeJ + BTH samples, which reached concentrations much higher than the other groups. At the 18-h sampling, this compound continued to increase in MeJ and BTH treatments, although to a lesser extent in the latter. Conversely, a marked decrease was recorded in the MeJ + BTH-treated plants, with a slight decrease in the control group. In the 24-h sampling, T-resveratrol concentration dramatically decreased in MeJ-treated samples, while in BTH-treated samples, it also declined but less markedly. Conversely, the control and MeJ + BTH groups showed a slight increase in T-resveratrol concentration. At the end of the study, T-resveratrol concentration levelled across all samples, except in MeJ + BTH samples, which showed the lowest concentration of this compound.
3.4.2. Phenolic Acids
- Coumaroyltartaric Acid and 3-O-p-Coumaroylquinic Acid: The evolution of these two compounds (Figure 3F,G) followed a similar pattern throughout the study, although the signal detected for 3-O-p-Coumaroylquinic acid was considerably higher than that for Coumaroyltartaric acid. In general, during the first 12 h, all samples except for the control group experienced a decrease in the concentration of these two compounds. At the 18-h sampling, this decreasing trend continued, except for the MeJ-treated plants, which reached their maximum concentration in both cases. At 24 h, the control, BTH, and MeJ + BTH groups increased their concentration of Coumaroyltartaric acid and 3-O-p-Coumaroylquinic acid, whereas MeJ-treated samples began to experience a decrease in the concentration of these compounds, similar to the majority of the treatments, until the end of the study (72 h).
- Ellagic Acid Pentoside: At the 12-h sampling, a general decrease in Ellagic acid pentoside concentration was observed across all samples (Figure 3H). After this point, this compound’s concentration continued to decline in most samples, except for MeJ-treated plants, where an increase was observed. Between the 18-h and the 24-h samplings, significant increases were noted in MeJ, BTH, and MeJ + BTH-treated samples. However, concentrations subsequently began to decrease, except in the control samples, which continued to increase. By the end of the study, the Ellagic acid pentoside concentration in the control samples was notably lower than in the other treatments.
- Dihydroxybenzoic acidhexosyl pentoside: At 12 h after the start of the trial, a general increase in the concentration of this compound was observed across all treatments (Figure 3I), except for those treated with MeJ. By the 18-h sampling, control group samples continued to show a significant increase in dihydroxybenzoic acid hexosyl pentoside concentration, with a slight rise also seen in MeJ-treated samples. However, samples from the BTH and MeJ + BTH groups exhibited a slight decrease in concentration. At the 24-h mark, control group samples displayed a notable increase in this compound’s concentration, whereas BTH and MeJ + BTH group samples began to show a slight rise, in contrast to the slight decrease observed in MeJ-treated samples. By the end of the trial, the concentration of dihydroxybenzoic acid hexosyl pentoside in control samples had nearly tripled its initial value. It is worth noting that although samples from the treated groups also experienced an increase in this compound’s concentration, the increase was lower compared to the control group. Specifically, samples from the MeJ + BTH group showed the least variation in concentration from the start of the trial.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsakirakis, A.; Tsatsakis, A.; Tsakalof, A.; Kasiotis, K.; Machera, K.; Charistou, A. Operator Exposure during Fungicide Applications in Vineyards. Toxicol. Lett. 2012, 211, S174. [Google Scholar] [CrossRef]
- Baša-Česnik, H.; Gregorčič, A.; Čuš, F. Pesticide Residues in Grapes from Vineyards Included in Integrated Pest Management in Slovenia. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Comitini, F.; Ciani, M. Grape Berry Yeast Communities: Influence of Fungicide Treatments. Int. J. Food Microbiol. 2013, 161, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Bony, S.; Gillet, C.; Bouchez, A.; Margoum, C.; Devaux, A. Genotoxic Pressure of Vineyard Pesticides in Fish: Field and Mesocosm Surveys. Aquat. Toxicol. 2008, 89, 197–203. [Google Scholar] [CrossRef]
- Belhadj, A.; Saigne, C.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.F.; Mérillon, J.M. Methyl Jasmonate Induces Defense Responses in Grapevine and Triggers Protection against Erysiphe necator. J. Agric. Food Chem. 2006, 54, 9119–9125. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Nascimento, R.; Monteiro, F.; Traquete, F.; Marques, A.P.; Cunha, J.; Eiras-Dias, J.E.; Cordeiro, C.; Figueiredo, A.; et al. Integrating Metabolomics and Targeted Gene Expression to Uncover Potential Biomarkers of Fungal/Oomycetes-Associated Disease Susceptibility in Grapevine. Sci. Rep. 2020, 10, 15688. [Google Scholar] [CrossRef]
- Adrian, M.; Lucio, M.; Roullier-Gall, C.; Héloir, M.-C.; Trouvelot, S.; Daire, X.; Kanawati, B.; Lemaître-Guillier, C.; Poinssot, B.; Gougeon, R.; et al. Metabolic Fingerprint of PS3-Induced Resistance of Grapevine Leaves against Plasmopara Viticola Revealed Differences in Elicitor-Triggered Defenses. Front. Plant Sci. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling Crop Diseases Using Induced Resistance: Challenges for the Future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Namdeo, A. Plant Cell Elicitation for Production of Secondary Metabolites: A Review. Rev. Lit. Arts Am. 2007, 1, 69–79. [Google Scholar]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Klarzynski, O.; Fritig, B. Stimulation of Plant Defense Responses. Comptes Rendus l’Acad. Sci. Ser. III 2001, 324, 953–963. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early Signaling Events Induced by Elicitors of Plant Defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of Methyl Jasmonate and Benzothiadiazole on the Composition of Grape Skin Cell Walls and Wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Application of Elicitors at Two Maturation Stages of Vitis Vinifera L. Cv Monastrell: Changes in Skin Cell Walls. Chemistry 2022, 4, 98–111. [Google Scholar] [CrossRef]
- Tariq, A.; Ahmed, A. Plant Phenolics Production: A Strategy for Biotic Stress Management. In Plant Phenolics in Biotic Stress Management; Springer: Singapore, 2024; pp. 441–454. [Google Scholar] [CrossRef]
- Dev, M.; Dev, V.; Singh, K.P.; Pant, P.; Rawat, S. Role of Phytoalexins in Plant Disease Resistance. In Biorationals and Biopesticides: Pest Management; De Gruyter: Berlin, Germany, 2024; pp. 127–140. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef]
- Xiao, K.; Zhang, H.J.; Xuan, L.J.; Zhang, J.; Xu, Y.M.; Bai, D.L. Stilbenoids: Chemistry and Bioactivities. Stud. Nat. Prod. Chem. 2008, 34, 453–646. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- del Río, J.A.; Gómez, P.; Baídez, A.; Fuster, M.D.; Ortuño, A.; Frías, V. Phenolic Compounds Have a Role in the Defence Mechanism Protecting Grapevine against the Fungi Involved in Petri Disease. Phytopathol. Mediterr. 2004, 43, 8794. [Google Scholar]
- Chitarrini, G.; Soini, E.; Riccadonna, S.; Franceschi, P.; Zulini, L.; Masuero, D.; Vecchione, A.; Stefanini, M.; Di Gaspero, G.; Mattivi, F.; et al. Identification of Biomarkers for Defense Response to Plasmopara viticola in a Resistant Grape Variety. Front. Plant Sci. 2017, 8, 1524. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Fernández, M.J.; Oliva, J.; Tomás-Barberán, F.A. Postharvest UV-C-Irradiated Grapes as a Potential Source for Producing Stilbene-Enriched Red Wines. J. Agric. Food Chem. 2003, 51, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Becatti, E.; Chkaiban, L.; Tonutti, P.; Forcato, C.; Bonghi, C.; Ranier, A.M. Short-Term Postharvest Carbon Dioxide Treatments Induce Selective Molecular and Metabolic Changes in Grape Berries. J. Agric. Food Chem. 2010, 58, 8012–8020. [Google Scholar] [CrossRef] [PubMed]
- De La Hera Orts, M.L.; Martínez-Cutillas, A.; López Roca, J.M.; Pérez-Prieto, L.J.; Gómez-Plaza, E. Effect of Deficit Irrigation on Anthocyanin Content of Monastrell Grapes and Wines. J. Int. Sci. Vigne Vin 2005, 39, 47–55. [Google Scholar] [CrossRef]
- Romero-Azorín, P.; García-García, J.; Fernández-Fernández, J.I.; Gil-Muñoz, R.; del Amor Saavedra, F.; Martínez-Cutillas, A. Improving Berry and Wine Quality Attributes and Vineyard Economic Efficiency by Long-Term Deficit Irrigation Practices under Semiarid Conditions. Sci. Hortic. 2016, 203, 69–85. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Pérez-Álvarez, E.P.; López-Urrea, R.; Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Intrigliolo, D.S.; Gil-Muñoz, R. Effects of Deficit Irrigation with Saline Water on Wine Color and Polyphenolic Composition of Vitis vinifera L. Cv. Monastrell. Sci. Hortic. 2021, 283, 110085. [Google Scholar] [CrossRef]
- Gil-Muñoz; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving Phenolic and Chromatic Characteristics of Monastrell, Merlot and Syrah Wines by Using Methyl Jasmonate and Benzothiadiazole. J. Int. Sci. Vigne Vin 2017, 51, 17–27. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abcisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of Benzothiadiazole and Methyl Jasmonate on the Volatile Compound Composition of Vitis vinifera L. Monastrell Grapes and Wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A.; Gómez-Plaza, E. Increasing Bioactive Phenolic Compounds in Grapes: Response of Six Monastrell Grape Clones to Benzothiadiazole and Methyl Jasmonate Treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Different Response of Proanthocyanidins from Vitis vinifera Cv. Monastrell Depending on Time of Elicitor Application. J. Sci. Food Agric. 2022, 103, 143–151. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Gómez-Martínez, J.C.; Martínez-Jiménez, J.A.; Gil-Muñoz, R. Application of Elicitors in Two Ripening Periods of Vitis vinifera L. Cv Monastrell: Influence on Anthocyanin Concentration of Grapes and Wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef] [PubMed]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and Pre-Fermentative Cold Maceration: Effects on Polyphenol Concentration in Monastrell Grapes and Wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in Plants under Abiotic Stresses: Crosstalk with Other Phytohormones Matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Repka, V.; Fischerová, I.; Šilhárová, K. Methyl Jasmonate Is a Potent Elicitor of Multiple Defense Responses in Grapevine Leaves and Cell-Suspension Cultures. Biol. Plant 2004, 48, 273–283. [Google Scholar] [CrossRef]
- Hamiduzzaman, M.M.; Jakab, G.; Barnavon, L.; Neuhaus, J.M.; Mauch-Mani, B. β-Aminobutyric Acid-Induced Resistance Against Downy Mildew in Grapevine Acts Through the Potentiation of Callose Formation and Jasmonic Acid Signaling. Mol. Plant-Microbe Interact. 2007, 18, 819–829. [Google Scholar] [CrossRef]
- FDA-EPA; 2013. Available online: https://www.federalregister.gov/documents/2013/04/17/2013-08829/methyl-jasmonate-exemption-from-the-requirement-of-a-tolerance (accessed on 1 January 2025).
- Kunz, W.; Schurter, R.; Maetzke, T. The Chemistry of Benzothiadiazole Plant Activators. Pestic. Sci. 1997, 50, 275–282. [Google Scholar] [CrossRef]
- Tomlin, C. The Pesticide Manual: A World Compendium, 10th ed.; British Crop Protection Council: Farnham Surrey, UK; Cambridge, UK, 2013; Volume 51, ISBN 9780948404795. [Google Scholar]
- Scarponi, L.; Buonaurio, R.; Martinetti, L. Persistence and Translocation of a Benzothiadiazole Derivative in Tomato Plants in Relation to Systemic Acquired Resistance against Pseudomonas Syringae Pv Tomato. Pest. Manag. Sci. 2001, 57, 262–268. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Borgo, M.; Ferrara, L.; Faoro, F. Induction of Resistance to Gray Mold with Benzothiadiazole Modifies Amino Acid Profile and Increases Proanthocyanidins in Grape: Primary versus Secondary Metabolism. J. Agric. Food Chem. 2005, 53, 9133–9139. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T.; Nedelcheva, D.V.; Parushev, S.P.; Atanassov, A.I.; Hvarleva, T.D.; Djakova, G.J.; Bankova, V.S.; Popov, S.S. Preliminary Study on Biomarkers for the Fungal Resistance in Vitis vinifera Leaves. J. Plant Physiol. 2008, 165, 791–795. [Google Scholar] [CrossRef]
- Ali, A.; Ali, S.; Husnain, M.; Saad Missen, M.M.; Samad, A.; Khan, M. Detection of Deficiency of Nutrients in Grape Leaves Using Deep Network. Math. Probl. Eng. 2022, 2022, 3114525. [Google Scholar] [CrossRef]
- Ge, X.; Hetzer, B.; Tisch, C.; Kortekamp, A.; Nick, P. Surface Wax in the Ancestral Grapevine Vitis sylvestris Correlate with Partial Resistance to Powdery Mildew. BMC Plant Biol. 2023, 23, 304. [Google Scholar] [CrossRef]
- Burdziej, A.; Da Costa, G.; Gougeon, L.; Le Mao, I.; Bellée, A.; Corio-Costet, M.F.; Mérillon, J.M.; Richard, T.; Szakiel, A.; Cluzet, S. Impact of Different Elicitors on Grapevine Leaf Metabolism Monitored by 1H NMR Spectroscopy. Metabolomics 2019, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Puertas, B.; Fernández, M.I.; Palma, M.; Cantos-Villar, E. Induction of Stilbenes in Grapes by UV-C: Comparison of Different Subspecies of Vitis. Innov. Food Sci. Emerg. Technol. 2010, 11, 231–238. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.; Cueva, C.; Gil-Muñoz, R.; Cenis, J.L.; Bartolomé, B.; Moreno-Arribas, M.V.; Lozano-Pérez, A.A. Preparation, Characterization and Gastrointestinal Stability of Silk Fibroin Nanoparticles Loaded with Red Wine Polyphenols. Food Biosci. 2023, 52, 102431. [Google Scholar] [CrossRef]
- Sumner, L.W.; Alexander, A.E.; Ae, A.; Ae, D.B.; Ae, M.H.B.; Beger, R.; Daykin, C.A.; Teresa, A.E.; Fan, W.-M.; Oliver, A.E.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Martins, V.; Billet, K.; Garcia, A.; Lanoue, A.; Gerós, H. Exogenous Calcium Deflects Grape Berry Metabolism towards the Production of More Stilbenoids and Less Anthocyanins. Food Chem. 2020, 313, 126123. [Google Scholar] [CrossRef] [PubMed]
- Barreto Peixoto, J.A.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Marti, G.; Schnee, S.; Andrey, Y.; Simoes-Pires, C.; Carrupt, P.A.; Wolfender, J.L.; Gindro, K. Study of Leaf Metabolome Modifications Induced by UV-C Radiations in Representative Vitis, Cissus and Cannabis Species by LC-MS Based Metabolomics and Antioxidant Assays. Molecules 2014, 19, 14004–14021. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and Characterization of Chlorogenic Acids, Chlorogenic Acid Glycosides and Flavonoids from Lonicera henryi L. (Caprifoliaceae) Leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Ares, A.M.; González, Y.; Nozal, M.J.; Bernal, J.L.; Higes, M.; Bernal, J. Development and Validation of a Liquid Chromatography with Mass Spectrometry Method to Determine Resveratrol and Piceid Isomers in Beeswax. J. Sep. Sci. 2015, 38, 197–204. [Google Scholar] [CrossRef]
- Malagoli, M.; Sut, S.; Kumar, G.; Dall’Acqua, S. Variations of Elements, Pigments, Amino Acids and Secondary Metabolites in Vitis vinifera (L.) Cv Garganega after 501 Biodynamic Treatment. Chem. Biol. Technol. Agric. 2022, 9, 36. [Google Scholar] [CrossRef]
- Shahaf, N.; Franceschi, P.; Arapitsas, P.; Rogachev, I.; Vrhovsek, U.; Wehrens, R. Constructing a Mass Measurement Error Surface to Improve Automatic Annotations in Liquid Chromatography/Mass Spectrometry Based Metabolomics. Rapid Commun. Mass Spectrom. 2013, 27, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Billet, K.; Malinowska, M.A.; Munsch, T.; Unlubayir, M.; Adler, S.; Delanoue, G.; Lanoue, A. Semi-Targeted Metabolomics to Validate Biomarkers of Grape Downy Mildew Infection Under Field Conditions. Plants 2020, 9, 1008. [Google Scholar] [CrossRef]
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The Influence of Storage on the “Chemical Age” of Red Wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Bick, J.A.; Lange, B.M. Metabolic Cross Talk between Cytosolic and Plastidial Pathways of Isoprenoid Biosynthesis: Unidirectional Transport of Intermediates across the Chloroplast Envelope Membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, S.H.; Park, S.C.; Kim, S.; Kim, T.H.; Lee, J.; Kim, S.W.; Ryu, Y.B.; Jeong, J.C.; Kim, C.Y. Induced Extracellular Production of Stilbenes in Grapevine Cell Culture Medium by Elicitation with Methyl Jasmonate and Stevioside. Bioresour. Bioprocess. 2020, 7, 38. [Google Scholar] [CrossRef]
- Burdziej, A.; Bellée, A.; Bodin, E.; Valls Fonayet, J.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.F. Three Types of Elicitors Induce Grapevine Resistance against Downy Mildew via Common and Specific Immune Responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Ton, J.; Van Loon, L.C. Cross-Talk Between Plant Defence Signalling Pathways: Boost or Burden? AgBiotechNet 2001, 3, 1–8. [Google Scholar]
- Benevenuto, R.F.; Seldal, T.; Hegland, S.J.; Rodriguez-Saona, C.; Kawash, J.; Polashock, J. Transcriptional Profiling of Methyl Jasmonate-Induced Defense Responses in Bilberry (Vaccinium myrtillus L.). BMC Plant Biol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Panda, S.; Kazachkova, Y.; Aharoni, A. Catch-22 in Specialized Metabolism: Balancing Defense and Growth. J. Exp. Bot. 2021, 72, 6027–6041. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Martínez-Márquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Nabavi, S.F.; et al. Phytostilbenes as Agrochemicals: Biosynthesis, Bioactivity, Metabolic Engineering and Biotechnology. Nat. Prod. Rep. 2021, 38, 1282–1329. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, T.; Patanita, M.; Félix, M.d.R.; Albuquerque, A.; Ribeiro, J.A.; Santos, F.; Basaloco, M.; da Rosa, A.M.; Campos, M.D. Organic vs. Integrated-Production Agriculture Farming: Which Grapevine Stress-Responsive Genes Are Affected by the Application of Resistance Inducers and Elicitors? Agronomy 2024, 14, 892. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant and Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619. [Google Scholar] [CrossRef] [PubMed]

| No | tR, min | Compound Name | Chemical Group | Molecular Formula | Level of Identification | Fragments | Wavelength (max. Absorbance) (nm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.923 | Myricetin 3-O-hexocide | Flavanols | C21H19O13- | 3 | 479-317 | 264-353-389 | [50,51] |
| 2 | 7.153 | Gallic acid hexoside | Phenolic Acids | C13H15O10- | 3 | 331 | 276-309 | [50] |
| 3 | 7.292 | unknown | 4 | 282-268 | ||||
| 4 | 7.86 | unknown | 4 | 274 | ||||
| 5 | 8.114 | unknown | 4 | 259 | ||||
| 6 | 8.307 | unknown | 4 | 278 | ||||
| 7 | 8.465 | unknown | 4 | 277-259 | ||||
| 8 | 8.564 | unknown | 4 | 262 | ||||
| 9 | 8.715 | Dihydroxybenzoic acid hexoside | Phenolic Acids | C13H15O9- | 3 | 315-153-135 | 319-241 | [50,52] |
| 10 | 8.879 | unknown | 4 | 263 | ||||
| 11 | 9.04 | unknown | 4 | 268 | ||||
| 12 | 9.265 | unknown | 4 | 277 | ||||
| 13 | 9.395 | unknown | 4 | 287 | ||||
| 14 | 9.543 | Astringin | Stilbenes | C20H22O9- | 3 | 405-243-159 | 324-295 | [53] |
| 15 | 9.942 | Miyabenol C | Stilbenes | C42H32O9- | 3 | 451-359-345 | 329-293-246 | |
| 16 | 10.23 | unknown | 4 | 323-289 | ||||
| 17 | 10.402 | unknown | 4 | 287 | ||||
| 18 | 10.706 | unknown | 4 | 290-312 | ||||
| 19 | 11.011 | unknown | 4 | 264 | ||||
| 20 | 11.3 | Coumaroyltartaric acid | Phenolic Acids | C13H11O8- | 3 | 295-163-140-119 | 311 | [50] |
| 21 | 11.486 | 3-O-p-Coumaroylquinic acid | Phenolic Acids | C16H17O8- | 3 | 337-163-119 | 314-289 | [50] |
| 22 | 11.866 | unknown | 4 | 277 | ||||
| 23 | 12.03 | Feruloyltartaric (fertaric) acid | Phenolic Acids | C14H13O9- | 3 | 325-193-149 | 327-296-249 | [50] |
| 24 | 12.408 | Ellagic acid pentoside | Phenolic Acids | C19H13O12- | 3 | 433-301-257-213 | 284-326 | [50] |
| 25 | 12.676 | Dihydroxybenzoic acidhexosyl pentoside | Phenolic Acids | C18H23O13- | 3 | 447-315-285 | 275 | [50] |
| 26 | 12.876 | Caffeoylshikimic acid | Phenolic Acids | C16H15O8- | 3 | 335-179-135 | 272-260 | [50] |
| 27 | 13.097 | unknown | 4 | 262-354 | ||||
| 28 | 13.346 | unknown | 4 | 272 | ||||
| 29 | 13.683 | unknown | 4 | 258 | ||||
| 30 | 13.786 | Chlorogenic acid hexoside | Phenolic Acids | C22H27O14- | 3 | 299-137-353-341 | 258 | [50,54] |
| 31 | 14.097 | Kaempferol 3-O-hexuronidemethyl ether | Flavanols | C22H19O12- | 3 | 475-327-285 | 259-354 | [50] |
| 32 | 14.392 | unknown | 4 | 264 | ||||
| 33 | 14.601 | T-piceid | Stilbenes | C20H22O8- | 1 | 227-210-225 | 329-302 | [55] |
| 34 | 14.894 | Quercetin | Flavanols | C15H9O7- | 1 | 301-271-179 | 256-355 | [50,56,57] |
| 35 | 15.458 | unknown | 4 | 265-352 | ||||
| 36 | 15.624 | unknown | 4 | 268-294 | ||||
| 37 | 15.916 | unknown | 4 | 265-297-354 | ||||
| 38 | 16.233 | unknown | 4 | 252-321-358 | ||||
| 39 | 16.355 | unknown | 4 | 264-349 | ||||
| 40 | 16.464 | Kaempferol 7-O-hexuronide | Flavanols | C21H17O12- | 3 | 461-285-267-239-241 | 265-347 | [50] |
| 41 | 16.641 | Piceatannol | Stilbenes | C14H12O4- | 1 | 243-159-227-213-202 | 323 | [58] |
| 42 | 16.923 | unknown | 4 | 257-355 | ||||
| 43 | 17.681 | unknown | 4 | 278 | ||||
| 44 | 17.796 | unknown | 4 | 251 | ||||
| 45 | 18.265 | unknown | 4 | 245-329 | ||||
| 46 | 18.956 | unknown | 4 | 288 | ||||
| 47 | 19.836 | T-resveratrol | Stilbenes | C14H11O3- | 1 | 227-185-159-143 | 311-317 | [50,58] |
| 48 | 20.499 | unknown | 4 | 291 | ||||
| 49 | 22.349 | Ellagic acid | 3 | 301 | 298-368 | [57,59] | ||
| 50 | 23.472 | unknown | 4 | 333 |
| Sampling | Generated Discriminant Functions | Percentage Variance Explained | ||
|---|---|---|---|---|
| Function 1 | Function 2 | Function 1 + Function 2 | ||
| 12 h | 3 | 77% Most significant standardised coefficients: - Coumaroyltartaric acid - Ellagic acid pentoside | 20.5% Most significant standardised coefficients: - Dihydroxybenzoic acidhexosyl pentoside - Compound No. 16 (unknown) | 97.5% |
| 18 h | 3 | 97.1% Most significant standardised coefficients: - Astringin - Compuesto nº16 (desconocido) | 2.9% Most significant standardised coefficients: - 3-O-p-Coumaroylquinic acid - Astringin | 100% |
| 24 h | 3 | 93.5% Most significant standardised coefficients: - Miyabenol - 3-O-p-Coumaroylquinic acid | 6.2% Most significant standardised coefficients: - Astringin - Miyabenol | 99.7% |
| 72 h | 3 | 88.3% Most significant standardised coefficients: - Astringin - 3-O-p-Coumaroylquinic acid | 11.2% Most significant standardised coefficients: - Astringin - Miyabenol | 99.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladines-Quezada, D.; Gil-Muñoz, R. Metabolic Response Induced by Methyl Jasmonate and Benzothiadiazole in Vitis vinifera cv. Monastrell Seedlings. Horticulturae 2025, 11, 277. https://doi.org/10.3390/horticulturae11030277
Paladines-Quezada D, Gil-Muñoz R. Metabolic Response Induced by Methyl Jasmonate and Benzothiadiazole in Vitis vinifera cv. Monastrell Seedlings. Horticulturae. 2025; 11(3):277. https://doi.org/10.3390/horticulturae11030277
Chicago/Turabian StylePaladines-Quezada, Diego, and Rocío Gil-Muñoz. 2025. "Metabolic Response Induced by Methyl Jasmonate and Benzothiadiazole in Vitis vinifera cv. Monastrell Seedlings" Horticulturae 11, no. 3: 277. https://doi.org/10.3390/horticulturae11030277
APA StylePaladines-Quezada, D., & Gil-Muñoz, R. (2025). Metabolic Response Induced by Methyl Jasmonate and Benzothiadiazole in Vitis vinifera cv. Monastrell Seedlings. Horticulturae, 11(3), 277. https://doi.org/10.3390/horticulturae11030277








