Influence of Habitat Factors on the Yield, Morphological Characteristics, and Total Phenolic/Flavonoid Content of Wild Garlic (Allium ursinum L.) in the Republic of Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.1.1. Soil Properties in the Habitat
2.1.2. Climate Conditions in Habitat
2.2. Collection of Plant Material
2.3. Yield Analysis
2.4. Morphological Analysis
2.5. Analysis of Bioactive Compounds
2.5.1. Extraction Procedure
2.5.2. Determination of Bioactive Compounds
Total Phenolic Content (TPC)
Total Flavonoid Content (TFC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Research Area
3.1.1. Soil Properties in the Habitat
3.1.2. Climatic Conditions in the Habitat
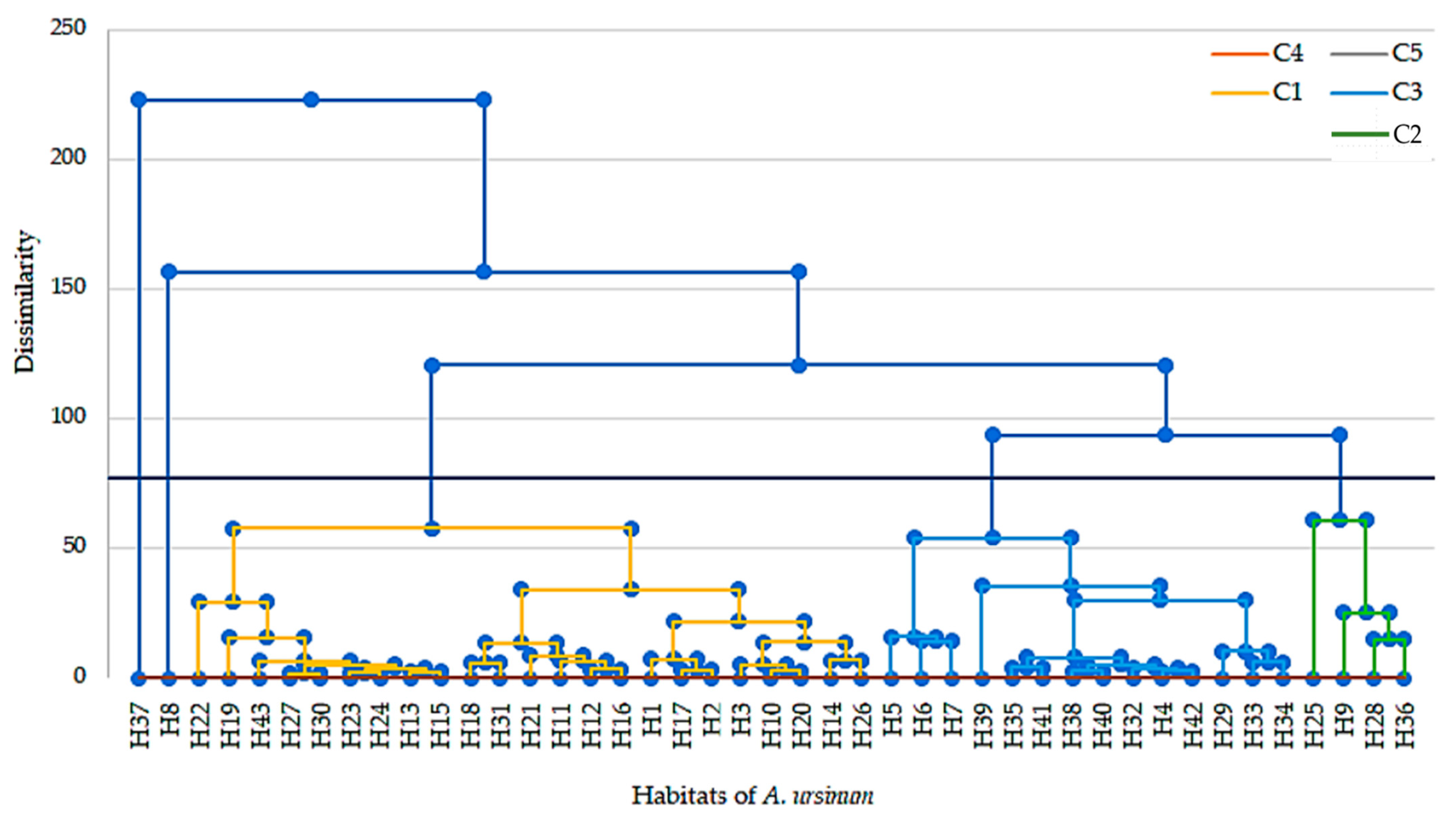
3.1.3. Relationship Between the Habitat of A. ursinum and Climatic and Soil Parameters
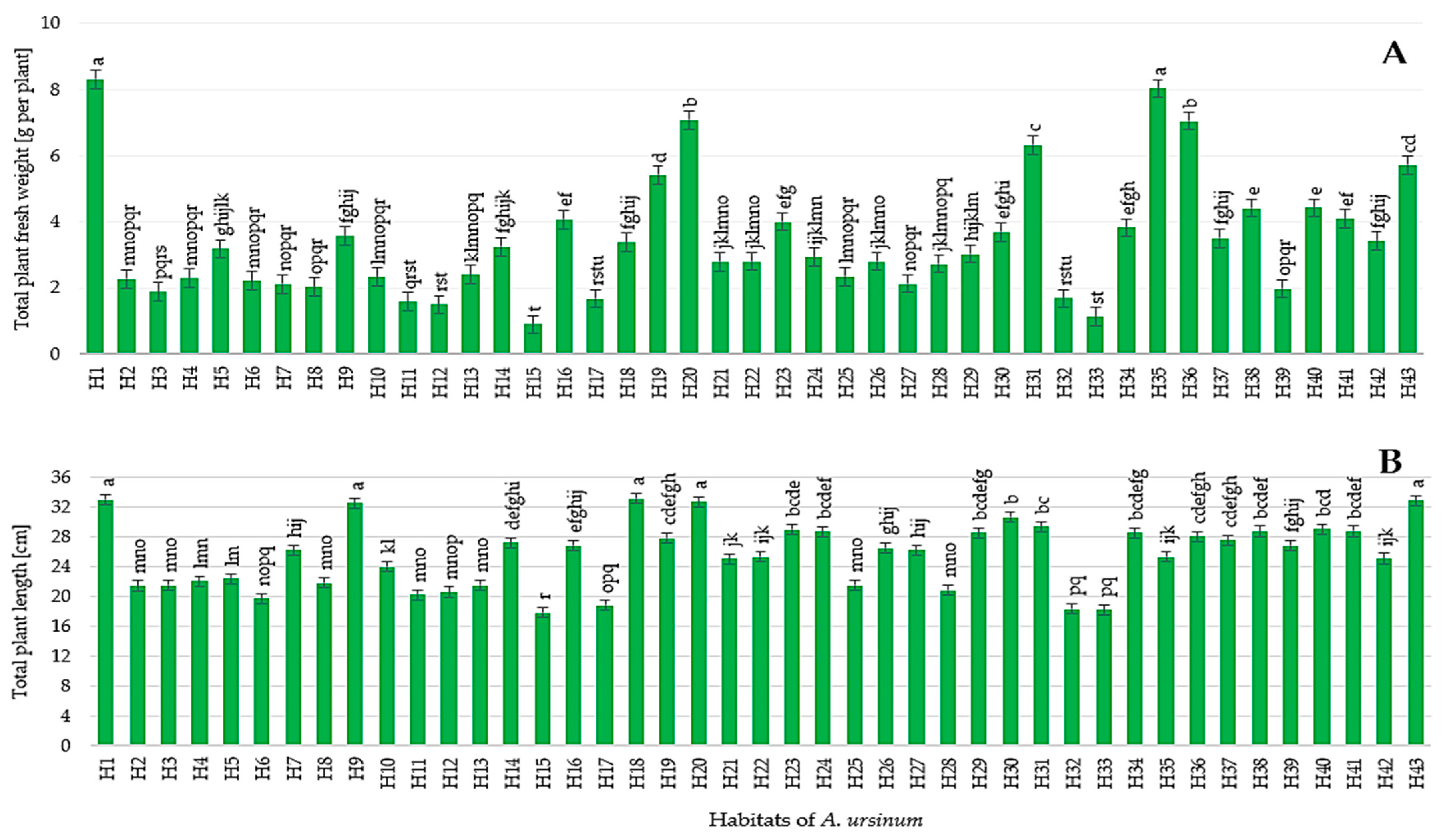
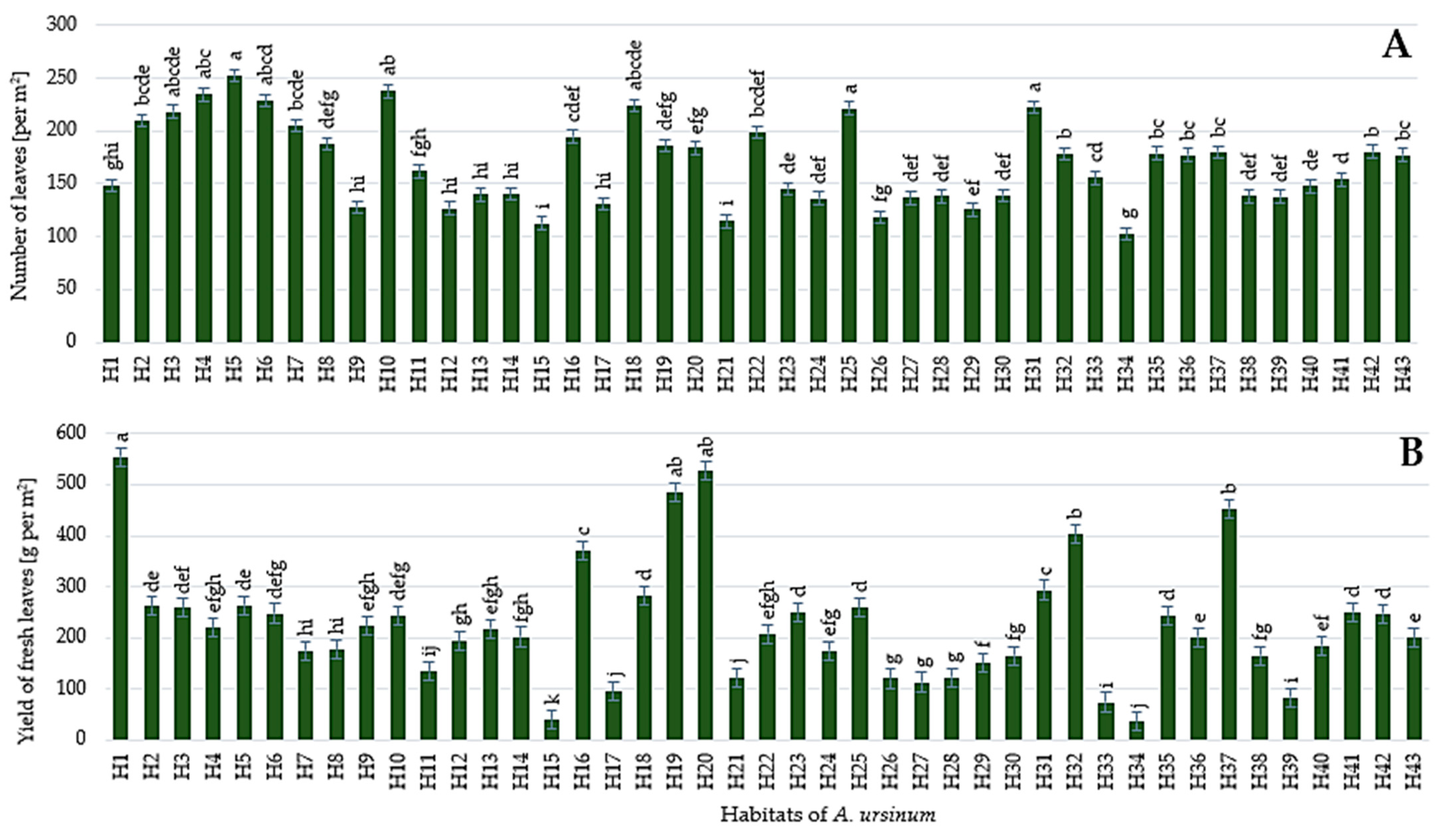
3.2. Morphology and Yield
3.3. Total Phenolic (TPC) and Flavonoid (TFC) Content
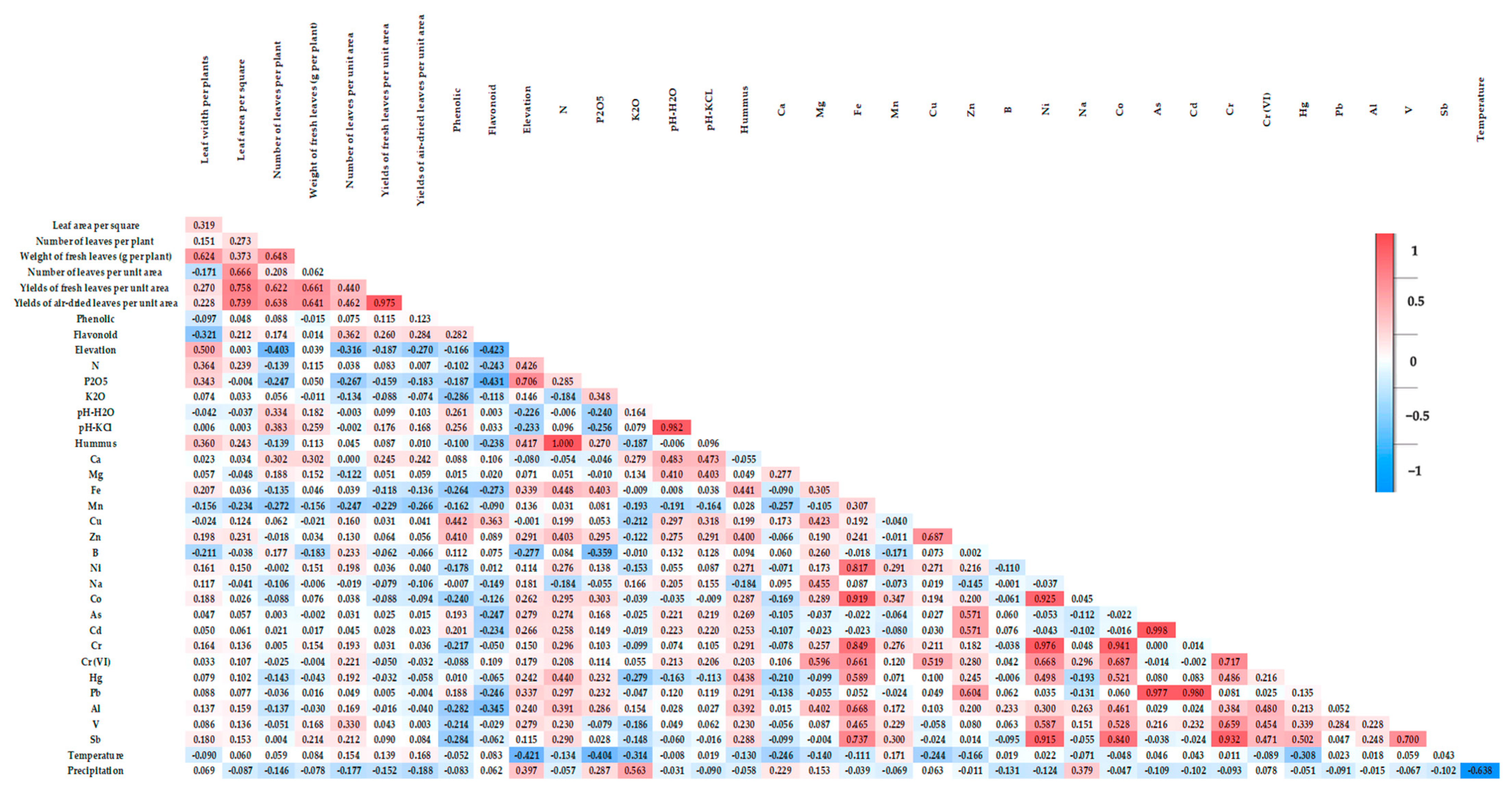
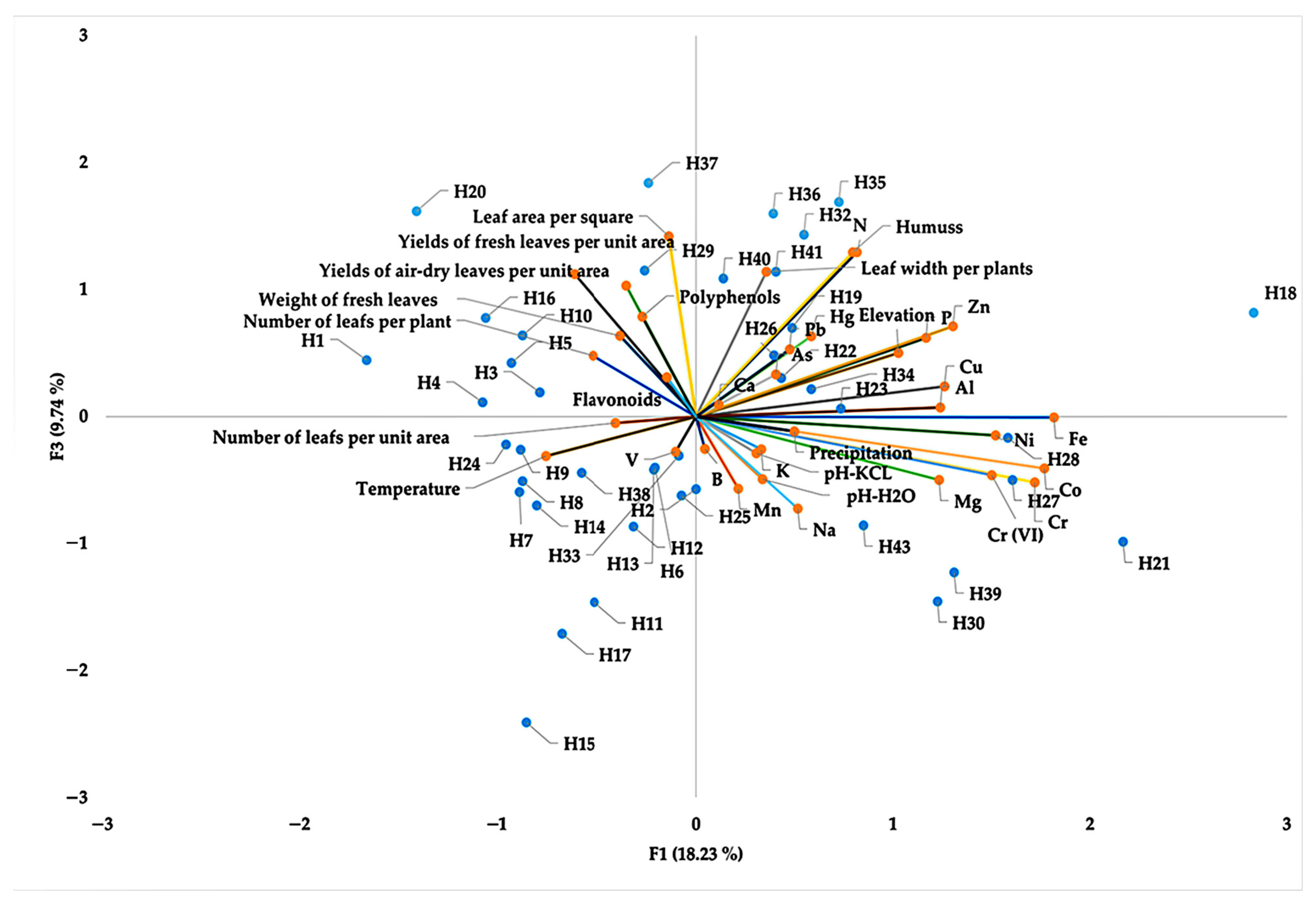
Correlation and Principal Component Analysis (PCA) of Habitat Factors, Yield, Morphological Traits, and Bioactive Compounds in A. ursinum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Herden, T.; Neuffer, B.; Friesen, N. Allium ursinum L. in Germany–surprisingly low genetic variability. Feddes Repert. 2021, 123, 81–95. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Schulz, H.; Storsberg, J.; Keusgen, M. Chemical characterization of Allium ursinum L. depending on harvesting time. J. Agric. Food Chem. 2005, 53, 7288–7294. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Wiśniewski, R. Determination of triterpenoids, carotenoids, chlorophylls, and antioxidant capacity in Allium ursinum L. at different times of harvesting and anatomical parts. Eur. Food Res. Technol. 2018, 244, 1269–1280. [Google Scholar] [CrossRef]
- Štajner, D.; Popović, B.M.; Čanadanović-Brunet, J.; Štajner, M. Antioxidant and scavenger activities of Allium ursinum. Fitoterapia 2008, 79, 303–305. [Google Scholar] [CrossRef]
- Dželetović, Z.; Simić, A.; Marković, J.; Andrejić, G.; Denader, T.; Babić, S. Fertility and chemical composition of forest soils covered with Allium ursinum L. in Serbia. Fresenius Environ. Bull. 2022, 31, 5197–5203. [Google Scholar] [CrossRef]
- Gordanić, S.V.; Kostić, A.Ž.; Krstić, Đ.; Vuković, S.; Kilibarda, S.; Marković, T.; Moravčević, Đ. A detailed survey of agroecological status of Allium ursinum across the republic of Serbia: Mineral composition and bioaccumulation potential. Heliyon 2023, 9, 11. [Google Scholar] [CrossRef]
- Pellissier, L.; Espíndola, A.; Pradervand, J.N.; Dubuis, A.; Pottier, J.; Ferrier, S.; Guisan, A. A probabilistic approach to niche-based community models for spatial forecasts of assemblage properties and their uncertainties. J. Biogeogr. 2013, 40, 1939–1946. [Google Scholar] [CrossRef]
- Wright, I.J.; Groom, P.K.; Lamont, B.B.; Poot, P.; Prior, L.D.; Reich, P.B.; Westoby, M. Leaf trait relationships in Australian plant species. Funct. Plant Biol. 2004, 31, 551–558. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- He, J.S.; Wang, Z.; Wang, X.; Schmid, B.; Zuo, W.; Zhou, M.; Fang, J. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol. 2006, 170, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Crosby, K.M.; Pike, L.M.; Yoo, K.S.; Leskovar, D.I. Impact of genetic and environmental variation on development of flavonoids and carotenoids in pepper (Capsicum spp.). Sci. Hortic. 2005, 106, 341–352. [Google Scholar] [CrossRef]
- Maliníková, E.; Kukla, J.; Kuklová, M.; Balážová, M. Altitudinal variation of plant traits: Morphological characteristics in Fragaria vesca L. (Rosaceae). Ann. For. Res. 2013, 56, 79–89. [Google Scholar]
- Trad, M.; Gaaliche, B.; Renard, C.M.G.C.; Mars, M. Inter- and intra-tree variability in quality of figs. Influence of altitude, leaf area and fruit position in the canopy. Sci. Hortic. 2013, 162, 49–54. [Google Scholar] [CrossRef]
- Bo, F.E.N.G.; Guanghua, X.U.; Xiufeng, W.A.N.G.; Xuejiao, S.U.; Jiayi, L.I.U.; Chenglong, L.I.; Shuyao, S.O.N.G. The relationship between habitat factors and the nutrient contents of wild Allium victorialis L. in the Changbai Mountains. Not. Bot. Horti Agrobo. 2023, 51, 13194. [Google Scholar] [CrossRef]
- Bodó, A.; Farkas, Á.; Nagy, D.U.; Rudolf, K.; Hoffmann, R.; Kocsis, M.; Morschhauser, T. Soil humus, iron, sulphate and magnesium content affect nectar traits of wild garlic (Allium ursinum L.). Plants 2021, 10, 597. [Google Scholar] [CrossRef]
- Blazewicz-Wozniak, M.; Michowska, A. The growth, flowering and chemical composition of leaves of three ecotypes of Allium ursinum L. Acta Agrobot. 2011, 64, 4. [Google Scholar] [CrossRef]
- Śmiecińska, K.; Gugołek, A.; Kowalska, D. Effects of garlic (Allium sativum L.) and ramsons (Allium ursinum L.) on lipid oxidation and the microbiological quality, physicochemical properties and sensory attributes of rabbit meat burgers. Animals 2022, 12, 1905. [Google Scholar] [CrossRef]
- Todorović, V.; Đekić, N.; Antić, M.; Bosančić, B.; Gidas, J.D.; Murtić, S. Morphological characteristics and antioxidant properties of Allium ursinum L. wild growing in the northwestern part of the Republic of Srpska (Bosnia and Herzegovina). JABFQ 2023, 96, 48–54. [Google Scholar] [CrossRef]
- Bernaś, E.; Słupski, J.; Gębczyński, P.; Ražná, K.; Žiarovská, J. Chemical Composition and Genome Pattern as a Means of Identifying the Origin of Preserved Wild Garlic (Allium ursinum L.) in Poland. Agriculture 2023, 14, 20. [Google Scholar] [CrossRef]
- Gordanić, S.; Simić, A.; Radanović, D.; Marković, T.; Mrđan, S.; Vuković, S.; Filipović, V.; Mikić, S.; Moravčević, Đ. Morphological definition populations of Allium ursinum L. from the western part of the Republic of Serbia. In Proceedings of the X International Symposium on Agricultural Sciences AgroReS, Trebinje, Bosnia and Herzegovina, 27–29 May 2021; pp. 104–112. [Google Scholar]
- Republic Hydrometeorological Institute of Serbia (RHMZS). Available online: https://www.hidmet.gov.rs/eng/meteorologija/agrometeorologija.php (accessed on 8 November 2024).
- Kolić, B. Forest Eco Climatology; Naučna knjiga: Belgrade, Serbia, 1988; pp. 1–397. (In Serbian) [Google Scholar]
- Milosavljević, M. Climatology; Naučna knjiga: Belgrade, Serbia, 1990; pp. 1–261. (In Serbian) [Google Scholar]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmiański, J.; Wiśniewski, R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. JFPP 2017, 41, e12921. [Google Scholar] [CrossRef]
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef]
- Ljevnaić-Mašić, B.; Konjević, A.; Džigurski, D. Principles of Ecology, Practical Handbook; Faculty of Agriculture, University of Novi Sad: Novi Sad, Serbia, 2020; p. 109. [Google Scholar]
- Tomšik, A.; Radojčin, M.; Stamenković, Z.; Kevrešan, Ž.; Mastilović, J.; Pavkov, I.; Vidović, S. Convective drying and preservation of functional ingridients of wild garlic (Allium ursinum L.). In Proceedings of the Dependence of Drying Temperature, III International Congress “Food Technology, Quality and Safety”, Novi Sad, Serbia, 25–27 October 2016; pp. 646–650. [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. APPS 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Gordanić, S.; Radanović, D.; Vuković, S.; Kolašinac, S.; Kilibarda, S.; Marković, T.; Kostić, A.Ž. Phytochemical characterization and antioxidant potential of Allium ursinum L. cultivated on different soil types-a preliminary study. EJFA 2022, 34, 904–914. [Google Scholar] [CrossRef]
- Ng, A.; Parker, M.L.; Parr, A.J.; Saunders, P.K.; Smith, A.C.; Waldron, K.W. Physicochemical characteristics of onion (Allium cepa L.) tissues. J. Agric. Food Chem. 2000, 48, 5612–5617. [Google Scholar] [CrossRef]
- Simin, N.; Orcic, D.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Anackov, G.; Beara, I.; Bozin, B. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp. flavum, Alliaceae). LWT-Food Sci. Technol. 2013, 54, 139–146. [Google Scholar] [CrossRef]
- Asemani, Y.; Zamani, N.; Bayat, M.; Amirghofran, Z. Allium vegetables for possible future of cancer treatment. Phytother. Res. 2019, 33, 3019–3039. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, M.J.; Shin, Y.; Jung, S.K.; Kim, Y.J. Green pepper (Piper nigrum L.) extract suppresses oxidative stress and LPS-induced inflammation via regulation of JNK signaling pathways. Appl. Sci. 2020, 10, 2519. [Google Scholar] [CrossRef]
- Škorić, A.; Filipovski, G.; Ćirić, M. Classification of Yugoslav Soils. Special Publications, Tome LXXXVIII; Department of Natural and Mathematical Sciences, Academy of Sciences and Arts of Bosnia and Herzegovina: Sarajevo, Bosnia and Herzegovina, 1985; Volume 13, p. 72. (In Serbian) [Google Scholar]
- Jovanović, D.; Petrović, N.; Marković, M.; Stanković, M. The Influence of Climatic, Geological, Hydrological, and Biological Factors on Soil Types in Serbia. Geol. J. 2017, 32, 45–60. [Google Scholar]
- Nikolić, D.; Petrović, S.; Jovanović, M.; Marković, M. The Role of Geological Factors in Soil Fertility and Their Impact on Wild Garlic Growth. J. Environ. Geol. 2015, 34, 210–219. [Google Scholar]
- Marković, M.; Jovanović, D.; Petrović, N.; Stanković, M. Impact of Rendzina and Ranker Soils on the Growth of Wild Garlic in Mountainous Regions of Serbia. JPNSS 2016, 56, 458–467. [Google Scholar]
- Radovanović, S.; Jovanović, D.; Petrović, S.; Marković, M. Climatic Factors and Their Influence on Soil Fertility and Plant Growth at Different Elevations. Environ. Res. J. 2020, 56, 175–185. [Google Scholar]
- Boltovskoy, E.; Scott, D.B.; Medioli, F.S. Morphological variations of benthic foraminiferal tests in response to changes in ecological parameters: A review. J. Paleontol. 1991, 65, 175–185. [Google Scholar] [CrossRef]
- Atif, M.J.; Amin, B.; Ghani, M.I.; Ali, M.; Cheng, Z. Variation in morphological and quality parameters in garlic (Allium sativum L.) bulb influenced by different photoperiod, temperature, sowing and harvesting time. Plants 2020, 9, 155. [Google Scholar] [CrossRef]
- Tadić, M.; Jovanović, D.; Petrović, N.; Stanković, M. Influence of Forest Ecosystems on Soil Fertility and Wild Garlic Growth. For. Ecol. Manag. 2019, 42, 115–124. [Google Scholar]
- Djordjević, A. Hydrological Factors Affecting Soil Moisture and the Growth of Wild Garlic in Serbia. Environ. Eng. Sci. 2021, 15, 123–134. [Google Scholar] [CrossRef]
- Todorović, V.; Lazić, B.; Igić, R.; Ðurovka, M. The characteristics of different populations of spring onion Allium ursinum L. In Proceedings of the 4 International Symposium of Agriculture, Opatija, Croatia, 16–20 February 2009; Book of Proceedings 44. pp. 454–458. [Google Scholar]
- Jarić, S.; Kostić, O.; Miletić, Z.; Marković, M.; Sekulić, D.; Mitrović, M.; Pavlović, P. Ethnobotanical and ethnomedicinal research into medicinal plants in the Mt Stara Planina region (south-eastern Serbia, Western Balkans). J. Ethnobiol. Ethnomed. 2024, 20, 7. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Mitić, B.; Miladinović, D.; Stefanović, M. Environmental Influences on the Growth and Morphology of Allium ursinum in Different Habitats. Environ. Res. Ecol. 2007, 28, 245–256. [Google Scholar]
- Jakovljević, M.; Petrović, N.; Marković, M.; Stanković, L. Adaptation Strategies of Allium ursinum in Shaded and Illuminated Areas. Ecol. Res. 2019, 45, 112–123. [Google Scholar]
- Lukinac, J.; Jukić, M. Influence of drying temperature on the organoleptic properties, antioxidant activity and polyphenol content in dried leaves of Allium ursinum L. subsp. ucrainicum. Ukr. Food J. 2022, 11, 1. [Google Scholar] [CrossRef]
- Kovarovič, J.; Bystrická, J.; Urminská, D.; Harangozo, Ľ.; Vollmannová, A.; Trebichalský, P.; Carbonell-Barrachina, A.A. Evaluation and comparison of total polyphenols content and antioxidant activity of wild garlic (Allium ursinum L.) in selected morphological parts. JMBFS 2021, 9, 492–495. [Google Scholar] [CrossRef]
- Pejatović Krivokapić, M.; Bradić, J.; Petković, A.; Popović, M. Phytochemical and pharmacological properties of Allium ursinum. SJECR 2018, 22, 357–362. [Google Scholar]
- Voća, S.; Šic Žlabur, J.; Fabek Uher, S.; Peša, M.; Opačić, N.; Radman, S. Neglected potential of wild garlic (Allium ursinum L.)-Specialized metabolites content and antioxidant capacity of wild populations in relation to location and plant phenophase. Horticulturae 2022, 8, 24. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Chandola, V.; Chandra, S.; Nautiyal, A.R.; Concenço, G. Antioxidant potential and impact of different extraction solvents on the free, esterified and insoluble-bound phenolics, flavonoid and tannin content of Trillium govanianum Wall ex D. Don, a rare Himalayan herb. Vegetos 2022, 35, 953–960. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Climate Variables of the Sites of Origin and Genotype Influence on Phenolic Compounds Accumulation in Cultivars of Myrtus communis L. Horticulturae 2022, 8, 928. [Google Scholar] [CrossRef]
- Cornara, L.; Sgrò, F.; Raimondo, F.M.; Ingegneri, M.; Mastracci, L.; D’Angelo, V.; Smeriglio, A. Pedoclimatic Conditions Influence the Morphological, Phytochemical and Biological Features of Mentha pulegium L. Plants 2022, 12, 24. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. TREE 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Deng, B.; Fang, S.; Yang, W.; Tian, Y.; Shang, X. Provenance variation in growth and wood properties of juvenile Cyclocarya paliurus. New For. 2014, 45, 625–639. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Nunez-Ramirez, F.; González-Mendoza, D.; Grimaldo-Juarez, O.; Díaz, L.C. Nitrogen fertilization effect on antioxidants compounds in fruits of habanero chili pepper (Capsicum chinense). IJAB 2011, 13, 827–830. [Google Scholar]
- Sinkovič, L.; Demšar, L.; Žnidarčič, D.; Vidrih, R.; Hribar, J.; Treutter, D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015, 166, 507–513. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Koc, M.; Kulak, M. Monitoring of mineral and polyphenol content in olive leaves under drought conditions: Application chemometric techniques. Ind. Crops Prod. 2016, 88, 78–84. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis, and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Saito, K.; Matsui, K. Plant Phenolics: Natural Sources and Their Applications. BBBIEJ 2013, 77, 1519–1530. [Google Scholar]
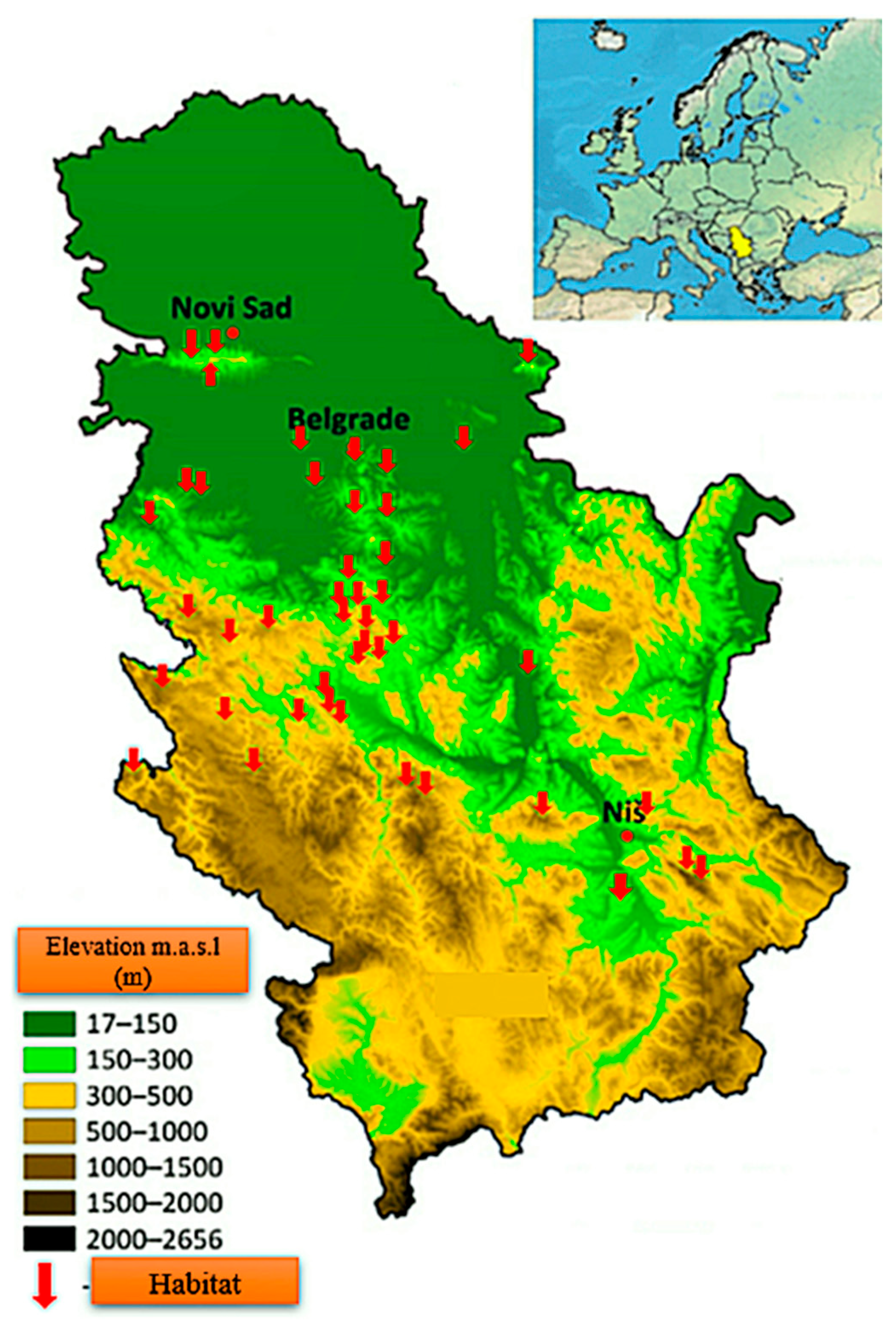








| Habitat [7] | GPS | Elevation a.s.l. (m) | Soil Type [21] | |
|---|---|---|---|---|
| N | E | |||
| H1 | 44°43′46.1″ | 21°01′46.0″ | 70 | Chernozem |
| H2 | 44°36′34.0″ | 20°12′02.8″ | 77 | Eutric cambisol |
| H3 | 44°44′14.3″ | 19°33′52.3″ | 82 | Euglei |
| H4 | 44°44′04.4″ | 19°37′20.1″ | 83 | Euglei |
| H5 | 44°44′41.2″ | 20°08′46.5″ | 83 | Eutric cambisol |
| H6 | 44°33′01.5″ | 20°23′54.4″ | 130 | Eutric cambisol |
| H7 | 44°14′08.9″ | 20°15′44.2″ | 139 | Luvisol |
| H8 | 45°10′03.1″ | 19°29′10.6″ | 200 | Eutric cambisol |
| H9 | 45°07′44.5″ | 19°31′58.8″ | 216 | Eutric cambisol |
| H10 | 44°06′10.7″ | 21°28′29.2″ | 221 | District cambisol |
| H11 | 44°34′43.1″ | 19°22′44.5″ | 222 | Luvisol |
| H12 | 45°09′14.8″ | 19°34′56.4″ | 264 | Eutric cambisol |
| H13 | 44°14′53.6″ | 20°23′55.3″ | 266 | Eutric cambisol |
| H14 | 44°15′46.9″ | 20°20′45.1″ | 268 | Eutric cambisol |
| H15 | 44°39′02.0″ | 20°24′23.0″ | 277 | Eutric cambisol |
| H16 | 45°07′45.2″ | 21°22′09.1″ | 287 | Ranker |
| H17 | 44°36′17.8″ | 20°33′18.2″ | 302 | Luvisol |
| H18 | 43°48′30.5″ | 20°00′41.8″ | 346 | Rendzina |
| H19 | 43°54′13.2″ | 20°12′04.6″ | 359 | Rendzina |
| H20 | 44°10′58.4″ | 20°32′47.6″ | 386 | Luvisol |
| H21 | 43°54′17.7″ | 20°13′20.5″ | 405 | Eutric cambisol |
| H22 | 43°14′37.0″ | 22°06′06.9″ | 502 | District cambisol |
| H23 | 44°05′57.4″ | 20°27′13.8″ | 508 | Eutric cambisol |
| H24 | 44°17′57.7″ | 20°32′08.2″ | 529 | District cambisol |
| H25 | 44°28′11.5″ | 20°34′17.0″ | 550 | Luvisol |
| H26 | 43°24′42.5″ | 21°22′56.1″ | 660 | Ranker |
| H27 | 43°50′33.6″ | 20°17′35.4″ | 662 | Ranker |
| H28 | 44°08′31.2″ | 20°30′29.1″ | 675 | District cambisol |
| H29 | 43°10′45.7″ | 21°32′52.7″ | 684 | Luvisol |
| H30 | 44°06′54.2″ | 19°55′19.2″ | 737 | Redness |
| H31 | 44°06′48.1″ | 20°08′58.8″ | 748 | Luvisol |
| H32 | 43°54′86.3″ | 20°73′88.4″ | 756 | District cambisol |
| H33 | 43°34′01.8″ | 19°20′09.5″ | 776 | Luvisol |
| H34 | 43°24′07.7″ | 21°56′58.3″ | 793 | Luvisol |
| H35 | 44°08′31.3″ | 20°33′20.3″ | 814 | Luvisol |
| H36 | 44°10′21.6″ | 19°37′11.1″ | 823 | Rendzina |
| H37 | 43°55′22.6″ | 20°73′64.9″ | 837 | District cambisol |
| H38 | 43°12′17.6″ | 22°09′12.2″ | 854 | District cambisol |
| H39 | 43°43′58.2″ | 19°42′36.8″ | 996 | Luvisol |
| H40 | 43°54′03.4″ | 19°22′41.0″ | 1021 | District cambisol |
| H41 | 43°36′24.9″ | 19°54′36.3″ | 1053 | Rendzina |
| H42 | 44°07′47.9″ | 20°32′40.1″ | 1061 | Rendzina |
| H43 | 44°07′34.1″ | 19°45′25.8″ | 1211 | Rendzina |
| Meteorological Stations | Nearby Habitats of A. ursinum Populations | |
|---|---|---|
| Place | Elevation a.s.l. (m) | |
| Zrenjanin | 80 | H16 |
| Sremska Mitrovica | 82 | H3; H4; H8; H9; H12 |
| Loznica | 121 | H11 |
| Ćuprija | 123 | H10 |
| Beograd | 132 | H1; H2; H5; H6; H15; H17; H25 |
| Valjevo | 176 | H7; H13; H14; H24; H30; H36; H43 |
| Kragujevac | 185 | H20; H23; H27; H28; H31; H35; H42 |
| Niš | 202 | H22; H26; H29; H38 |
| Kraljevo | 215 | H32; H34; H37 |
| Požega | 310 | H18; H19; H21 |
| Zlatibor | 1028 | H39; H40; H41 |
| Sjenica | 1038 | H33 |
| Elevation a.s.l. Range | Sampling Period | Habitats of A. ursinum | |
|---|---|---|---|
| <500 | March | 2nd week | H1–H8 |
| 3rd week | H9–H14 | ||
| 4th week | H15–H21 | ||
| >500 | April | 1st week | H22–H30 |
| 2nd week | H31–H38 | ||
| 4th week | H39–H43 | ||
| Habitats | Bulb Weight: Total Leaf Weight | Habitats | Bulb Weight: Total Leaf Weight |
|---|---|---|---|
| H1 | 2.0: 1.1 ± 0.235 | H23 | 2.0: 1.1 ± 0.233 |
| H2 | 2.2: 1.4 ± 0.249 | H24 | 2.9: 1.6 ± 0.244 |
| H3 | 1.8: 1.0 ± 0.221 | H25 | 2.3: 1.4 ± 0.229 |
| H4 | 2.3: 1.2 ± 0.239 | H26 | 2.8: 1.7 ± 0.240 |
| H5 | 3.1: 1.9 ± 0.218 | H27 | 2.1: 1.3 ± 0.244 |
| H6 | 2.2: 1.5 ± 0.228 | H28 | 2.7: 1.8 ± 0.246 |
| H7 | 2.1: 1.2 ± 0.237 | H29 | 3.0: 1.8 ± 0.223 |
| H8 | 2.0: 1.0 ± 0.213 | H30 | 3.7: 2.3 ± 0.234 |
| H9 | 3.5: 1.8 ± 0.233 | H31 | 3.1: 2.0 ± 0.258 |
| H10 | 2.3: 1.3 ± 0.247 | H32 | 1.6: 1.2 ± 0.202 |
| H11 | 1.5: 0.9 ± 0.242 | H33 | 1.1: 0.7 ± 0.221 |
| H12 | 1.5: 0.7 ± 0.240 | H34 | 3.8: 2.4 ± 0.235 |
| H13 | 2.4: 1.3 ± 0.221 | H35 | 8.0: 5.9 ± 0.250 |
| H14 | 3.2: 1.8 ± 0.215 | H36 | 7.0: 4.5 ± 0.220 |
| H15 | 1.0: 0.5 ± 0.227 | H37 | 3.5: 2.3 ± 0.239 |
| H16 | 2.0: 1.0 ± 0.245 | H38 | 4.3: 1.7 ± 0.242 |
| H17 | 1.6: 1.1 ± 0.230 | H39 | 1.9: 1.3 ± 0.228 |
| H18 | 3.3: 2.1 ± 0.241 | H40 | 4.4: 3.1 ± 0.223 |
| H19 | 5.4: 2.8 ± 0.237 | H41 | 2.0: 1.2 ± 0.229 |
| H20 | 7.0: 4.2 ± 0.250 | H42 | 3.4: 2.0 ± 0.233 |
| H21 | 2.8: 1.7 ± 0.228 | H43 | 5.7: 4.0 ±0.237 |
| H22 | 2.8: 1.8 ± 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordanić, S.V.; Kostić, A.Ž.; Moravčević, Đ.; Vuković, S.; Kilibarda, S.; Dragumilo, A.; Prijić, Ž.; Lukić, M.; Marković, T. Influence of Habitat Factors on the Yield, Morphological Characteristics, and Total Phenolic/Flavonoid Content of Wild Garlic (Allium ursinum L.) in the Republic of Serbia. Horticulturae 2025, 11, 118. https://doi.org/10.3390/horticulturae11020118
Gordanić SV, Kostić AŽ, Moravčević Đ, Vuković S, Kilibarda S, Dragumilo A, Prijić Ž, Lukić M, Marković T. Influence of Habitat Factors on the Yield, Morphological Characteristics, and Total Phenolic/Flavonoid Content of Wild Garlic (Allium ursinum L.) in the Republic of Serbia. Horticulturae. 2025; 11(2):118. https://doi.org/10.3390/horticulturae11020118
Chicago/Turabian StyleGordanić, Stefan V., Aleksandar Ž. Kostić, Đorđe Moravčević, Sandra Vuković, Sofija Kilibarda, Ana Dragumilo, Željana Prijić, Milan Lukić, and Tatjana Marković. 2025. "Influence of Habitat Factors on the Yield, Morphological Characteristics, and Total Phenolic/Flavonoid Content of Wild Garlic (Allium ursinum L.) in the Republic of Serbia" Horticulturae 11, no. 2: 118. https://doi.org/10.3390/horticulturae11020118
APA StyleGordanić, S. V., Kostić, A. Ž., Moravčević, Đ., Vuković, S., Kilibarda, S., Dragumilo, A., Prijić, Ž., Lukić, M., & Marković, T. (2025). Influence of Habitat Factors on the Yield, Morphological Characteristics, and Total Phenolic/Flavonoid Content of Wild Garlic (Allium ursinum L.) in the Republic of Serbia. Horticulturae, 11(2), 118. https://doi.org/10.3390/horticulturae11020118







