In Vitro Propagation of Peumus boldus Molina Using a Temporary Immersion System
Abstract
1. Introduction
2. Materials and Methods
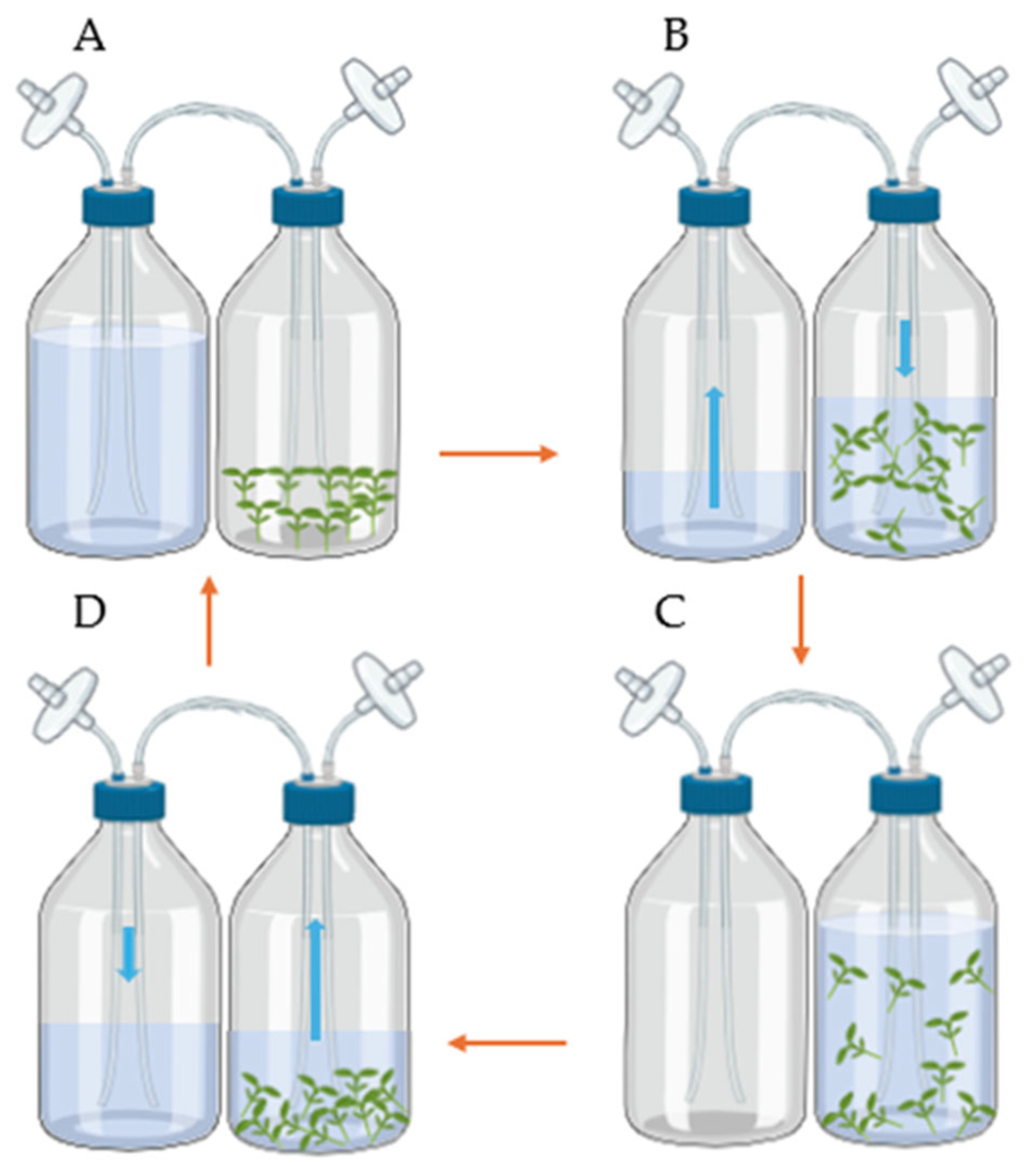
2.1. In Vitro Multiplication Using a Temporary Immersion System (TIS)
2.2. Ex Vitro Rooting
2.3. Ex Vitro Acclimatization
- Cold Greenhouse: Plantlets were placed on a bench with basal heating (25 °C) within a cold greenhouse. Temperatures inside the greenhouse fluctuated between 18 and 30 °C.
- Growth Chamber: Plantlets were maintained in a growth chamber at 25 ± 1 °C under a 16 h photoperiod with a light intensity of 400–700 nm (PPFD 9.96 μmol m−2 s−1) (fluorescent lamps Philips TL-D 36W/54 brand) (Shanghai, China).
3. Results
3.1. In Vitro Multiplication Using a Temporary Immersion System (TIS)
3.2. Ex Vitro Rooting
3.3. Ex Vitro Acclimatization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grau, J.; Zizka, G. Chiles Pflanzenwelt; Palmengarten Sonderheft 19: Fráncfort del Meno, Germany, 1992; p. 42. [Google Scholar]
- Montenegro, G. Chile Nuestra Flora útil. Guía Para Uso Apícola, Medicinal, Folclórica, Artesanal y Ornamental; Universidad Católica de Chile: Santiago, Chile, 2000; p. 267. [Google Scholar]
- Doll, U.; Aedo, D.; Lopez, P. Caracterización morfológica de tres procedencias de boldo (Peumus boldus) en una plantación joven de 6 años. Bosque 2005, 26, 45–54. [Google Scholar] [CrossRef]
- Mcwethy, D.B.; Pauchard, A.; García, R.A.; Holz, A.; González, M.E.; Veblen, T.T.; Stahl, J.; Currey, B. Landscape drivers of recent fire activity (2001–2017) in south-central chile. PLoS ONE 2018, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Farga, C.; Lastra, J.; Veghazi, E. Plantas Medicinales de Uso Común en Chile, 3rd ed.; Fundación Claudio Gray: Santiago, Chile, 2003; p. 275. [Google Scholar]
- Barros, S.; Benedetti, S. Boldo (Peumus boldus Mol.) Rescate de Un Patrimonio Forestal Chileno. Manejo Sustentable y Valorización de Sus Productos; Instituto Forestal: Santiago, Chile, 2011; p. 235. [Google Scholar]
- Salehi, B.; Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.; Delporte, C.; Barra, G.; Cazar, M.E.; López, M.; Ramírez-Alarcón, K.; Cruz-Martins, N.; et al. Ethnopharmacology, phytochemistry and biological activities of native chilean plants. Curr. Pharm. Des. 2020, 27, 953–970. [Google Scholar] [CrossRef]
- Speisky, H.; Cassels, B. Boldo and boldine: An emerging case of natural drug development. Pharmacol. Res. 1994, 29, 1–12. [Google Scholar] [CrossRef]
- Kubinova, R.; Machala, M.; Minksova, K.; Neca, J.; Suchý, V. Chemoprotective activity of boldine: Modulation of drug-metabolizing enzymes. Pharmazie 2001, 56, 242–243. [Google Scholar]
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its antioxidant or health-promoting properties. Chem. Biol. Interact 2006, 159, 1–17. [Google Scholar] [CrossRef]
- Verdeguer, M.; García-Rellán, D.; Boira, H.; Pérez, E.; Gandolfo, S.; Blázquez, M. Herbicidal activity of Peumus boldus and Drimys winteri essential oils from Chile. Molecules 2011, 16, 403–411. [Google Scholar] [CrossRef]
- Uquiche, E.; Huerta, E.; Sandoval, A.; Del Valle, J.M. Effect of boldo (Peumus boldus M.) pretreatment on kinetics of supercritical CO2 extraction of essential oil. J. Food Eng. 2012, 109, 230–237. [Google Scholar] [CrossRef]
- Soto, C.; Caballero, E.; Pérez, E.; Zúñiga, M.E. Effect of extraction conditions on total phenolic content and antioxidant capacity of pretreated wild Peumus boldus leaves from Chile. Food Bioprod. Process. 2014, 92, 328–333. [Google Scholar] [CrossRef]
- Castro-Saavedra, S.; Fuentes-Barros, G.; Tirapegui, C.; Acevedo-Fuentes, W.; Cassels, B.K.; Barriga, A.; Vilches-Herrera, M. Phytochemical analysis of alkaloids from the chilean endemic tree Cryptocarya alba. J. Chil. Chem. Soc. 2016, 61, 3076–3080. [Google Scholar] [CrossRef]
- Alaniz, A.J.; Smith-Ramírez, C.; Rendón-Funes, A.; Hidalgo-Corrotea, C.; Carvajal, M.A.; Vergara, P.M.; Fuentes, N. Multiscale spatial analysis of headwater vulnerability in South-Central Chile reveals a high threat due to deforestation and climate change. Sci. Total. Environ. 2022, 849, 157930. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Rasmilic, I.; San Martín, J.; Doll, U.; González, B. Plantas Medicinales Chilenas. Experiencias de Domesticación y Cultivo de Boldo, Matico, Bailahuén, Canelo, Peumo y Maqui; Universidad de Talca: Talca, Chile, 2005; p. 194. [Google Scholar]
- Lara, A.; Little, C.; Urrutia, R.; Mcphee, J.; Alvarez-Garretón, C.; Oyarzún, C.; Soto, D.; Donoso, P.; Nahuelhual, L.; Pino, M.; et al. Assessment of ecosystem services as an opportunity for the conservation and management of native forests in Chile. For. Ecol. Manag. 2009, 258, 415–424. [Google Scholar] [CrossRef]
- Castañeda, L.E.; Godoy, K.; Manzano, M.; Marquet, P.A.; Barbosa, O. Comparison of soil microbial communities inhabiting vineyards and native sclerophyllous forests in central Chile. Ecol. Evol. 2015, 5, 3857–3868. [Google Scholar] [CrossRef]
- Santelices, R.; Bobadilla, S. Arraigamiento de estacas de Quillaja saponaria Mol. y Peumus boldus Mol. Rev. Bosque 1997, 18, 77–85. [Google Scholar] [CrossRef]
- Figueroa, J.; Jaksic, F. Latencia y banco de semillas en plantas de la región mediterránea de Chile central. Rev. Chil. Hist. Nat. 2004, 77, 201–215. [Google Scholar] [CrossRef]
- Guerra, F.; Badilla, L.; Cautín, R.; Castro, M. In Vitro Propagation of Peumus boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile. Horticulturae 2023, 9, 1032. [Google Scholar] [CrossRef]
- Ríos, D.; Sandoval, D.; Gómez, D. In vitro culture of Peumus boldus Molina via direct organogenesis. J. Med. Chem. 2010, 2, 70–72. [Google Scholar]
- Koch, Z.; González, J.; Benedetti, S. Regeneración de plantas in vitro de Peumus boldus Mol. (Boldo) mediante organogénesis de brotes epicórmicos de árboles maduros. Cienc. Investig. For. 2018, 24, 57–74. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary Immersion System for Micropropagation of Tree Species: A Bibliographic and Systematic Review. Not. Bot. Horti Agrobot. 2019, 47, 269–277. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Grąbkowska, R.; Piątczak, E. Plant Liquid Cultures as a Source of Bioactive Metabolites. Plant Cell Tissue Differ. Second. Metab. Fundam. Appl. 2021, 743–771. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Aguilar, M.E.; Ortiz, J.L.; Mesén, F.; Jiménez, L.D.; Altmann, F. Cafe arabica Coffea arabica L. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants: Volume II; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 39–62. [Google Scholar]
- Barry-Etienne, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Comparison of somatic embryogenesis-derived coffee (Coffea arabica L.) plantlets regenerated in vitro or ex vitro: Morphological, mineral and water characteristics. Ann. Bot. 2002, 90, 77–85. [Google Scholar] [CrossRef] [PubMed]
- McAlister, B.; Finnie, J.; Watt, M.P.; Blakeway, F. Use of temporary immersion system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). Plant Cell Tissue Organ Cult. 2005, 81, 347–358. [Google Scholar] [CrossRef]
- Debnath, S.C. A scale-up system for lowbush blueberry micropropagation using a bioreactor. HortScience 2009, 44, 1962–1966. [Google Scholar] [CrossRef]
- Moreno, R.J.; Morales, A.V.; Daquinta, M.; Gómez, L. Towards scaling-up the micropropagation of Juglans major (Torrey) Heller var. 209 x J. regia L., a hybrid walnut of commercial interest. In Proceedings of the IUFRO Working Party 2.09.02 Conference “Integrating vegetative propagation, biotechnologies and genetic improvement for tree production and sustainable forest management”, Brno, Czech Republic, 25–28 June 2012; pp. 80–91. [Google Scholar]
- Akdemir, H.; Süzerer, V.; Onay, A.; Tilkat, E.; Ersali, Y.; Çiftçi, Y.O. Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ Cult. 2014, 117, 65–76. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Hahn, E.J.; Yoon, Y.S.; Paek, K.Y. Micropropagation of apple root stock ‘M9 EMLA’ using bioreactor. J. Hortic. Sci. Biotechnol. 2003, 78, 377–388. [Google Scholar] [CrossRef]
- Zhu, L.H.; Li, X.Y.; Welander, M. Optimisation of growing conditions of the apple rootstock M26 grown in RITA® containers using temporary immersion principle. Plant Cell Tissue Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 651–656. [Google Scholar] [CrossRef]
- Roels, S.; Noceda, C.; Escalona, M.; Sandoval, J.; Canal-Villanueva, M.J.F.; Rodriguez, R.; DeBergh, P. The effect of headspace renewal in a Temporary Immersion Bioreactor on plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tissue Organ. 2005, 84, 155–163. [Google Scholar] [CrossRef]




| Immersion Duration | Proliferation Rate | Shoot Length (cm) |
|---|---|---|
| 1 min | 3.2 ± 0.3 c * | 6.1 ± 0.3 a * |
| 2 min | 7.2 ± 0.2 b | 6.4 ± 0.3 a |
| 3 min | 10.8 ± 0.3 a | 4.0 ± 0.2 b |
| F Immersion Duration | p = 0.000 | p = 0.000 |
| F Immersion Frequency | p = 0.510 | p = 0.861 |
| F Immersion Duration × Immersion Frequency | p = 0.441 | p = 0.620 |
| Treatment | IBA Concentration | % Rooting |
|---|---|---|
| IBA1 | 0 | 17.4 ± 3.1 d * |
| IBA2 | 492 μM | 36.4 ± 4.3 c |
| IBA3 | 984 μM | 47.1 ± 4.1 b |
| IBA4 | 1476 μM | 75.4 ± 4.1 a |
| F Substrate | p = 0.349 | |
| F IBA | p = 0.000 | |
| F Substrate × IBA | p = 0.697 | |
| Substrate | Acclimatization Treatment | % Acclimatization |
|---|---|---|
| Perlite | Bench with basal heat | 77.1 ± 4.1 a |
| Perlite | Growth chamber | 77.1 ± 4.2 a |
| Vermiculite | Bench with basal heat | 80.0 ± 3.1 a |
| Vermiculite | Growth chamber | 83.3 ± 4.3 a |
| Peat moss | Bench with basal heat | 80.0 ± 3.2 a |
| Peat moss | Growth chamber | 80.0 ± 3.0 a |
| Peat moss, perlite, and vermiculite (1:1:1 v/v/v) | Bench with basal heat | 80.0 ± 3.1 a |
| Peat moss, perlite, and vermiculite (1:1:1 v/v/v) | Growth chamber | 80.0 ± 3.0 a |
| F Substrate | p = 0.740 | |
| F Acclimatization condition | p= 0.800 | |
| F Substrate x Acclimatization condition | p = 0.977 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, F.; Badilla, L.; Cautín, R.; Castro, M. In Vitro Propagation of Peumus boldus Molina Using a Temporary Immersion System. Horticulturae 2025, 11, 142. https://doi.org/10.3390/horticulturae11020142
Guerra F, Badilla L, Cautín R, Castro M. In Vitro Propagation of Peumus boldus Molina Using a Temporary Immersion System. Horticulturae. 2025; 11(2):142. https://doi.org/10.3390/horticulturae11020142
Chicago/Turabian StyleGuerra, Francesca, Loreto Badilla, Ricardo Cautín, and Mónica Castro. 2025. "In Vitro Propagation of Peumus boldus Molina Using a Temporary Immersion System" Horticulturae 11, no. 2: 142. https://doi.org/10.3390/horticulturae11020142
APA StyleGuerra, F., Badilla, L., Cautín, R., & Castro, M. (2025). In Vitro Propagation of Peumus boldus Molina Using a Temporary Immersion System. Horticulturae, 11(2), 142. https://doi.org/10.3390/horticulturae11020142








