Differential Metabolite Analysis of Anthocyanins in Variously Colored Petal Types During Different Developmental Stages of Sophora japonica L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Plant Materials
2.2. Measurement of Petal Color Difference Values
2.3. Extraction of S. japonica ‘AM’ Anthocyanin Metabolite
2.4. Conditions of UPLC-MS/MS Analysis
2.5. Quantitative and Qualitative Metabolite Analysis
2.6. Data Analysis
3. Results
3.1. Analysis of Floral Color Phenotypic Differences
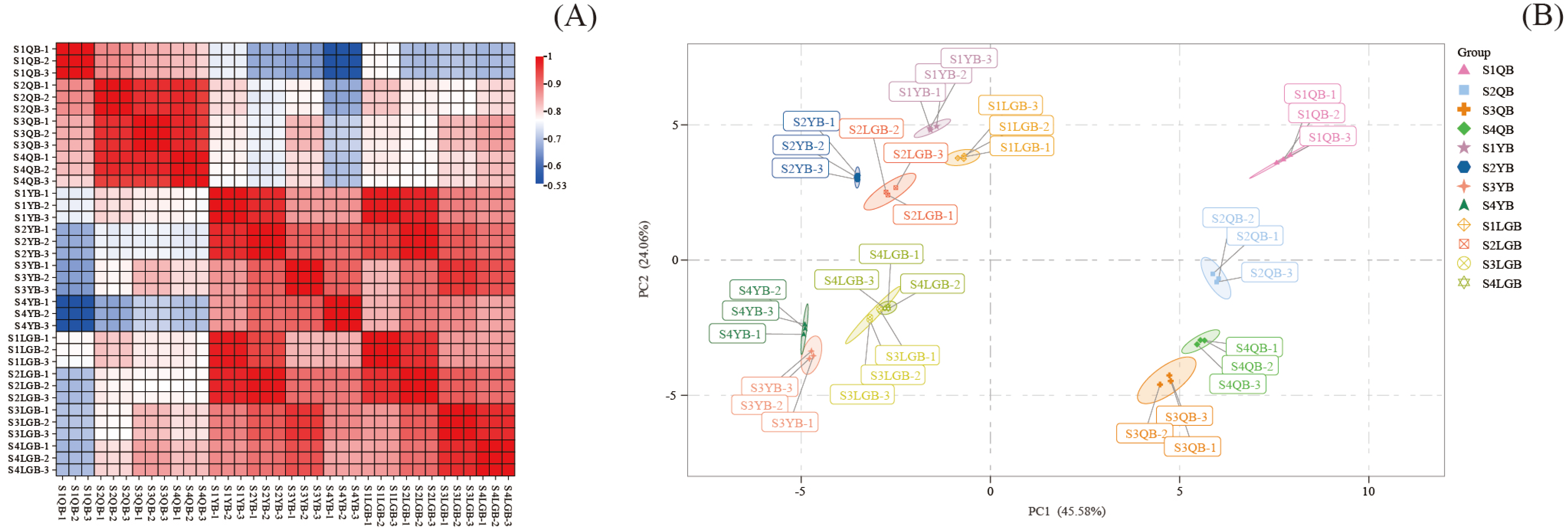
3.2. Analysis of Sample Quality Control
3.3. OPLS-DA
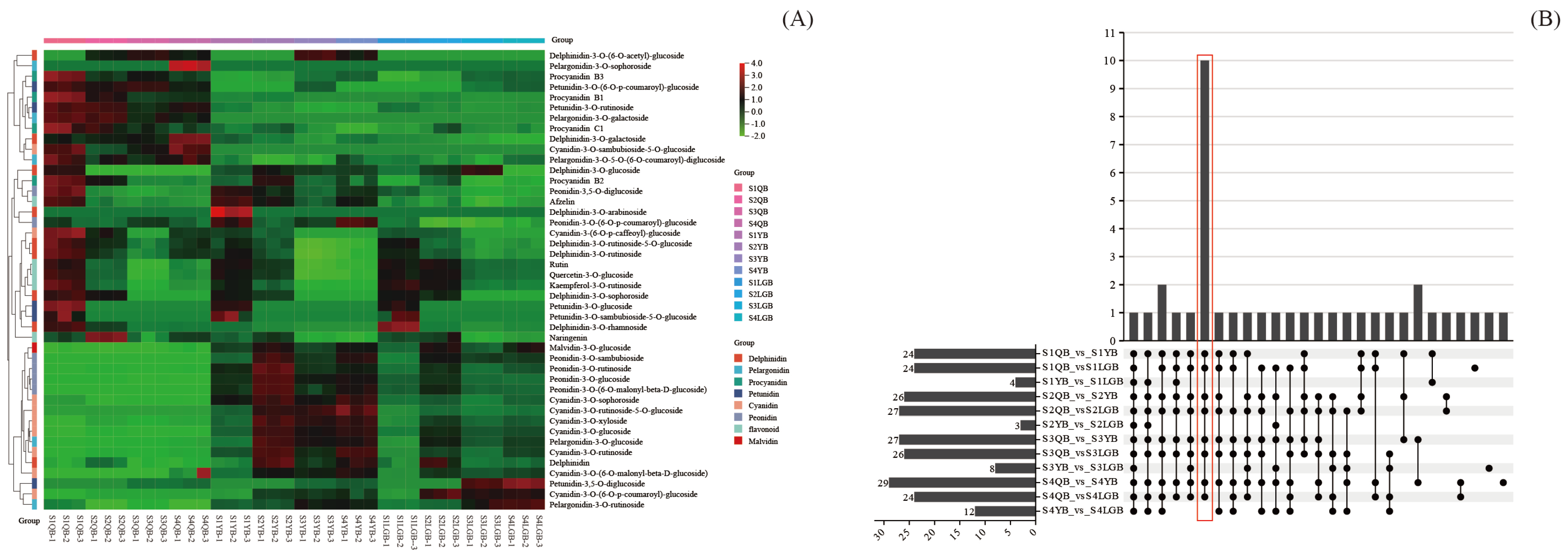
3.4. Cluster Analysis of Anthocyanin-like Metabolites
3.5. Screening for Differential Metabolites
3.6. Specific Analysis of Metabolites Affecting Petal Color in Different Types of S. japonica ‘AM’
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Peng, Z.; Liu, Y. Phenotypic difference and comprehensive evaluation of Sophora japonica in Beijing urban area. J. Beijing For. Univ. 2022, 44, 23–33. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Z. Insights into history culture and value of Sophora japonica. J. Beijing For. Univ. (Soc. Sci.) 2018, 17, 23–31. [Google Scholar] [CrossRef]
- Qu, B.; Liu, Y.; Sun, X.; Li, S.; Wang, X.; Xiong, K.; Yun, B.; Zhang, H. Effect of various mulches on soil physico—Chemical properties and tree growth (Sophora japonica) in urban tree pits. PLoS ONE 2019, 14, e0210777. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Qu, L.; Ma, K.; Lin, L. Effects of road dust on the growth characteristics of Sophora japonica L. seedlings. J. Environ. Sci. 2016, 46, 147–155. [Google Scholar] [CrossRef]
- Zan, W.; Wu, Q.; Dou, S.; Wang, Y.; Zhu, Z.; Xing, S.; Yu, Y. Analysis of flower color diversity revealed the co-regulation of cyanidin and peonidin in the red petals coloration of Rosa rugosa. Plant Physiol. Biochem. 2024, 216, 109126. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, Y.J.; Gao, F.P.; Li, Y.K.; Sun, W. Advances in the biosynthesis and influencing factors of anthocyanins. Jiangsu J. Agric. Sci. 2019, 35, 1246–1253. [Google Scholar] [CrossRef]
- Li, L.; Zhai, Y.; Luo, X.; Zhang, Y.; Shi, Q. Comparative transcriptome analyses reveal genes related to pigmentation in the petals of red and white Primula vulgaris cultivars. Physiol. Mol. Biol. Plants 2019, 25, 1029–1041. [Google Scholar] [CrossRef]
- Sun, W.; Yan, Y.; Muhammad, Z.; Zhang, G. Transcriptomic analysis reveals the formation mechanism of anthocyanins light-independent synthesis in Chrysanthemum. Tech Sci. Press 2024, 10, 791. [Google Scholar] [CrossRef]
- Fu, M.; Yang, X.; Zheng, J.; Wang, L.; Yang, X.; Tu, Y.; Ye, J.; Zhang, W.; Liao, Y.; Cheng, S.; et al. Unraveling the regulatory mechanism of color diversity in Camellia japonica petals by integrative transcriptome and metabolome analysis. Front. Plant Sci. 2021, 12, 685136. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, P.; Jiang, Y.; Li, J.; Chang, J.; Zhang, H.; Shao, H.; Zhou, Y. Integrated metabolome and transcriptome analysis of petal anthocyanin accumulation mechanism in Gloriosa superba ‘Rothschildiana’ during different flower development stages. Int. J. Mol. Sci. 2023, 24, 15034. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, G.; Sang, L.; Deng, Y.; Gao, L.; Yu, Y.; Liu, J. Mitochondrial citrate synthase plays important roles in anthocyanin synthesis in petunia. Plant Sci. Int. J. Exp. Plant Biol. 2021, 305, 110835. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of anthocyanin biosynthesis. Acta Hortic. Sin. 2013, 40, 2295–2306. [Google Scholar] [CrossRef]
- Sun, C.; Huang, H.; Xu, C.; Li, X.; Chen, K. Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): A Review. Plant Foods Hum. Nutr. 2013, 68, 97–106. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Augustijn, D.; De Groot, H.J.M.; Alia, A. HR-MAS NMR applications in plant metabolomics. Molecules 2021, 26, 931. [Google Scholar] [CrossRef]
- Ramautar, R. CE-MS in metabolomics: Status quo and the way forward. Bioanalysis 2016, 8, 371–374. [Google Scholar] [CrossRef]
- Rontani, J.-F. Use of gas chromatography-mass spectrometry techniques (GC-MS, GC-MS/MS and GC-QTOF) for the characterization of photooxidation and autoxidation products of lipids of autotrophic organisms in environmental samples. Molecules 2022, 27, 1629. [Google Scholar] [CrossRef]
- Wu, W.; Jiao, C.; Li, H.; Ma, Y.; Jiao, L.; Liu, S. LC-MS based metabolic and metabonomic studies of Panax ginseng. Phytochem. Anal. PCA 2018, 29, 331–340. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; Shi, T.; Chen, M.; Li, Y.; Du, J.; Jiang, H.; Yang, X.; Hu, H.; Wang, L. Integrating transcriptomic and GC-MS metabolomic analysis to characterize color and aroma formation during tepal development in Lycoris longituba. Plants 2019, 8, 53. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.-W.; Liu, J.-G.; Chen, T.; Shi, Y.; Qi, Y.; Xiao, S. Target metabolomic analysis of material composition using a pair of near isogenic lines with different floral colors in sunflower. J. Nanjing Agric. Univ. 2021, 44, 437–446. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. Metabolite profiling reveals distinct modulation of complex metabolic networks in non-pigmented, black, and red rice (Oryza sativa L.) cultivars. Metabolites 2021, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of flavonoids and carotenoids and their contributions to various colors of rose cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 123. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, S.; Liu, X.; Su, L.; Wu, Y.; Zhang, W.; Li, Y.; Jiang, Y.; Wang, H.; Fu, R.; et al. SlWRKY35 positively regulates carotenoid biosynthesis by activating the MEP pathway in tomato fruit. New Phytol. 2022, 234, 164–178. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Yang, W.; Pan, Q.; Li, C.; Sun, Q.; Zeng, Q.; Li, B.; Zhang, L. Comparative metabolic study of two contrasting Chinese cabbage genotypes under mild and severe drought stress. Int. J. Mol. Sci. 2022, 23, 5947. [Google Scholar] [CrossRef]
- Kanehisa, M.S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhu, Z.; Xu, Z.; Qi, S.; Xing, S.; Yu, Y.; Wu, Q. Transcriptome and chemical analyses revealed the mechanism of flower color formation in Rosa rugosa. Front. Plant Sci. 2022, 13, 1021521. [Google Scholar] [CrossRef]
- Horiuchi, R.; Nishizaki, Y.; Okawa, N.; Ogino, A.; Sasaki, N. Identification of the biosynthetic pathway for anthocyanin triglucoside, the precursor of polyacylated anthocyanin, in red cabbage. J. Agric. Food Chem. 2020, 68, 9750–9758. [Google Scholar] [CrossRef]
- Tian, J.; Xu, R.; Chang, K.; Yuan, S.; Huang, C.; Wang, J.; Li, S.; Liu, F.; Zhong, F. Identification of PAL gene in purple cabbage and functional analysis related to anthocyanin synthesis. Horticulturae 2023, 9, 469. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Yusuyin, A.; Xu, Y.; Yuan, H.; Liu, C. Transcriptome analysis provides insights into anthocyanin synthesis in Blueberry. Horticulturae 2023, 9, 1036. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, Y.; Xu, Y.; Shi, Y.; Tang, D. Anthocyanin profiles in petals of different hyacinthus orientalis. Acta Hortic. Sin. 2015, 42, 301–310. [Google Scholar] [CrossRef]
- Yu, J.-J.; Tang, D.-Q.; Li, X. Anthocyanin compositions in petals of Freesia hybrida. Guihaia 2020, 40, 687–695. [Google Scholar] [CrossRef]
- Lan, W.; Xue-Qin, W.; Hao, Z. Analysis of flavonoids of flower of Robiniapseu Doacacia L. by HPLC-DAD-ESI-MS/MS. Food Drug 2013, 15, 240–242. [Google Scholar]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. Cell Mol. Biol. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef]
- Xue, L. Study on Germplasm Innovation and Molecular Mechanism of Flower Color Formation in Red-Flowered Strawberry; Shengyang Agricultural University: Shenyang, China, 2016. [Google Scholar]
- Liu, G.; Liu, H.; Shi, G.; Xu, N.; Niu, Z.; Wang, L.; Zhao, R.; Wang, L.; Fan, L. Multi-omics analysis of Iris sanguinea with distinctive flower colors provides insights into petal coloration. Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Du, H.; Lai, L.; Wang, F.; Sun, W.; Zhang, L.; Li, X.; Wang, L.; Jiang, L.; Zheng, Y. Characterisation of flower colouration in 30 Rhododendron species via anthocyanin and flavonol identification and quantitative traits. Plant Biol. 2018, 20, 121–129. [Google Scholar] [CrossRef]
- Shao, D.; Liang, Q.; Wang, X.; Zhu, Q.-H.; Liu, F.; Li, Y.; Zhang, X.; Yang, Y.; Sun, J.; Xue, F. Comparative metabolome and transcriptome analysis of anthocyanin biosynthesis in white and pink petals of cotton (Gossypium hirsutum L.). Int. J. Mol. Sci. 2022, 23, 10137. [Google Scholar] [CrossRef]
- Zhou, C.; Mei, X.; Rothenberg, D.O.; Yang, Z.; Zhang, W.; Wan, S.; Yang, H.; Zhang, L. Metabolome and transcriptome analysis reveals putative genes involved in anthocyanin accumulation and coloration in white and pink tea (Camellia sinensis) flower. Molecules 2020, 25, 190. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, R.; Zhang, T.; Wang, H.; Gao, J.; Li, Y.; Jiang, H.; Wang, L.; Yuan, X.; Li, Y.; et al. Identification of F3’5’H Gene in petunia and the roles in petal coloration. J. Henan Agric. Sci. 2021, 50, 121–127. [Google Scholar] [CrossRef]
- Abid, M.A.; Wei, Y.; Meng, Z.; Wang, Y.; Ye, Y.; Wang, Y.; He, H.; Zhou, Q.; Li, Y.; Wang, P.; et al. Increasing floral visitation and hybrid seed production mediated by beauty mark in Gossypium hirsutum. Plant Biotechnol. J. 2022, 20, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-F.; Zhang, Q.-X.; Cheng, T.-R.; Yan, X.-L.; Pan, H.-T.; Wang, J. Substantial epigenetic variation causing flower color chimerism in the ornamental tree Prunus mume revealed by single base resolution methylome detection and transcriptome sequencing. Int. J. Mol. Sci. 2018, 19, 2315. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.-Y.; Wang, S.-L.; Niu, X.-Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol. Biochem. 2016, 104, 250–256. [Google Scholar] [CrossRef]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Guo, L.-P. Transcriptome Analysis Revealing Flower Coloration of Sophora japonica Linn. and Function Analysis for Key Genes Involved in Anthycayanin Accumulation; Beijing Forestry University: Beijing, China, 2020. [Google Scholar]
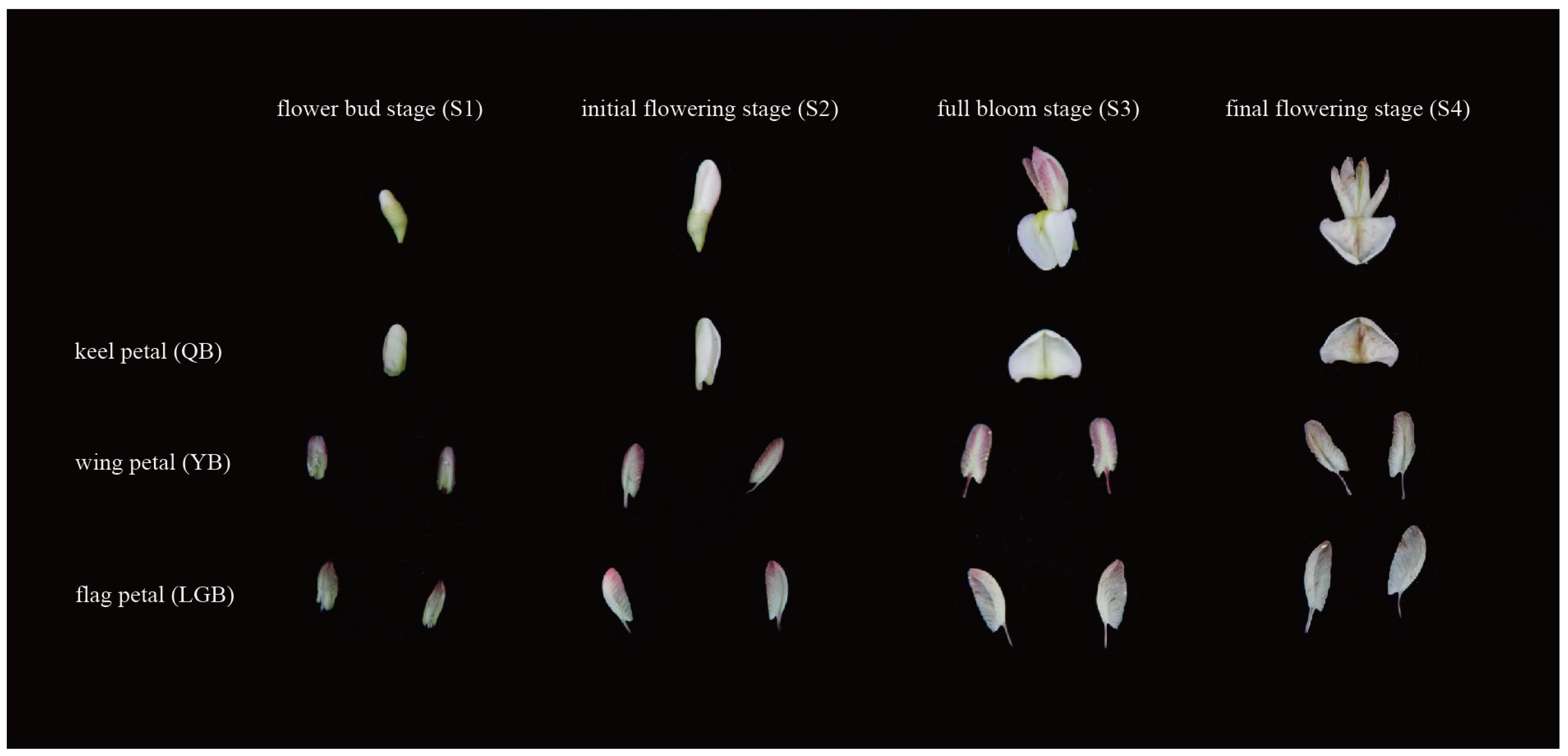


| Petal Type | L* | a* | b* |
|---|---|---|---|
| S1QB | 90.76 ± 0.46 ab | −2.62 ± 0.09 e | 37.31 ± 1.54 a |
| S2QB | 83.60 ± 4.60 cd | −3.16 ± 0.99 e | 32.58 ± 2.03 b |
| S3QB | 80.74 ± 3.49 de | −2.04 ± 0.79 e | 27.34 ± 1.59 c |
| S4QB | 85.05 ± 2.15 cd | −3.64 ± 1.08 e | 21.57 ± 1.87 d |
| S1YB | 80.02 ± 1.73 def | 1.29 ± 3.54 cd | 17.42 ± 0.27 ef |
| S2YB | 71.93 ± 1.74 h | 7.46 ± 0.03 a | 17.06 ± 1.79 f |
| S3YB | 74.07 ± 2.38 gh | 5.87 ± 0.92 ab | 18.12 ± 1.43 ef |
| S4YB | 86.52 ± 2.73 bc | 3.36 ± 1.13 bc | 22.96 ± 0.93 d |
| S1LGB | 84.32 ± 3.81 cd | −1.26 ± 2.92 de | 32.43 ± 2.69 b |
| S2LGB | 74.91 ± 2.23 fgh | 3.82 ± 0.56 bc | 28.71 ± 0.69 c |
| S3LGB | 77.62 ± 1.89 ef | 4.06 ± 0.52 bc | 20.48 ± 2.23 de |
| S4LGB | 93.03 ± 1.06 a | −3.59 ± 0.14 e | 20.52 ± 0.96 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, L.; Ji, X.; Sun, T.; Mu, Y.; Wang, Y.; Han, Y.; Sun, Y.; Li, X.; Xie, K.; Zhang, S.; et al. Differential Metabolite Analysis of Anthocyanins in Variously Colored Petal Types During Different Developmental Stages of Sophora japonica L. Horticulturae 2025, 11, 143. https://doi.org/10.3390/horticulturae11020143
Guan L, Ji X, Sun T, Mu Y, Wang Y, Han Y, Sun Y, Li X, Xie K, Zhang S, et al. Differential Metabolite Analysis of Anthocyanins in Variously Colored Petal Types During Different Developmental Stages of Sophora japonica L. Horticulturae. 2025; 11(2):143. https://doi.org/10.3390/horticulturae11020143
Chicago/Turabian StyleGuan, Lingshan, Xinyue Ji, Tao Sun, Yanjuan Mu, Yan Wang, Yi Han, Yanguo Sun, Xinhui Li, Kongan Xie, Shuxin Zhang, and et al. 2025. "Differential Metabolite Analysis of Anthocyanins in Variously Colored Petal Types During Different Developmental Stages of Sophora japonica L." Horticulturae 11, no. 2: 143. https://doi.org/10.3390/horticulturae11020143
APA StyleGuan, L., Ji, X., Sun, T., Mu, Y., Wang, Y., Han, Y., Sun, Y., Li, X., Xie, K., Zhang, S., Song, C., & Lu, Y. (2025). Differential Metabolite Analysis of Anthocyanins in Variously Colored Petal Types During Different Developmental Stages of Sophora japonica L. Horticulturae, 11(2), 143. https://doi.org/10.3390/horticulturae11020143






