Effect of Propagation Techniques on Growth, Development, Oil Yield, and Quality of Medicinal Cannabis (Cannabis sativa) Found in Lusikisiki, Eastern Cape, South Africa
Abstract
1. Introduction
2. Materials and Methods
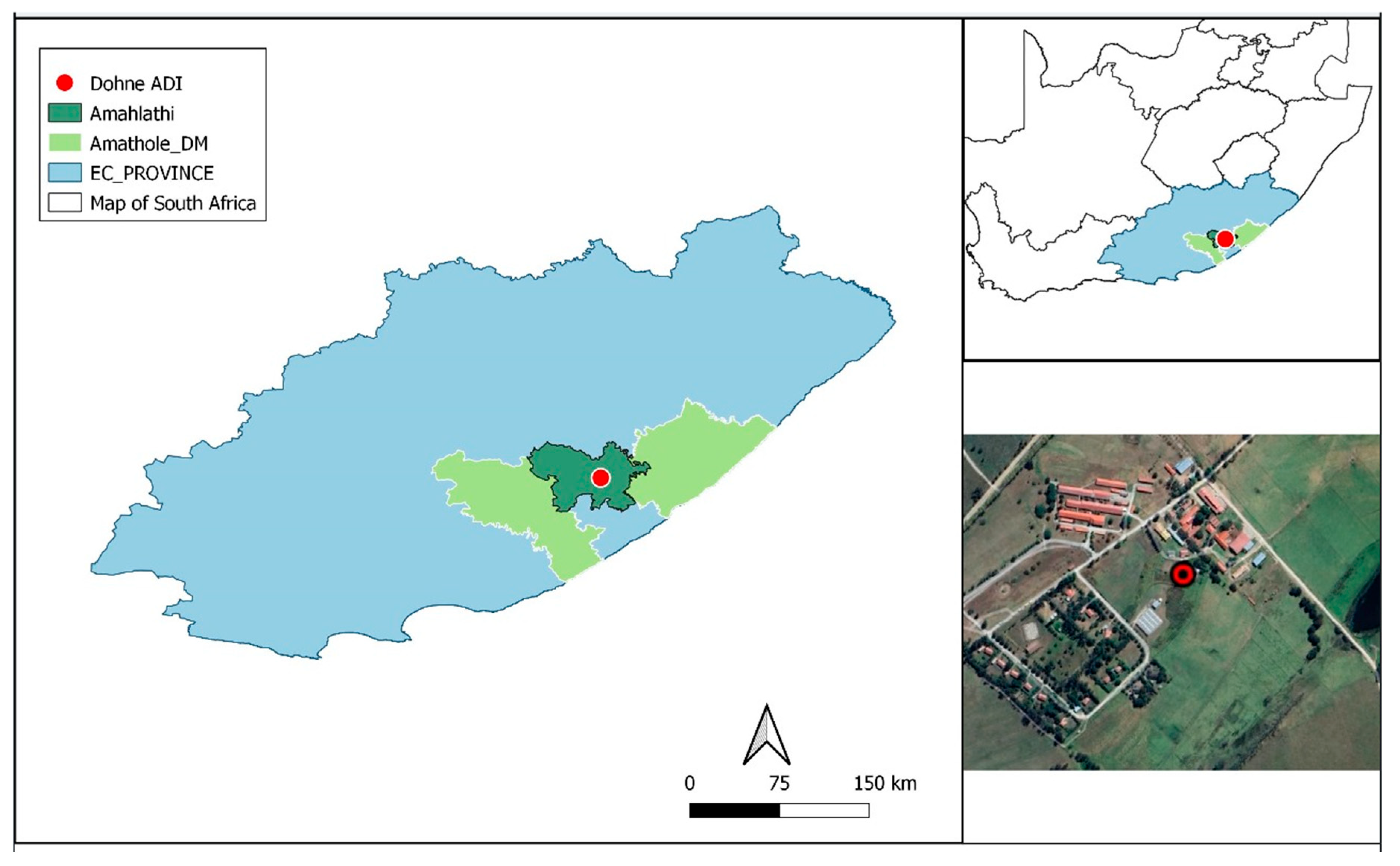
2.1. Location of the Experiment
2.2. Planting Material and Treatments
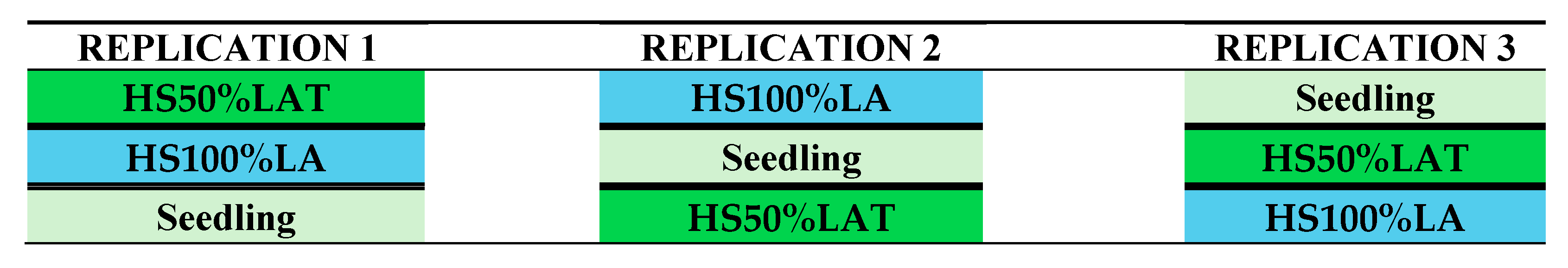
2.3. Experimental Design
- Herbaceous shoot with 50% leaf area trimmed (HS50%LAT);
- Herbaceous shoot with 100% leaf area retained/not trimmed (HS100%LA);
- Sexually produced seedlings (seedling).
2.4. Experimental Procedure
2.5. Data Collection
- (i)
- Vegetative growth and development: Plant height (cm), the number of lateral branches on one plant, and stem girth were measured using a flexible but non-elastic measuring tape, manual counting, and a Venier calliper (cm), respectively. Data were taken at two-week intervals after transplanting (AT) until the crop was harvested 12 weeks after transplanting. A total of five (5) plants per treatment were selected and tagged to measure the aforementioned vegetative growth parameters.
- (ii)
- Flower yield:
- The number of weeks to first flowering was determined;
- The number of flowers and the flower sex determination per plant were performed through a physical count;
- The number of weeks taken by plants to reach 50% amber coloration was recorded.
- (iii)
- At harvest, five plants were destructively sampled, with plant stems cut at the first node above ground level, and the following biological yield parameters were obtained using an Adam ACBplus-6000g balanced scale (LBB6001e, Adam equipment, UK): (a) fresh plant weight (g) and (b) the leaves and flowers were removed from stems with the aid of secateurs and their fresh weight was measured. Samples were then dried and cured in dark-cool room conditions for 14 days, following the standards recommended by Jin et al. [18], and their dry weights were determined.
- (iv)
- Oil yield and chemical composition: The oil yield and chemical composition were determined using gas chromatography (GC-MS) (Thermo Scientific, TriPlus RSH Smart, Switzerland) at Dohne Analytical Laboratory.
- The oil yield was determined using an analytical balance (Mettler Toledo, MS104TS, Greifensee, Switzerland), which determined their mass with an accuracy of 0.001 g using the following formula (Demirel et al. [19]):
- The oil chemical composition was determined following the protocol by [20,21,22]. For instance, 310 grams (g) of dried flower was used for oil extraction through a hydro-distillation method using a Clevenger apparatus (WITEG, India). The vapour–oil mixture was passed through the condenser, where, after being condensed, was collected into a flask, and the oil was separated from water through a process known as decantation. The distillation period was 3 h, and the temperature of the heating mantle was 100 °C. Then, 10 µL of the sample was pipetted and diluted with 1 ml of ethanol. The sample was injected into the injector port. Gas chromatography–mass spectrometry (GC-MS) on a Thermo Fischer ISQ TM7610 single quadrupole with a fused silica polar capillary column (30 m × 0.25 mm × 0.25 µm film thickness) was used to analyze the essential oil. The oven temperature programming was from 50 to 250 °C and kept for 10 min at a rate of 2 °C per minute. The injector temperature of 250 °C and transfer line temperature of 280 °C were used. The mass spectrometer’s ion source and the analyzer were maintained at 280 °C and 100 °C, respectively. The mass spectrometer was conducted under full scan mode in electron impact ionisation (EI) positive mode, and the data was gathered from 40 to 600 m/z. The carrier gas was hydrogen at a rate of 1.2 mL/min. The chemical components were identified by comparing their relative retention times and mass spectra with data from the NIST library.
2.6. Data Analysis
3. Results
3.1. Influence of Propagation Techniques on the Growth and Development of Cannabis sativa
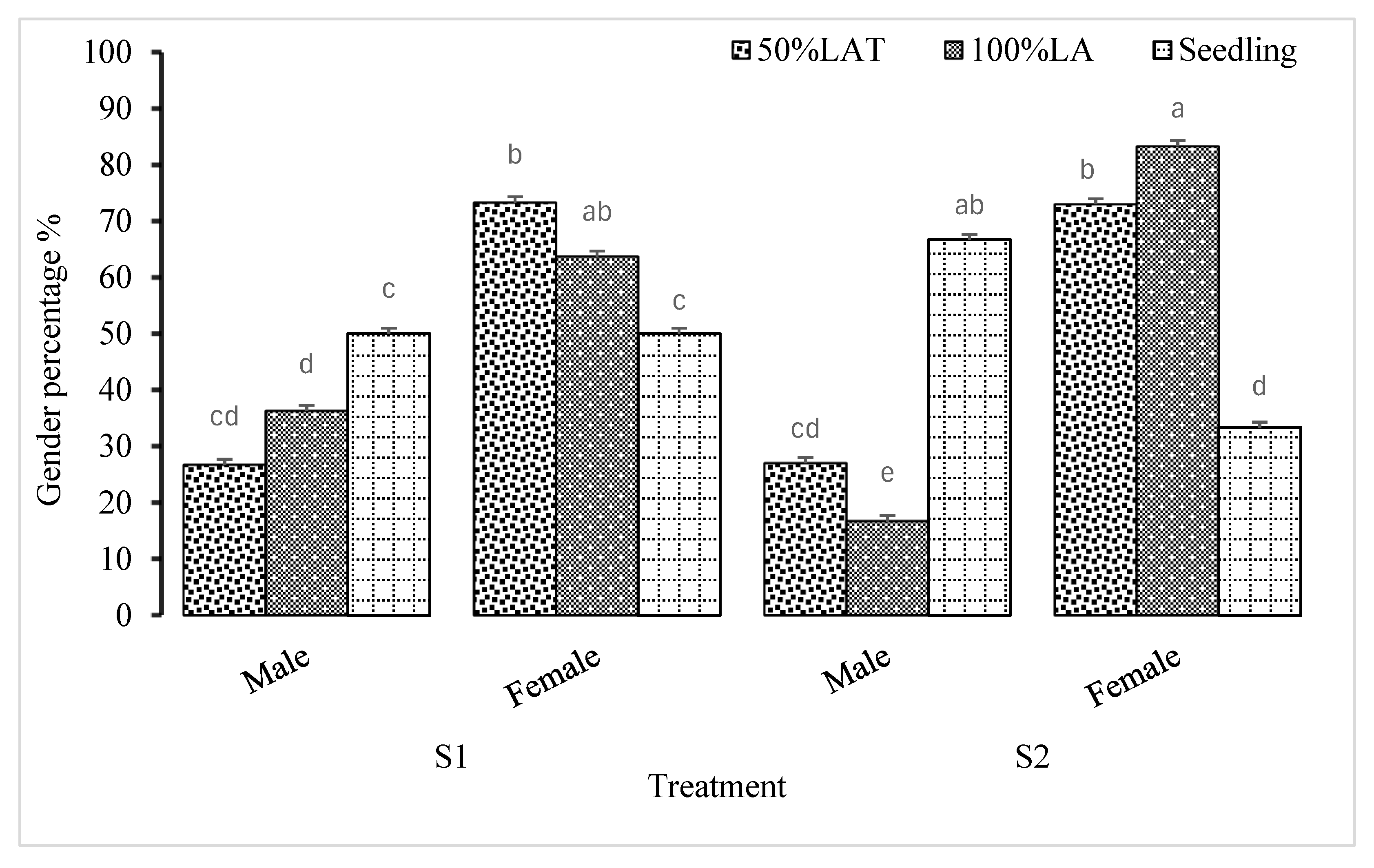
3.2. Determination of Plant Gender as Influenced by Propagation Techniques Used in Cannabis sativa Production
3.3. Effect of Propagation Techniques on Biological Yield and Yield of Cannabis sativa
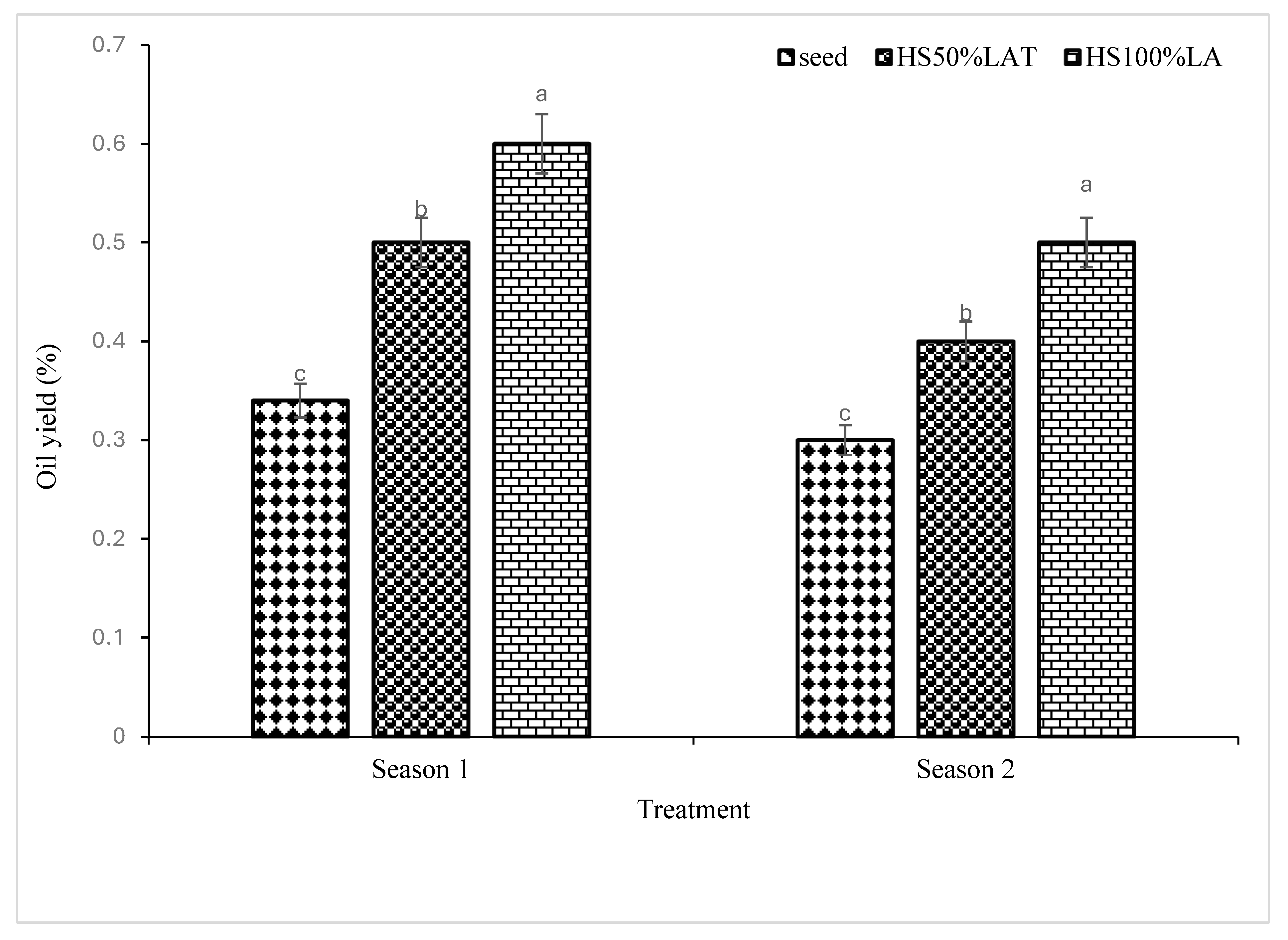
3.4. Influence of Propagation Techniques on Oil Yield of Cannabis sativa
3.5. Influence of Propagation Techniques on the Oil Quality of Cannabis sativa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacGowan, C.; Martynenko, A. Electrostatic separator of cannabis trichomes: An innovative approach to extraction. J. Cannabis Res. 2025, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Nabil, E.L.; Metouekel, A.; Serondo, H.U.; El Koufi, C.; ELAmri, H.; El Fahime, E.; El Kazzouli, S. Cannabis unveiled: From history to innovation, exploring the world of cannabis strains, chemistry, industry and economic impact. Chem. Rev. Lett. 2024, 7, 827–845. [Google Scholar] [CrossRef]
- Busta, L.; Dweikat, I.; Sato, S.J.; Qu, H.; Xue, Y.; Zhou, B.; Gan, L.; Yu, B.; Clemente, T.E.; Cahoon, E.B.; et al. Chemical and genetic variation in feral Cannabis sativa populations across the Nebraska climate gradient. Phytochemistry 2022, 200, 113206. [Google Scholar] [CrossRef] [PubMed]
- Aramendiz-Tatis, H.; Cardona-Ayala, C.; Espitia-Camacho, M.; Herrera-Contreras, A.; Villalba-Soto, A. Agronomic evaluation of Cannabis sativa (L.) cultivars in northern Colombia. Rev. Colomb. Cienc. Hortícolas 2023, 17. [Google Scholar] [CrossRef]
- Lipson Feder, C.; Cohen, O.; Shapira, A.; Katzir, I.; Peer, R.; Guberman, O.; Procaccia, S.; Berman, P.; Flaishman, M.; Meiri, D. Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in cannabis inflorescences. Front. Plant Sci. 2021, 12, 753847. [Google Scholar] [CrossRef]
- Potter, D.J.; Hammond, K.; Tuffnell, S.; Walker, C.; Di Forti, M. Potency of Δ9–tetrahydrocannabinol and other cannabinoids in cannabis in England in 2016: Implications for public health and pharmacology. Drug Test. Anal. 2018, 10, 628–635. [Google Scholar] [CrossRef]
- Timoteo Junior, A.A.; Oswald, I.W. Optimized guidelines for feminized seed production in high-THC Cannabis cultivars. Front. Plant Sci. 2024, 15, 1384286. [Google Scholar] [CrossRef]
- Porras-García, B.; Pinzón-Sandoval, E.H.; Almanza-Merchán, P.J. Propagation of Cannabis sativa (L.) plants through cuttings and use of auxin phytoregulators. Rev. Colomb. Cienc. Hortícolas 2023, 17, 41–90. [Google Scholar] [CrossRef]
- Das, R. Establishing Robust Tools for Cannabis Propagation and Variety Improvement. Ph.D. Thesis, Southern Cross University, East Lismore, Australia, 2024. [Google Scholar] [CrossRef]
- Sanz Gallego, M.; Tomás Gascón, M.; Esteban Pascual, L.S. Optimization of Vegetative Propagation Techniques for Juniperus communis L. Under Greenhouse Conditions. Int. J. Plant Biol. 2025, 16, 57. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Borlaug, B.A.; Gersh, B.J. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation 2022, 146, 339–357. [Google Scholar] [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.P.; et al. Medical cannabis and industrial hemp tissue culture: Present status and future potential. Front. Plant Sci. 2021, 12, 627240. [Google Scholar] [CrossRef]
- Nahar, K. Propagation Technologies: Seedling, Stem Cutting, and Grafting. In Jatropha curcas L: A Potential 2G Energy Crop to Produce Biofuel in Bangladesh: Agronomy, Biotechnology, Biodiesel and Byproducts; Springer Nature: Cham, Switzerland, 2025; pp. 33–55. [Google Scholar] [CrossRef]
- Athwal, A.K. Strategic Analysis & Recommendations for Southwest Garden Supplies’s Nursery Operations. Ph.D. Thesis, University of Northern British Columbia, Prince George, BC, Canada, 2020. [Google Scholar] [CrossRef]
- Sheat, S.; Mushi, E.; Gwandu, F.; Sikirou, M.; Baleke, P.; Kayondo, S.I.; Kulembeka, H.; Adetoro, N.; Winter, S. Cut, root, and grow: Simplifying cassava propagation to scale. Plants 2024, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Odieka, A.E.; Obuzor, G.U.; Oyedeji, O.O.; Gondwe, M.; Hosu, Y.S.; Oyedeji, A.O. The medicinal natural products of Cannabis sativa Linn.: A review. Molecules 2022, 27, 1689. [Google Scholar] [CrossRef] [PubMed]
- Hartman, H.T.; Kester, D.E.; Davies, F.T., Jr.; Geneve, R.L. Plant Propagation: Principles and Practices, 5th ed.; Prentice-Hall Inc.: Englewood, NJ, USA, 2002; p. 647. [Google Scholar]
- Jin, D.; Jin, S.; Chen, J. Cannabis indoor growing conditions, management practices, and post-harvest treatment: A review. Am. J. Plant Sci. 2019, 10, 925. [Google Scholar] [CrossRef]
- Demirel, C.; Kabutey, A.; Herák, D.; Hrabě, P.; Mizera, Č.; Dajbych, O. Optimizing uniaxial oil extraction of bulk rapeseeds: Spectrophotometric and chemical analyses of the extracted oil under pretreatment temperatures and heating intervals. Processes 2021, 9, 1755. [Google Scholar] [CrossRef]
- Micalizzi, G.; Cucinotta, L.; Chiaia, V.; Alibrando, F.; Cannizzaro, F.; Branca, G.; Maida, P.; Oliveri, P.; Mondello, L.; Sciarrone, D. Profiling of seized Cannabis sativa L. flowering tops by means of microwave-assisted hydro distillation and gas chromatography analyses. J. Chromatogr. A 2024, 1727, 464994. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Maggi, F. Valorization of CBD-hemp through distillation to provide essential oil and an improved cannabinoid profile. Sci. Rep. 2021, 11, 19890. [Google Scholar] [CrossRef]
- Aljamali, N.M.; Salih, N.S. Review on chemical separation of crude oil and analysis of its components. J. Pet. Eng. Technol. 2021, 11, 35–49. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Propagation of cannabis for clinical research: An approach towards a modern herbal medicinal product development. Front. Plant Sci. 2020, 11, 958. [Google Scholar] [CrossRef]
- Awotedu, B.F.; Omolola, T.O.; Akala, A.O.; Awotedu, O.L.; Olaoti-Laaro, S.O. Vegetative propagation: A unique technique of improving plants growth. World News Nat. Sci. 2021, 35, 83–101. [Google Scholar]
- Caplan, D.M. Propagation and Root Zone Management for Controlled Environment Cannabis Production. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2018. [Google Scholar]
- Srikanth, S.; Choong, T.W.; Yan, A.; He, J.; Chen, Z. An efficient method for adventitious root induction from stem segments of Brassica species. Front. Plant Sci. 2016, 7, 943. [Google Scholar] [CrossRef]
- Weingarten, M.; Mattson, N.; Grab, H. Evaluating Propagation Techniques for Cannabis sativa L. Cultivation: A Comparative Analysis of Soilless Methods and Aeroponic Parameters. Plants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Sierra Cornejo, N.; Hertel, D.; Becker, J.N.; Hemp, A.; Leuschner, C. Biomass, morphology, and dynamics of the fine root system across a 3,000-m elevation gradient on Mt. Kilimanjaro. Front. Plant Sci. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Amaducci, S.; Zatta, A.; Raffanini, M.; Venturi, G. Characterisation of hemp (Cannabis sativa L.) roots under different growing conditions. Plant Soil 2008, 313, 227–235. [Google Scholar] [CrossRef]
- Gu, J.; Struik, P.C.; Evers, J.B.; Lertngim, N.; Lin, R.; Driever, S.M. Quantifying differences in plant architectural development between hybrid potato (Solanum tuberosum) plants grown from two types of propagules. Ann. Bot. 2024, 133, 365–378. [Google Scholar] [CrossRef]
- Ioannidis, K.; Tomprou, I.; Mitsis, V. An alternative In vitro propagation protocol of Cannabis sativa L. (Cannabaceae) presenting efficient rooting, for commercial production. Plants 2022, 11, 1333. [Google Scholar] [CrossRef]
- Jones, M.; Monthony, A.S. Cannabis propagation. In Handbook of Cannabis Production in Controlled Environments; CRC Press: Boca Raton, FL, USA, 2022; pp. 91–121. [Google Scholar] [CrossRef]
- Spitzer-Rimon, B.; Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and florogenesis in female Cannabis sativa plants. Front. Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef]
- Gaudreau, S.; Missihoun, T.; Germain, H. Early topping: An alternative to standard topping increases yield in cannabis production. Plant Sci. Today 2020, 7, 627–630. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Jones, A.M.P. Morphological characterization of Cannabis sativa L. throughout its complete life cycle. Plants 2023, 12, 3646. [Google Scholar] [CrossRef]
- Lazare, S.; Golshmid, P.; Krassin, A.; Simhon, E.; Cohen, T.L.; Dag, A. Grafting of Cannabis–The effect of the rootstock on vegetative and reproductive indices of the scion. Plant Sci. 2024, 348, 112210. [Google Scholar] [CrossRef]
- Kurtz, L.E.; Borbas, L.N.; Brand, M.H.; Lubell-Brand, J.D. Ex vitro rooting of cannabis sativa microcuttings and their performance compared to retip and stem cuttings. HortScience 2022, 57, 1576–1579. [Google Scholar] [CrossRef]
- Dunn, E. Cultivation, propagation, morphology, and phytochemistry of Cannabis sativa. 2022. Available online: https://www.electricveg.com/cannabaceae/cannabis-sativa.html (accessed on 17 October 2025).
- Clarke, R.C. Marijuana Botany: An Advanced Study: The Propagation and Breeding of Distinctive Cannabis; Ronin Publishing: Berkeley, CA, USA, 2024; Ebook; Available online: www.roninpub.com (accessed on 23 May 2025).
- Pinsard, L. From Cultivars to Textile Application: Insight Into the Textile Hemp Value Chain. Ph.D. Thesis, Institut National Polytechnique de Toulouse-INPT, Toulouse, France, 2023. [Google Scholar]
- Babaei, M.; Nemati, H.; Arouiee, H.; Torkamaneh, D. Characterization of indigenous populations of cannabis in Iran: A morphological and phenological study. BMC Plant Biol. 2024, 24, 151. [Google Scholar] [CrossRef] [PubMed]
- Flajsman, M.; Slapnik, M.; Murovec, J. Production of feminized seeds of high CBD Cannabis sativa L. by manipulation of sex expression and its application to breeding. Front. Plant Sci. 2021, 12, 718092. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Chaudhary, N.; Shanker, K.; Kumar, B.; Kumar, N. Monoecious Cannabis sativa L. discloses the organ-specific variation in glandular trichomes, cannabinoids content and antioxidant potential. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100476. [Google Scholar] [CrossRef]
- Trancoso, I.; de Souza, G.A.; dos Santos, P.R.; dos Santos, K.D.; de Miranda, R.M.D.S.N.; da Silva, A.L.P.M.; Santos, D.Z.; García-Tejero, I.F.; Campostrini, E. Cannabis sativa L.: Crop management and abiotic factors that affect phytocannabinoid production. Agronomy 2022, 12, 1492. [Google Scholar] [CrossRef]
- Campbell, S.M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Evaluation of substrate composition and exogenous hormone application on vegetative propagule rooting success of essential oil hemp (Cannabis sativa L.). PLoS ONE 2021, 16, e0249160. [Google Scholar] [CrossRef]
- Albrecht, U.; Bordas, M.; Lamb, B.; Meyering, B.; Bowman, K.D. Influence of propagation method on root architecture and other traits of young citrus rootstock plants. HortScience 2017, 52, 1569–1576. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; Bassiri Rad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H.; et al. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Change Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef]
- Dorken, M.E.; van Kleunen, M.; Stift, M. Costs of reproduction in flowering plants. New Phytologist. 2025, 247, 55–70. [Google Scholar] [CrossRef]
- Klimesova, J.; Martinkova, J. Clonal growth, resprouting, and vegetative propagation of weeds. In Persistence Strategies of Weeds; Wiley: Hoboken, NJ, USA, 2022; pp. 200–218. [Google Scholar] [CrossRef]
- Kumari, R.; Hamal, U.; Sharma, N. Resource allocation in Flowering Plants: Concept and implications. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Springer: Singapore, 2020; pp. 157–171. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Kim, J.G. The optimal balance between sexual and asexual reproduction in variable environments: A systematic review. J. Ecol. Environ. 2016, 40, 12. [Google Scholar] [CrossRef]
- Stancheva, I.; Geneva, M.; Hristozkova, M.; Zayova, E. Comparison of bioactive compounds in Hyssopus officinalis plants collected from natural habitats with those propagated from seed and in vitro. J. Herbs Spices Med. Plants 2019, 25, 104–113. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Injamum-Ul-Hoque, M.; Das, A.K.; Shaffique, S.; Hasan, M.; Kang, S.M.; Lee, I.J.; Choi, H.W. Tuning Up In Vitro Growth and Development of Cannabis sativa: Recent Advances in Micropropagational Approach. Appl. Biosci. 2025, 4, 12. [Google Scholar] [CrossRef]
- Small, E. Genetics and plant breeding of Cannabis sativa for controlled environment production. In Handbook of Cannabis Production in Controlled Environments; Zheng, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 41–90. [Google Scholar]
- Ahsan, S.M.; Injamum-Ul-Hoque, M.; Shaffique, S.; Ayoobi, A.; Rahman, M.A.; Rahman, M.M.; Choi, H.W. Illuminating Cannabis sativa L.: The power of light in enhancing C. sativa growth and secondary metabolite production. Plants 2024, 13, 2774. [Google Scholar] [CrossRef] [PubMed]
- Druege, U. Overcoming physiological bottlenecks of leaf vitality and root development in cuttings: A systemic perspective. Front. Plant Sci. 2020, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.S.; MacPherson, S.; Lefsrud, M. Light quality impacts vertical growth rate, phytochemical yield and cannabinoid production efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Biteznik, L.; Štukelj, R.; Flajšman, M. The efficiency of CBD production using grafted Cannabis sativa L. Plants is highly dependent on the type of rootstock: A study. Plants 2024, 13, 1117. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Kerrigan, S. Differentiation of hemp from marijuana using a qualitative decision-point assay. Forensic Chem. 2024, 37, 100541. [Google Scholar] [CrossRef]
- Talei, D.; Shams, A.; Khayam Nekouei, M. A Comprehensive Review of Cannabis as a Crucial Pharmaceutical Plant and Its Efficient Propagation Methods. ACS Agric. Sci. Technol. 2025, 5, 1191–1214. [Google Scholar] [CrossRef]
- Seaman, C. Cultivation stress techniques and the production of secondary metabolites in Cannabis sativa. In Recent Advances in the Science of Cannabis; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–30. [Google Scholar]
- Rana, G.; Dhiman, P.; Kumar, A.; Selvaraj, S.; Chauhan, A.; Sharma, G. Phytomediated synthesis of Fe3O4 nanoparticles using Cannabis sativa root extract: Photocatalytic activity and antibacterial efficacy. Biomass Convers. Bioref. 2024, 15, 10275–10292. [Google Scholar] [CrossRef]
| Nutrient Element | Value |
|---|---|
| Phosphorus (P) (mg/L) | 514 |
| Potassium (K) (mg/L) | 381 |
| Calcium (Ca) (mg/L) | 2154 |
| Magnesium (Mg) (mg/L) | 336 |
| Zinc (Zn) (mg/L) | 17.8 |
| pH (kcl) | 5.8 |
| Treatment | Plant Height (cm) | No. of Branches - 1 | Stem Girth (mm) | No. of Weeks to Flowering | No. of Weeks to Flower Amber Coloration |
|---|---|---|---|---|---|
| S1 | |||||
| HS50%LAT | 73.3 (±16.4) | 58 (±33.5) | 11.8 (±1.53) | 5 (±0.46) | 8 (±0.57) |
| HS100%LA | 88.2 (±23.1) | 64.1 (±29.1) | 12.9 (± 1.81) | 4 (±0.35) | 6 (±0.69) |
| Seedling | 92.8 (±7.5) | 49.3 (±14.6) | 16.4 (±2.04) | 9 (±0.81) | 12 (±1.04) |
| Mean | 84.8 | 46.5 | 13.7 | 6 | 8.7 |
| CV% | 26.6 | 49.1 | 13.8 | 9.4 | 8.5 |
| p-Value | 0.05 * | 0.001 ** | 0.05 * | 0.06 ns | 0.03 ** |
| S2 | |||||
| HS50%LAT | 129.2 (±30.4) | 53 (±8.1) | 10.9 (±5.03) | 8 (±0.57) | 9 (±1.4) |
| HS100%LA | 131.1 (±37.6) | 71.3 (±17) | 11.4 (±4.9) | 8 (±0.57) | 9 (±1.4) |
| Seedling | 156.4 (±13.7) | 58.2 (±8.6) | 12.7 (±5.4) | 10 (±1.2) | 12 (±2.23) |
| Mean | 138.9 | 60.8 | 11.7 | 8.6 | 10 |
| CV% | 23.7 | 24 | 15.2 | 9.7 | 9.4 |
| p-Value | 0.05 * | 0.05 * | 0.001 ** | 0.07 ns | 0.05 * |
| Treatment | Means | |||||
|---|---|---|---|---|---|---|
| Fresh Plant Weight (g) | No of Flowers | Fresh Flower Weight (g) | Fresh Leaf Weight (g) | Dry Flower Weight (g) | Dry Leaf Weight (g) | |
| S1 | ||||||
| HS50%LAT | 166.8 (±38) | 163.5 (±44.3) | 75.9 (±23) | 50.9 (±28) | 25.8 (±11) | 15.6 (±7.2) |
| HS100%LA | 167.5 (±37) | 202 (±81.2) | 99.5 (±22) | 53.8 (±24) | 45.3 (±7.4) | 18.3 (±7) |
| Seedling | 236.1 (±101) | 125 (±35) | 55.2 (±11) | 106.9 (±31) | 18.9 (±8) | 71.2 (±26) |
| Mean | 190.1 | 163 | 77 | 70.5 | 30 | 35 |
| Cv% | 35.3 | 28 | 33 | 37.5 | 40 | 24.2 |
| p-Value | 0.05 * | 0.01 ** | 0.01 ** | 0.05 * | 0.04 * | 0.05 * |
| S2 | ||||||
| HS50%LAT | 415 (±92) | 177.8 (±27) | 111.9 (±53) | 103 (±42) | 49 (±19) | 48 (±14.2) |
| HS100%LA | 469 (±57) | 183.1 (±30.4) | 113.9 (±58.3) | 111 (±52) | 49.8 (±20) | 49.9 (±18) |
| Seedling | 476 (±91) | 131.6 (±50) | 68.7 (±40.2) | 127.6 (±52) | 35.3 (±16.3) | 54.4 (±19) |
| Mean | 453.4 | 164.2 | 98.2 | 115.8 | 44.7 | 50.7 |
| Cv% | 17.4 | 21.3 | 24.5 | 41.4 | 41.4 | 33 |
| p-Value | 0.07 ns | 0.02 ** | 0.05 * | 0.6 ns | 0.05 * | 0.57 ns |
| Treatment | Cannabidiol (%) | Cannabicyclol (%) | Cannabichromene (%) | Δ8-THC (%) | Δ9-THC (%) | Δ11-THC (%) |
|---|---|---|---|---|---|---|
| S1 | ||||||
| HS50% LAT | 28 | 0.75 | 0.13 | 43.3 | 11.9 | 8.4 |
| SH100% LA | 26.1 | 0.5 | 0.07 | 35 | 15.8 | 6.26 |
| Seedling | 26.1 | 1.36 | 0.18 | 53.8 | 7.6 | 7 |
| S2 | ||||||
| HS50% LAT | 25.7 | 1.2 | 0.15 | 47 | 11.7 | 8.25 |
| HS100% LA | 24.3 | 0.7 | 0.03 | 24.3 | 4.9 | 5.68 |
| Seedling | 29.6 | 2 | 0.27 | 48.8 | 7.9 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumani, A.; Silwana, T.T.; Egbichi, I.M.; Oyedeji, A.O.; Mpambani, B.; Seepe, H.A. Effect of Propagation Techniques on Growth, Development, Oil Yield, and Quality of Medicinal Cannabis (Cannabis sativa) Found in Lusikisiki, Eastern Cape, South Africa. Horticulturae 2025, 11, 1428. https://doi.org/10.3390/horticulturae11121428
Dumani A, Silwana TT, Egbichi IM, Oyedeji AO, Mpambani B, Seepe HA. Effect of Propagation Techniques on Growth, Development, Oil Yield, and Quality of Medicinal Cannabis (Cannabis sativa) Found in Lusikisiki, Eastern Cape, South Africa. Horticulturae. 2025; 11(12):1428. https://doi.org/10.3390/horticulturae11121428
Chicago/Turabian StyleDumani, Azile, Tembakazi Theodora Silwana, Ifeanyi Moses Egbichi, Adebola Omowunmi Oyedeji, Babalwa Mpambani, and Hlabana Alfred Seepe. 2025. "Effect of Propagation Techniques on Growth, Development, Oil Yield, and Quality of Medicinal Cannabis (Cannabis sativa) Found in Lusikisiki, Eastern Cape, South Africa" Horticulturae 11, no. 12: 1428. https://doi.org/10.3390/horticulturae11121428
APA StyleDumani, A., Silwana, T. T., Egbichi, I. M., Oyedeji, A. O., Mpambani, B., & Seepe, H. A. (2025). Effect of Propagation Techniques on Growth, Development, Oil Yield, and Quality of Medicinal Cannabis (Cannabis sativa) Found in Lusikisiki, Eastern Cape, South Africa. Horticulturae, 11(12), 1428. https://doi.org/10.3390/horticulturae11121428






