Quality Responses of Sweet Pepper Varieties Under Irrigation and Fertilization Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Materials and Sample Preparation
2.3. Chemicals and Materials Used in the Experiment
2.4. Hydrophilic Extraction
2.5. Antioxidant Activity
2.6. Analysis of Condensed Tannins
2.7. Pigment Extraction and Analysis
2.8. Protein Content Analysis
2.9. Determination of δ15N via EA-IRMS
2.10. Statistical Analysis
3. Results
3.1. Influence of Variety on Sweet Pepper Quality
3.2. Influence of Fertilizers on Sweet Pepper Quality
3.3. Influence of Irrigation on Sweet Pepper Quality
3.4. Interaction Effect of Variety and Fertilization on Sweet Pepper Quality
3.5. Interaction Effect of Variety and Irrigation on Sweet Pepper Quality
3.6. Interaction Effect of Fertilization and Irrigation on Sweet Pepper Quality
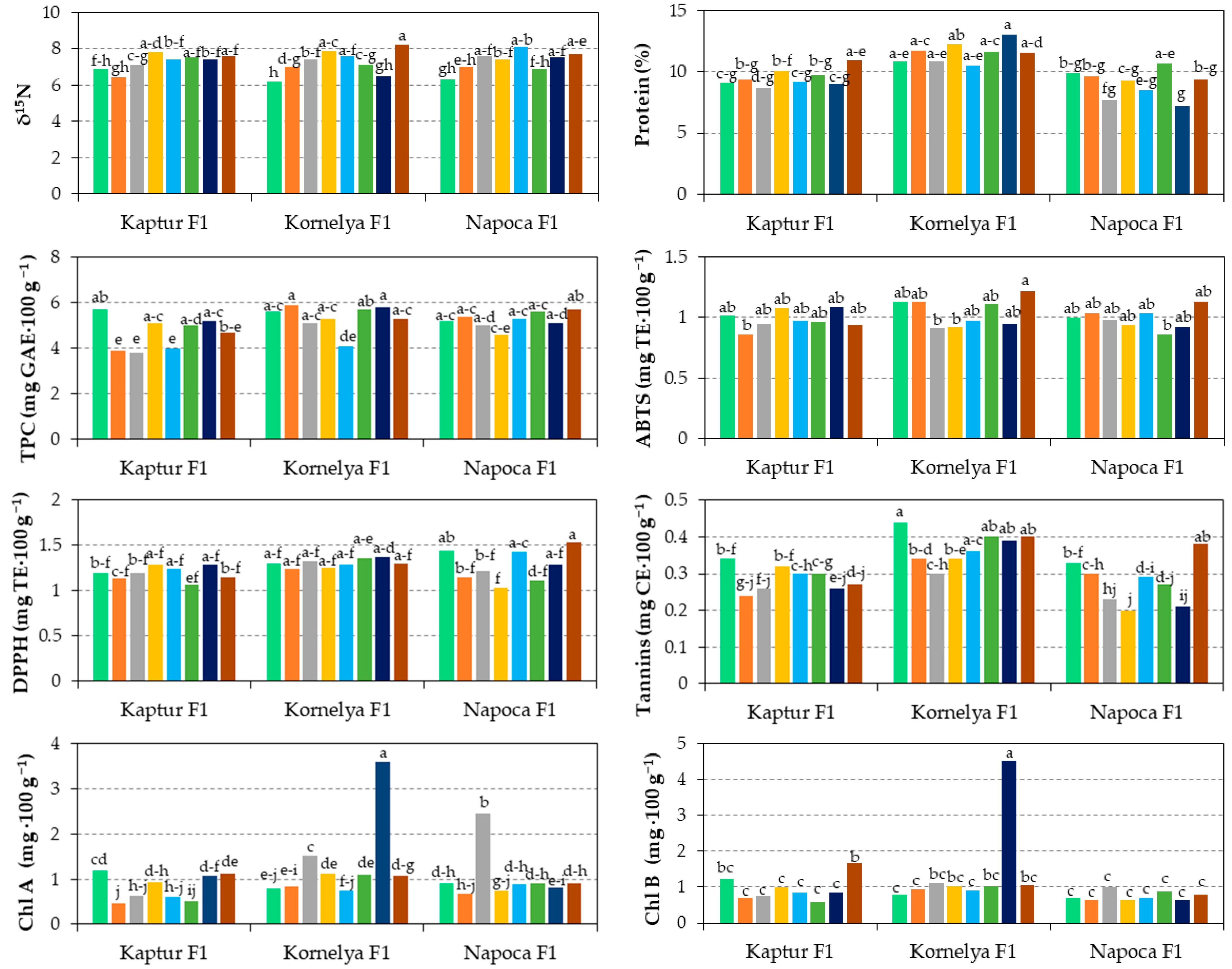
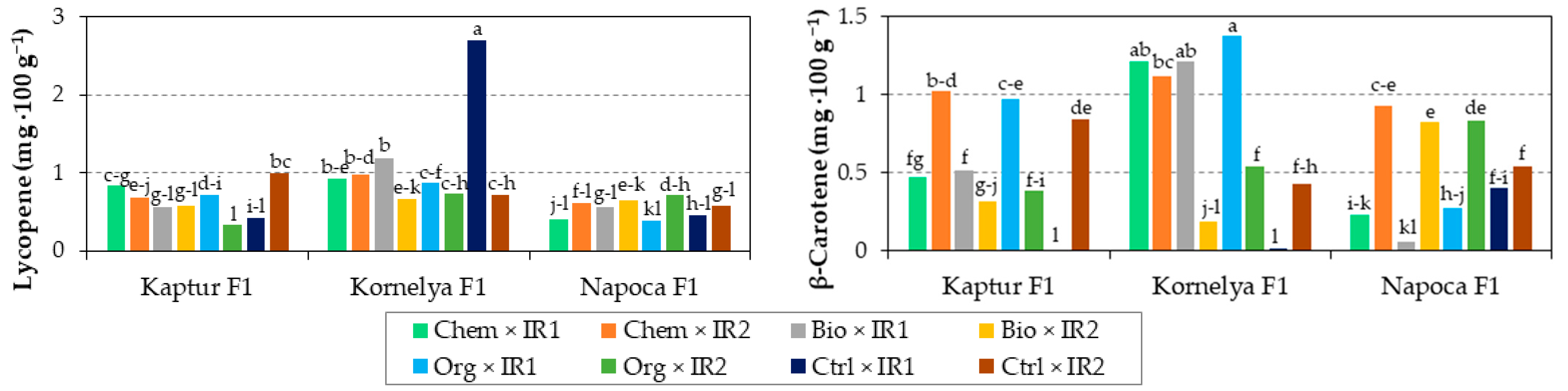
3.7. Interaction Effect of Variety, Fertilization, and Irrigation on Sweet Pepper Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brezeanu, C.; Brezeanu, P.M.; Stoleru, V.; Irimia, L.M.; Lipșa, F.D.; Teliban, G.-C.; Ciobanu, M.M.; Murariu, F.; Puiu, I.; Branca, F. Nutritional Value of New Sweet Pepper Genotypes Grown in Organic System. Agriculture 2022, 12, 1863. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Mirzaalian Dastjerdi, A.; Jamali, B. Microbial-Based Biological Treatments Improved the Nutritional, Nutraceutical and Functional Properties of Greenhouse Sweet Pepper (Capsicum annuum L.). Front. Sustain. Food Syst. 2023, 7, 1145972. [Google Scholar] [CrossRef]
- Krasnow, C.; Ziv, C. Non-Chemical Approaches to Control Postharvest Gray Mold Disease in Bell Peppers. Agronomy 2022, 12, 216. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Martínez-Cuenca, M.-R.; Marsal, J.I.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Calatayud, Á. Bioactive Compounds and Antioxidant Capacity of Valencian Pepper Landraces. Molecules 2021, 26, 1031. [Google Scholar] [CrossRef]
- Souza, C.S.; Daood, H.G.; Duah, S.A.; Vinogradov, S.; Palotás, G.; Neményi, A.; Helyes, L.; Pék, Z. Stability of Carotenoids, Carotenoid Esters, Tocopherols and Capsaicinoids in New Chili Pepper Hybrids during Natural and Thermal Drying. LWT 2022, 163, 113520. [Google Scholar] [CrossRef]
- Żurawik, A.; Jadczak, D.; Panayotov, N.; Żurawik, P. Antioxidant Properties of Pepper (Capsicum annuum L.) Depending on Its Cultivar and Fruit Colouration. Plant Soil Environ. 2021, 67, 653–659. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Young, J.E.; Zhao, X.; Carey, E.E.; Welti, R.; Yang, S.-S.; Wang, W. Phytochemical Phenolics in Organically Grown Vegetables. Mol. Nutr. Food Res 2005, 49, 1136–1142. [Google Scholar] [CrossRef]
- World Health Organization Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 25 October 2024).
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental Composition and Some Nutritional Parameters of Sweet Pepper from Organic and Conventional Agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef]
- Sahu, B.; Pradhan, M. Organic Farming: A Sustainable Approach of Agriculture. In Rural Development: Possibilities and Challenges in the Present Perspectives; Ganga Prakashan Sadatpur Extension: Delhi, India, 2023; pp. 8–28. ISBN 978-93-5514-103-3. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/TCL (accessed on 22 August 2024).
- Mamphogoro, T.P.; Babalola, O.O.; Aiyegoro, O.A. Sustainable Management Strategies for Bacterial Wilt of Sweet Peppers (Capsicum annuum) and Other Solanaceous Crops. J. Appl. Microbiol. 2020, 129, 496–508. [Google Scholar] [CrossRef]
- Inculet, C.-S.; Mihalache, G.; Sellitto, V.M.; Hlihor, R.-M.; Stoleru, V. The Effects of a Microorganisms-Based Commercial Product on the Morphological, Biochemical and Yield of Tomato Plants under Two Different Water Regimes. Microorganisms 2019, 7, 706. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial Use of Pepper (Capsicum Annum L.) Derived Products: Technological Benefits and Biological Advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; Gómez-García MD, R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (Hot Pepper): An Ancient Latin-American Crop with Outstanding Bioactive Compounds and Nutraceutical Potential. A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Lima GP, P.; Vianello, F.; Corrêa, C.R.; Campos RA, D.S.; Borguini, M.G. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Saleh, B.; Omer, A.; Teweldemedhin Keleta, B. Medicinal Uses and Health Benefits of Chili Pepper (Capsicum Spp.): A Review. MOJ Food Process. Technol. 2018, 6, 325–328. [Google Scholar] [CrossRef]
- Mateusz, W.; Jeremi, K.; Katarzyna, S.; Magdalena, L.-R.; Małgorzata, K. Iron biofortification in four non-heterotic red sweet cultivars of bell pepper. Food Res. Int. 2024, 196, 115050. [Google Scholar]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, D.; Raghunath, M. Antioxidant Activity and Phenolic Content of Roots, Tubers and Vegetables Commonly Consumed in India. Food Res. Int. 2010, 43, 1017–1020. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P.; Viskelis, J.; Jankauskiene, J.; Bobinas, C. Lycopene and β-Carotene in Non-Blanched and Blanched Tomatoes. J. Food Agric. Environ. 2012, 10, 142–146. [Google Scholar] [CrossRef]
- Atanassova, M.; Christova-Bagdassarian, V. Determination of Tannins Content by Titrimetric Method for Comparison of Different Plant Species. J. Univ. Chem. Technol. Metall. 2009, 44, 413–415. [Google Scholar]
- Fess, T.L.; Benedito, V.A. Organic versus Conventional Cropping Sustainability: A Comparative System Analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef]
- Rahmann, G.; Ardakani, M.R.; Bàrberi, P.; Böhm, H.; Canali, S.; Chander, M.; David, W.; Dengel, L.; Nuutila, J. Organic Agriculture 3.0 Is Innovation with Research. Org. Agric. 2017, 7, 169–197. [Google Scholar] [CrossRef]
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant Activity of the Phenolic and Oily Fractions of Different Sweet Bell Peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Boyhan, G.E.; McGregor, C.; O’Connell, S.; Biang, J.; Berle, D. A Comparison of 13 Sweet Pepper Varieties under an Organic Farming System. HortTechnology 2020, 30, 135–143. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting New Peach and Plum Genotypes Rich in Phenolic Compounds and Enhanced Functional Properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Guilherme, R.; Aires, A.; Rodrigues, N.; Peres, A.M.; Pereira, J.A. Phenolics and Antioxidant Activity of Green and Red Sweet Peppers from Organic and Conventional Agriculture: A Comparative Study. Agriculture 2020, 10, 652. [Google Scholar] [CrossRef]
- Ye, S.; Peng, B.; Liu, T. Effects of Organic Fertilizers on Growth Characteristics and Fruit Quality in Pear-Jujube in the Loess Plateau. Sci. Rep. 2022, 12, 13372. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Ali, S.; Izni, A.; Harun, R. Impact of Excessive Nitrogen Fertilizers on the Environment and Associated Mitigation Strategies. Asian J. Microbiol. Biotechnol. Environ. Sci. 2013, 15, 213–221. [Google Scholar]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial Fertilizers: A Comprehensive Review of Current Findings and Future Perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yazar, A.; Tekin, S. Physiological Response of Red Pepper to Different Irrigation Regimes under Drip Irrigation in the Mediterranean Region of Turkey. Sci. Hortic. 2019, 245, 280–288. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Pereira-Caro, G.; Cardeñosa, V.; Muriel, J.L.; Moreno-Rojas, J.M. Study of the Quality Attributes of Selected Blueberry (Vaccinium corymbosum L.) Varieties Grown under Different Irrigation Regimes and Cultivation Systems. Appl. Sci. 2020, 10, 8459. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant Constituents in Some Sweet Pepper (Capsicum annuum L.) Genotypes during Maturity. LWT-Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Rusu, O.R.; Mangalagiu, I.; Amăriucăi-Mantu, D.; Teliban, G.C.; Cojocaru, A.; Burducea, M.; Mihalache, G.; Roșca, M.; Caruso, G.; Sekara, A.; et al. Interaction Effects of Cultivars and Nutrition on Quality and Yield of Tomato. Horticulturae 2023, 9, 541. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and Validation of UHPLC-HRMS Methodology for the Determination of Flavonoids, Amino Acids and Organosulfur Compounds in Black Onion, a Novel Derived Product from Fresh Shallot Onions (Allium Cepa Var. Aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Cuevas, F.J.; Pradas, I.; Ruiz-Moreno, M.J.; Arroyo, F.T.; Perez-Romero, L.F.; Montenegro, J.C.; Moreno-Rojas, J.M. Effect of Organic and Conventional Management on Bio-Functional Quality of Thirteen Plum Cultivars (Prunus salicina Lindl.). PLoS ONE 2015, 10, e0136596. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Muñoz-Redondo, J.M.; Montenegro, J.C.; Moreno-Rojas, J.M. Using Nitrogen Stable Isotopes to Authenticate Organically and Conventionally Grown Vegetables: A New Tracking Framework. Agronomy 2023, 13, 131. [Google Scholar] [CrossRef]
- Tulipani, S.; Marzban, G.; Herndl, A.; Laimer, M.; Mezzetti, B.; Battino, M. Influence of Environmental and Genetic Factors on Health-Related Compounds in Strawberry. Food Chem. 2011, 124, 906–913. [Google Scholar] [CrossRef]
- Chełpiński, P.; Skupień, K.; Ochmian, I. Effect of Fertilization on Yield and Quality of Cultivar Kent Strawberry Fruit. J. Elemntology 2010, 15, 251–257. [Google Scholar] [CrossRef]
- Stoleru, V.; Mangalagiu, I.; Amăriucăi-Mantu, D.; Teliban, G.-C.; Cojocaru, A.; Rusu, O.-R.; Burducea, M.; Mihalache, G.; Rosca, M.; Caruso, G.; et al. Enhancing the Nutritional Value of Sweet Pepper through Sustainable Fertilization Management. Front. Nutr. 2023, 10, 1264999. [Google Scholar] [CrossRef]
- Al-Omran, A.; Al-Harbi, A.; Wahb-Allah, M.; Nadeem, M.; Al-Eter, A. Impact of Irrigation Water Quality, Irrigation Systems, Irrigation Rates and Soil Amendments on Tomato Production in Sandy Calcareous Soil. Turk. J. Agric. For. 2010, 34, 59–73. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wu, H.; Yuan, Q.; Wang, J.; Cui, J.; Lin, A. Effects of Selenium Fertilizer Application and Tomato Varieties on Tomato Fruit Quality: A Meta-Analysis. Sci. Hortic. 2022, 304, 111242. [Google Scholar] [CrossRef]
- Ariza, M.T.; Miranda, L.; Gómez-Mora, J.A.; Medina, J.J.; Lozano, D.; Gavilán, P.; Soria, C.; Martínez-Ferri, E. Yield and Fruit Quality of Strawberry Cultivars under Different Irrigation Regimes. Agronomy 2021, 11, 261. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.V.; Munteanu, N.C.; Sellitto, V.M.; Teliban, G.C.; Burducea, M.; Tenu, I.; Morano, G.; Butnariu, M. Quality Performances of Sweet Pepper under Farming Management. Not. Bot. Horti Agrobot. 2019, 47, 458–464. [Google Scholar] [CrossRef][Green Version]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early Growth Stage Characterization and the Biochemical Responses for Salinity Stress in Tomato. Plants 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, S.; Maggio, A.; Orsini, F.; Barbieri, G. Cultivar, soil type, nitrogen source and irrigation regime as quality determinants of organically grown tomatoes. Sci. Hort. 2016, 199, 88–94. [Google Scholar] [CrossRef]
| Variable | δ15N | Protein (%) | TPC (mg GAE·100 g−1 d.w.) | ABTS (mg TE·100 g−1 d.w.) | DPPH (mg TE·100 g−1 d.w.) | Chlorophyll A (mg·100 g−1 d.w.) | Chlorophyll B (mg·100 g−1 d.w.) | Lycopene (mg·100 g−1 d.w.) | β-Carotene (mg·100 g−1 d.w.) | Tannins (mg CE·100 g−1 d.w.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Variety effects: | ||||||||||
| Kaptur F1 | 7.3 | 9.5 b | 4.7 b | 0.98 | 1.19 b | 0.82 c | 0.96 b | 0.64 b | 0.56 b | 0.29 b |
| Kornelya F1 | 7.2 | 11.5 a | 5.4 a | 1.04 | 1.30 a | 1.35 a | 1.43 a | 1.10 a | 0.76 a | 0.37 a |
| Napoca F1 | 7.3 | 9.0 b | 5.2 a | 0.99 | 1.27 a | 1.04 b | 0.76 c | 0.55 c | 0.51 c | 0.28 b |
| p-value | ns | *** | *** | ns | *** | *** | *** | *** | *** | *** |
| Fertilization effects: | ||||||||||
| Chemical | 6.6 b | 10.1 | 5.3 a | 1.03 | 1.24 ab | 0.81 c | 0.84 b | 0.74 b | 0.83 a | 0.33 a |
| Biological | 7.5 a | 9.8 | 4.8 b | 0.96 | 1.22 b | 1.23 b | 0.93 b | 0.70 bc | 0.51 c | 0.27 b |
| Organic | 7.4 a | 10.0 | 4.9 b | 0.98 | 1.24 ab | 0.79 c | 0.83 b | 0.63 c | 0.73 b | 0.32 a |
| Control | 7.5 a | 10.2 | 5.3 a | 1.04 | 1.32 a | 1.43 a | 1.59 a | 0.98 a | 0.37 d | 0.32 a |
| p-value | *** | ns | *** | ns | * | *** | *** | *** | *** | *** |
| Irrigation regimes: | ||||||||||
| IR1 | 7.2 b | 9.5 b | 5.0 b | 0.99 | 1.29 a | 1.27 a | 1.18 a | 0.84 a | 0.56 b | 0.31 |
| IR2 | 7.4 a | 10.5 a | 5.2 a | 1.01 | 1.22 b | 0.86 b | 0.92 b | 0.69 b | 0.66 a | 0.31 |
| p-value | *** | *** | * | ns | ** | *** | *** | *** | *** | ns |
| Variable | δ15N | Protein (%) | TPC (mg GAE·100 g−1 d.w.) | ABTS (mg TE·100 g−1 d.w.) | DPPH (mg TE·100 g−1 d.w.) | Chlorophyll A (mg·100 g−1 d.w.) | Chlorophyll B (mg·100 g−1 d.w.) | Lycopene (mg·100 g−1 d.w.) | β-Carotene (mg·100 g−1 d.w.) | Tannins (mg CE·100 g−1 d.w.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaptur F1 × Chemical | 6.6 | 9.3 de | 4.8 cd | 0.94 b | 1.16 bc | 0.82 e | 0.97 bc | 0.75 cde | 0.75 c | 0.29 bc |
| Kaptur F1 × Biologic | 7.5 | 9.4 de | 4.4 d | 1.01 ab | 1.24 abc | 0.79 e | 0.89 bc | 0.57 fg | 0.41 g | 0.29 bc |
| Kaptur F1 × Organic | 7.5 | 9.5 de | 4.5 d | 0.97 ab | 1.15 bc | 0.56 f | 0.73 c | 0.52 g | 0.68 cd | 0.30 bc |
| Kaptur F1 × Control | 7.5 | 10.0 bcde | 5.0 bcd | 1.01 ab | 1.21 bc | 1.10 cd | 1.26 b | 0.71 def | 0.42 g | 0.27 c |
| Kornelya F1 × Chemical | 6.6 | 11.3 abc | 5.8 a | 1.13 a | 1.27 abc | 0.82 e | 0.87 bc | 0.95 b | 1.17 a | 0.39 a |
| Kornelya F1 × Biologic | 7.6 | 11.5 ab | 5.2 abc | 0.91 b | 1.29 abc | 1.32 c | 1.08 bc | 0.92 bc | 0.70 cd | 0.32 b |
| Kornelya F1 × Organic | 7.3 | 11.1 abcd | 4.9 bcd | 1.04 ab | 1.32 ab | 0.93 de | 0.98 bc | 0.81 bcd | 0.96 b | 0.38 a |
| Kornelya F1 × Control | 7.4 | 12.2 a | 5.6 a | 1.09 ab | 1.33 ab | 2.33 a | 2.79 a | 1.71 a | 0.22 h | 0.39 a |
| Napoca F1 × Chemical | 6.6 | 9.8 cde | 5.3 abc | 1.01 ab | 1.28 abc | 0.79 e | 0.68 c | 0.51 g | 0.58 de | 0.31 bc |
| Napoca F1 × Biologic | 7.5 | 8.5 e | 4.8 cd | 0.96 ab | 1.12 c | 1.59 b | 0.83 c | 0.61 efg | 0.43 fg | 0.21 d |
| Napoca F1 × Organic | 7.5 | 9.6 cde | 5.4 ab | 0.94 b | 1.26 abc | 0.90 de | 0.79 c | 0.55 fg | 0.55 ef | 0.28 bc |
| Napoca F1 × Control | 7.6 | 8.3 e | 5.4 abc | 1.03 ab | 1.41 a | 0.86 e | 0.73 c | 0.51 g | 0.47 efg | 0.30 bc |
| p-value | ns | * | ** | * | ** | *** | *** | *** | *** | *** |
| Variable | δ15N | Protein (%) | TPC (mg GAE·100 g−1 d.w.) | ABTS (mg TE·100 g−1 d.w.) | DPPH (mg TE·100 g−1 d.w.) | Chlorophyll A (mg·100 g−1 d.w.) | Chlorophyll B (mg·100 g−1 d.w.) | Lycopene (mg·100 g−1 d.w.) | β-Carotene (mg·100 g−1 d.w.) | Tannins (mg CE·100 g−1 d.w.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaptur F1 × IR1 | 7.2 bc | 9.0 | 4.7 | 1.01 ab | 1.22 | 0.87 d | 0.93 bc | 0.63 c | 0.49 d | 0.29 b |
| Kaptur F1 × IR2 | 7.3 ab | 10.1 | 4.7 | 0.96 b | 1.16 | 0.76 d | 0.99 bc | 0.65 c | 0.64 c | 0.28 b |
| Kornelya F1 × IR1 | 6.9 c | 11.3 | 5.2 | 0.99 ab | 1.32 | 1.66 a | 1.84 a | 1.43 a | 0.95 a | 0.37 a |
| Kornelya F1 × IR2 | 7.6 a | 11.8 | 5.5 | 1.10 a | 1.29 | 1.03 c | 1.03 b | 0.77 b | 0.57 c | 0.37 a |
| Napoca F1 × IR1 | 7.4 ab | 8.3 | 5.2 | 0.98 b | 1.34 | 1.27 b | 0.77 c | 0.45 d | 0.24 e | 0.26 b |
| Napoca F1 × IR2 | 7.2 b | 9.8 | 5.3 | 0.99 ab | 1.20 | 0.80 d | 0.75 c | 0.64 c | 0.78 b | 0.29 b |
| p-value | *** | ns | ns | * | ns | *** | *** | *** | *** | * |
| Variable | δ15N | Protein (%) | TPC (mg GAE·100 g−1 d.w.) | ABTS (mg TE·100 g−1 d.w.) | DPPH (mg TE·100 g−1 d.w.) | Chlorophyll A (mg·100 g−1 d.w.) | Chlorophyll B (mg·100 g−1 d.w.) | Lycopene (mg·100 g−1 d.w.) | β-Carotene (mg·100 g−1 d.w.) | Tannins (mg CE·100 g−1 d.w.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical F1 × IR1 | 6.5 e | 9.9 | 5.5 a | 1.05 | 1.31 abc | 0.96 cd | 0.91 bc | 0.72 bcd | 0.64 c | 0.37 a |
| Chemical × IR2 | 6.8 de | 10.3 | 5.1 abc | 1.01 | 1.17 c | 0.66 f | 0.77 c | 0.76 bc | 1.03 a | 0.29 cde |
| Biologic × IR1 | 7.3 bc | 9.1 | 4.6 cd | 0.94 | 1.24 abc | 1.53 b | 0.97 bc | 0.77 b | 0.59 c | 0.26 e |
| Biologic F1 × IR2 | 7.7 ab | 10.5 | 5.0 bc | 0.98 | 1.19 abc | 0.93 cd | 0.90 bc | 0.63 cd | 0.43 d | 0.29 de |
| Organic × IR1 | 7.7 ab | 9.4 | 4.5 d | 0.99 | 1.31 ab | 0.75 ef | 0.82 c | 0.66 bcd | 0.87 b | 0.32 bcd |
| Organic × IR2 | 7.2 cd | 10.7 | 5.4 ab | 0.98 | 1.18 bc | 0.84 de | 0.84 c | 0.60 d | 0.59 c | 0.32 bc |
| Control × IR1 | 7.1 cd | 9.7 | 5.4 ab | 0.99 | 1.31 ab | 1.83 a | 2.01 a | 1.19 a | 0.14 e | 0.29 cde |
| Control × IR2 | 7.9 a | 10.6 | 5.3 ab | 1.10 | 1.32 a | 1.03 c | 1.18 b | 0.77 b | 0.60 c | 0.35 ab |
| p-value | *** | ns | *** | ns | * | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhawary, S.M.A.; Ordóñez-Díaz, J.L.; Nicolaie, F.; Montenegro, J.C.; Teliban, G.-C.; Cojocaru, A.; Moreno-Rojas, J.M.; Stoleru, V. Quality Responses of Sweet Pepper Varieties Under Irrigation and Fertilization Regimes. Horticulturae 2025, 11, 128. https://doi.org/10.3390/horticulturae11020128
Elhawary SMA, Ordóñez-Díaz JL, Nicolaie F, Montenegro JC, Teliban G-C, Cojocaru A, Moreno-Rojas JM, Stoleru V. Quality Responses of Sweet Pepper Varieties Under Irrigation and Fertilization Regimes. Horticulturae. 2025; 11(2):128. https://doi.org/10.3390/horticulturae11020128
Chicago/Turabian StyleElhawary, Saad Masooud Abdelnaby, Jose Luis Ordóñez-Díaz, Florentina Nicolaie, Jose Carlos Montenegro, Gabriel-Ciprian Teliban, Alexandru Cojocaru, Jose Manuel Moreno-Rojas, and Vasile Stoleru. 2025. "Quality Responses of Sweet Pepper Varieties Under Irrigation and Fertilization Regimes" Horticulturae 11, no. 2: 128. https://doi.org/10.3390/horticulturae11020128
APA StyleElhawary, S. M. A., Ordóñez-Díaz, J. L., Nicolaie, F., Montenegro, J. C., Teliban, G.-C., Cojocaru, A., Moreno-Rojas, J. M., & Stoleru, V. (2025). Quality Responses of Sweet Pepper Varieties Under Irrigation and Fertilization Regimes. Horticulturae, 11(2), 128. https://doi.org/10.3390/horticulturae11020128








