Novel Alleles of the Potato Leaf Gene Identified in Italian Traditional Varieties Conferring Potato-like Leaf Shape in Tomato
Abstract
1. Introduction
2. Materials and Methods
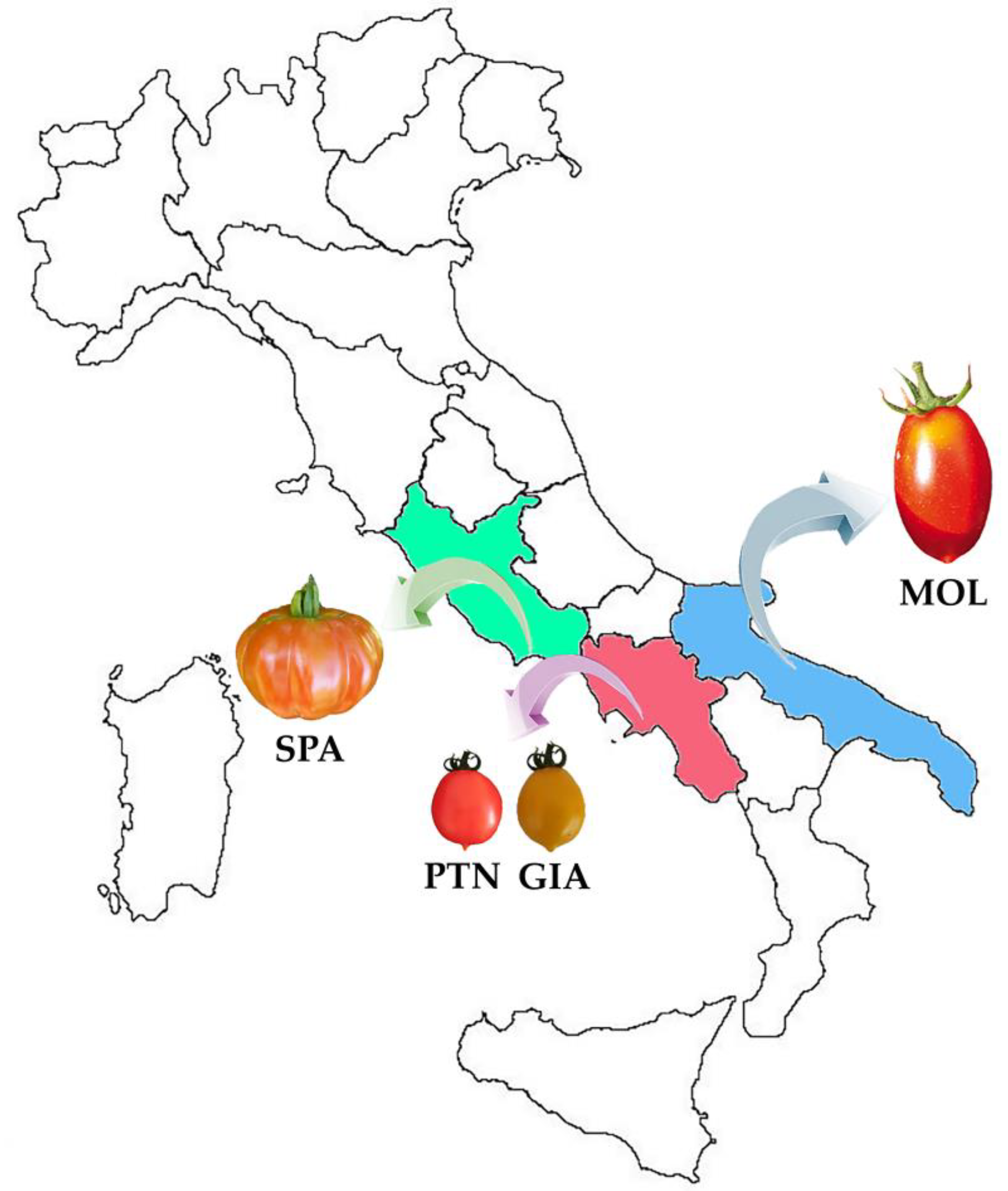
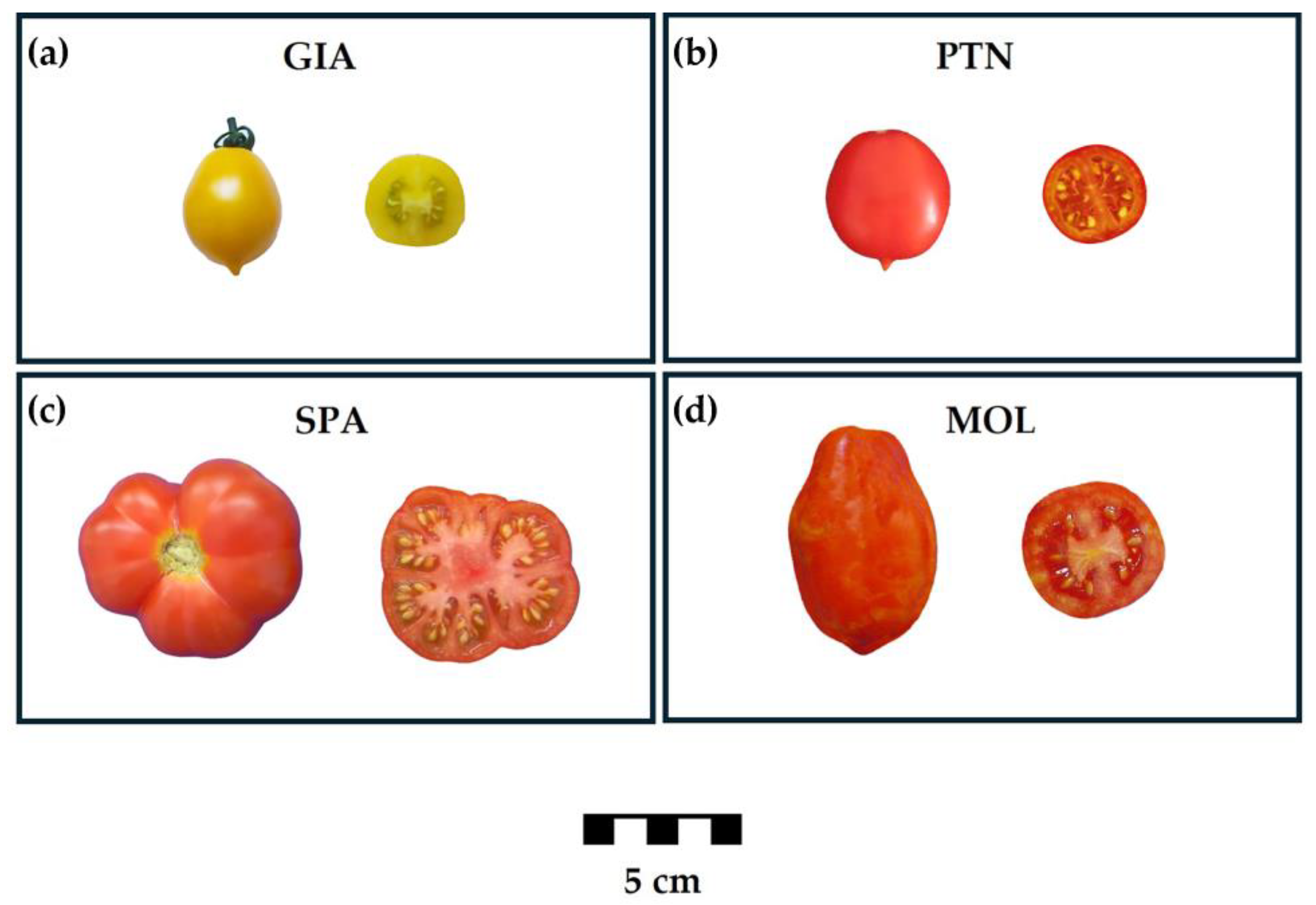
2.1. Plant Material
2.2. Growth Conditions and Allelism Test
2.3. DNA Extraction
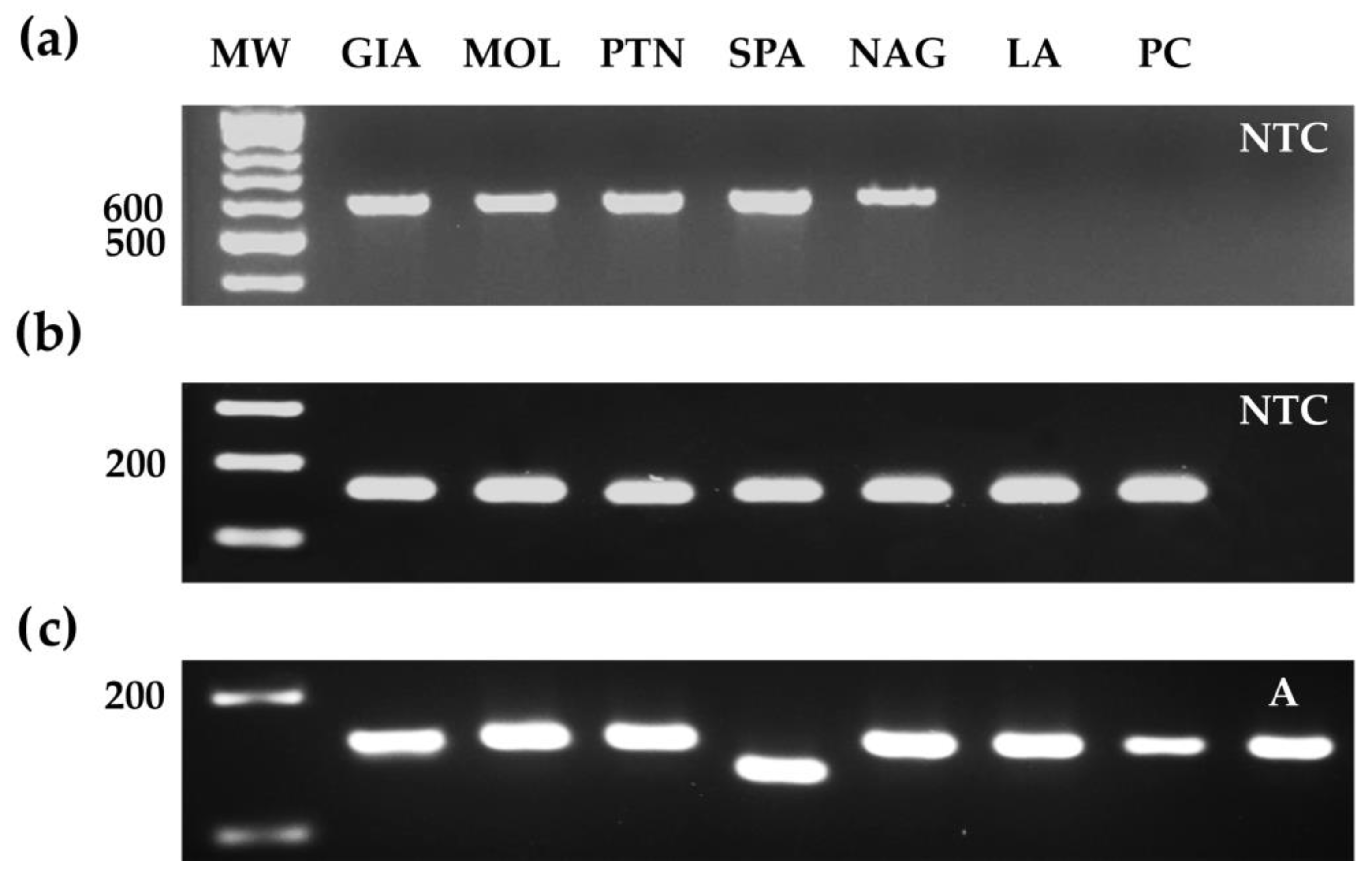
2.4. Design of SCAR and dCAPS Molecular Markers
2.5. Sanger DNA Sequencing
2.6. In Silico Gene and Protein Structure Analysis
3. Results and Discussion
3.1. Allelism Test Assessing C Involvement in the PL Phenotype
3.2. Molecular Integrated Approaches Revealed Novel SPA Private PL Alleles
3.3. Sanger Sequencing Analysis Highlighted Two Other Novel PL Alleles in Italian Landraces
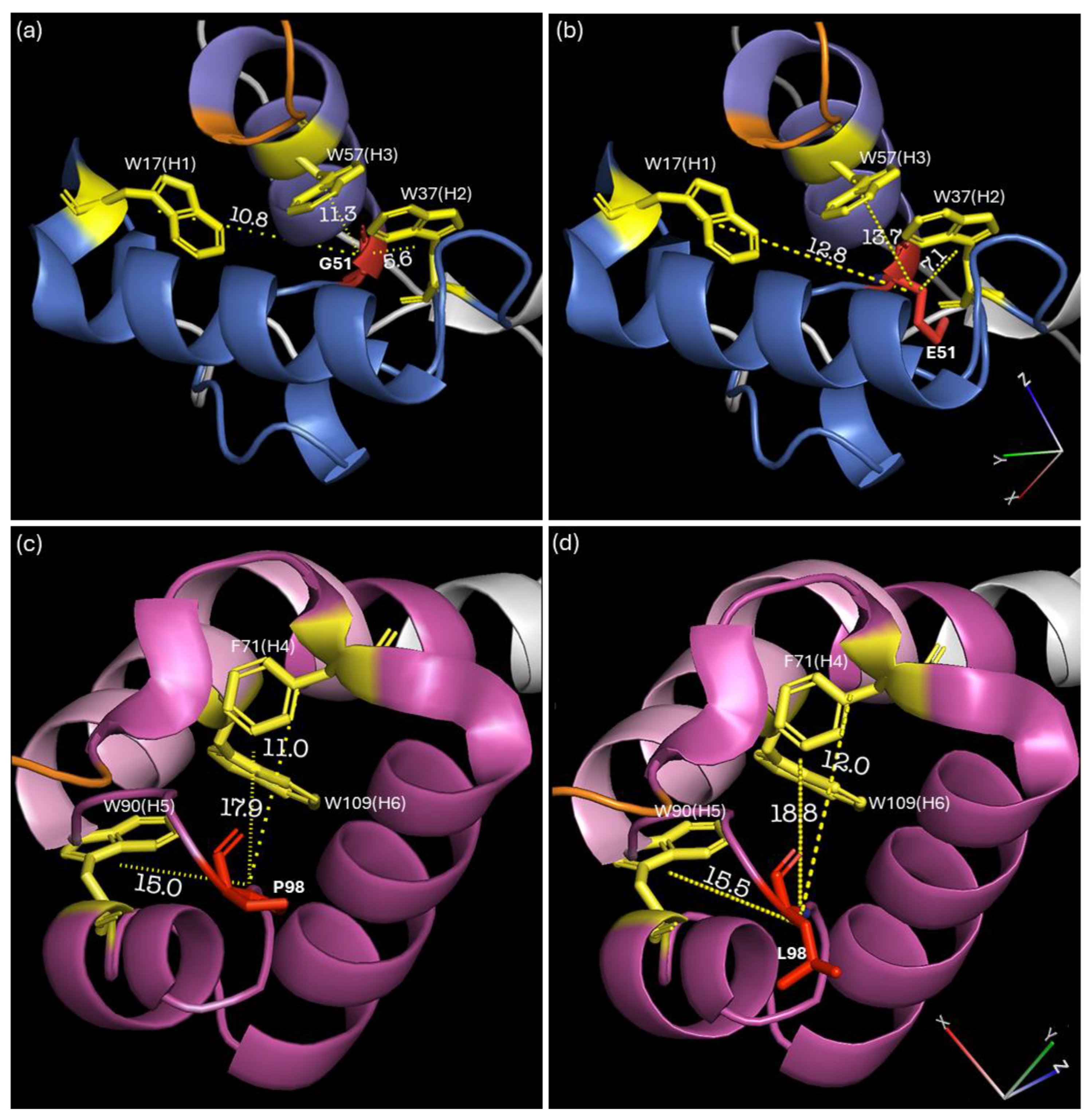
3.4. SNP Mutations Affect the R2R3 Domain of the Myb Factor
3.5. Importance of Preserving and Protecting Elite Landraces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Agricultural Production Statistics 2000–2022; FAO: Rome, Italy, 2023. [Google Scholar]
- Nakayama, H.; Ichihashi, Y.; Kimura, S. Diversity of Tomato Leaf Form Provides Novel Insights into Breeding. Breed. Sci. 2023, 73, 22061. [Google Scholar] [CrossRef] [PubMed]
- Pons, C.; Casals, J.; Palombieri, S.; Fontanet, L.; Riccini, A.; Rambla, J.L.; Ruggiero, A.; Figás, M.d.R.; Plazas, M.; Koukounaras, A.; et al. Atlas of Phenotypic, Genotypic and Geographical Diversity Present in the European Traditional Tomato. Hortic. Res. 2022, 9, uhac112. [Google Scholar] [CrossRef] [PubMed]
- Mazzucato, A.; Ficcadenti, N.; Caioni, M.; Mosconi, P.; Piccinini, E.; Reddy Sanampudi, V.R.; Sestili, S.; Ferrari, V. Genetic Diversity and Distinctiveness in Tomato (Solanum lycopersicum L.) Landraces: The Italian Case Study of ‘A Pera Abruzzese’. Sci. Hortic. 2010, 125, 55–62. [Google Scholar] [CrossRef]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a Tomato Landraces Collection for Fruit-Related Traits by the Aid of a High-Throughput Genomic Platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef]
- Farinon, B.; Picarella, M.E.; Siligato, F.; Rea, R.; Taviani, P.; Mazzucato, A. Phenotypic and Genotypic Diversity of the Tomato Germplasm from the Lazio Region in Central Italy, with a Focus on Landrace Distinctiveness. Front. Plant Sci. 2022, 13, 931233. [Google Scholar] [CrossRef]
- Ministero Dell’agricoltura, Della Sovranità Alimentare e Delle Foreste Prodotti Agroalimentari Tradizionali (PAT). Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/21121 (accessed on 16 December 2024).
- Rowland, S.D.; Zumstein, K.; Nakayama, H.; Cheng, Z.; Flores, A.M.; Chitwood, D.H.; Maloof, J.N.; Sinha, N.R. Leaf Shape Is a Predictor of Fruit Quality and Cultivar Performance in Tomato. New Phytol. 2020, 226, 851–865. [Google Scholar] [CrossRef]
- Busch, B.L.; Schmitz, G.; Rossmann, S.; Piron, F.; Ding, J.; Bendahmane, A.; Theres, K. Shoot Branching and Leaf Dissection in Tomato Are Regulated by Homologous Gene Modules. Plant Cell 2011, 23, 3595–3609. [Google Scholar] [CrossRef]
- Benoit, M.; Drost, H.G.; Catoni, M.; Gouil, Q.; Lopez-Gomollon, S.; Baulcombe, D.; Paszkowski, J. Environmental and Epigenetic Regulation of Rider Retrotransposons in Tomato. PLoS Genet. 2019, 15, e1008370. [Google Scholar] [CrossRef]
- Mazzucato, A.; Cellini, F.; Bouzayen, M.; Zouine, M.; Mila, I.; Minoia, S.; Petrozza, A.; Picarella, M.E.; Ruiu, F.; Carriero, F. A TILLING Allele of the Tomato Aux/IAA9 Gene Offers New Insights into Fruit Set Mechanisms and Perspectives for Breeding Seedless Tomatoes. Mol. Breed. 2015, 35, 22. [Google Scholar] [CrossRef]
- Abe-Hara, C.; Yamada, K.; Wada, N.; Ueta, R.; Hashimoto, R.; Osakabe, K.; Osakabe, Y. Effects of the Sliaa9 Mutation on Shoot Elongation Growth of Tomato Cultivars. Front. Plant Sci. 2021, 12, 627832. [Google Scholar] [CrossRef]
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X.; et al. Genomic Analyses Provide Insights into the History of Tomato Breeding. Nat. Genet. 2014, 46, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)—From Genotype to Phenotype to Breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef] [PubMed]
- Bhagyawant, S.S. RAPD-SCAR Markers: An Interface Tool for Authentication of Traits. J. Biosci. Med. 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Graci, S.; Olivieri, F.; Barone, A. Phenotypic and Genotypic Characterization to Authenticate and Trace a High-Quality Yellow Tomato Ecotype through the Processing Chain. Sci. Hortic. 2022, 306, 111449. [Google Scholar] [CrossRef]
- Neff, M.M.; Neff, J.D.; Chory, J.; Pepper, A.E. DCAPS, a Simple Technique for the Genetic Analysis of Single Nucleotide Polymorphisms: Experimental Applications in Arabidopsis Thaliana Genetics. Plant J. 1998, 14, 387–392. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Kiribuchi-Otobe, C.; Hirano, H.; Suzuki, Y.; Fujita, M. Detection of Single Nucleotide Polymorphism (SNP) Controlling the Waxy Character in Wheat by Using a Derived Cleaved Amplified Polymorphic Sequence (DCAPS) Marker. Theor. Appl. Genet. 2003, 107, 84–88. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Olivieri, F.; Calafiore, R.; Francesca, S.; Schettini, C.; Chiaiese, P.; Rigano, M.M.; Barone, A. High-Throughput Genotyping of Resilient Tomato Landraces to Detect Candidate Genes Involved in the Response to High Temperatures. Genes 2020, 11, 626. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- SIB. Swiss Institute of Bioinformatics ExPASy: SIB Bioinformatics Resource Portal. Available online: https://web.expasy.org/translate/ (accessed on 17 November 2024).
- European Molecular Biology Laboratory. The AlphaFold Database of Protein Structures: A Biologist’s Guide. Available online: https://alphafold.ebi.ac.uk (accessed on 19 November 2024).
- Phoenix Bioinformatics. The Arabidopsis Information Resource. Available online: https://www.arabidopsis.org/tools/blast/ (accessed on 17 December 2024).
- Wang, B.; Luo, Q.; Li, Y.; Yin, L.; Zhou, N.; Li, X.; Gan, J.; Dong, A. Structural Insights into Target DNA Recognition by R2R3-MYB Transcription Factors. Nucleic Acids Res. 2019, 48, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL. Available online: https://www.pymol.org/pymol (accessed on 17 November 2024).
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, D.B. DynaMut2: Assessing Changes in Stability and Flexibility upon Single and Multiple Point Missense Mutations. Protein Sci. 2021, 30, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Torella, A.; Zanobio, M.; Zeuli, R.; del Vecchio Blanco, F.; Savarese, M.; Giugliano, T.; Garofalo, A.; Piluso, G.; Politano, L.; Nigro, V. The Position of Nonsense Mutations Can Predict the Phenotype Severity: A Survey on the DMD Gene. PLoS ONE 2020, 15, e0237803. [Google Scholar] [CrossRef]
- Sivakumar, K.; Subbiah, U. Computational Analysis of Non-Synonymous SNPs in the Human LCN2 Gene. Egypt. J. Med. Hum. Genet. 2024, 25, 94. [Google Scholar] [CrossRef]
- Li, Z.; Peng, R.; Tian, Y.; Han, H.; Xu, J.; Yao, Q. Genome-Wide Identification and Analysis of the MYB Transcription Factor Superfamily in Solanum lycopersicum. Plant Cell Physiol. 2016, 57, 1657–1677. [Google Scholar] [CrossRef]
- Deber, C.M.; Brodsky, B.; Rath, A. Proline Residues in Proteins. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Biedermannova, L.; Riley, K.E.; Berka, K.; Hobza, P.; Vondrasek, J. Another Role of Proline: Stabilization Interactions in Proteins and Protein Complexes Concerning Proline and Tryptophane. Phys. Chem. Chem. Phys. 2008, 10, 6350. [Google Scholar] [CrossRef]
- Krieger, F.; Möglich, A.; Kiefhaber, T. Effect of Proline and Glycine Residues on Dynamics and Barriers of Loop Formation in Polypeptide Chains. J. Am. Chem. Soc. 2005, 127, 3346–3352. [Google Scholar] [CrossRef]
- Batkhishig, D.; Enkhbayar, P.; Kretsinger, R.H.; Matsushima, N. A Crucial Residue in the Hydrophobic Core of the Solenoid Structure of Leucine Rich Repeats. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2021, 1869, 140631. [Google Scholar] [CrossRef]
- Karvonen, M.K.; Pesonen, U.; Koulu, M.; Niskanen, L.; Laakso, M.; Rissanen, A.; Dekker, J.M.; Hart, L.M.; Valve, R.; Uusitupa, M.I.J. Association of a Leucine(7)-to-Proline(7) Polymorphism in the Signal Peptide of Neuropeptide Y with High Serum Cholesterol and LDL Cholesterol Levels. Nat. Med. 1998, 4, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Srivastava, A.K.; Schwartz, C.E.; Alexov, E.; Wang, L. Structural Assessment of the Effects of Amino Acid Substitutions on Protein Stability and Protein Protein Interaction. Int. J. Comput. Biol. Drug Des. 2010, 3, 334. [Google Scholar] [CrossRef] [PubMed]
- Pancotti, C.; Benevenuta, S.; Birolo, G.; Alberini, V.; Repetto, V.; Sanavia, T.; Capriotti, E.; Fariselli, P. Predicting Protein Stability Changes upon Single-Point Mutation: A Thorough Comparison of the Available Tools on a New Dataset. Brief. Bioinform. 2022, 23, bbab555. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, I.; Cantarella, C.; Fasano, C.; Cardi, T.; Mennella, G.; D’Agostino, N. Liquid-Phase Sequence Capture and Targeted Re-Sequencing Revealed Novel Polymorphisms in Tomato Genes Belonging to the MEP Carotenoid Pathway. Sci. Rep. 2017, 7, 5616. [Google Scholar] [CrossRef] [PubMed]
- Pizzolongo, F.; Sorrentino, G.; Graci, S.; Barone, A.; Romano, R. Improved Antioxidant Properties and Sustainability of Whole-fruit Yellow Tomato Puree. Int. J. Food Sci. Technol. 2024, 59, 7668–7678. [Google Scholar] [CrossRef]
- Cammareri, M.; Sinesio, F.; Peparaio, M.; Pons, C.; Romero del Castillo, R.; Saggia Civitelli, E.; Vitiello, A.; Granell, A.; Casals, J.; Grandillo, S. Local Agro-Environmental Conditions Impact Fruit Quality, Sensory Properties and Consumer Acceptance of Long Shelf-Life Tomatoes. Agronomy 2023, 13, 1265. [Google Scholar] [CrossRef]
- Renna, M.; Montesano, F.F.; Signore, A.; Gonnella, M.; Santamaria, P. Biodiverso: A Case Study of Integrated Project to Preserve the Biodiversity of Vegetable Crops in Puglia (Southern Italy). Agriculture 2018, 8, 128. [Google Scholar] [CrossRef]
- Accogli, R.; Conversa, G.; Ricciardi, L.; Sonnante, G.; Santamaria, P. Nuovo Almanacco BiodiverSO; Università degli Studi di Bari Aldo Moro: Bari, Italy, 2018; ISBN 978-88-6629-024-7. [Google Scholar]
- Roohanitaziani, R.; de Maagd, R.A.; Lammers, M.; Molthoff, J.; Meijer-Dekens, F.; van Kaauwen, M.P.W.; Finkers, R.; Tikunov, Y.; Visser, R.G.F.; Bovy, A.G. Exploration of a Resequenced Tomato Core Collection for Phenotypic and Genotypic Variation in Plant Growth and Fruit Quality Traits. Genes 2020, 11, 1278. [Google Scholar] [CrossRef]
- Heuvelink, E.; Bakker, M.J.; Elings, A.; Kaarsemaker, R.C.; Marcelis, L.F.M. Effect of leaf area on tomato yield. Acta Hortic. 2005, 691, 43–50. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Rozhon, W. The Effect of Salinity on Fruit Quality and Yield of Cherry Tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; El-Nakhel, C.; Fiorentino, N.; Pelosi, M.E.; Rouphael, Y.; Mori, M. Impact of Salinity and Biostimulants on Cherry Tomato Yield and Quality. Horticulturae 2024, 10, 1239. [Google Scholar] [CrossRef]
- Raiola, A.; Pizzolongo, F.; Manzo, N.; Montefusco, I.; Spigno, P.; Romano, R.; Barone, A. A Comparative Study of the Physico-chemical Properties Affecting the Organoleptic Quality of Fresh and Thermally Treated Yellow Tomato Ecotype Fruit. Int. J. Food Sci. Technol. 2018, 53, 1219–1226. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, L.; Farinon, B.; Fumelli, L.; Picarella, M.E.; Mazzucato, A.; Olivieri, F. Novel Alleles of the Potato Leaf Gene Identified in Italian Traditional Varieties Conferring Potato-like Leaf Shape in Tomato. Horticulturae 2025, 11, 129. https://doi.org/10.3390/horticulturae11020129
Mancini L, Farinon B, Fumelli L, Picarella ME, Mazzucato A, Olivieri F. Novel Alleles of the Potato Leaf Gene Identified in Italian Traditional Varieties Conferring Potato-like Leaf Shape in Tomato. Horticulturae. 2025; 11(2):129. https://doi.org/10.3390/horticulturae11020129
Chicago/Turabian StyleMancini, Lorenzo, Barbara Farinon, Ludovica Fumelli, Maurizio Enea Picarella, Andrea Mazzucato, and Fabrizio Olivieri. 2025. "Novel Alleles of the Potato Leaf Gene Identified in Italian Traditional Varieties Conferring Potato-like Leaf Shape in Tomato" Horticulturae 11, no. 2: 129. https://doi.org/10.3390/horticulturae11020129
APA StyleMancini, L., Farinon, B., Fumelli, L., Picarella, M. E., Mazzucato, A., & Olivieri, F. (2025). Novel Alleles of the Potato Leaf Gene Identified in Italian Traditional Varieties Conferring Potato-like Leaf Shape in Tomato. Horticulturae, 11(2), 129. https://doi.org/10.3390/horticulturae11020129










