Advances in Growing Degree Days Models for Flowering to Harvest: Optimizing Crop Management with Methods of Precision Horticulture—A Review
Abstract
1. Introduction
2. Methodology
3. Growing Degree Days Model
3.1. Concept
- d: day
- D: end of growing stage
3.2. Starting Date
3.3. Base Temperature
3.4. Simple Average Method
3.5. Sine Wave Approximation (Baskerville-Emin Method)
4. Using Growing Degree Days to Guide Harvest and Quality Decisions
4.1. Flowering
| Crop | Climatic Region | °C | Starting Date/Duration | GDD (°C) | Reference |
|---|---|---|---|---|---|
| Olive | Mediterranean | 5.0 | 1 January | 1434 | [133] |
| Apple | Mediterranean | 4.0 | February | 7471–9690 * | [120] |
| Sweet cherry | Temperate | 2.0 | 1 March | 254 | [119] |
| Sweet cherry | Temperate | 4.5 | 3 Feb–28 March 11 Feb–14 April | 3233–4343 * 5444–6988 * | [134] |
| Sour cherry | Temperate | 2.0 | 1 January | 492 | [135] |
| Sour cherry | Temperate | 4.0 | 1 March | 125 | [38] |
| Grapevine | Temperate | 10.0 | 1 January | 1600–1850 | [136] |
| Grapevine | Temperate | 10.0 | 1 January | 318–552 | [131] |
| Mango | Subtropical | 10.0 | from floral bud swelling-floral opening | 623 | [122] |
4.2. Fruit Development and Maturity
| Crop | Climatic Region | °C | Starting Date/Measurement Duration | GDD (°C) | Reference |
|---|---|---|---|---|---|
| Peach | Sub-temperate | 7.0 | January | 814–1894 | [140] |
| Peach | Temperate | 7.0 | May–August | 1651–1826 | [141] |
| Grapevine | Temperate | 10.0 | April–September | 1204–1940 | [142] |
| Grapevine | Temperate | 10.0 | After bloom | 556–800 | [131] |
| Grapevine | Humid continental | 10.0 | 1 April–31 Oct. | 1070–1700 | [143] |
| Mango | Tropical | 17.9 | July–November | 1062.71–1309.41 | [121] |
| Mango | Tropical | 12.0 | Asparagus stage of flower | 1740–2185 | [144] |
| Mango | Subtropical | 10.0 | Floral bud swelling to commercial maturity | 2399 | [122] |
| Papaya | Subtropical | 15.0 | Flowering to harvest | 1293–1488 | [34] |
| Orange | Subtropical (Humid) | 12.8 | 9 August | 4462–5090 | [145] |
| Eggplant | Subtropical | 15.6 | June–November | 892–1078 | [146] |
4.3. Fruit Quality
5. Remote Sensing Approaches
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greer, D.H.; Weedon, M. The impact of high temperatures on Vitis vinifera cv. Semillon grapevine performance and berry ripening. Front. Plant Sci. 2013, 4, 491. [Google Scholar] [CrossRef]
- Marklein, A.; Elias, E.; Peter, N.; Steenwerth, K. Projected temperature increases may require shifts in the growing season of cool-season crops and the growing locations of warm-season crops. Sci. Total Environ. 2020, 746, 140918. [Google Scholar] [CrossRef]
- Cesaraccio, C.; Spano, D.; Duce, P.; Snyder, R.L. An improved model for determining degree-day values from daily temperature data. Int. J. Biometeorol. 2001, 45, 161–169. [Google Scholar] [CrossRef]
- Nahas, A.; Abou-Saleh, H.; Baydoun, S. Temperature effect on plant pigments, enzymes and essential oil content. Adv. Crop Sci. Technol. 2019, 7, 420. [Google Scholar]
- Grigorieva, E.; Matzarakis, A.; De Freitas, C. Analysis of growing degree-days as a climate impact indicator in a region with extreme annual air temperature amplitude. Clim. Res. 2010, 42, 143–154. [Google Scholar] [CrossRef]
- Knapp, B.D.; Huang, K.C. The effects of temperature on cellular physiology. Annu. Rev. Biophys. 2022, 51, 499–526. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Sung, D.Y.; Kaplan, F.; Lee, K.J.; Guy, C.L. Acquired tolerance to temperature extreme. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Yamori, W. Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. BiophysActa Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Bernáth, S.; Juraszovich, B.; Kocsis, I.; Végh, B. Influence of climate warming on grapevine (Vitis vinifera L.) phenology. Plants 2021, 10, 1020. [Google Scholar] [CrossRef]
- Licurici, M.; Vlăduț, A.Ș.; Burada, C.D. A study of observed climate change effects on grapevine suitability in Oltenia (Romania). Horticulturae 2025, 11, 591. [Google Scholar] [CrossRef]
- Baskerville, G.L.; Emin, P. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology 1969, 50, 514–521. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- De Réaumur, R.A.F. Observations du thermomètre, faites à Paris pendant l’année 1735, comparées avec celles qui ont été faites sous la ligne, à l’île de France, à Alger et quelques-unes des nos îles de l’Amérique. Mém. Acad. Sci. Paris 1735. Available online: https://www.academie-sciences.fr/pdf/dossiers/Reaumur/Reaumur_pdf/p545_576_vol3532m.pdf (accessed on 20 November 2024).
- Wang, J.Y. Critique of the heat unit approach to plant response studies. Ecology 1960, 41, 785–787. [Google Scholar] [CrossRef]
- Russelle, M.P.; Wilhelm, W.W.; Olson, R.A.; Power, J.F. Growth analysis based on degree days. Crop Sci. 1984, 24, 28–32. [Google Scholar] [CrossRef]
- Pawasut, A.; Fujishige, N.; Yamane, K.; Yamaki, Y.; Honjo, H. Relationships between chilling and heat requirement for flowering in ornamental peaches. Engei Gakkai Zasshi 2004, 73, 519–523. [Google Scholar] [CrossRef]
- Ruiz, D.; Campoy, J.A.; Egea, J. Chilling and heat requirements of apricot cultivars for flowering. Environ. Exp. Bot. 2007, 61, 254–263. [Google Scholar] [CrossRef]
- Souza, A.P.R.; Carvalho, C.M.; Lima, A.D.; Florentino, H.O.; Escobedo, J.F. Comparison of methodologies for degree-day estimation using numerical methods. Acta Sci. Agron. 2011, 33, 347–353. [Google Scholar] [CrossRef]
- Ritchie, J.; Nesmith, D. Temperature and crop development. In Modeling Plant and Soil Systems; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1991; pp. 31–45. [Google Scholar]
- Kukal, M.S.; Irmak, S.U.S. Agro-climate in the 20th centuryagro: Growing degree days, first and last frost, growing season length, and impacts on crop yields. Sci. Rep. 2018, 8, 6977. [Google Scholar] [CrossRef]
- Gu, S. Growing degree hours: A simple, accurate, and precise protocol to approximate growing heat summation for grapevines. Int. J. Biometeorol. 2016, 60, 1123–1134. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Bonhomme, R. Bases and limits to using “degree-day” units. Eur. J. Agron. 2000, 13, 1–10. [Google Scholar] [CrossRef]
- Bollero, G.A.; Bullock, D.G.; Hollinger, S.E. Soil temperature and planting date effects on corn yield, leaf area, and plant development. Agron. J. 1996, 88, 385–390. [Google Scholar] [CrossRef]
- Whiting, M.D.; Salazar, M.R.; Hoogenboom, G. Development of bloom phenology models for tree fruits. Acta Hortic. 2015, 1068, 107–112. [Google Scholar] [CrossRef]
- Salinas, I.; Hueso, J.; Cuevas, J. Fruit growth model, thermal requirements, and fruit size determinants in papaya cultivars grown under subtropical conditions. Sci. Hortic. 2019, 246, 1022–1027. [Google Scholar] [CrossRef]
- Zalom, F.J.; Goodell, P.B.; Wilson, L.T.; Barnett, W.W.; Bentley, W.J. Degree-Days: The Calculation and Use of Heat Units in Pest Management; DANR Leaflet 21373; University of California: Oakland, CA, USA, 1983. [Google Scholar]
- Pruess, K. Day-degree methods for pest management. Environ. Entomol. 1983, 12, 613–619. [Google Scholar] [CrossRef]
- Tao, M.; Adler, P.R.; Larsen, A.E.; Suh, S. Pesticide application rates and their toxicological impacts: Why do they vary so widely across the U.S.? Environ. Res. Lett. 2020, 15, 124049. [Google Scholar] [CrossRef]
- Zavalloni, C.; Andresen, J.A.; Flore, J.A. Phenological models of flower bud stages and fruit growth of Montmorency sour cherry based on growing degree-day accumulation. J. Am. Soc. Hort. Sci. 2006, 131, 601. [Google Scholar] [CrossRef]
- Hernández-Martínez, N.R. Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 2023, 290, 110515. [Google Scholar] [CrossRef]
- Tao, F.; Zhao, Z.; Zhang, S.; Rötter, R.P. Heat stress impacts on wheat growth and yield were reduced in the Huang-Huai-Hai Plain of China in the past three decades. Eur. J. Agron. 2015, 71, 44–52. [Google Scholar] [CrossRef]
- Yuri, J.A.; Moggia, C.; Torres, C.A.; Sepulveda, A.; Lepe, V.; Vásquez, J.L. Performance of apple (Malus × domestica Borkh.) cultivars grown in different Chilean regions on a six-year trial. Part I: Vegetative growth, yield, and phenology. HortScience 2011, 46, 365–370. [Google Scholar] [CrossRef]
- Forshey, C.G.; Elfving, D.C. The relationship between vegetative growth and fruiting in apple trees. Hortic. Rev. 1989, 11, 229–287. [Google Scholar]
- James, R.; Pitts-Singer, T. Bee Pollination in Agricultural Ecosystems; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Rodrigues, E.J.; da Silva Caldana, N.F.; Ferreira, L.G.B.; da Silva, C.M.; Nitsche, P.R.; Alves, D.S.; de Aguiar e Silva, M.A. Accumulation of degree-days and chilling hours for ‘Eva’ apple tree production in temperate climate. Austral. J. Crop Sci. 2022, 16, 676–681. [Google Scholar] [CrossRef]
- Warrington, I.J.; Fulton, T.A.; Halligan, E.A.; de Silva, H.N. Apple fruit growth and maturity are affected by early season temperatures. J. Am. Soc. Hortic. Sci. 1999, 124, 468–477. [Google Scholar] [CrossRef]
- Ruiz-Nieves, J.M.; Ayala-Garay, O.J.; Serra, V.; Dumont, D.; Vercambre, G.; Génard, M.; Gautier, H. The effects of diurnal temperature rise on tomato fruit quality. Can the management of the greenhouse climate mitigate such effects? Sci. Hortic. 2021, 278, 109847. [Google Scholar] [CrossRef]
- Sikhandakasmita, P.; Panawat, S.; Kataoka, I.; Mochioka, R.; Beppu, K. Impact of temperatures during fruit development on fruit growth rate and qualities of ‘KU-PP2’ Peach. Hortic. J. 2022, 91, 149–158. [Google Scholar] [CrossRef]
- Wielgolaski, F.E. Starting dates and basic temperatures in phenological observations of plants. Int. J. Biometeorol. 1999, 42, 158–168. [Google Scholar] [CrossRef]
- Kläring, H.; Schmidt, A. Diurnal temperature variations significantly affect cucumber fruit growth. HortScience 2017, 52, 60–64. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q. A new nonlinear method for calculating growing degree days. Sci. Rep. 2018, 8, 10149. [Google Scholar] [CrossRef]
- Keramitsoglou, I.; Sismanidis, P.; Sykioti, O.; Pisinaras, V.; Tsakmakis, I.; Panagopoulos, A.; Argyriou, A.; Kiranoudis, C.T. SENSE-GDD: A satellite-derived temperature monitoring service to provide growing degree days. Agriculture 2023, 13, 1108. [Google Scholar] [CrossRef]
- Priyanka, B. Machine learning-driven precision agriculture: Enhancing farm management through predictive insights. Int. J. Intell. Syst. Appl. Eng. 2024, 12, 195–201. [Google Scholar]
- Miller, P.; Lanier, W.; Brandt, S. Using Growing Degree Days to Predict Plant Stages; MT200103 AG 7/2001; Montana State University: Bozeman, MT, USA, 2001. [Google Scholar]
- Arnold, C.Y. The development and significance of the base temperature in a linear heat unit system. Proc. Am. Soc. Hortic. Sci. 1959, 74, 430–445. [Google Scholar]
- Snyder, R.L.; Spano, D.; Cesaraccio, C.; Duce, P. Determining degree-day thresholds from field observations. Int. J. Biometeorol. 1999, 42, 177–182. [Google Scholar] [CrossRef]
- Nuttonson, M.Y. Wheat-Climate Relationships and the Use of Phenology in Ascertaining the Thermal and Photo-Thermal Requirements of Wheat. e-book. 1955. Available online: https://babel.hathitrust.org/cgi/pt?id=mdp.39015007513594&seq=7 (accessed on 10 November 2024).
- Arnold, C.Y. Maximum-minimum temperatures as a basis for computing heat units. Proc. Am. Soc. Hortic. Sci. 1960, 76, 682–692. [Google Scholar]
- Davidson, H.R.; Campbell, C.A. The effect of temperature, moisture, and nitrogen on the rate of development of spring wheat as measured by degree days. Can. J. Plant Sci. 1983, 63, 833–846. [Google Scholar] [CrossRef]
- Sevacherian, V.; Stern, V.M.; Mueller, A.J. Heat accumulation for timing Lygus control measures in a safflower-cotton complex. J. Econ. Entomol. 1977, 70, 399–402. [Google Scholar] [CrossRef]
- Ometto, J.C. Bioclimatologia Vegetal; Agronômica CERES: São Paulo, Brazil, 1981; 440p. [Google Scholar]
- Snyder, R.L. Hand calculating degree days. Agric. For. Meteorol. 1985, 35, 353–358. [Google Scholar] [CrossRef]
- Roltsch, W.J.; Zalom, F.G.; Strawn, A.J.; Strand, J.F.; Pitcairn, M.J. Evaluation of several degree-day estimation methods in California climates. Int. J. Biometeorol. 1999, 42, 169–176. [Google Scholar] [CrossRef]
- Yang, S.; Logan, J.; Coffey, D.L. Mathematical formulae for calculating the base temperature for growing degree-days. Agric. For. Meteorol. 1995, 75, 11–28. [Google Scholar] [CrossRef]
- Myking, T.; Heide, O.M. Dormancy release and chilling requirement of buds of latitudinal ecotypes of Betula pendula and B. pubescens. Tree Physiol. 1995, 15, 697–704. [Google Scholar] [CrossRef]
- Ununger, J.; Ekberg, I.; Kang, H. Genetic control and age-related changes of juvenile growth characters in Picea abies. Scand. J. For. Res. 1988, 3, 55–66. [Google Scholar] [CrossRef]
- Arzt, T.; Ludwig, W. Alte Probleme der Phänologie in neuer Beleuchtung. Naturw. Rundsch. 1949, 2, 450–459. [Google Scholar]
- Besselat, B.; Drouet, G.; Palagos, B. Méthodologie pour déterminer le besoin thermique nécessaire au départ de la floraison de la vigne. J. Int. Sci. Vigne Vin 1995, 29, 171–182. [Google Scholar]
- Schwartz, C.J.; Doyle, M.R.; Manzaneda, A.J.; Rey, P.J.; Mitchell-Olds, T.; Amasino, R.M. Natural variation of flowering time and vernalization responsiveness in Brachypodium distachyon. Bioenerg. Res. 2010, 3, 38–46. [Google Scholar] [CrossRef]
- Bradley, N.L.; Leopold, A.C.; Ross, J.; Huffaker, W. Phenological changes reflect climate change in Wisconsin. Proc. Natl. Acad. Sci. USA 1999, 96, 9701–9704. [Google Scholar] [CrossRef]
- Inouye, D.W.; Saavedra, F.; Lee-Yang, W. Environmental influences on the phenology and abundance of flowering by Androsace septentrionalis (Primulaceae). Am. J. Bot. 2003, 90, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Miller-Rushing, A.J.; Primack, R.B. Global warming and flowering times in Thoreau’s Concord: A community perspective. Ecology 2008, 89, 332–341. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Elzinga, J.A.; Atlan, A.; Biere, A.; Gigord, L.; Weis, A.E.; Bernasconi, G. Time after time: Flowering phenology and biotic interactions. Trends Ecol. Evol. 2007, 22, 432–439. [Google Scholar] [CrossRef]
- Tooke, F.; Battey, N. Temperate flowering phenology. J. Exp. Bot. 2010, 61, 2853–2862. [Google Scholar] [CrossRef]
- Ruml, M.; Vuković, A.; Milatović, D. Evaluation of different methods for determining growing degree-day thresholds in apricot cultivars. Int. J. Biometeorol. 2010, 54, 411–422. [Google Scholar] [CrossRef]
- Kramer, K. Modelling comparison to evaluate the importance of phenology for the effects of climate change in growth of temperate-zone deciduous trees. Clim. Res. 1995, 5, 119–130. [Google Scholar] [CrossRef]
- Vachůn, Z. Phenophases of blossoming and picking maturity and their relationships in twenty apricot genotypes for a period of six years. Hortic. Sci. 2003, 30, 43–50. [Google Scholar] [CrossRef]
- Parker, A.K.; De Cortázar-Atauri, I.G.; Van Leeuwen, C.; Chuine, I. General phenological model to characterise the timing of flowering and veraison of Vitis vinifera L. Aust. J. Grape Wine Res. 2011, 17, 206–216. [Google Scholar] [CrossRef]
- Malyshev, A.V.; van der Maaten, E.; Garthen, A.; Maß, D.; Schwabe, M.; Kreyling, J. Inter-individual budburst variation in Fagus sylvatica is driven by warming rate. Front. Plant Sci. 2022, 13, 853521. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; van den Boom, T.; et al. The BBCH system for coding the phenological growth stages of plants—History and publications. J. Kult. Pflanz. 2009, 61, 14–21. [Google Scholar]
- Chen, L.; Zhang, H.; Yang, J. Effects of flowering mode and pollinator sharing on reproductive success in a mixed-mating system. Sci. Total Environ. 2024, 869, 161690. [Google Scholar] [CrossRef]
- Thornley, J.H.; Johnson, I.R. Plant and Crop Modelling; Clarendon: Oxford, UK, 1990. [Google Scholar]
- Caradonna, P.; Iler, A.; Inouye, D. Shifts in flowering phenology reshape a subalpine plant community. Proc. Natl. Acad. Sci. USA 2014, 111, 4916–4921. [Google Scholar] [CrossRef]
- Williams, D.W.; Andris, H.L.; Beede, R.H.; Luvisi, D.A.; Norton, M.V.K.; Williams, L.E. Validation of a model for the growth and development of the Thompson Seedless grapevine. II. Phenology. Am. J. Enol. Vitic. 1985, 36, 283–289. [Google Scholar] [CrossRef]
- Nuttonson, M.Y. Some preliminary observations of phenological data as a tool in the study of photoperiodic and thermal requirements of various plant material. In Vernalization and Photoperiodism; Murneek, A.E., Whyte, R.O., Eds.; Chronica Botanica: Waltham, MA, USA, 1948; pp. 129–143. [Google Scholar]
- Murray, M.B.; Cannell, M.G.R.; Smith, R.I. Date of budburst of fifteen tree species in Britain following climate warming. J. Appl. Ecol. 1989, 26, 693–700. [Google Scholar] [CrossRef]
- Hunter, A.; Lechowicz, M. Predicting the timing of budburst in temperate trees. J. Appl. Ecol. 1992, 29, 297–304. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Bonhomme, R. Predicting maize phenology. In Predicting Crop Phenology; Springer: Berlin/Heidelberg, Germany, 1991; pp. 5–131. [Google Scholar]
- Magoon, C.A.; Culpepper, C.W. Response of Sweet Corn to Varying Temperatures from Time of Planting to Canning Maturity; Technical Bulletin 312; US Department of Agriculture: Washington, DC, USA, 1932. [Google Scholar]
- Stier, H.S. A Physiological Study of Growth and Fruiting in the Tomato (Lycopersicum esculentum L.) with Reference to the Effect of Climatic and Edaphic Conditions. Ph.D. Dissertation, University of Maryland, College Park, MD, USA, 1939. [Google Scholar]
- Nuttonson, M.Y. The role of bioclimatology in agriculture with special reference to the use of thermal and photo-thermal requirements of pure-line varieties of plants as a biological indicator in ascertaining climatic analogues (Homoclimes). Int. Soc. Bioclimatol. Biometeorol. 1958, 2, 129–148. [Google Scholar] [CrossRef]
- Hoover, M.W. Some effects of temperature on the growth of southern peas. Proc. Am. Soc. Hort. Sci. 1955, 66, 308–312. [Google Scholar]
- Perry, K.B.; Wehner, T.C.; Johnson, G.L. Comparison of 14 methods to determine heat unit requirements for cucumber harvest. HortScience 1986, 21, 419–423. [Google Scholar] [CrossRef]
- Wilson, L.T.; Barnett, W.W. Degree days: An aid in crop and pest management. Calif. Agric. 1983, 37, 4–7. [Google Scholar]
- Milatovic, D.; Ruml, M.; Vulić, T. Heat requirement from blooming to maturing in apricot cultivars. Acta Hortic. 2010, 862, 245–250. [Google Scholar] [CrossRef]
- Richardson, E.; Seeley, S.D.; Walker, D.R. A model for estimating the completion of rest for “Redhaven” and “Elberta” peach trees. HortScience 1974, 9, 331–332. [Google Scholar] [CrossRef]
- White, L.M. Relationships between meteorological measurements and flowering of index species to flowering of 53 plant species. Agric. Meteorol. 1979, 20, 189–204. [Google Scholar] [CrossRef]
- Allen, J.C. A modified sine wave method for calculating degree days. Environ. Entomol. 1976, 5, 388–396. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, F.; Zheng, J.; Chen, L.; Hänninen, H.; Wu, J. Temperature sum models in plant spring phenology studies: Two commonly used methods have different fields of application. J. Exp. Bot. 2024, 75, 6011–6016. [Google Scholar] [CrossRef]
- Ashcroft, G.L.; Richardson, E.A.; Seeley, S.D. A statistical method for determining chill unit and growing degree hour requirements for deciduous fruit trees. HortScience 1977, 12, 347–348. [Google Scholar] [CrossRef]
- Anderson, J.L.; Richardson, E.A.; Kesner, C.D. Validation of chill unit and flower bud phenology models for ‘Montmorency’ sour cherry. Acta Hortic. 1986, 184, 71–78. [Google Scholar] [CrossRef]
- VÀvra, R.; Litschmann, T. Apricot flowering time prediction using growing degree hours. Acta Hortic. 2022, 1342, 111–114. [Google Scholar] [CrossRef]
- Hachisuca, A.M.M.; de Souza, E.G.; Oliveira, W.K.M.; Bazzi, C.L.; Donato, D.G.; Mendes, I.S.; Abdala, M.C.; Mercante, E. AgDataBox-IoT—Application development for agrometeorological stations in smart. MethodsX 2023, 11, 102419. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Smith, R.I. Thermal time, chill days and prediction of budburst in Picea sitchensis. J. Appl. Ecol. 1983, 20, 951–963. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Nonlinear effects of weather on corn yields. Rev. Agric. Econ. 2006, 28, 391–398. [Google Scholar] [CrossRef]
- Lowry, W.P. Water and Life: An Introduction to Biometeorology; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Thompson, R.; Clark, R.M. Spatio-temporal modelling and assessment of within-species phenological variability using thermal time methods. Int. J. Biometeorol. 2006, 50, 312–322. [Google Scholar] [CrossRef]
- Fitter, A.H.; Fitter, R.S.R.; Harris, I.T.B.; Williamson, M.H. Relationships between first flowering date and temperature in the flora of a locality in central England. Funct. Ecol. 1995, 9, 55–60. [Google Scholar] [CrossRef]
- Robertson, G.W. A biometeorological time scale for a cereal crop involving day and night temperatures and photoperiod. Int. J. Biometeorol. 1968, 12, 191–223. [Google Scholar] [CrossRef]
- Barriball, S.; Han, A.; Schlautman, B. Effect of growing degree days, day of the year, and cropping systems on reproductive development of Kernza in Kansas. Agrosyst. Geosci. Environ. 2022, 5, e20286. [Google Scholar] [CrossRef]
- Farbo, A.; Sarvia, F.; De Petris, S.; Borgogno-Mondino, E. Preliminary concerns about agronomic interpretation of NDVI time series from Sentinel-2 data: Phenology and thermal efficiency of winter wheat in Piemonte (NW Italy). In The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Proceedings of the XXIV ISPRS Congress, Nice, France, 6–11 June 2022; Copernicus Publications: Göttingen, Germany, 2022; Volume XLIII-B3-2022, pp. 6–11. [Google Scholar]
- Zhang, Z.; Li, Y.; Harder, P.; Helgason, W.; Famiglietti, J.; Valayamkunnath, P.; He, C.; Li, Z. Developing spring wheat in the Noah-MP land surface model (v4.4) for growing season dynamics and responses to temperature stress. Geosci. Model Dev. 2023, 16, 3809–3825. [Google Scholar] [CrossRef]
- Lim, M.H.; Lee, H.; Yoe, H. A study on the design of a melon fruit size prediction system using GDD and integrated solar radiation. In Proceedings of the 2024 International Conference on Artificial Intelligence in Information and Communication (ICAIIC), Osaka, Japan, 19–22 February 2024; pp. 868–871. [Google Scholar]
- Milech, C.; Dini, M.; Scariotto, S.; Santos, J.; Herter, F.; Raseira, M. Chilling requirement of ten peach cultivars estimated by different models. J. Exp. Agric. Int. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Mathematical analysis of a two-step model involving a cooperative transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Luedeling, E.; Schiffers, K.; Fohrmann, T.; Urbach, C. PhenoFlex—An integrated model to predict spring phenology in temperate fruit trees. Agric. For. Meteorol. 2021, 307, 108491. [Google Scholar] [CrossRef]
- Guo, L.; Dai, J.; Ranjitkar, S.; Yu, H.; Xu, J.; Luedeling, E. Chilling and heat requirements for flowering in temperate fruit trees. Int. J. Biometeorol. 2014, 58, 1195–1206. [Google Scholar] [CrossRef]
- Citadin, I.; Raseira, M.C.B.; Herter, F.G.; Silva, J.B. Heat requirement for blooming and leafing in peach. HortScience 2001, 36, 305–307. [Google Scholar] [CrossRef]
- Meland, M.; Frøynes, O.; Coop, L.; Kaiser, C. Modeling of sweet cherry flowering based on temperature and phenology in a mesic Nordic climate. Acta Hortic. 2017, 1162, 19–22. [Google Scholar] [CrossRef]
- Díez-Palet, I.; Funes, I.; Savé, R.; Biel, C.; de Herralde, F.; Miarnau, X.; Vargas, F.; Àvila, G.; Carbó, J.; Aranda, X. Blooming under Mediterranean climate: Estimating cultivar-specific chill and heat requirements of almond and apple trees using a statistical approach. Agronomy 2019, 9, 760. [Google Scholar] [CrossRef]
- Kanzaria, D.; Chovatia, R.; Polara, N.; Varu, D. Impact of GDD on phenology of mango (Mangifera indica). Indian J. Agric. Sci. 2015, 85, 1114–1117. [Google Scholar] [CrossRef]
- Lemos, L.M.C.; Salomão, L.C.C.; Siqueira, D.L.; Pereira, O.L.; Cecon, P.R. Heat unit accumulation and inflorescence and fruit development in ‘Ubá’ mango trees grown in Visconde do Rio Branco-MG. Rev. Bras. Frutic. 2018, 40, e-491. [Google Scholar] [CrossRef]
- Menzel, C.M. A review of fruit development in strawberry: High temperatures accelerate flower development and decrease the size of the flowers and fruit. J. Hortic. Sci. Biotechnol. 2023, 98, 409–431. [Google Scholar] [CrossRef]
- Parker, L.E.; Zhang, N.; Abatzoglou, J.T.; Kisekka, I.; McElrone, A.J.; Ostoja, S.M. A variety-specific analysis of climate change effects on California winegrapes. Int. J. Biometeorol. 2024, 68, 1559–1571. [Google Scholar] [CrossRef]
- Atagul, O.; Calle, A.; Demirel, G.; Lawton, J.M.; Bridges, W.C.; Gasic, K. Estimating heat requirement for flowering in peach germplasm. Agronomy 2022, 12, 1002. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Rötzer, T. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Taylor, L. Phenological sensitivity of temperate perennial fruit crops to climate change. Horticulturae 2019, 5, 45. [Google Scholar]
- Funes, I.; Aranda, X.; Biel, C.; Carbó, J.; Camps, F.; Molina, A.J.; de Herralde, F.; Grau, B.; Savé, R. Future climate change impacts on apple flowering date in a Mediterranean subbasin. Agric. Water Manag. 2016, 164, 19–27. [Google Scholar] [CrossRef]
- Rodrigo, J.; Herrero, M. Effects of pre-blossom temperatures on flower development and fruit set in apricot. Sci. Hortic. 2002, 92, 125–135. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Brennan, R.M.; Jones, H.G. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Zapata, D.; Salazar-Gutierrez, M.; Chaves, B.; Keller, M.; Hoogenboom, G. Predicting key phenological stages for 17 grapevine cultivars (Vitis vinifera L.). Am. J. Enol. Vitic. 2016, 67, 224–233. [Google Scholar] [CrossRef]
- Parker, A.K.; García de Cortázar-Atauri, I.; Gény, L.; Spring, J.-L.; Destrac, A.; Schultz, H.; Molitor, D.; Lacombe, T.; Graça, A.; Monamy, C.; et al. Temperature-based grapevine sugar ripeness modelling for a wide range of Vitis vinifera L. cultivars. Agric. For. Meteorol. 2020, 285, 107913. [Google Scholar] [CrossRef]
- Orlandi, F.; Ruga, L.; Romano, B.; Fornaciari, M. Olive flowering as an indicator of local climatic changes. Theor. Appl. Climatol. 2005, 81, 169–176. [Google Scholar] [CrossRef]
- Fadón, E.; Do, H.; Blanke, M.; Rodrigo, J.; Luedeling, E. Apparent differences in agroclimatic requirements for sweet cherry across climatic settings reveal shortcomings in common phenology models. Agric. For. Meteorol. 2023, 333, 109387. [Google Scholar] [CrossRef]
- Matzneller, P.; Blümel, K.; Chmielewski, F.-M. Models for the beginning of sour cherry blossom. Int. J. Biometeorol. 2014, 58, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Mallucci, S.; Bignardi, A.; Varner, M.; Bertamini, M. Four decades in the vineyard: The impact of climate change on grapevine phenology and wine quality in northern Italy. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Saini, M.K.; Capalash, N.; Kaur, C.; Singh, S.P. Targeted metabolic profiling indicates differences in primary and secondary metabolites in Kinnow mandarin (C. nobilis × C. deliciosa) from different climatic conditions. J. Food Compos. Anal. 2019, 83, 103278. [Google Scholar] [CrossRef]
- Palmer, J.W.; Dennis, F.G.; Luby, J.J. Climate change and apple production in the Pacific Northwest: Implications for bloom timing and fruit maturity. HortScience 2003, 38, 1044–1048. [Google Scholar]
- Nawaza, R.; Akhtar Abbasia, N.; Ahmad Hafiza, I.; Khalid, A. Impact of climate variables on growth and development of Kinnow fruit (Citrus nobilis Lour × Citrus deliciosa Tenora) grown at different ecological zones under climate change scenario. Sci. Hortic. 2020, 260, 108868. [Google Scholar] [CrossRef]
- Verma, P.; Singh, J.; Sharma, S.; Thakur, H. Phenological growth stages and growing degree days of peach [Prunus persica (L.) Batsch] in sub-temperate climatic zone of North-Western Himalayan region using BBCH scale. Ann. Appl. Biol. 2023, 182, 284–294. [Google Scholar] [CrossRef]
- Roh, Y.H.; Choi, I.-L.; Lee, J.H.; Kwon, Y.B.; Yoon, H.S.; Jeong, H.N.; Kang, H.-M. Physiological responses and determination of harvest maturity in ‘Daehong’ peach according to days after full bloom. Horticulturae 2025, 11, 1013. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Garnier, C.; Agut, C.; Baculat, B.; Barbeau, G.; Besnard, E.; Bois, B.; Boursiquot, J.-M.; Chuine, I.; Dessup, T.; et al. Heat requirements for grapevine varieties is essential information to adapt plant material in a changing climate. In Proceedings of the VIIth International Terroir Congress, Nyon, Switzerland, 19 May 2008; Agroscope Changins: Wädenswil, Switzerland, 2008; pp. 222–227. [Google Scholar]
- Sabbatini, P.; Dami, I.; Howell, G.S. Predicting Harvest Yield in Juice and Wine Grape Vineyards; Extension Bulletin 3186; Michigan State University: East Lansing, MI, USA, 2012. [Google Scholar]
- Amaral, M.H.; McConchie, C.; Dickinson, G.; Walsh, K.B. Growing degree day targets for fruit development of Australian mango cultivars. Horticulturae 2023, 9, 489. [Google Scholar] [CrossRef]
- Colauto Stenzel, N.M.; Neves, C.S.V.J.; Marur, C.J.; Scholz, M.B.S.; Gomes, J.C. Maturation curves and degree-days accumulation for fruits of “folha murcha” orange trees. Sci. Agric. 2006, 63, 219–225. [Google Scholar] [CrossRef]
- León Pacheco, R.I.; Correa Álvarez, E.M.; Ferrer, J.L.R.; Bonilla, H.A.; Gómez-Correa, J.C.; Hernández, M.J.Y.; Artiles, L.P. Accumulation of degree days and their effect on potential yield of eggplant. Rev. Fac. Nac. Agron. Medellín 2019, 72, 8917–8926. [Google Scholar] [CrossRef]
- Reighard, G.L.; Rauh, B. Predicting peach fruit size potential from GDD 30 days post-bloom. Acta Hortic. 2015, 1084, 753–758. [Google Scholar] [CrossRef]
- De la Fuente, M.; Linares, R.; Baeza, P.; Miranda, C.; Lissarrague, J.R. Comparison of different methods of grapevine yield prediction in the time window between fruitset and veraison. OENO One 2015, 49, 27–35. [Google Scholar] [CrossRef]
- Yang, C.; Pinto, J.G.; Garcia-Herrera, R. Performance of seasonal forecasts for the flowering and veraison of two major Portuguese grapevine varieties. Agric. For. Meteorol. 2023, 331, 109342. [Google Scholar] [CrossRef]
- Naito, H.; Kawasaki, Y.; Hidaka, K.; Higashide, T.; Misumi, M.; Ota, T.; Lee, U.; Takahashi, M.; Hosoi, F.; Nakagawa, J. Effect of air temperature on each fruit growth and ripening stage of strawberry ‘Koiminori’. Int. Agrophys. 2024, 38, 195–202. [Google Scholar] [CrossRef]
- Azarenko, V.; Chozinski, A.; Brewer, L. Fruit growth curve analysis of seven sweet cherry cultivars. Acta Hortic. 2008, 795, 101–108. [Google Scholar] [CrossRef]
- Perry, K.B.; Wehner, T.C. A heat unit accumulation method for predicting cucumber harvest date. HortTechnology 1996, 6, 27–29. [Google Scholar] [CrossRef]
- Kovaleski, A.P.; Williamson, J.G.; Olmstead, J.W.; Darnell, R.L. Inflorescence bud initiation, development, and bloom in two Southern Highbush blueberry cultivars. J. Am. Soc. Hortic. Sci. 2015, 140, 38–44. [Google Scholar] [CrossRef]
- Hamdani, A.; Bouda, S.; Hssaini, L.; Adiba, A.; Kouighat, M.; Razouk, R. The effect of heat stress on yield, growth, physiology, and fruit quality in Japanese plum ‘Angelino’. Vegetos 2022, 37, 1061–1070. [Google Scholar] [CrossRef]
- Thole, V.; Vain, P.; Martin, C. Effect of elevated temperature on tomato post-harvest properties. Plants 2021, 10, 2359. [Google Scholar] [CrossRef]
- de Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Sadras, V.; Moran, M. Elevated temperature decouples anthocyanins and sugar in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Souza, F.; Alves, E.; Pio, R.; Castro, E.; Reighard, G.; Freire, A.I.; Mayer, N.A.; Pimentel, R. Influence of temperature on the development of peach fruit in a subtropical climate region. Agronomy 2019, 9, 20. [Google Scholar] [CrossRef]
- Haggerty, L.L. Understanding the ripening chemistry of cold-hardy wine grapes to predict optimal harvest time. North. Grapes News 2012, 1, 1–2. [Google Scholar]
- Leisso, R.; Hanrahan, I.; Mattheis, J. Assessing preharvest field temperature and at-harvest fruit quality for prediction of soft scald risk of ‘Honeycrisp’ apple fruit during cold storage. HortScience 2019, 54, 910–915. [Google Scholar] [CrossRef]
- Cepeda, A.; Vélez-Sánchez, J.E.; Balaguera-López, H.E. Analysis of growth and physicochemical changes in apple cv. Anna in a high-altitude tropical climate. Rev. Colomb. Cienc. Hortíc. 2021, 15, e12508. [Google Scholar] [CrossRef]
- Łysiak, G. The sum of active temperatures as a method of determining the optimum harvest date of ‘Sampion’ and ‘Ligol’ apple cultivars. Acta Sci. Pol. Hortorum Cultus 2012, 11, 3–13. [Google Scholar]
- Budde, C.O.; Polenta, G.; Lucangeli, C.D.; Murray, R.E. Air and immersion heat treatments affect ethylene production and organoleptic quality of “Dixiland” peaches. Postharvest Biol. Technol. 2006, 41, 32–37. [Google Scholar] [CrossRef]
- Rachma, D.F.; Munyanont, M.; Maeda, K.; Lu, N.; Takagaki, M. Estimation of Harvest Time Based on Cumulative Temperatures to Produce High-Quality Cherry Tomatoes in a Plant Factory. Agronomy 2024, 14, 3074. [Google Scholar] [CrossRef]
- Lokesha, A.N.; Shivashankara, K.; Hunashikatti, L.; Geetha, G.A.; Shankar, A. Effect of high temperature on fruit quality parameters of contrasting tomato genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1019–1029. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Shaji, S.; Beena, R.; Sarada, S.; Sajitha Rani, T.; Stephen, R.; Manju, R.V.; Viji, M.M. High temperature induced changes in quality and yield parameters of tomato (Solanum lycopersicum L.) and similarity coefficients among genotypes using SSR markers. Heliyon 2021, 7, e05988. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.A.; Wilson, L.T.; Hoffmann, M.P.; Zalom, F.G. Comparisons of temperature measurements from local weather stations and the tomato plant canopy: Implications for crop and pest forecasting. J. Am. Soc. Hortic. Sci. 1990, 115, 861–869. [Google Scholar] [CrossRef]
- Arx, G.; Dobbertin, M.; Rebetez, M. Detecting and correcting sensor drifts in long-term weather data. Environ. Monit. Assess. 2013, 185, 4483–4489. [Google Scholar] [CrossRef]
- Lee, M.A.; Monteiro, A.; Barclay, A.; Marcar, J.; Miteva-Neagu, M.; Parker, J. A framework for predicting soft-fruit yields and phenology using embedded, networked microsensors, coupled weather models, and machine-learning techniques. Comput. Electron. Agric. 2020, 168, 105103. [Google Scholar] [CrossRef]
- González, Y.; Sepúlveda, Á.; Yuri, J.A. Harvest date estimation of ‘Gala’ apples based on environment temperature using artificial intelligence. Chil. J. Agric. Res. 2023, 83, 272–280. [Google Scholar] [CrossRef]
- Ärje, J.; Milioris, D.; Tran, D.T.; Jepsen, J.U.; Raitoharju, J.; Gabbouj, M.; Iosifidisf, A.; Høye, T.T. Automatic flower detection and classification system using a light-weight convolutional neural network. In Proceedings of the EUSIPCO Workshop on Signal Processing, Computer Vision, and Deep Learning for Autonomous Systems, A Coruna, Spain, 2–6 September 2019. [Google Scholar]
- Tran, D.T.; Høye, T.T.; Gabbouj, M.; Iosifidis, A. Automatic flower and visitor detection system. In Proceedings of the European Signal Processing Conference, Rome, Italy, 3–7 September 2018; pp. 405–409. [Google Scholar] [CrossRef]
- Mann, H.; Iosifidis, A.; Jepsen, J.; Welker, J.; Loonen, M.; Høye, T. Automatic flower detection and phenology monitoring using time-lapse cameras and deep learning. Remote Sens. Ecol. Conserv. 2022, 8, 765–777. [Google Scholar] [CrossRef]
- Jolly, B.; Dymond, J.R.; Shepherd, J.D.; Greene, T.; Schindler, J. Detection of Southern Beech heavy flowering using Sentinel-2 imagery. Remote Sens. 2022, 14, 1573. [Google Scholar] [CrossRef]
- Génard, M.; Gouble, B. ETHY: A theory of fruit climacteric ethylene emission. Plant Physiol. 2005, 139, 531–545. [Google Scholar] [CrossRef]
- Saudreau, M.; Marquier, A.; Adam, B.; Monney, P.; Sinoquet, H. Experimental study of fruit temperature dynamics within apple tree crowns. Agric. For. Meteorol. 2009, 149, 275–284. [Google Scholar] [CrossRef]
- Hellebrand, H.J.; Beuche, H.; Linke, M. Thermal Imaging. In Physical Methods in Agriculture; Blahovec, J., Kutílek, M., Eds.; Springer: Boston, MA, USA, 2002; pp. 157–176. [Google Scholar]
- Li, L.; Peters, T.; Zhang, Q.; Zhang, J.; Huang, D. Modeling apple surface temperature dynamics based on weather data. Sensors 2014, 14, 20217–20234. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, A.J.P.; Keller, M.; Gutierrez, M.R.S.; Khot, L.; Hoogenboom, G. Comparison between grapevine tissue temperature and air temperature. Sci. Hortic. 2019, 247, 407–420. [Google Scholar] [CrossRef]
- Allegro, G.; Filippetti, I.; Pastore, C.; Sangiorgio, D.; Valentini, G.; Bortolotti, G.; Kertész, I.; Phuong Nguyen, L.; Baranyai, L. Prediction of berry sunburn damage with machine learning: Results on grapevine (Vitis vinifera L.). Biosyst. Eng. 2025, 250, 62–67. [Google Scholar] [CrossRef]
- Coetzee, C.; Dobson, R. A Simple Numerical Model for the Prediction of Apple Temperatures under Evaporative Cooling. Res. Agric. Agron. 2017, 2017, 884279. [Google Scholar] [CrossRef]
- Amogi, B.R.; Khot, L.R.; Sallato, B.V. Impact of summer heat and mitigation strategies on apple (Cosmic Crisp®) fruit color dynamics quantified using crop physiology sensing system. J. Agric. Food Res. 2025, 23, 102163. [Google Scholar] [CrossRef]
- Tapia Zapata, N.; Regen, C.; Quero-Garcia, J.; Fountas, S.; Alchanatis, V.; Zude-Sasse, M. Temperature Annotated 3D Point Clouds Measured in Various Climate Conditions, Providing the Fruit Surface Temperature and Local Weather Data. ATB. 2025. Available online: https://technologygarden.atb-potsdam.de/cracksense/ (accessed on 1 October 2025).
- Osroosh, Y.; Peters, R.T. Detecting fruit surface wetness using a custom-built low-resolution thermal-RGB imager. Comput. Electron. Agric. 2019, 157, 509–517. [Google Scholar] [CrossRef]
- Müller, K.; Keller, M.; Stoll, M.; Friedel, M. Wind speed, sun exposure and water status alter sunburn susceptibility of grape berries. Front. Plant Sci. 2023, 14, 1145274. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Khot, L.R.; Peters, R.T.; Salazar-Gutierrez, M.R.; Shi, G. In-field crop physiology sensing aided real-time apple fruit surface temperature monitoring for sunburn prediction. Comput. Electron. Agric. 2020, 175, 105558. [Google Scholar] [CrossRef]
- Ranjan, R.; Sinha, R.; Khot, L.R.; Whiting, M. Thermal-RGB imagery and in-field weather-sensing derived sweet cherry wetness prediction model. Sci. Hortic. 2022, 294, 110782. [Google Scholar] [CrossRef]
- Tapia-Zapata, N.; Winkler, A.; Zude-Sasse, M. Occurrence of wetness on the fruit surface modeled using spatio-temporal temperature data from sweet cherry tree canopies. Horticulturae 2024, 10, 757. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Ye, Z.; Hoegner, L.; Stilla, U. Fusion of urban 3D point clouds with thermal attributes using MLS data and TIR image sequences. Infrared Phys. Technol. 2021, 113, 103622. [Google Scholar] [CrossRef]
- Yandún Narváez, F.J.; Salvo del Pedregal, J.; Prieto, P.A.; Torres-Torriti, M.; Auat Cheein, F.A. LiDAR and thermal images fusion for ground-based 3D characterisation of fruit trees. Biosyst. Eng. 2016, 151, 12–23. [Google Scholar] [CrossRef]
- Jörissen, S.; Regen, C.; Zude-Sasse, M. Code for Data Fusion of LiDAR 3D Point Clouds and Thermal Images. 2024. Available online: https://gitlab-extern.atb-potsdam.de/cracksense/3d_thermal_annotation (accessed on 1 October 2025).
- Tsoulias, N.; Paraforos, D.S.; Xanthopoulos, G.; Zude-Sasse, M. Apple shape detection based on geometric and radiometric features using a LiDAR laser scanner. Remote Sens. 2020, 12, 2481. [Google Scholar] [CrossRef]
- Tsoulias, N.; Khosravi, A.; Herppich, W.B.; Zude-Sasse, M. Fruit water stress index of apple measured by means of temperature-annotated 3D point cloud. Plant Phenomics 2024, 6, 0252. [Google Scholar] [CrossRef]
- Tapia-Zapata, N.; Saha, K.K.; Tsoulias, N.; Zude-Sasse, M. A geometric modelling approach to estimate apple fruit size by means of LiDAR 3D point clouds. Int. J. Food Prop. 2024, 27, 566–583. [Google Scholar] [CrossRef]
- Bortolotti, G.; Piani, M.; Mengoli, D.; Franceschini, C.; Omodei, N.; Rossi, S.; Manfrini, L. Development of a consumer-grade scanning platform for fruit thermal and position data collection. In Proceedings of the 2023 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Pisa, Italy, 6–8 November 2023; pp. 357–362. [Google Scholar] [CrossRef]
- Saha, K.K.; Weltzien, C.; Bookhagen, B.; Zude-Sasse, M. Chlorophyll content estimation and ripeness detection in tomato fruit based on NDVI from dual wavelength LiDAR point cloud data. J. Food Eng. 2024, 383, 112218. [Google Scholar] [CrossRef]
- Gunawan, K.C.; Lie, Z.S. Apple ripeness level detection based on skin color features with convolutional neural network classification method. In Proceedings of the 2021 7th International Conference on Electrical, Electronics and Information Engineering (ICEEIE), Malang, Indonesia, 2 October 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Aherwadi, N.; Mittal, U.; Singla, J.; Jhanjhi, N.Z.; Yassine, A.; Hossain, M.S. Prediction of fruit maturity, quality, and its life using deep learning algorithms. Electronics 2022, 11, 4100. [Google Scholar] [CrossRef]
- Wang, C.; Han, Q.; Li, J.; Li, C.; Zou, X. YOLO-BLBE: A novel model for identifying blueberry fruits with different maturities using the I-MSRCR method. Agronomy 2024, 14, 658. [Google Scholar] [CrossRef]


| Method | Variants of Base Temperature, in °C | Criteria, Minimized to Select for GDD Calculation |
|---|---|---|
| Least standard deviation in GDD | (2) N: number of plantings per year | (3) GDDi: GDD of the observed timing of the phenological stage for i plantings : overall mean GDD of the phenological event across all plantings N: number of plantings/years. is given in °C |
| Least standard deviation in days | (4) , where overall mean of temperature in all plantings : mean temperature of ith planting/year | (5) : standard deviation in GDD : overall mean temperature of the developmental period of all years or all plantings is given in days |
| Coefficient of variation in days | (6) | (7) Mean d is the number of days observed to reach the phenological stage across all plantings is given as percentage |
| Linear regression | (8) | (9) : Predicted number of d in the ith year. : Observed number of d in the ith year |
| Crop | Cultivar | in °C | in °C | in °C |
|---|---|---|---|---|
| Apricot | Harcot | 6.4 (925) | 3.0 (1248) | 4.3 (1117) |
| Bergeron | 5.0 (1311 | 2.6 (1578) | 3.7 (1472) | |
| Stella | 3.5 (1280) | 2.0 (1417) | 2.5 (1367) | |
| Cherry | Burlat C1 | −3 (887) | - | 1 (650) |
| Burlat | 1 (649) | - | 4 (475) | |
| Forli | 2 (673) | - | 3 (585) |
| Diagram | Equation | |
|---|---|---|
| 1 |  | GDD = |
| 2 |  | GDD = [()] |
| 3 |  | GDD = {π/2} : upper threshhold |
| 4 | 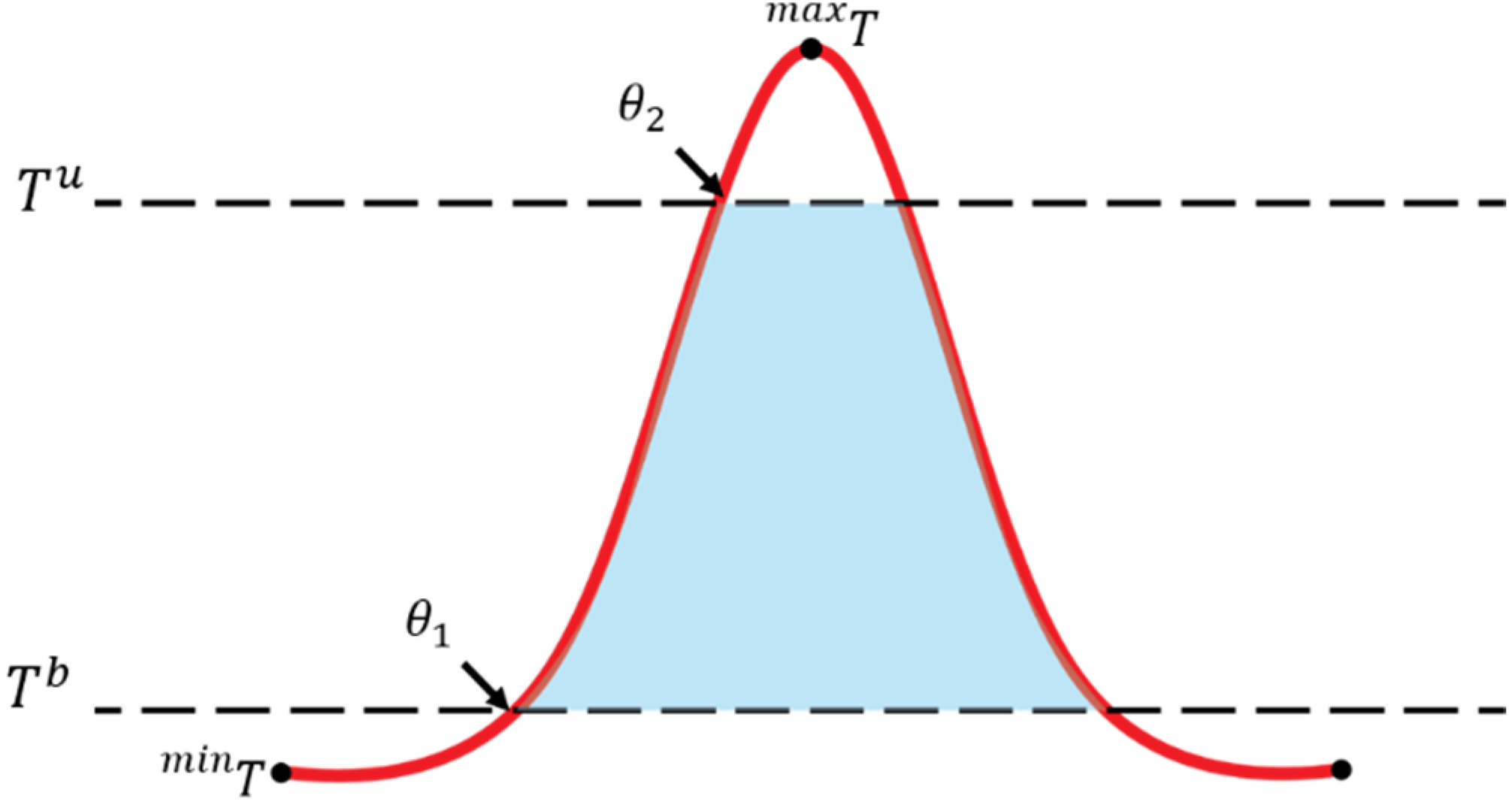 | GDD = {} |
| Crop | Climatic Region | °C | Starting Date/Measurement Duration | GDD (°C) | Fruit Quality Attributes Related to GDD | Reference |
|---|---|---|---|---|---|---|
| Mango | Subtropical | 12.0 | From asparagus stage of flowering | 1728–2185 | Flesh color, dry matter content, SSC:TA ratio | [144] |
| Peach | Subtropical | 7.0 | From full bloom to fruit senescence/harvest | 1106–1547 | Fruit size and mass, mesocarp cell number | [158] |
| Grape | Humid continental | 10.0 | August–October | 1330–1500 | SSC, timing of ripening | [159] |
| Apple | Humid continental | 10.0 | Seasonal accumulation up to harvest (29 Aug–3 Oct. 2012, 11 Sept–26 Sept. 2013) | 1444–2406 | SSC, TA, starch index, firmness, internal ethylene and soft-scald susceptibility | [160] |
| Apple | Temperate | 7.2 | From anthesis to harvest | 892 | SSC, firmness, diameter | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotouo Makouate, H.; Zude-Sasse, M. Advances in Growing Degree Days Models for Flowering to Harvest: Optimizing Crop Management with Methods of Precision Horticulture—A Review. Horticulturae 2025, 11, 1415. https://doi.org/10.3390/horticulturae11121415
Fotouo Makouate H, Zude-Sasse M. Advances in Growing Degree Days Models for Flowering to Harvest: Optimizing Crop Management with Methods of Precision Horticulture—A Review. Horticulturae. 2025; 11(12):1415. https://doi.org/10.3390/horticulturae11121415
Chicago/Turabian StyleFotouo Makouate, Helene, and Manuela Zude-Sasse. 2025. "Advances in Growing Degree Days Models for Flowering to Harvest: Optimizing Crop Management with Methods of Precision Horticulture—A Review" Horticulturae 11, no. 12: 1415. https://doi.org/10.3390/horticulturae11121415
APA StyleFotouo Makouate, H., & Zude-Sasse, M. (2025). Advances in Growing Degree Days Models for Flowering to Harvest: Optimizing Crop Management with Methods of Precision Horticulture—A Review. Horticulturae, 11(12), 1415. https://doi.org/10.3390/horticulturae11121415









