1. Introduction
Lilium (
Lilium sp.) is a famous cut flower that is grown commercially all over the world [
1] as one of the most important cut flowers in the international market [
2]. In the process of lily production, postharvest quality parameters, such as the color of the flower, flower size, stem length and diameter, and flower longevity, can be greatly influenced by the type of cultivar, application of plant nutrients, and growing media as preharvest factors [
3]. The quality and quantity of cut lily flowers also depend on the composition of the culture medium [
4], ensuring good aeration, water retention, drainage, and physical structure. Poorly drained heavy soils hinder root development and increase susceptibility to soil diseases [
5].
In the future, more focus will be needed on how to use natural resources for more efficient production. However, about a quarter of agricultural land worldwide has been declared unproductive [
6]. The factors behind this unproductivity are inadequate and improper crop management, soil degradation, rapid changes in regional climate, rapid urbanization, industrialization, continuous cultivation, frequent droughts, water pollution, and groundwater depletion [
7]. Currently, several hydroponic and aeroponic soilless cultivation techniques are used [
8]. It has been supposed that the characteristics of the cultivation medium, directly and indirectly, affect plant growth and crop production [
9].
For optimal plant growth, the substrate must supply enough water and air, as well as macro- and micronutrients [
10]. A hydroponic system uses a nutrient-rich solution to supply essential nutrients, usually with inert substrates to sustain plant growth [
10,
11,
12,
13]. Aeroponic is a type of hydroponic system with an air–water growing system where plant roots are freely suspended in the air under controlled conditions inside a closed container in the dark, and nutrient solution is provided by atomizing nozzles. Nozzles intermittently or continuously create a fine spray mist of different droplet sizes [
7]. This system is commonly used in plant physiology research but is not yet widely used on a commercial scale to the extent of other hydroponic methods [
12]. Recently, in a type of aeroponic system called ultrasonic, water is sprayed on the roots in the form of a mist using ultrasound waves [
13]. Some researchers have reported that ultrasonic waves can change the physical and chemical properties of the water containing the nutrient solution, which can ultimately affect plant performance [
14,
15,
16,
17,
18,
19]. In a piece of research, it was reported that in the ultrasonic atomization system, the roots of lettuce uniformly absorbed nutrients and moisture, and also increased root growth and yield, and reduced water consumption [
18]. In this regard, our previous research revealed that the best pulses for
Lilium performance were 50% or 10% time function for ultrasonic or aeroponic systems, respectively [
19].
Nutritional studies about
Lilium are of interest because geophytes store nutrients and carbohydrates in the bulb, and this may influence the plant’s response to external nutrient concentrations [
20]. Also, lily has a moderate demand for nitrogen and a small demand for calcium. This low requirement may be due to the redistribution of nutrients stored in the bulbs [
20]. It is clear that calcium plays a key role in the growth of plant structures, including the bulbs and tubers. In research on two lily cultivars, “Serda” and “Navona”, with the application of 40 g per square meter of nitrogen from two sources (calcium nitrate and urea), the relative growth rate of plants fed with calcium nitrate was higher than that of plants fed with urea. This was attributed to the N absorbed in the nitrate form and the absorption of calcium from calcium nitrate, which increases nitrogen absorption and meristem activities. Nitrogen in the form of nitrate in calcium nitrate significantly enhanced plant growth rate, leaf surface area, dry matter accumulation, and bulb yield [
21]. It has been reported that some characteristics of Tuberose affected by preharvest application of calcium chloride and preharvest foliar spraying of gladiolus with 200 mM calcium increased its corm size [
22,
23]. Foliar application of calcium nano fertilizer on the
Gerbera flowers showed that the diameter, height, and fresh weight of cut
Gerbera flowers were higher compared to the control [
24].
Based on the effectiveness of nutritional balance and the adaptation of plant physiology to meet the demands for beneficial lily cut flower production, a detailed understanding of the physiological changes in the lily is required [
25]. On the other hand, the influence of ultrasonic technology on plant nutrition and its response or performance has not been studied or compared with aeroponics or hydroponics yet. So, in this aspect, different soilless cultivation systems (aeroponic, ultrasonic, hydroponics in pots and containers) were evaluated using nutrient solutions differing in two variables: the ratio of ammonium to total nitrogen and the ratio of calcium to total cations in this experiment, and the morpho-physiological and biochemical responses of the lily were evaluated. Based on the results, the best system and treatment were selected. Finally, the biochemical and anatomical characteristics of the treated bulbs in the superior system were compared with the primary bulbs that had been stored and untreated.
2. Materials and Methods
In this experiment,
Lilium bulbs (
Lilium Oriental × Trumpet (Orienpet; OT) Hybrid cv. ‘Zambesi’) were planted in four soilless cultivation systems (hydroponics in pots and containers, aeroponics, and ultrasonic), and fed with three nutrient solution formulations (focusing on two variables, the ratio of ammonium to total nitrogen and the ratio of calcium to total cations) (NS1 as control based on Hoagland, NS2, NS3) (
Table 1). Also, all nutrient solutions (NSs) contained similar amounts of microelements (
Table 2). Nutrient solutions were prepared in distilled water based on mM accurate weight calculation and measurement of elements. The experiment was performed in a growth chamber with automatic control of environmental conditions regarding temperature, light, and humidity in the workshop building of the Department of Agricultural Machinery Engineering, Faculty of Agriculture, University of Tehran, Iran. Growing conditions included day/night temperatures of 23/18 ± 2 °C, relative humidity of 70%, light intensity of 150 μmol m
−2 s
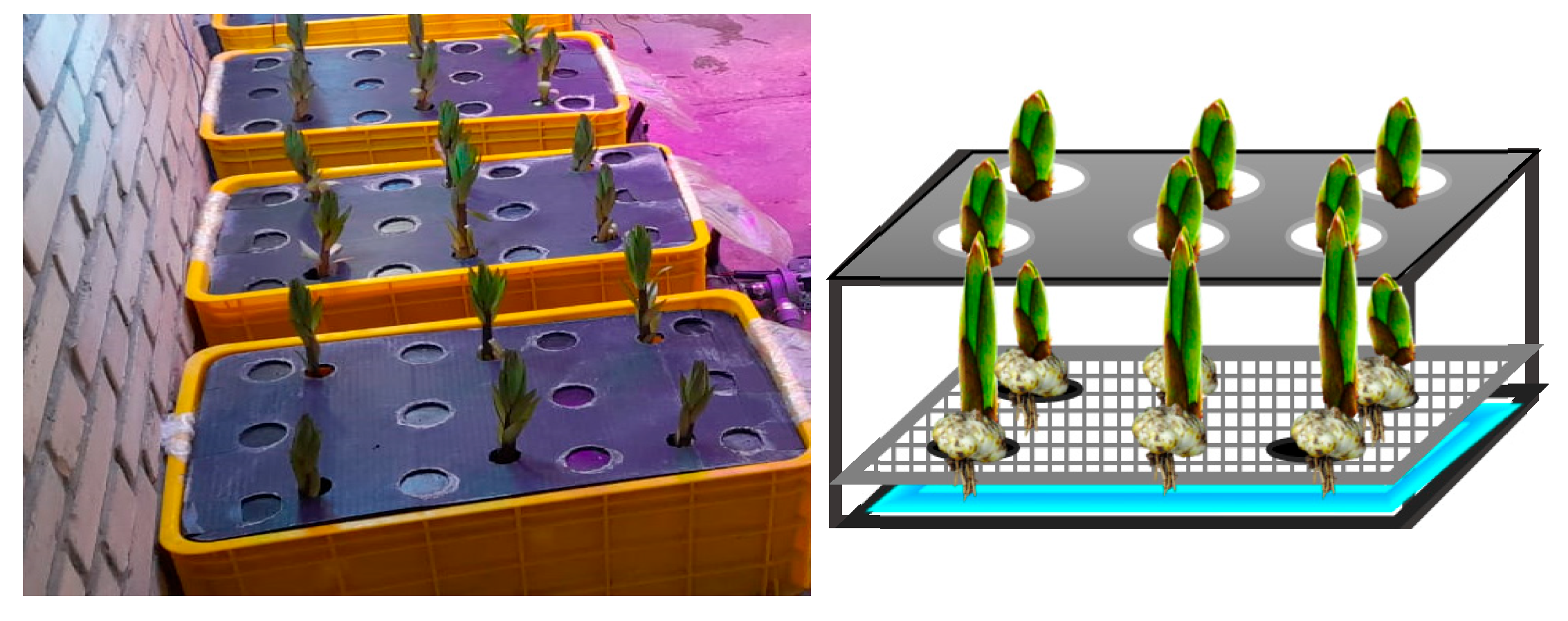
−1 (artificial light supplied by LED lamps with a red/blue ratio of 4:1), and 16/8 h of day/night photoperiod. Cultivation containers (for ultrasonic and aeroponics systems) were used with a length, width, and height of 55, 32, and 25 cm, respectively. Black polycarbonate sheets were installed on the containers. There were six holes (2 × 3) on this plate sheet to stabilize the growing stems (
Figure 1). Before cultivation, hybrid lily bulbs were disinfected with 1 mL L
−1 Mancozeb fungicide (manganese/zinc ethylene bisdithiocarbamate as active ingredient) (Dithane M-45, Golsam Gorgan Chemicals Co., Gorgan, Iran), and were placed at a depth of 8 cm below the surface of these holes, on an isolated plastic basket above the nutrient solution, so that the bulbs and roots were not immersed in the nutrient solution.
Leveling floats and drain lines were considered for the proper functioning of ultrasonic and aeroponic systems and for preventing upturn of the surface of the nutrient solution, since solution delivery was continuous and closed in these systems (
Figure 2). In this study, the ultrasonic fogging system was designed based on the piezoelectric phenomenon. Piezoelectric ceramics were installed on the lower surface of the containers, and for each container, four piezoelectric ceramics were used. As a result of the impact of these ceramics, small water particles (approximately 5 to 150 microns in size) were suspended, and with increasing the contact surface area and the possibility of surface evaporation, the relative humidity of the plant root environment increased significantly to over 95%. In the ultrasonic system, the frequency was 1.3 MHz, and the level of fogging on/off time occurred in seconds of 10 to 10 or 50% system time operation [
19]. In the aeroponic system, there were six centrifugal nozzles in each container, and the level of fogging on/off time occurred in seconds of 3 to 27 or as 10% of system time operation [
19]. The solution pipelines were arranged separately, and the containers were placed precisely. The length distance of the containers from the composition was 33 cm, and their width distance was 11 cm. The automatic nutrient solution operation schedule was established based on maintaining a constant volume of the nutrient solution in each container. The nutrient solution was changed based on a recording of the pH and EC of the nutrient solution during the experiment. Accordingly, a pH of 5.7 ± 0.2 remained constant, and EC was adjusted to 2000 ± 100 μS cm
−1 when dropping below 1100 μS cm
−1 [
19]. After changing the nutrient solution, EC values in NS1, NS2, and NS3 were 2000, 2100, and 2000 μS cm
−1, and the pH values were 7.1, 7.2, and 7, respectively, and adjusted by phosphoric acid to the range of 5.7 ± 0.2. After about 10 days, it was necessary to change the nutrient solution again.
In the hydroponic systems, pots with an opening of 20 cm and containers with a length, width, and height of 55, 32, and 25 cm, respectively, were used. Also, the culture medium contained an equal volume ratio of cocopeat and perlite (size was 1.5 to 2 mm). Bulbs after disinfection (with the fungicide mancozeb at a concentration of 1:1000) were planted at a depth of 8 cm in both systems, and the nutrient solution applications were based on maintaining the water-holding capacity of the medium. The water-holding capacity of the medium was determined by maintaining the humidity of the medium by weight [
26]. The medium moisture was maintained at 60% (
v/
v) of its water-holding capacity by weighing and watering (fertigation) the pots and hydroponic containers before and after. They were weighed daily, and the decreased amount of water was re-added to maintain a constant substrate moisture content [
26]. Then, sampling was conducted, and traits were evaluated when flower buds reached the commercial harvest index [
27]. Finally, plant growth responses were evaluated as affected by different systems and nutrient solutions.
Experimental design was planned as a factorial, based on a randomized complete block design with a total of 12 treatments, including four soilless cultivation systems (hydroponics in pots and containers, aeroponics, and ultrasonic) × three nutrient solution formulations (NS1, NS2, NS3) and six plants in each container (three replications and two observations) = 72 plants). Analysis of variance was conducted, and post hoc comparisons were performed using the Tukey test at the 5% probability level using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Factor analysis was performed with SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA), and evaluation of bulbs was carried out with a t-test.
2.1. Measurement of the Quantitative Traits of Lily
The stem height was measured from the collar to the branch tip in centimeters. To measure root length, the fresh roots were cut into 1 cm segments, and 0.3 g were randomly poured into a mesh tray (dimensions of 2 × 2 cm) containing 0.5 cm of water. Then, the number of roots that intersected the horizontal and vertical lines was counted separately. Finally, the root length was calculated from the following equation:
where X is the root length for 0.3 g, 11/14 is a calculated and shortened coefficient (indeed it was converted from π/4 in which π is equal to 22/7 and if we multiply that with ¼ the result is 11/14), r is the dimensions of the squares of the grid plate (here 2 cm), H is the number of roots that intersected the horizontal lines, and U is the number of roots that intersected the vertical lines. Then, the number obtained from the formula (X) was multiplied by the fresh weight of the whole root, and the length of the whole root was calculated [
28]. The diameter of the opened flower was calculated by measuring the ends of the opened petals in centimeters. Regarding the size of the bulbs and bulblets, their circumference was measured in centimeters. To determine vase life, the samples were placed in containers containing 400 mL of distilled water at room temperature (average temperature 18 ± 2 °C, relative humidity of 45 ± 5% and 16/8 h light/dark photoperiod), and when 50% of the flowers of a branch were lost (dropped, withered, or turned brown), it was considered the end of the vase life [
27].
2.2. Evaluation of Growth Indices of Lily
To evaluate the growth indices of relative growth rate (RGR), net assimilation rate (NAR), leaf area index (LAI), and leaf area ratio (LAR), fresh and dry weights of leaves and shoots were calculated in two stages. The first stage was the 16th DAP (days after planting), and the second stage was the 66th DAP. Fresh weight was measured in grams with a digital scale (accuracy of 0.01 g). Then, each of the parts was placed separately in paper envelopes, dried in an oven at 75 °C until a constant weight was reached, and then its dry weight was measured. To measure the leaf area, the leaves were photographed next to an index (ruler). Then, the leaf area of the samples was calculated using Digimizer software version 9.1(MedCalc Software Ltd., Ostend, Belgium). The following formulas were used to calculate growth components:
RGR = Relative Growth Rate, NAR = Net Assimilation Rate, LAI = Leaf Area Index, LAR = Leaf Area Ratio, Ln= natural logarithm, t1 = initial time (16th DAP), t2 = later time (66th DAP), W1 = dry weight of aerial parts at t1 (g), W2 = dry weight of aerial parts at t2 (g), LA1 = leaf area of the whole plant at t1 (m
2), LA2 = the leaf area of the whole plant at t2 (m
2), E = area of land occupied by the plant at t2 (m
2).
2.3. Measuring of the Biochemical Traits of Lily
Leaf anthocyanins were measured by Wagner’s method [
29]. To measure anthocyanin content, 0.10 g of leaf was soaked in 10 mL of methanol/hydrochloric acid (99:1) in darkness for 24 h. Absorbance was then measured at 550 nm, pigment concentration was calculated according to Wagner (1979), and the measurement of carotenoids was conducted by Arnon’s method [
30]. Total carotenoid concentration in leaves was measured after extraction with 80% acetone. Absorbance was read at 645, 663, and 470 nm, and pigment concentrations were calculated according to Arnon.
2.4. Measurement of Chlorophyll Fluorescence Indices of Lily
Chlorophyll fluorescence imaging of leaves attached to the plant near the harvest stage of each treatment enabled biophysical measurements after placing the plants in darkness for 20 min. The temperature for these measurements was the same as during the experiment. The leaves were subjected to Fv/Fm assessment using a fluorometer equipped with an imager (Handy FluorCam FC 1000–H, Photon Systems Instruments, Drásov, Czech Republic). The calculation of FV/FM occurred on a custom-made protocol [
31]. For details of photosynthetic parameters, see
Table 3.
2.5. Evaluation of Changes in Biochemical and Anatomical Characteristics of Hybrid Lily Bulbs Cultivated in the Superior System with Primary Bulbs
The evaluated traits included root length, bulb size, anthocyanins, soluble carbohydrate content, inhibition of DPPH free radicals, hydrogen peroxide, and malondialdehyde. For measuring soluble carbohydrates, 0.1 g of bulb tissue was placed in a closed tube with 5 milliliters of 95% ethanol ((Kimia Alcohol Co., Tehran, Iran) and heated in a bain-marie bath at 80 °C for 1 h. After cooling, 1 mL of these samples was removed, and 1 mL of 0.5% phenol and 5 mL of 98% sulfuric acid were added. Finally, absorbance at 483 nm was read with a spectrophotometer, and soluble carbohydrate content was calculated as mg glucose per g of fresh weight [
32]. To evaluate DPPH free radical inhibition rate, bulb extracts were prepared at a concentration of 100 mg mL
−1 in 80% methanol (Merck; distributed by Kimia Pardazesh Pars Co., Tehran, Iran). In this method, 1 g of fresh scales of each bulb was rubbed with 10 mL of 80% methanol and then filtered. DPPH free radicals were used to measure the antioxidant activity of the extracts. A 1:1 mixture of DPPH solution (100 mL of pure methanol plus 0.004 g of DPPH powder) and the bulb extracts was prepared. The absorbance was measured after 40 min in the dark and at room temperature using a spectrophotometer at 517 nm. The inhibition percentage was calculated with the following equation [
33]:
R = DPPH free radical inhibition percentage, AD = absorbance of DPPH solution at 517 nm, AS = absorbance of sample at 517 nm.
Hydrogen peroxide content was estimated by observing the absorbance of the titanium peroxide compound at 410 nm. 0.2 g of fresh bulb scales was homogenized with 5 mL of acetone and centrifuged at 3000 rpm for 10 min. The reaction mixture contained 0.1 mL of a titanium reagent (50 μL of 20% titanium tetrachloride in concentrated hydrochloric acid) (Sigma-Aldrich; distributed by Parto Shimi Co., Tehran, Iran), 0.2 mL of ammonia and 1 mL of the supernatant. It was centrifuged for 10 min at 3000 rpm. The precipitate was washed five times with acetone and centrifuged at 10,000 rpm for 5 min. The resulting sediment was dissolved in 3 mL of sulfuric acid (1 M), and its absorbance was read at 410 nm. The hydrogen peroxide content was calculated based on a standard curve [
34].
Lipid peroxidation was evaluated by measuring malondialdehyde (MDA) using the thiobarbituric acid test at wavelengths of 450, 532, and 600 nm. For MDA, 0.2 g of fresh bulb scales was ground in 5 mL of 1% trichloroacetic acid and centrifuged at 5000 rpm for 5 min at 4 °C. Then, 1 mL of the supernatant was added to 4 mL of 20% tricarboxylic acid (containing 0.5% thiobarbituric acid). The mixture was heated at 95 °C for 30 min. To stop the reaction, the mixture was cooled quickly in an ice bath. The absorbance of the supernatant was measured at wavelengths of 450, 532, and 600 nm (OD450, OD532, and OD600), respectively. Eventually, the MDA content was calculated from the following formula [
35]:
where MDA is malondialdehyde (mol g
−1FW), and OD450, OD532, and OD600 are measured absorbance at wavelengths of 450, 532, and 600 nm, respectively.
2.6. Statistical Analysis
This experimental design was planned as a factorial, based on a randomized complete block design with a total of 12 treatments, including four soilless cultivation systems (hydroponics in pots and containers, aeroponics, and ultrasonic) × three nutrient solution formulations (NS1, NS2, NS3)) and six plants in each container (three replications and two observations) = 72 plants. Analysis of variance was conducted, and post hoc comparisons were performed using the Tukey test at the 5% probability level using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). The least significant difference (LSD) test was used to compare the means (p = 0.05). Cramer–Von Mises was used to test the normality of the data, and all data were analyzed under normal distribution conditions. Factor analysis was performed with SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA), and evaluation of bulbs was carried out with a t-test (with three treatments in three replications and two observations).
3. Results
The analysis of variance revealed that the nutrient solution significantly influenced plant height, root length, bulblet size and number, days to flowering, flower diameter, vase life, minimum, maximum, and variable fluorescence (Fo, Fm, Fv), Fv/Fm ratio, net assimilation rate (NAR), leaf area index (LAI), and leaf area ratio (LAR). However, no significant differences were observed regarding bulb size, number of buds, number of hairy roots, anthocyanins, carotenoids, and relative growth rate (RGR). Similarly, the type of cultivation system significantly affected height, root length, number and size of bulblets, days to flowering, flower diameter, number of hairy roots, vase life, carotenoid content, minimum, maximum, and variable fluorescence, Fv/Fm ratio, RGR, NAR, LAI, and LAR, whereas bulb size, number of buds, and anthocyanin content remained unaffected (
Table 4).
3.1. Characteristics of Lily Roots
3.1.1. Root Length
The comparison of means indicated the greatest root length in hydroponic cultivation (pot system) using nutrient solution NS1. Conversely, the shortest root length was recorded in the aeroponic system with NS2. Additionally, hydroponic container systems and aeroponic systems, both with NS1, showed root lengths statistically similar to the maximum observed length (
Table 5).
3.1.2. Number of Hairy Roots
The results indicated that the ultrasonic system using NS3 produced the highest number of hairy roots. In contrast, the hydroponic system (container) with NS2 resulted in the lowest number of hairy roots. Moreover, the ultrasonic and aeroponic systems generally had higher numbers of hairy roots compared to both hydroponic systems (
Table 5).
3.2. Characteristics of Lily Bulbs
Number and Size of Bulblets
The comparison of means demonstrated the highest number of bulblets in hydroponic pots with NS2. Regarding bulblet size, the hydroponic container system with NS2 yielded the largest bulblets. In contrast, aeroponic systems (across all nutrient solutions), ultrasonic systems with NS3, and hydroponic container systems with NS1 and NS3 showed significantly lower numbers and sizes of bulblets (
Table 5).
3.3. Characteristics of Lily Flowers
3.3.1. Stem Height
The tallest lilies (115 cm) were produced by the aeroponic system using NS3, while the shortest stems (88.16 cm) were observed in hydroponic pots with NS1 (
Table 6). Intermediate stem heights were recorded for other treatments.
3.3.2. Days to Flowering
The fewest days to flowering (66–67 days) were noted in the aeroponic system with NS2 and NS3, in the ultrasonic system with NS1, NS2, and NS3, in the hydroponic (container) system with NS2, and the hydroponic (pot) system with NS1. The longest flowering period (81 days) was found in the hydroponic system (container) with NS3 (
Table 6).
3.3.3. Number of Buds
No statistically significant differences in bud numbers were observed across treatments (
Table 5). However, ultrasonic systems, especially with NS1 and NS3, tended to produce slightly higher bud numbers, which is noteworthy for cut-flower quality.
3.3.4. Opened Flower Diameter
The largest flower diameter was recorded in the hydroponic (pot) system of NS1 and NS3, in the hydroponic (container) system of NS3 and in the ultrasonic and aeroponic systems with NS2. Also, in the aeroponic system, NS2 showed better results than NS1 or NS3 (
Table 6).
3.3.5. Vase Life
The longest vase life (6 days) was achieved in the aeroponic system using NS1 and NS3, and in hydroponic containers using NS2. The shortest vase life (3 days) occurred in ultrasonic systems and hydroponic pots, especially those using NS2 (
Table 6).
3.4. Growth Indices of Lily
3.4.1. Relative Growth Rate (RGR)
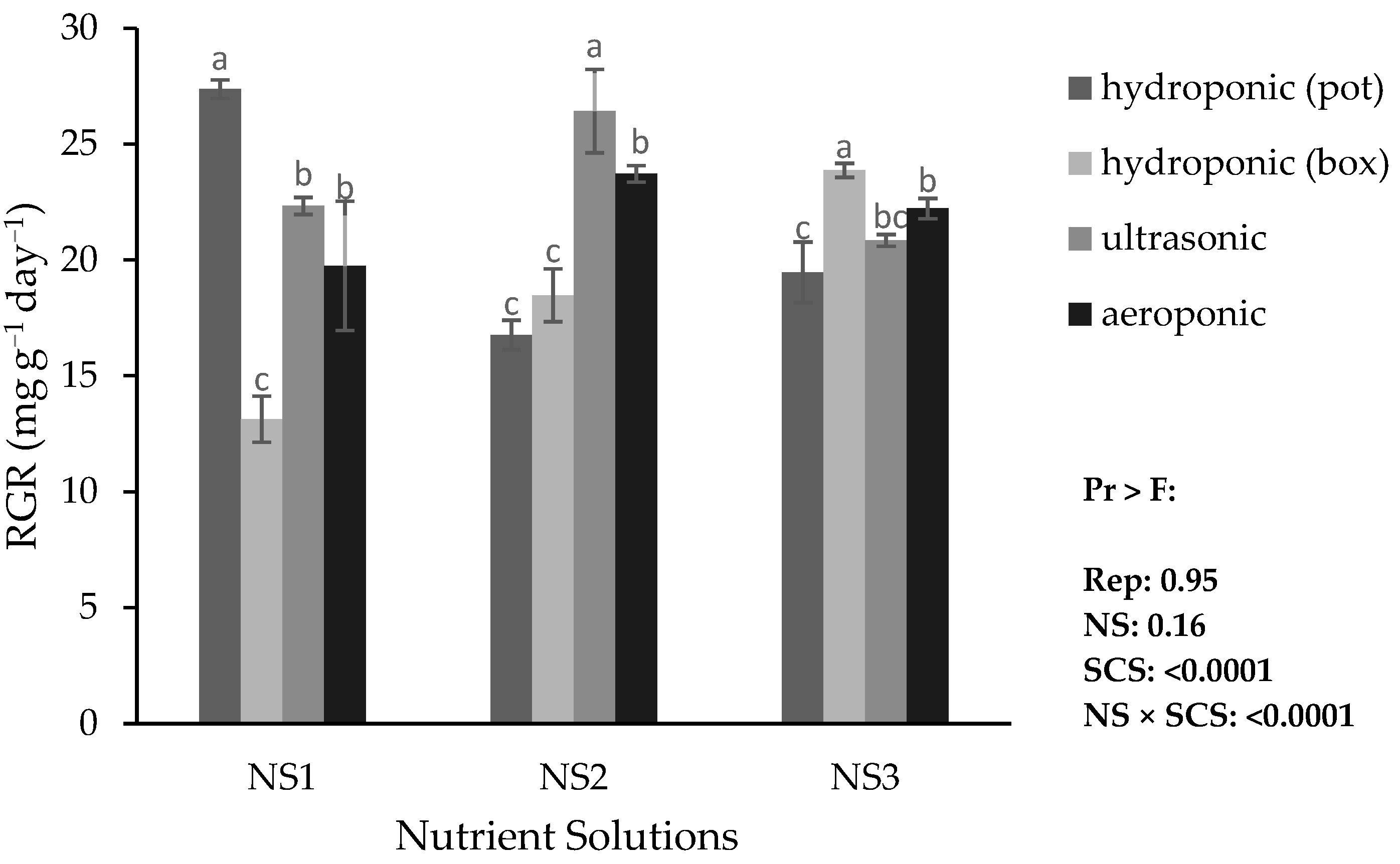
The highest RGR was recorded in hydroponic pots with NS1 (with a ratio of calcium to cations of 0.5 and ammonium nitrogen to total nitrogen of 0.1, control based on Hoagland solution) and in the ultrasonic system in NS2 (with a ratio of Ca to cations of 0.7 and ammonium nitrogen to total N of 0.15). The lowest RGR was obtained in the hydroponic system (container) with NS1 (
Figure 3). The highest RGR in the hydroponic system (container) was in NS3, and in the aeroponic system was in NS2. In other words, in hydroponic pots with NS1, in the ultrasonic system with NS2, and in hydroponic containers with NS3, plants had better growth. Different systems showed different results in this research.
3.4.2. Net Assimilation Rate (NAR)
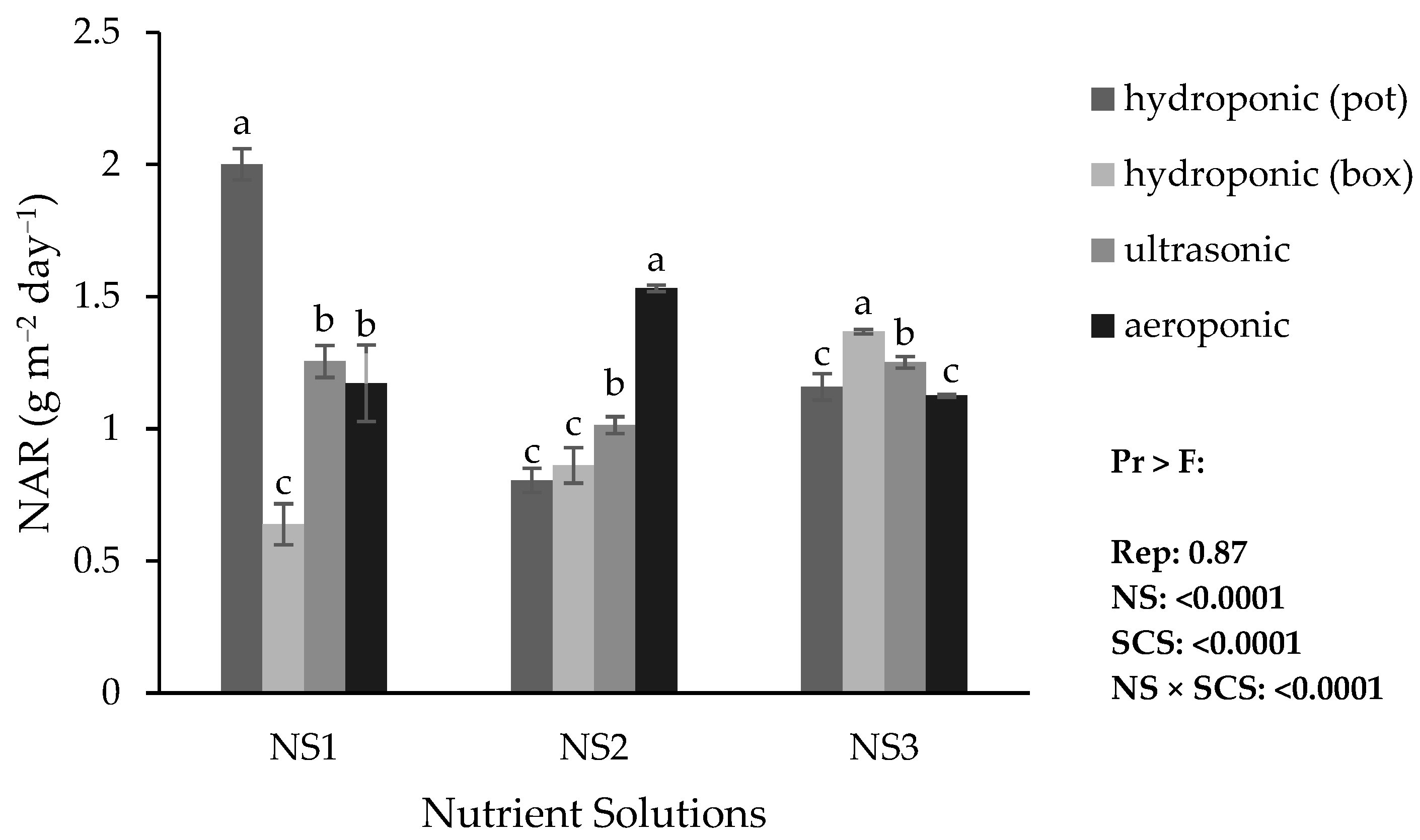
The highest NAR was obtained in the hydroponic (pot) system with NS1, whereas the lowest NAR occurred in the hydroponic (container) system with the same nutrient solution (NS1) (
Figure 4). In addition, the highest NAR within the hydroponic (container) system was observed with NS3, within the ultrasonic system with NS1 and NS3, and within the aeroponic system with NS2. In other words, with NS1, the hydroponic (pot) system had the better NAR; with NS2, the aeroponic system had the better NAR; and with NS3, the hydroponic (container) system had the better NAR.
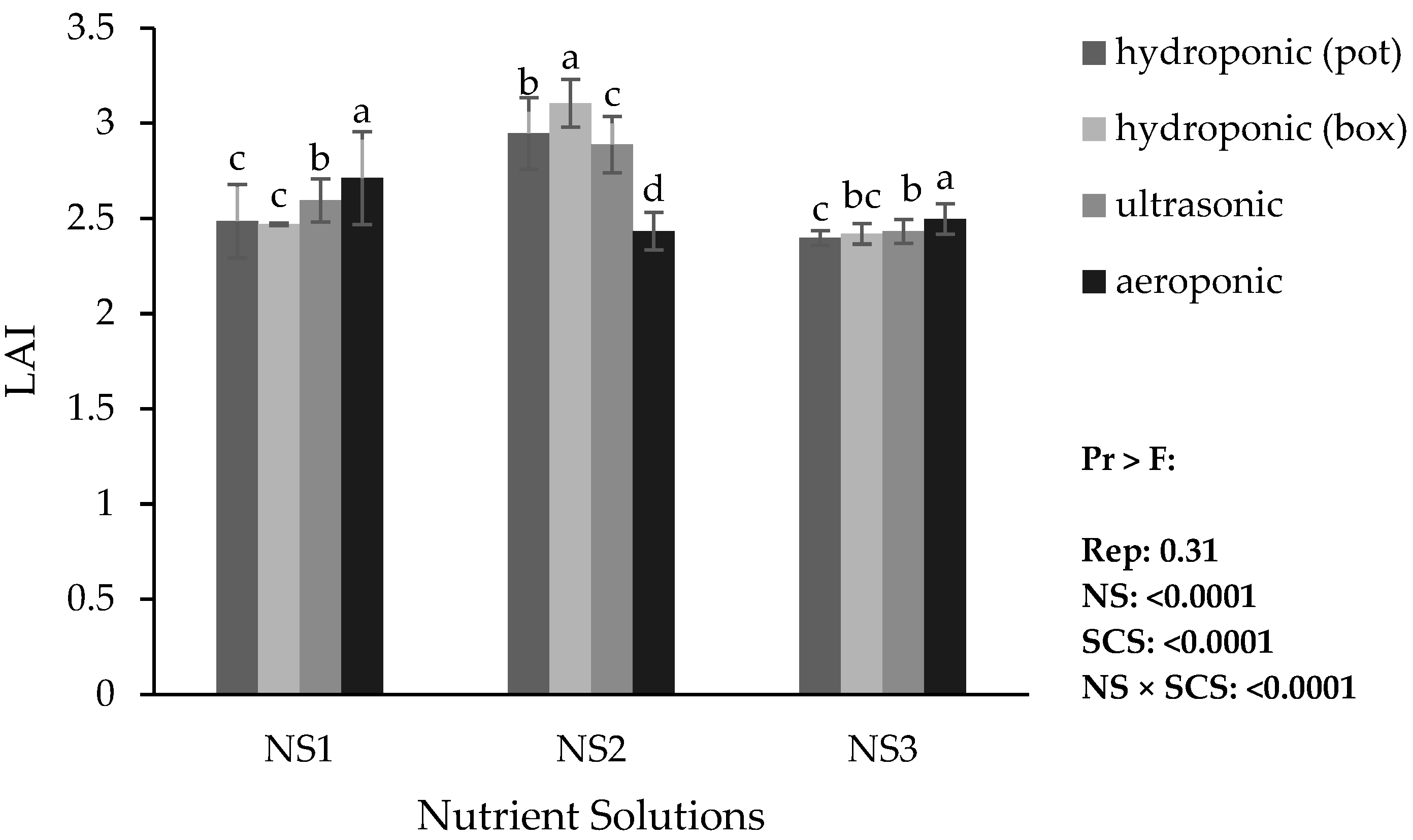
3.4.3. Leaf Area Index (LAI)
Based on the comparison of means, the aeroponic system showed higher LAI in NS1 and NS3, while the hydroponic (container) system showed higher LAI in NS2. The highest LAI overall occurred in the hydroponic (container) system with NS2. By contrast, the lowest LAI values were recorded in both hydroponic systems (pot and container), with NS3. Furthermore, the highest LAI within the hydroponic (pot) and ultrasonic systems occurred with NS2, whereas in the aeroponic system, it occurred with NS1 (
Figure 5).
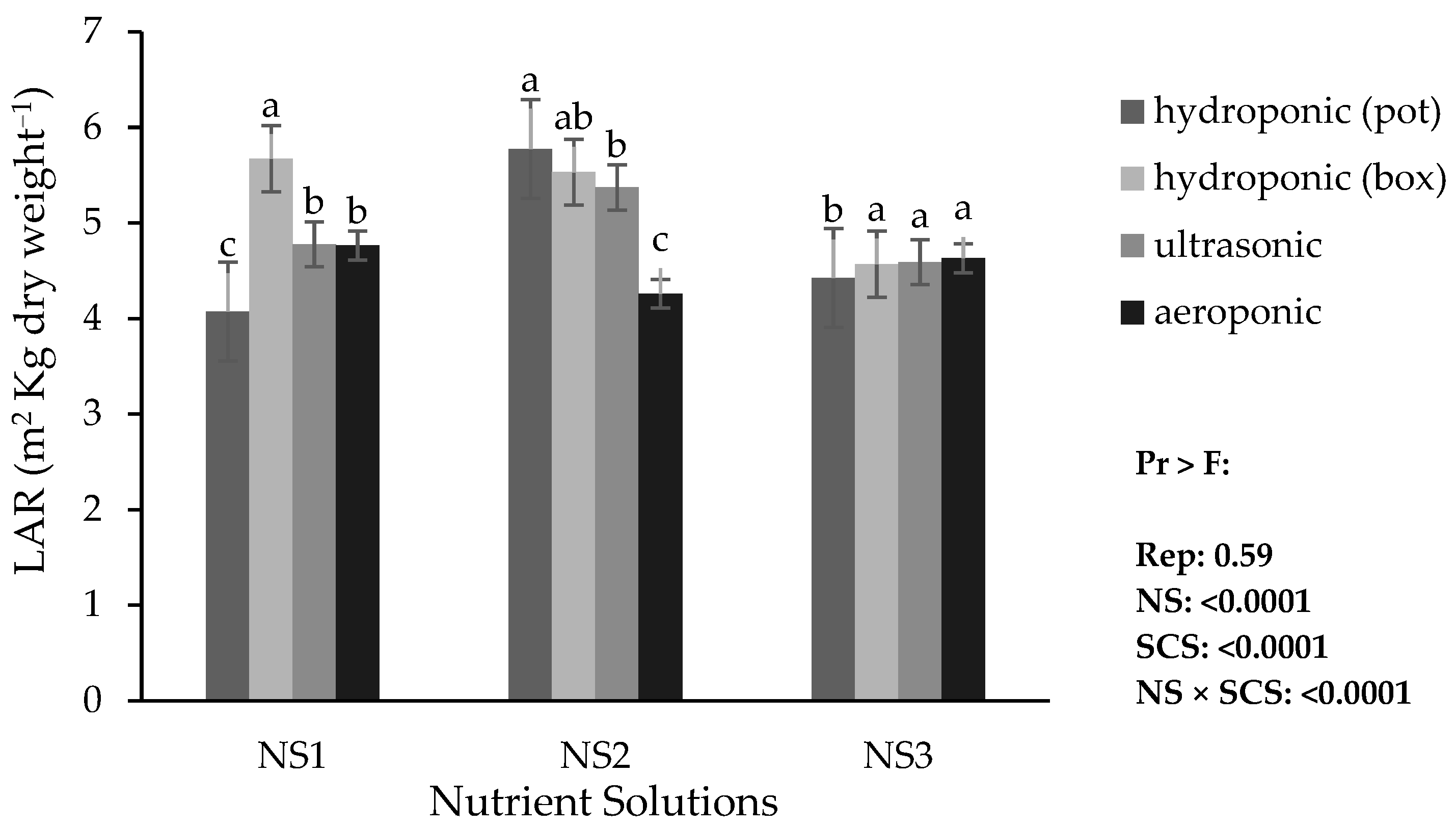
3.4.4. Leaf Area Ratio (LAR)
Based on the results, in NS1, the hydroponic (container) system showed higher LAR; in NS2, the hydroponic (pot) system showed higher LAR; and in NS3, the hydroponic (container), ultrasonic, and aeroponic systems showed higher LAR (
Figure 6). Among these, the highest LAR values were obtained in the hydroponic (pot) system with NS2 and in the hydroponic (container) system with NS1, while the lowest LAR was recorded in the hydroponic (pot) system with NS1.
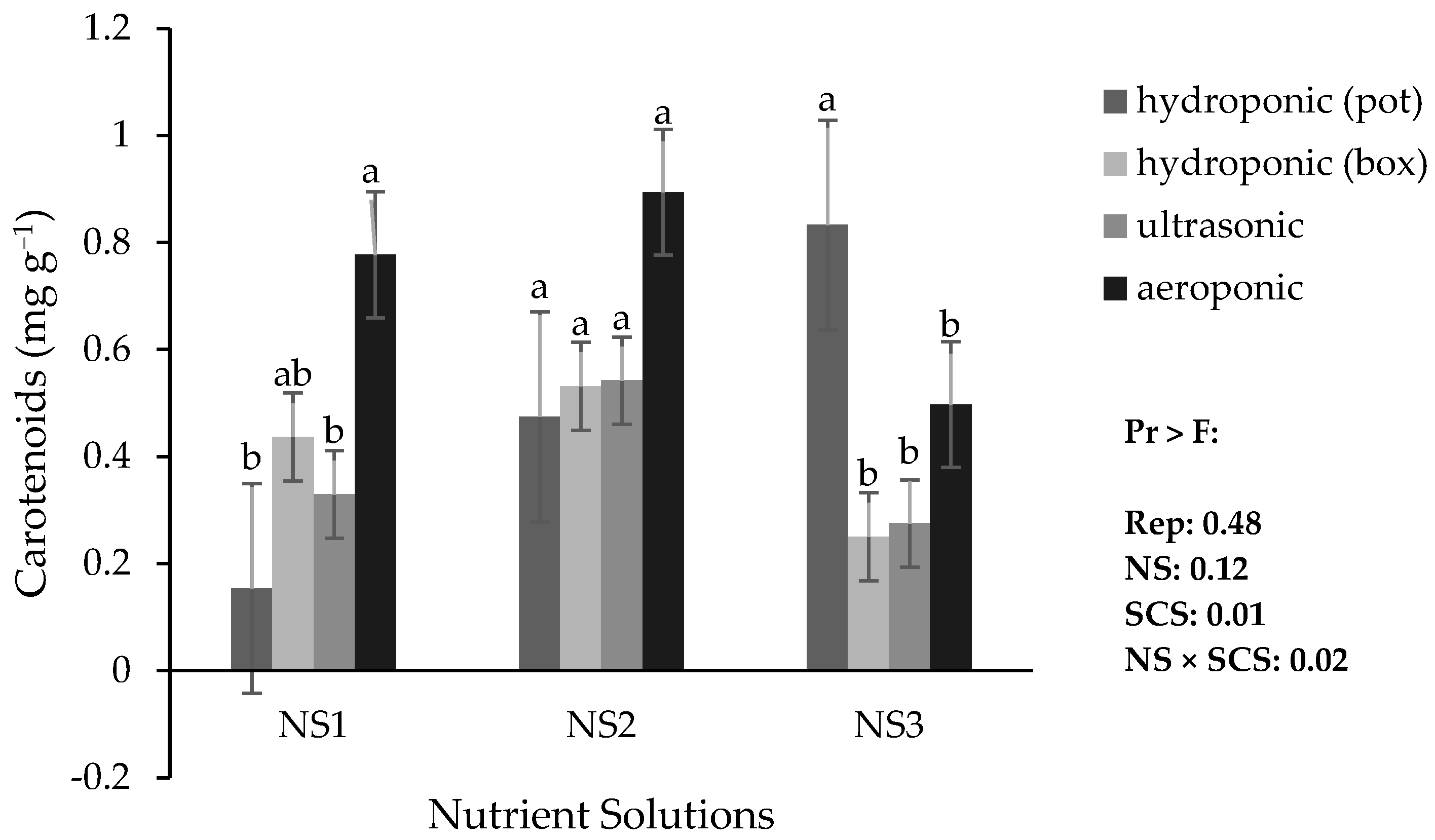
3.5. Photosynthetic Pigments in Lily
The comparison of means indicated that the highest amount of leaf carotenoids in NS1 and NS2 occurred in the aeroponic system, while in NS3, the highest amount occurred in the hydroponic (pot) system. The lowest carotenoid content was obtained in the hydroponic (pot) system with NS1 (
Figure 7).
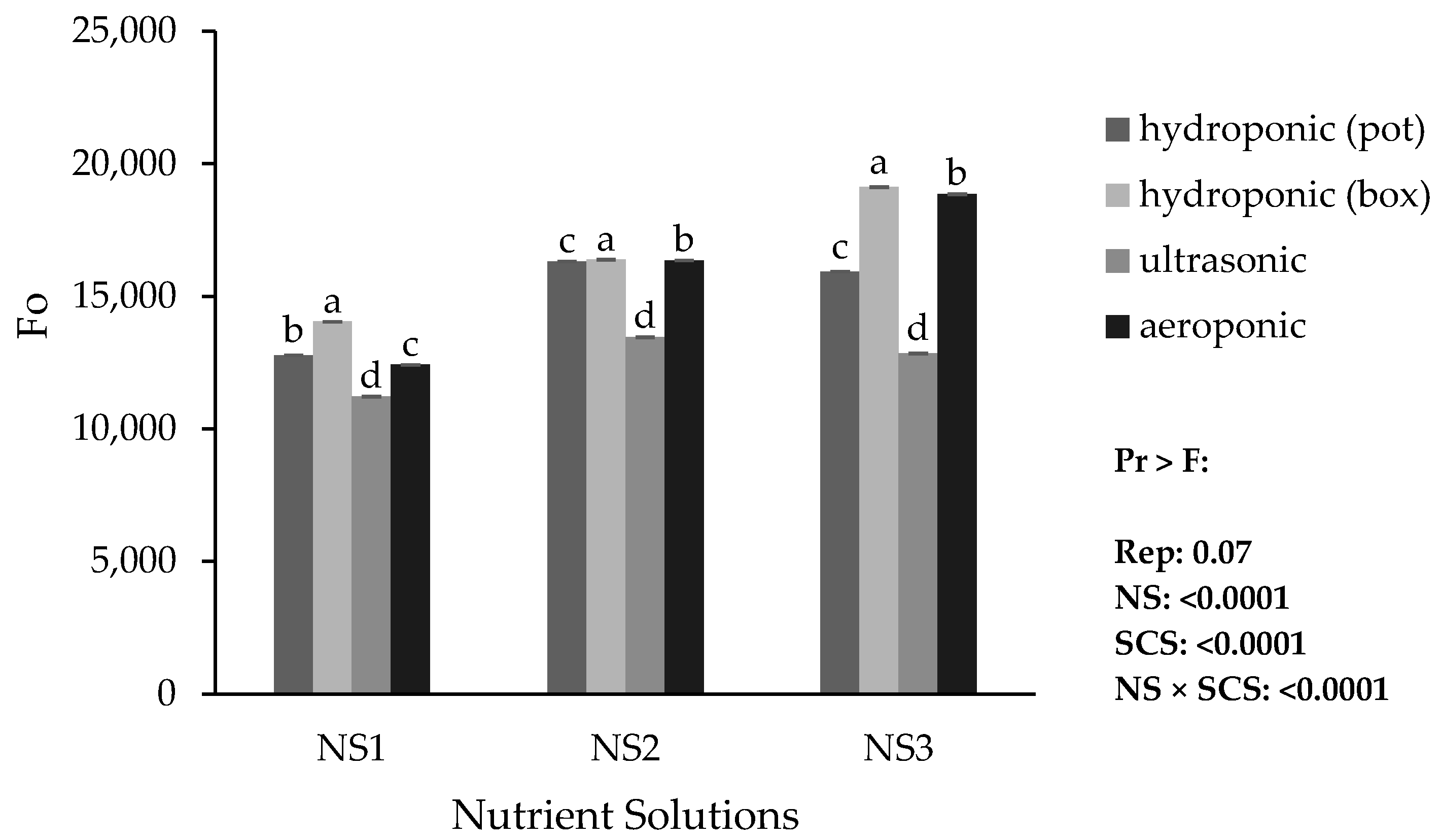
3.6. Photosynthetic Parameters in Lily
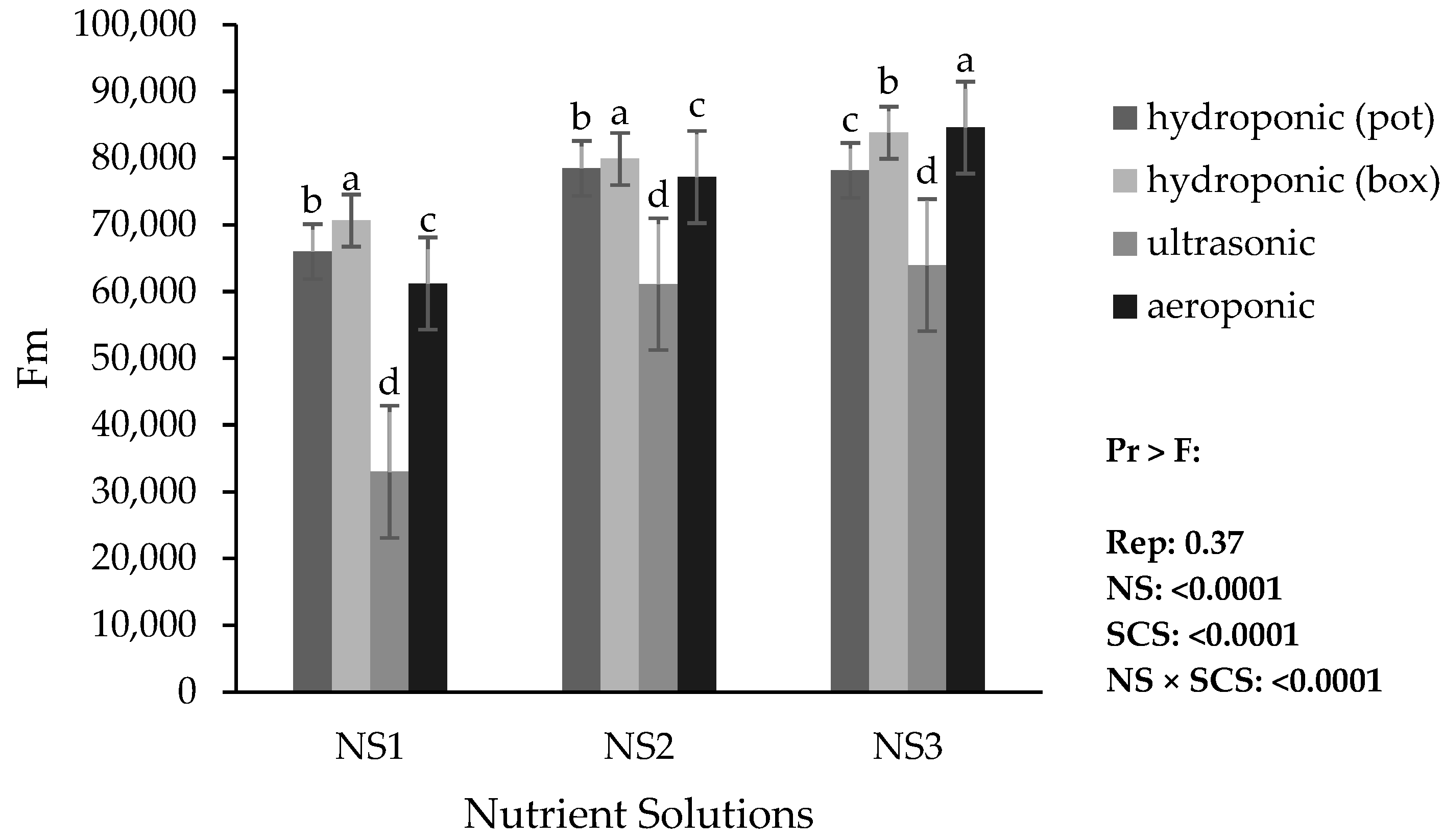
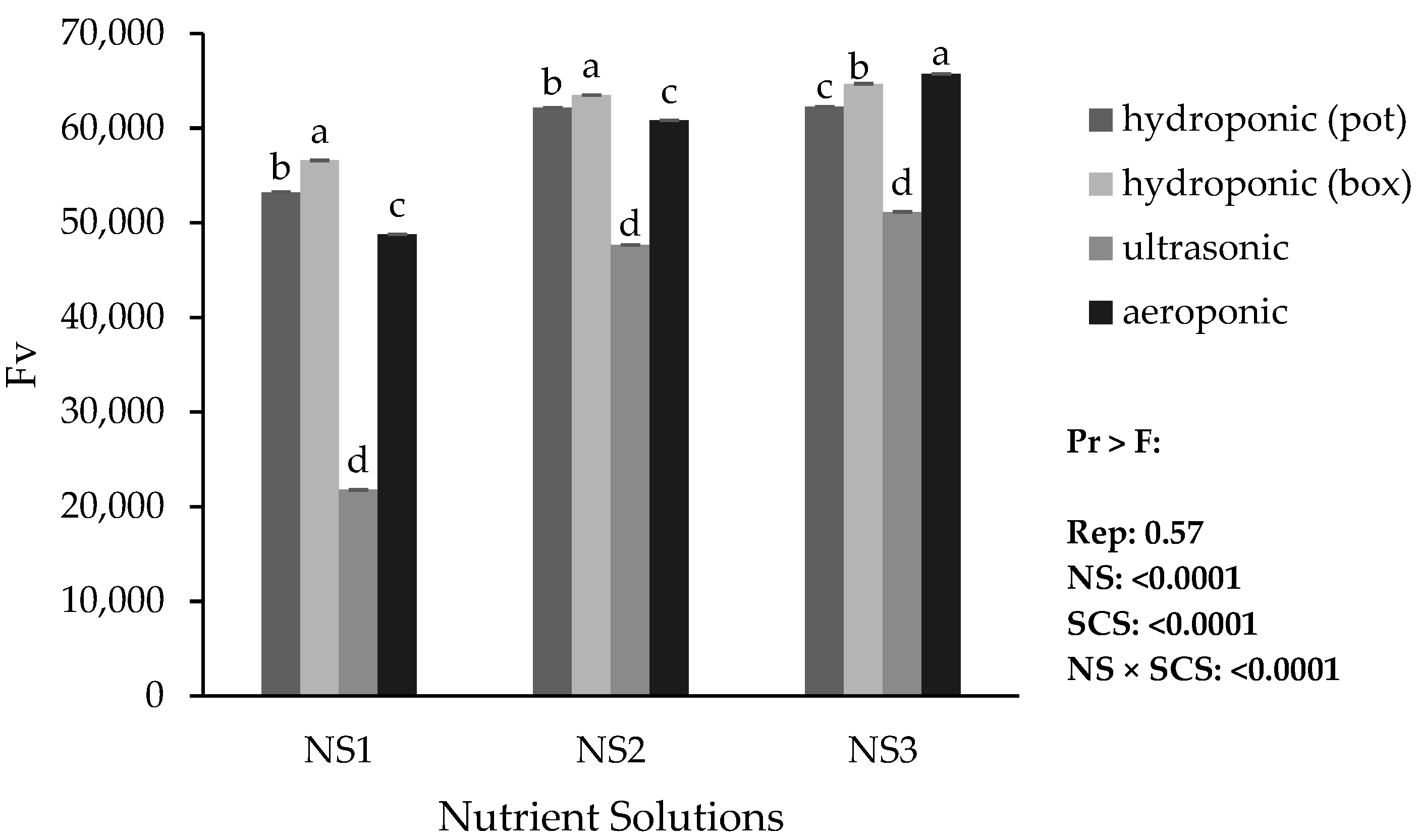
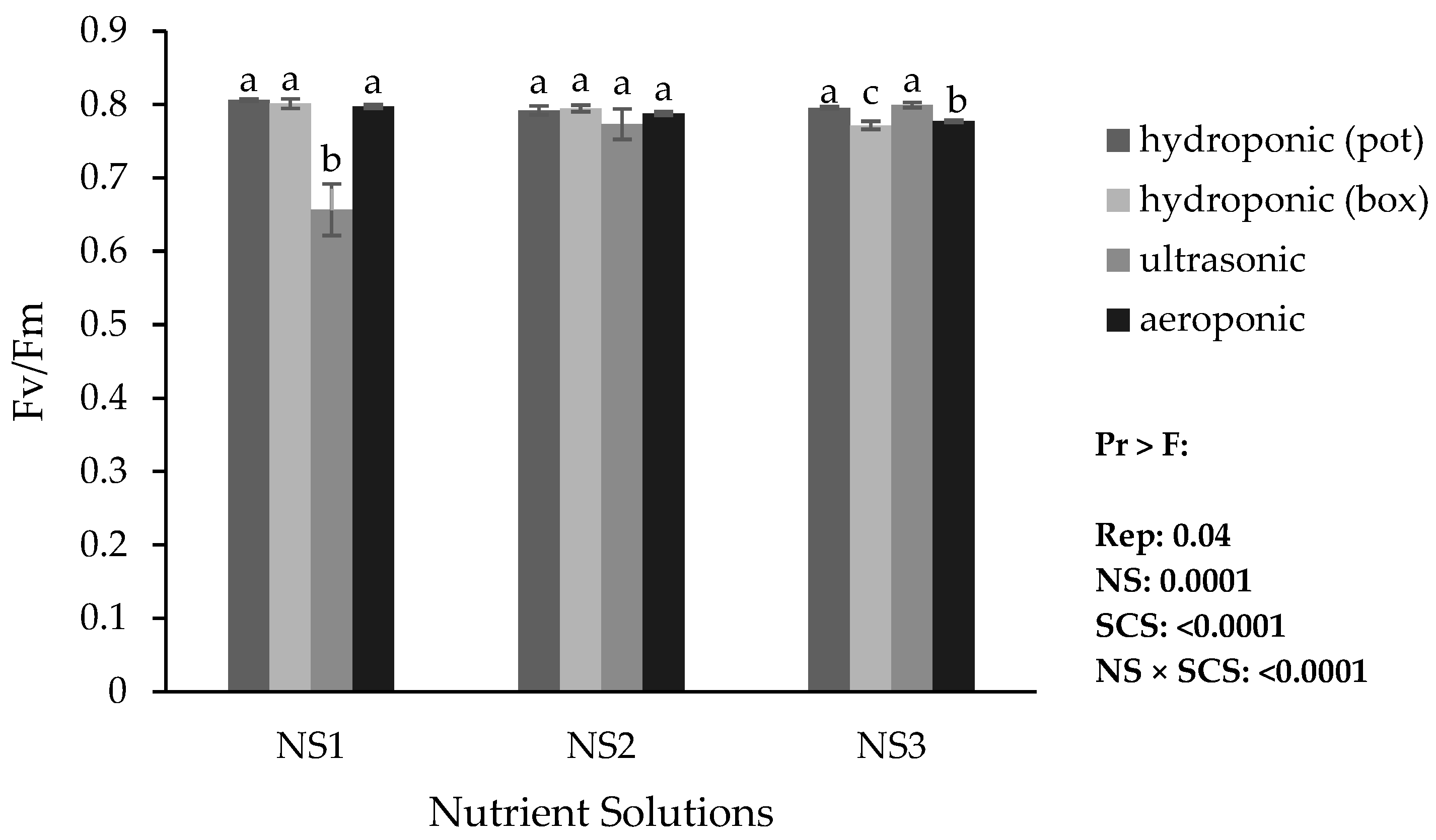
Based on the results, the lowest minimum fluorescence (Fo), maximum fluorescence (Fm), and variable fluorescence (Fv) were obtained in the ultrasonic system with NS1. The highest Fo was obtained in the hydroponic (container) system with NS3, while the highest Fm and Fv were obtained in the aeroponic system with NS3 (
Figure 8,
Figure 9 and
Figure 10). The Fv/Fm ratio decreased only in the ultrasonic system with NS1 (
Figure 11).
3.7. Factor Analysis
In this research, according to the nature and importance of the traits, and based on the results of factor analysis, a set of traits was selected. The factor analysis showed that the selected traits can be evaluated in the form of three factors (
Table 7). The naming of these factors was made according to the traits with the greatest loading of each factor.
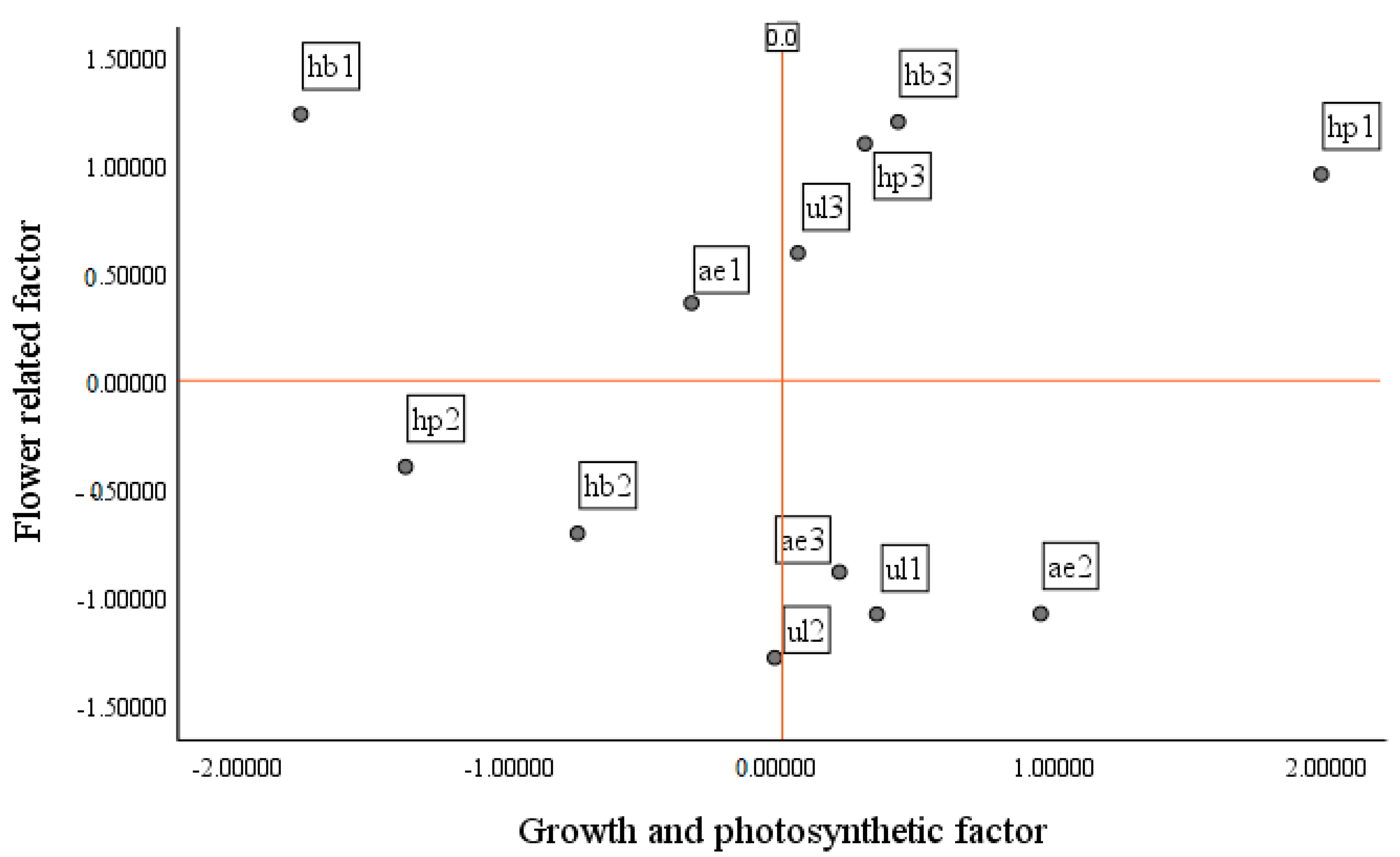
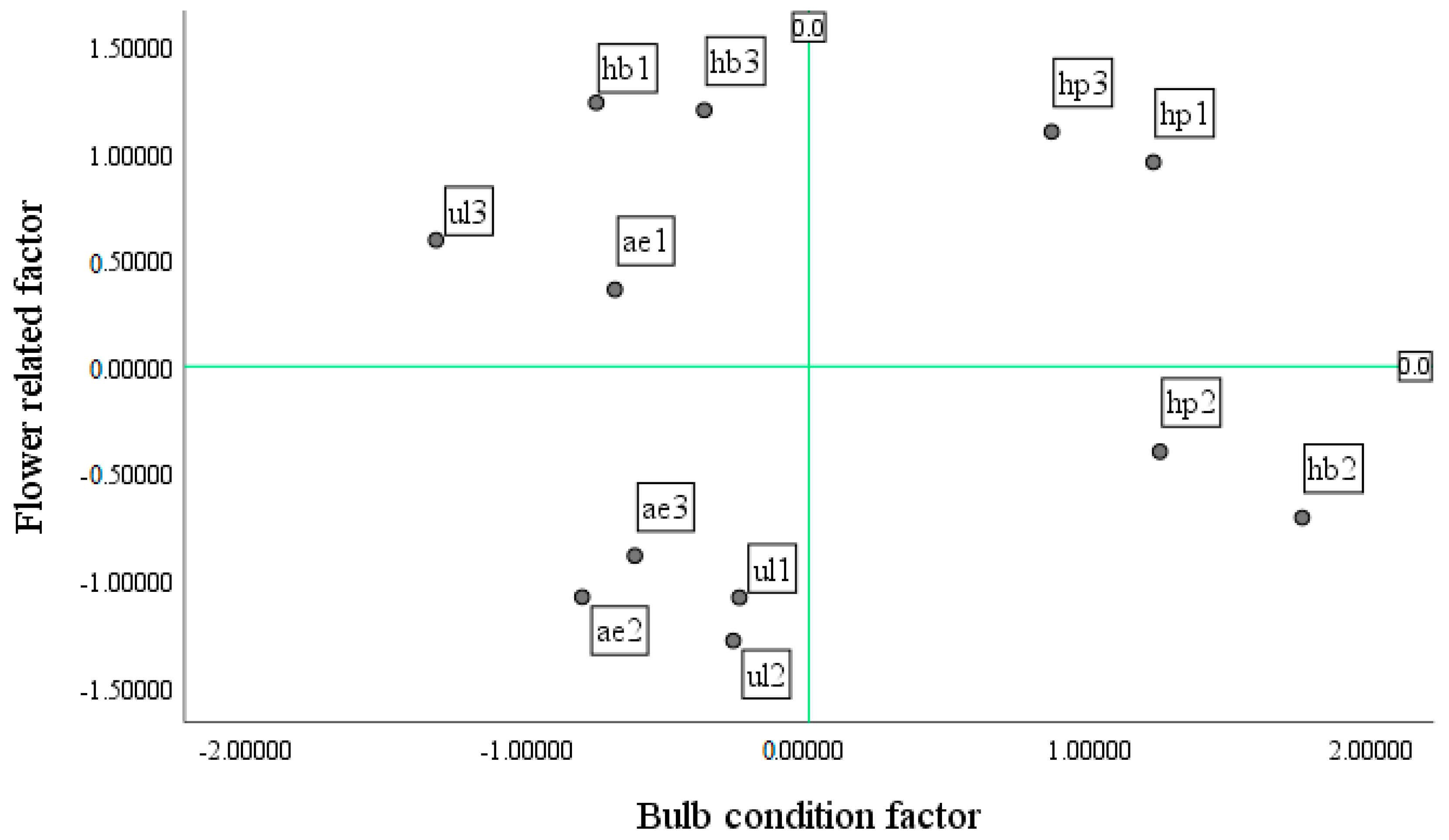
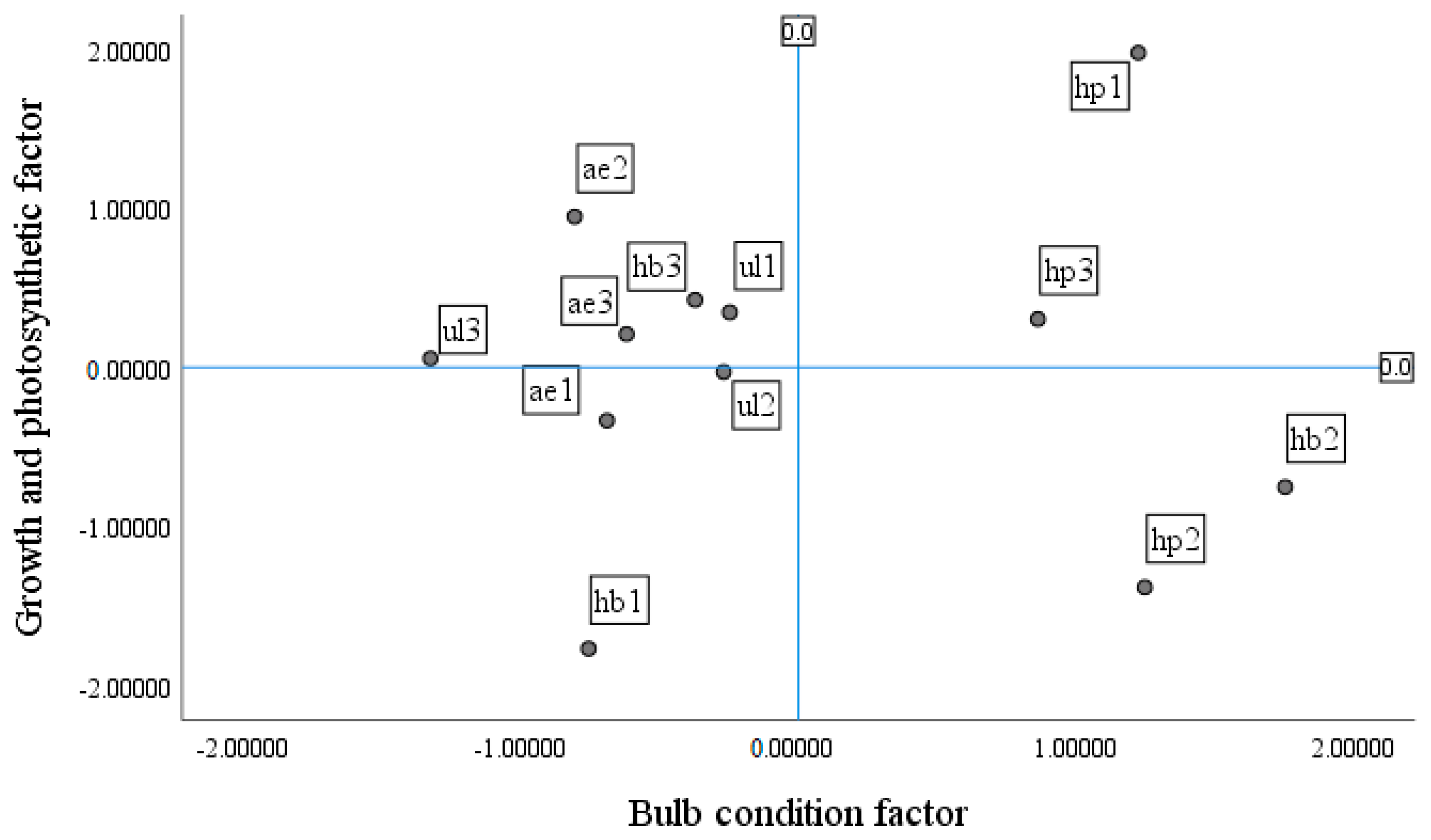
Investigating the value of growth and photosynthesis, bulb, and flower factors across treatments (
Figure 12,
Figure 13 and
Figure 14) showed that the hydroponic (pot) system with NS1 resulted in suitable growth and photosynthesis as well as flower and bulb characteristics; as a result of the higher RGR and NAR, traits such as root length, number and size of bulblets, and flower diameter were improved. It should be mentioned that LAI, LAR, and the number of hairy roots were low in this treatment. In the hydroponic (container) system with NS1, the flower diameter was suitable, but the days to flowering were slightly longer than in the hydroponic (pot) system. In addition, root length, number of hairy roots, and LAR were high, whereas RGR and NAR were low, and both the number and size of bulblets as well as LAI decreased. In the ultrasonic system with NS1, growth was less than optimal compared with other treatments, and flower and bulb characteristics were also not suitable. Flower diameter, root length, and days to flowering decreased, whereas LAI increased. In the aeroponic system with NS1, growth and photosynthetic characteristics and flower traits were not proper; the number and size of bulblets and LAI were low, but the number of hairy roots increased.
In the hydroponic (pot) system with NS2, flower characteristics were weak, but the number and size of bulblets, LAI, and LAR were high; by contrast, RGR, NAR, and the number of hairy roots decreased. In the hydroponic (container) system with NS2, bulblet characteristics had a positive response, while growth and flower characteristics had a negative response. In this treatment, the number and size of bulblets and LAI and LAR increased, whereas flower diameter, root length, number of hairy roots, days to flowering, RGR, and NAR decreased. In the ultrasonic system with NS2, photosynthetic capacity and bulb condition were very low, although LAI was relatively high; in this treatment, some buds abscised. The aeroponic system with NS2 showed good conditions in terms of growth and photosynthesis (RGR and NAR), and both LAI and the number of hairy roots were high; however, flower diameter, root length, number and size of bulblets, and LAR were low, and days to flowering were high. The hydroponic (pot) system with NS3 had better flower and bulb conditions than growth and photosynthesis; flower diameter, number and size of bulblets, and root length were high, while LAI and days to flowering were relatively high, and the number of hairy roots was low.
In the hydroponic (container) system with NS3, the flower condition was favorable, whereas growth and bulb conditions were unfavorable. Days to flowering were longer in this system than in the other systems, while flower diameter and root length were high, and LAI remained low. Bulbs treated with NS3 nutrient solution under the ultrasonic system resulted in good growth traits; however, photosynthetic and flowering characteristics, as well as bulb-related traits, were not satisfactory. Accordingly, flower diameter, root length, RGR, NAR, and the number of hairy roots were high, days to flowering decreased, and LAI, LAR, and the number and size of bulblets decreased.
Based on the results, in the aeroponic system with NS3, growth, flowering, and bulb conditions were not suitable, so flower diameter, root length, number and size of bulblets, and days to flowering were low, while the number of hairy roots, LAI, and LAR were high.
In this experiment, the best system was the hydroponic (pot) system with NS1 (control). In this system, traits such as number and size of bulblets, days to flowering, flower diameter, root length, RGR, NAR (production of photosynthetic dry matter), and chlorophyll fluorescence were suitable. Regarding the vase life, compared with the maximum vase life (6 days), an average vase life (4 days) was obtained in this system. Also, a plant height of 93 cm in this treatment has commercial value and is suitable for a premium bouquet in the cut-flower market. High-quality flowers were also obtained in a hydroponic (pot) system with NS3. Therefore, the bulbs from both nutrient solutions (NS1 and NS3) in this system were evaluated against the primary bulbs (i.e., bulbs that have been stored at room temperature until the end of the experiment).
3.8. Evaluating Changes in the Biochemical and Anatomical Characteristics of Hybrid Lily Bulbs Cultivated in the Superior System with Untreated Bulbs
3.8.1. Quantitative Traits
The results showed that in the hydroponic system, both NS1 (7393.80) and NS3 (6455.80) increased root length compared with the initial bulbs (66.830); this increase was greater with NS1. Bulb size in both nutrient solution treatments decreased to the same extent compared with the initial bulbs (
Table 8 and
Table 9).
3.8.2. Biochemical Traits
Anthocyanin content in bulbs was very low and close to zero in all treatments, and no obvious differences were observed among the treatments. Soluble carbohydrates increased in both NS1 (6.45) and NS3 (6.4) compared with the initial bulbs (6.41); this increase was greater with NS1, although it was not statistically significant (
Table 8 and
Table 9).
3.8.3. Amount of Antioxidant Capacity, Free Radicals, and Malondialdehyde
In this research, antioxidant capacity increased significantly in the hydroponic (pot) system with both NS1 and NS3, and this increase was greater with NS1. For hydrogen peroxide, a slight but statistically significant increase was observed in bulbs fed with NS1 (0.05) and NS3 (0.05), which was slightly higher with NS1. Malondialdehyde content in the bulbs increased in both nutrient solutions (NS1: 0.29 and NS2: 0.19), compared with the initial bulbs (0.15), with a higher increase in the bulbs fed with NS1 (
Table 8 and
Table 9).
4. Discussion
Root growth was higher in the aeroponic system than in the hydroponic systems that were treated with NS1, similar to the results of Li et al. on lettuce [
12], but this trend was not observed for other nutrient solutions. In NS2, the ratio of calcium to total cations (0.7) was high, and the ratio of ammonium to total nitrogen (0.15) was moderate; the high calcium may have disturbed ionic balance and negatively affected root growth. The composition of the nutrient solution plays an important role in root uptake due to nutrient interactions and internal regulation [
20]. Lily has a moderate demand for nitrogen and a small demand for calcium, likely due to the redistribution of reserves stored in the bulb [
20]. Also, it stated that a well-aerated root environment in aeroponics promoted root initiation and growth in woody (
Ficus) and herbaceous (
Chrysanthemum) cuttings [
36]. In aeroponics, the nutrient solution was only sprayed as fine droplets at intervals, which may limit shoot growth and improve root growth, as the plant’s response may be to adapt to the relative deficit of water and nutrients during the intervals. Thus, the droplet size and the misting interval will have a great effect on plant growth in aeroponic culture [
37].
The highest number of hairy roots was obtained in the ultrasonic system with NS3. The number of hairy roots in ultrasonic and then aeroponic systems exceeded more than two-fold from those in hydroponic systems, consistent with Eldridge et al. [
38], who point out that root architecture and anatomy differ between hydroponic and aeroponic cultivation; aeroponic roots exhibit more root hairs than hydroponic roots. As it is known, the main function of the plant root is the absorption of water and nutrients; plant stability in the soil and interaction with soil organisms, and its growth depends on the root environment, including substrate porosity, humidity, temperature, and frequency of watering, which was challenged in the examined systems. Root hairs, unicellular tubes developed from root epidermal cells, enhance water and nutrient absorption, anchorage, and biotic interactions. Their development adapts dynamically to the root environment [
39]. Dynamic modification of root and hairy root growth, including length, density, and morphology, allows the plant to meet its nutritional demands under different soil conditions (cultivation medium) [
40,
41]. In this experiment, in the ultrasonic system, adaptation to fog particles around the roots was manifested as an increase in the number of hairy roots. Because secondary-metabolite production in plants is slow and their biotechnological production is expensive, the ultrasonic system could be considered an alternative method for producing root biomass for medicinal purposes.
The number of bulblets was maximal with NS2, where the calcium ratio (0.7) was high and the ammonium ratio (0.15) was moderate. A high calcium ratio combined with a balanced ammonium fraction may have increased bulblet number. It has been reported that increasing the ratio of ammonium to total nitrogen from 0 to 0.14 increased the number of bulblets in tulips, which is in accordance with our results in hydroponic systems [
42]. Nitrogen status strongly affects quantitative and qualitative plant indices, and the nitrogen source is also important [
43]. In lily, calcium nitrate compared with urea/ammonium nitrogen increased both the number and size of bulblets, likely via positive effects of calcium on meristem growth [
44], which is consistent with our results in the ultrasonic system. The same results were reported by Haadi-e-Vincheh et al. [
45] using 200 kg ha
−1 of ammonium nitrate as a source of nitrogen in
Narcissus bulbs. In addition, in research on
Gladiolus, it was reported that foliar spraying with 200 mM of calcium increased the size of the corm [
23], which is consistent with our results in hydroponic systems with NS2 (with a high ratio of calcium to total cations of 0.7). Askar [
46] also stated that the most effective system for producing bulbs and bulblets is the hydroponic system, which is in accordance with the results of this research.
Based on the results, increasing the ammonium ratio increased stem length (
Figure 3), in accordance with the report of Abbasi et al. [
42] on tulips. The authors reported for tulips that increasing the ammonium ratio (ammonium: total nitrogen ≈ 0.038) increased phosphorus content, which in turn positively affected plant size via enhanced phosphorus uptake.
The shortest days to flowering were associated with the aeroponic system under NS2 and NS3 treatment; with the ultrasonic system under NS1, NS2, and NS3 treatment; with the hydroponic (container) system under NS2 treatment; and with the hydroponic (pot) system under NS1 treatment; however, the longest period occurred in the hydroponic (container) system under NS3 treatment. Because shortening the growth period reduces greenhouse costs, this trait is economically important. In tulips, days to flowering decreased as the ammonium ratio increased from 0 to 0.14, attributed to enhanced phosphorus absorption [
42]; this order was not observed in the current research.
The number of buds was not significantly affected by the treatments. However, calcium (e.g., 6 mM under hydroponics) has been reported to increase bud number in lily [
47]. Nitrogen can also increase the translocation of carbohydrates from vegetative to reproductive organs, thereby increasing the bud number [
48,
49]. Increasing calcium concentration in the nutrient solution, via increased phosphorus uptake, can substantially affect reproductive growth and increase flower buds; overall, reproductive growth health is influenced by calcium, likely due to elevated phosphorus in above-ground organs [
47]. It seems that the increased ratio of calcium to total cations or the ratio of ammonium to total nitrogen, as the main variables of the current research, were not effective in improving the reproductive traits of lily in the present study.
The largest flower sizes were obtained in the hydroponic (pot) system with NS1 and NS3, in the hydroponic (container) system with NS1, and in the ultrasonic system with NS3. In the aeroponic system, NS1 performed better than NS2 or NS3. Calcium contributes to the synthesis of mitochondrial proteins; because mitochondria participate in aerobic respiration and active nutrient uptake, there is a positive relationship between nutrient absorption and calcium. Calcium also increases growth and dry-matter content by inhibiting chlorophyll and protein breakdown [
47,
50]. Nitrogen plays a key role in protoplasm synthesis and amino-acid formation, which increases auxin activities and leads to increased meristem activities and thus increased growth [
49]; nitrogen application also increases carbohydrate transfer to reproductive parts, potentially enlarging flowers [
48]. In addition, the increase in flower size may be attributed to the increase in the absorption of nitrogen required for growth, which is due to the increase in the amount of nitrogen and, as a result, the use and transfer of metabolites [
49]. In this research, optimum levels and ratios of these elements (cations and anions) differed among systems: hydroponic (pot and container) with NS1 and NS3, ultrasonic with NS3, and aeroponic with NS1 showed better results.
Vase life increased in the aeroponic system with NS1 and NS3 and in the hydroponic (container) system with NS2; the lowest vase life was observed in the aeroponic and ultrasonic systems with NS2 and in the hydroponic (pot) system with NS2 and NS3 (
Table 4). In aeroponics, better oxygen availability may have enhanced water absorption, while specific calcium and ammonium ratios (NS1 and NS3) increased vase life. In the hydroponic (container) system, the high calcium ratio (NS2) may have increased vase life. An increase in postharvest vase life has been positively correlated with tissue calcium concentration [
42].
Overall, in the hydroponic (pot) system with NS1, better results in growth traits were observed than in other treatments. In NS1, total nitrogen (15 mM) and total cations (11 mM) were the highest compared with other solutions; ammonium (0.1) was the lowest relative to nitrate, and calcium (0.5) was intermediate. The optimal composition of the nutrient solution appears to be different for each system, which in turn affects dry matter accumulation. Previous research indicates that differences in nutrient concentrations, pH, EC, and NO
3:NH
4 ratios cause different growth outcomes in flowers and bulbs; these factors strongly influence growth [
51,
52,
53]. The growth medium itself can directly and indirectly affect plant growth and production [
10]. Li et al. [
12] observed increased shoot growth rates in hydroponic cultivation, and aeroponic lettuce had greater root dry weight and root/shoot ratio which was similar to this research. In lily, plants fed with nitrate showed higher RGR in hydroponics, attributed to nitrate uptake and calcium availability from calcium nitrate, which enhances nitrogen uptake and meristem activity [
21]. Although optimal nitrogen increases vegetative growth and yield, concentrations above the optimum can decrease growth by reducing phosphate uptake [
54]. In the present research, there was a positive correlation between RGR and NAR in hydroponic and aeroponic systems, and a positive correlation between RGR and LAR in the ultrasonic system; a positive correlation between LAI and LAR was observed in all systems except the hydroponic (container) system. Calcium nitrate has been reported to significantly improve dry matter accumulation per unit area throughout the growth period, and positive correlations between leaf area, LAI, and NAR with available nitrogen have been found in lily [
55,
56]. Adequate calcium contributes to cell-wall strength and membrane stability and may improve chlorophyll retention; improved nitrogen uptake resulting from adequate calcium availability may also help plants remain greener [
21]. Calcium significantly improved the fresh and dry weight of lily plants as well as the leaf surface under the treatment of 2 mM calcium nitrate [
57]. The results of the research also indicated that calcium nitrate feeding significantly increased the leaf area compared with urea/ammonium nitrogen; rapid nitrate availability from calcium nitrate likely increases the leaf area even at early growth stages, improving photosynthetic activity across the growth cycle. It has also been confirmed that the relationship between nitrogen availability and leaf surface in lily [
44]. These results were also reported for lily cv “Riongro” [
55]. The nitrate form of nitrogen, as calcium nitrate, was superior in improving growth, leaf area, dry-matter accumulation, and bulb yield [
21].
The Fv/Fm value decreased only in the ultrasonic system with NS1, which could reflect dehydration or senescence stress that reduced the quantum efficiency of photosystem II. In all systems, Fo in the control (NS1) was relatively favorable (low). By contrast, Fm and Fv were minimal in the control across systems, and their values increased with increasing ammonium. Considering that, in the ultrasonic system, Fv/Fm increased with increasing ammonium in the nutrient solutions, lily may be relatively ammonium tolerant; further research is needed. A decrease in Fv/Fm generally occurs when the PSII function and structure are compromised by stress, increasing the likelihood of photoinhibition; increased initial fluorescence can indicate damage to PSII reaction centers, structural changes, and pigment alterations under stress (likely dehydration or senescence), consistent with the present observations [
58]. This is consistent with the results of the present study. As mentioned, the value of Fv/Fm was reduced in the ultrasonic system in NS1. This probably indicates that PSII changed in efficiency among different systems and nutrient solutions. The stress intensity in the experiment is such that it can destroy PSII centers, which means that the increase in the first fluorescence can indicate the destruction of the reaction center of PSII, structural changes, and changes in the pigments of PSII under stress conditions [
59] (probably dehydration or senescence).
Finally, we selected the hydroponic system with NS1 and NS3 as the best treatments. Evaluating bulb characteristics in these treatments compared with the primary bulbs showed that the decrease in bulb size likely reflects the loss of lower leaves as plants approached senescence, reducing NAR and photosynthetic dry-matter production [
21], and, consequently, the translocation of photo-assimilates to the bulb. Regarding the increase in carbohydrates, nitrogen fertilization increases assimilate partitioning to generative plant organs [
60], and minerals such as calcium also increase carbohydrate accumulation; because NS1 contains higher calcium and nitrogen, these outcomes are predictable.
In this research, antioxidant capacity increased significantly with both NS1 and NS3 in the hydroponic (pot) system, with a greater increase under NS1 (highest nitrate, lowest ammonium). Hydrogen peroxide showed a slight but statistically significant increase with NS1 and NS3, slightly higher with NS1. Antioxidant capacity functions to remove reactive oxygen species and trap ROS in plant cells; under stress, plants activate antioxidant systems to mitigate adverse effects, reducing the density of free radicals in tissues.
Malondialdehyde (MDA) increased in bulbs under both nutrient solutions compared with the initial bulbs, with a higher increase in NS1. MDA, a lipid-peroxidation product, is an indicator of membrane damage; thus, stress and aging likely increased MDA. This probably indicates that aging led to peroxidation of fatty acids in the cell membrane and, finally, to the accumulation of MDA. Increased MDA indicates oxidative damage to membrane lipids and other biomolecules (proteins, DNA, RNA); elevated lipid peroxidation increases membrane fluidity and ion leakage [
61]. Yin et al. stated that in lily plants, heat stress-induced oxidative damage is linked to changes in antioxidant enzyme activities and antioxidants [
62]. Also, a close link has been reported between flower growth, antioxidant capacity, enzymatic antioxidant activity, and mineral content in roses [
63]. In the hydroponic system, bulbs fed with NS3 showed lower MDA and membrane-lipid peroxidation; this reduction likely reflects delayed senescence with higher ammonium. These results align with findings for lily, where increasing ammonium delayed senescence [
64].
The evaluation showed that, in the hydroponic system, bulbs fed with NS1 had increased root length and, possibly via increased absorption, accumulated more carbohydrates and showed higher antioxidant capacity, although free radicals, MDA, and membrane lipid peroxidation were also higher than in primary bulbs. In bulbs fed with NS3, MDA and membrane-lipid peroxidation decreased with higher ammonium, indicating delayed aging. Therefore, in addition to NS1 (Hoagland-based), NS3 (highest ammonium: total N ratio lowest calcium: total cations ratio) is also recommended for lily cultivation.
Regarding the limitations of the experiment, in the case of the ultrasonic system, the piezoelectric has a relatively short useful life, and we had to replace some of them in the last two weeks of the cultivation period; in the case of radiation, depending on the growth chamber facilities, the minimum acceptable light of 150 μmol m−2 s−1 was applied. Subsequently, it is expected that if high levels of light are applied, inconsistent results may be observed. Therefore, further experiment is recommended to validate the obtained results.