Towards Introgression Between Watermelon (Citrullus lanatus) and Its Wild Relative, Bitter Apple (C. colocynthis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
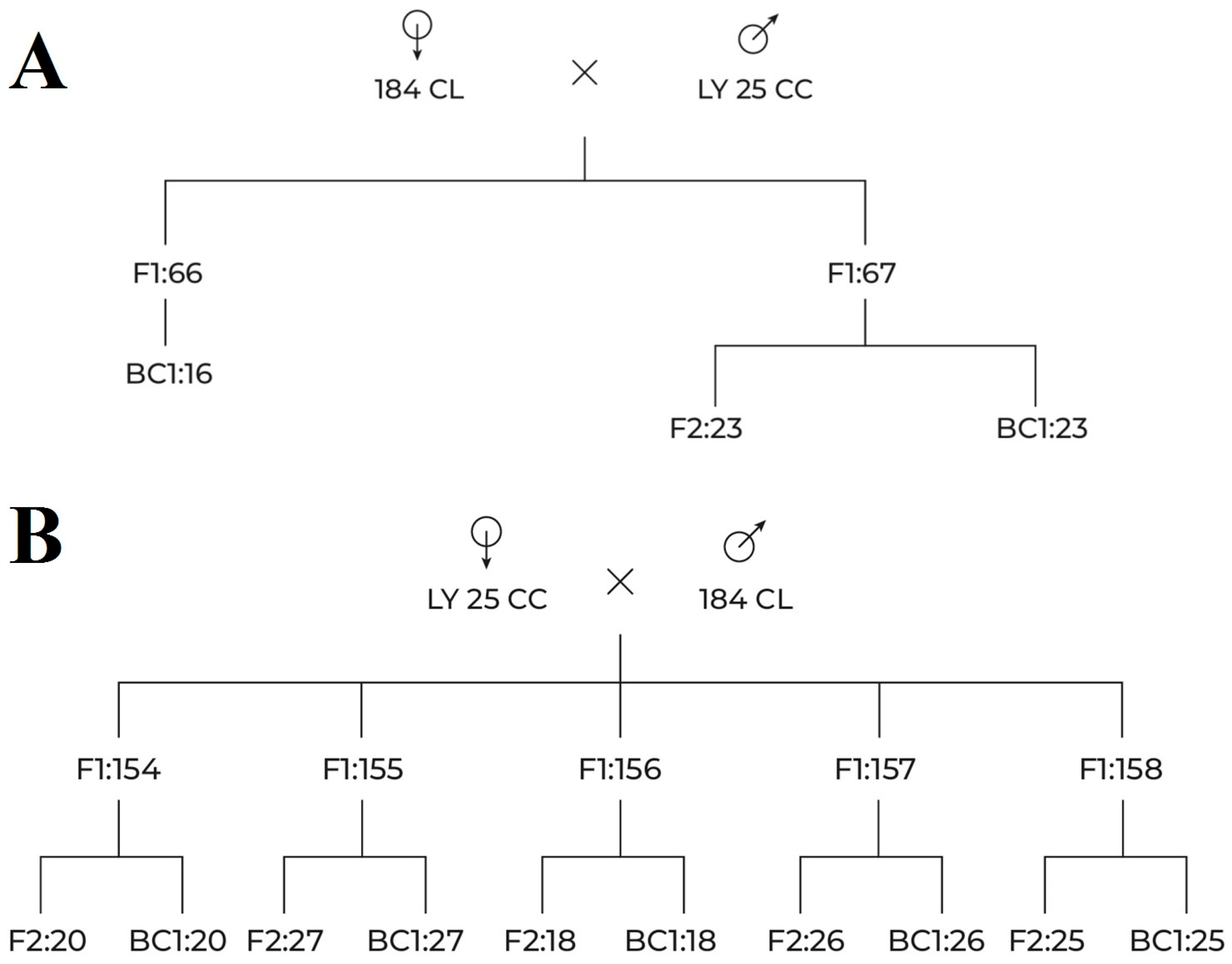
2.2. Crossing and Breeding Scheme
2.3. Data Collection
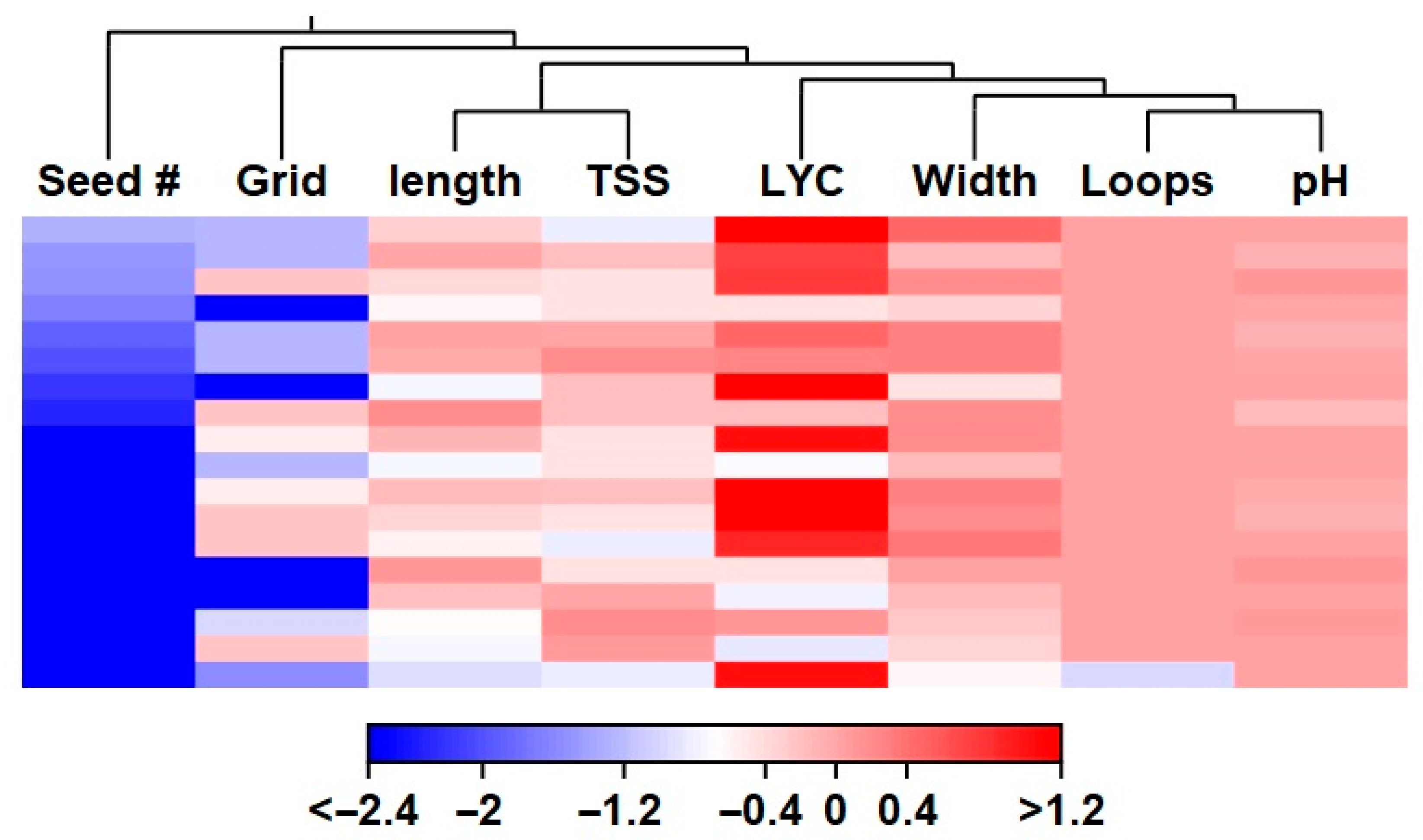
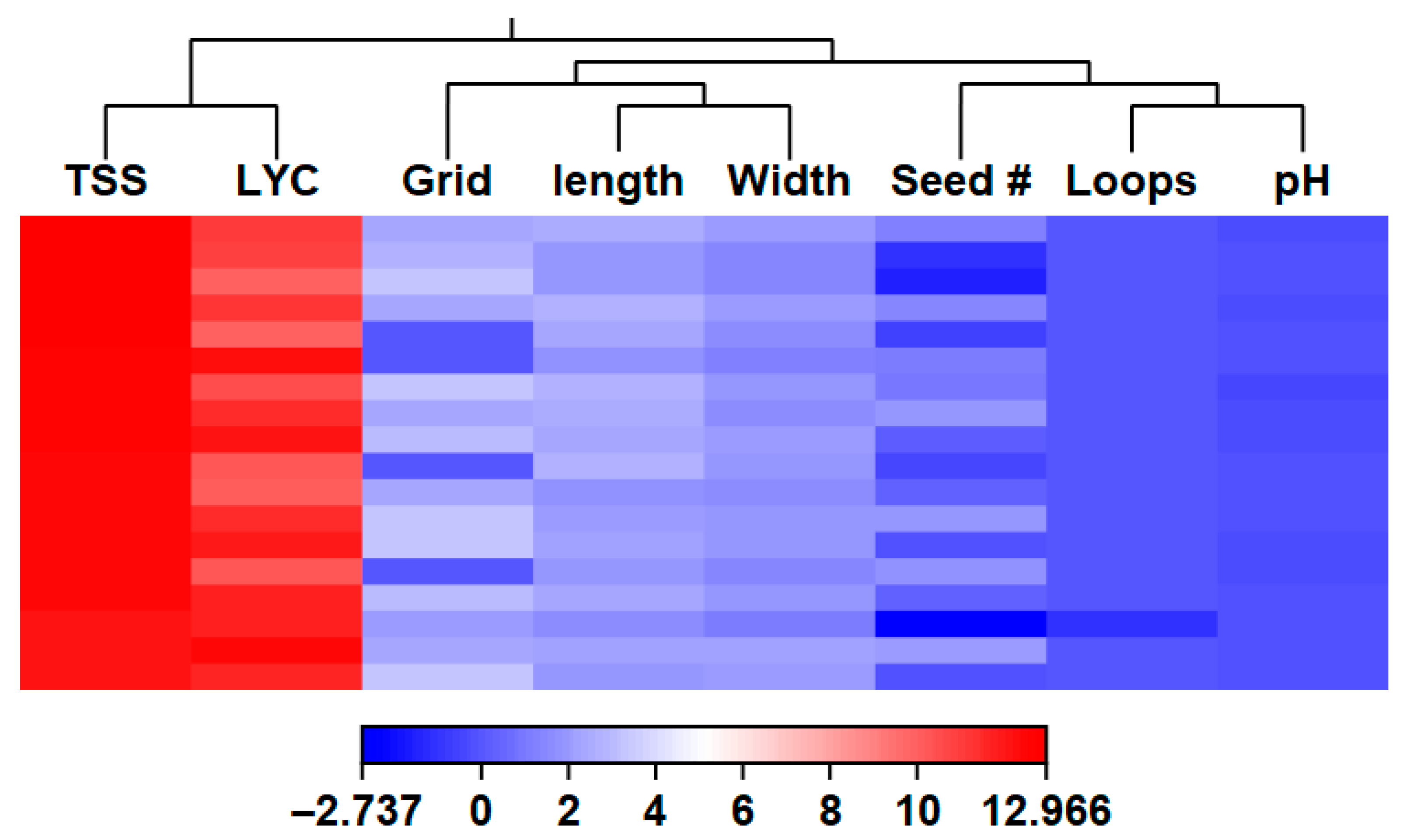
3. Results
3.1. Growing Plants and Crossings
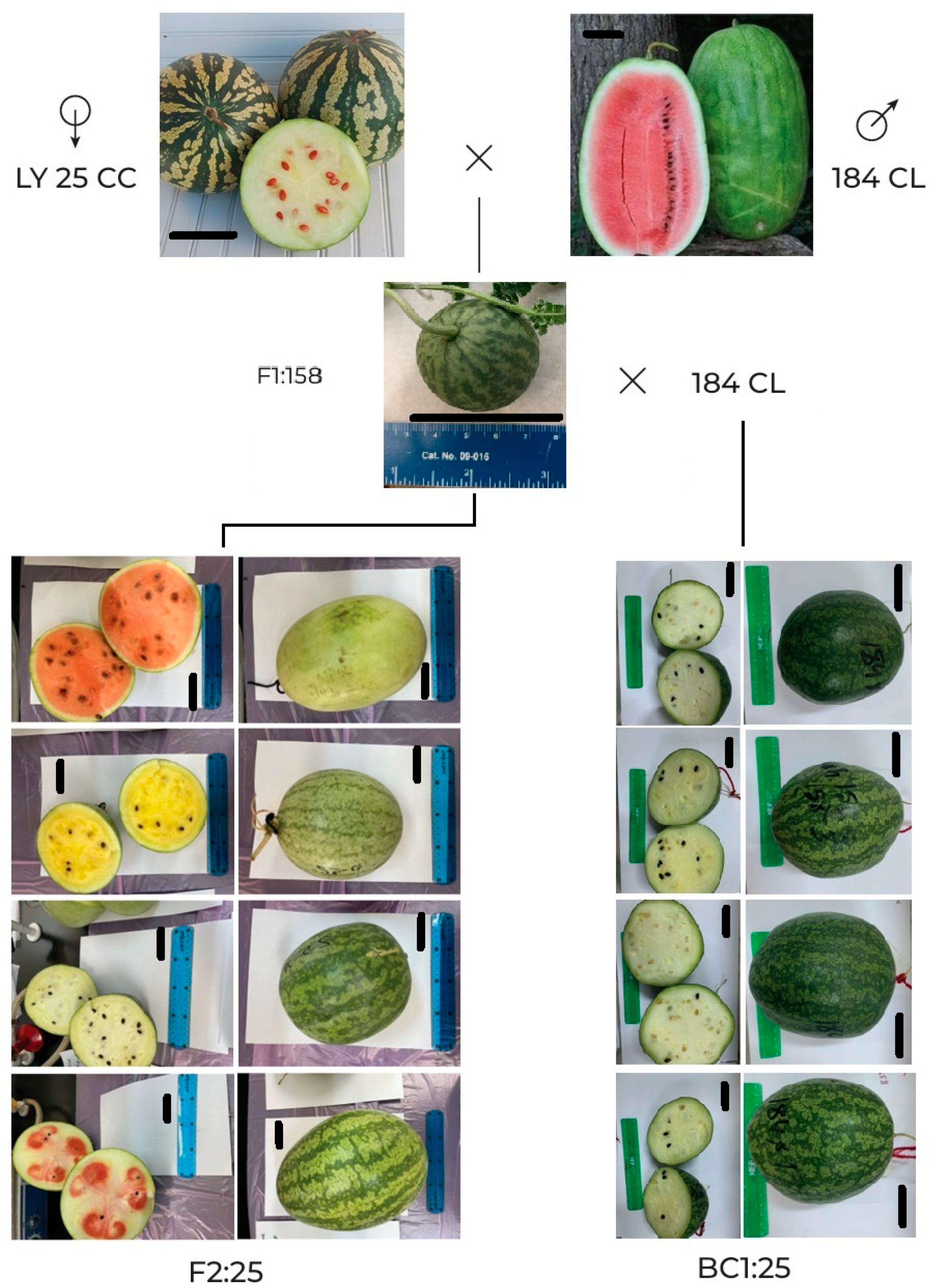
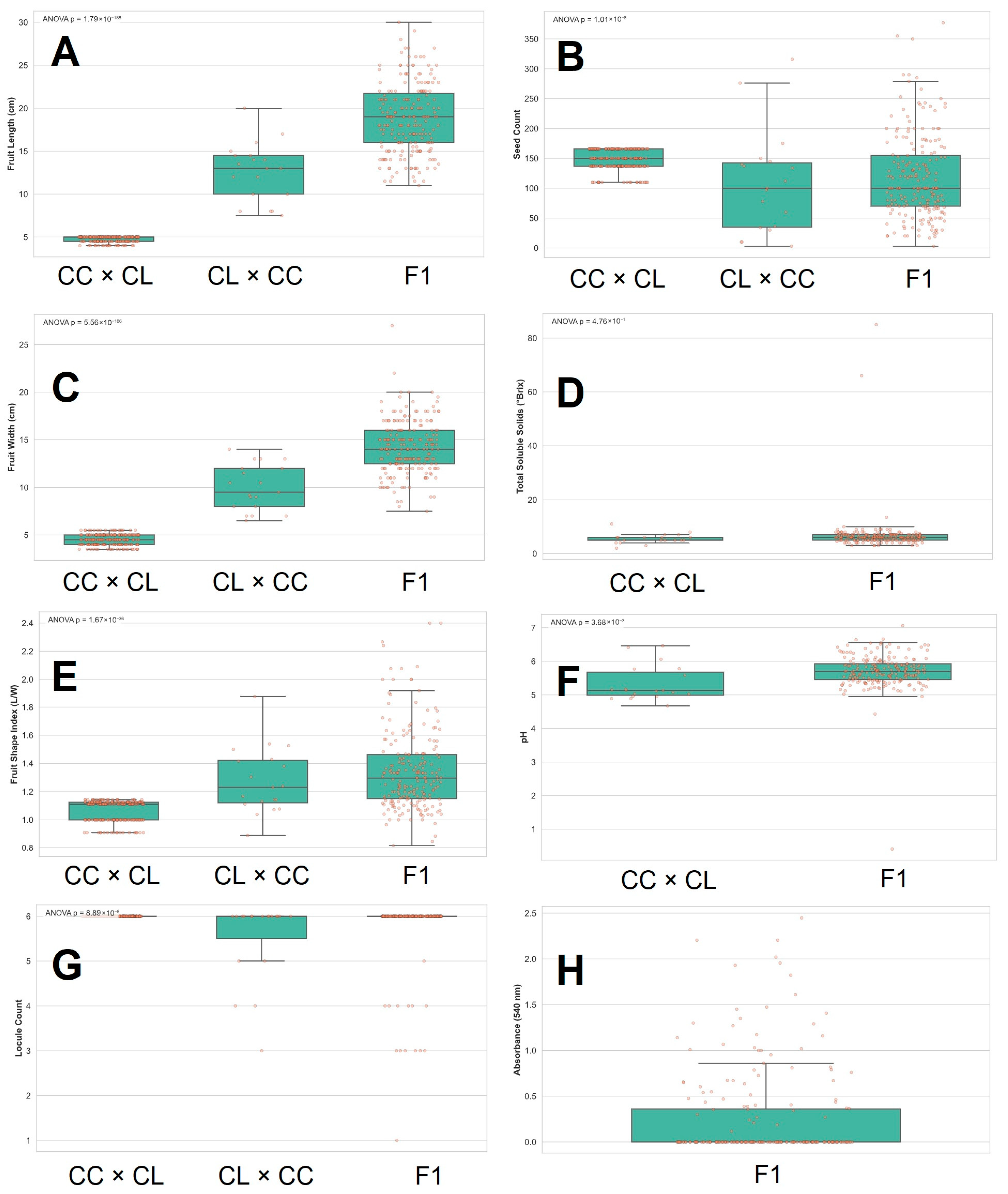
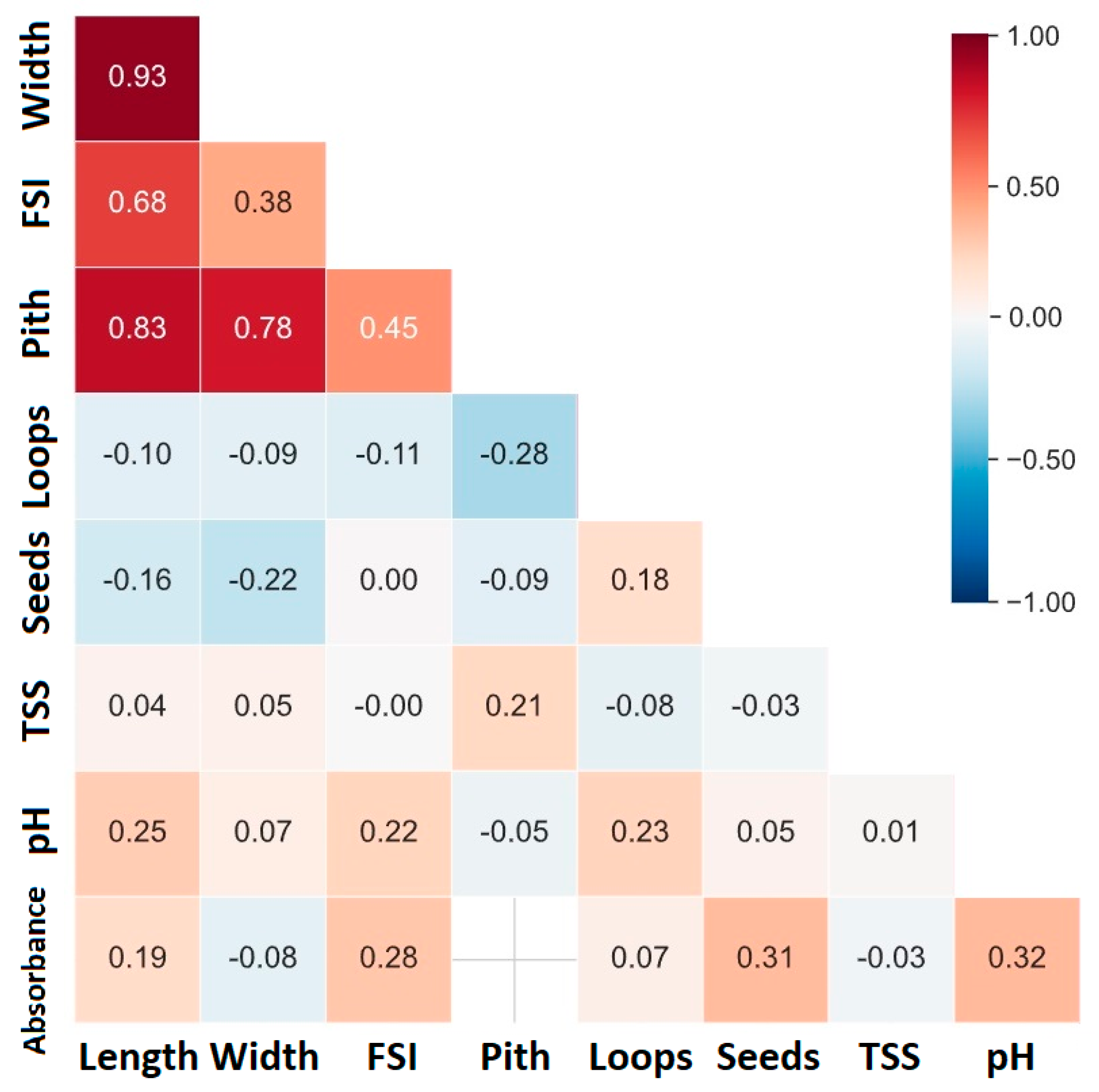
3.2. Fruit Traits
4. Discussion
4.1. Novel Watermelon Breeding Progenies
4.2. Crossability Between Watermelon and Bitter Apple
4.3. Fruit Evaluation Introgression Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Backcross |
| CC | Citrullus colocynthis |
| CL | Citrullus lanatus |
| CLL | Citrullus lanatus var. lanatus |
| CLC | Citrullus lanatus var. citron |
| CWR | Crop wild relative |
| F | Filial generation |
| TSS | Total soluble solids |
References
- Ricroch, A. The evolution of agriculture and tools for plant innovation. In Plant Biotechnology: Experience and Future Prospects; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–15. [Google Scholar]
- Conrady, M.; Lampei, C.; Bossdorf, O.; Hölzel, N.; Michalski, S.; Durka, W.; Bucharova, A. Plants cultivated for ecosystem restoration can evolve toward a domestication syndrome. Proc. Natl. Acad. Sci. USA 2023, 120, e2219664120. [Google Scholar] [CrossRef]
- Sofi, P.A.; Mir, R.A.; Bhat, K.A.; Mir, R.R.; Fatima, S.; Rani, S.; Mahajan, S.; Shafi, S.; Zaffar, A.; Ahmad, R.; et al. From domestication syndrome to breeding objective: Insights into unwanted breakup in common beans to improve shattering. Crop Pasture Sci. 2022, 74, 944–960. [Google Scholar] [CrossRef]
- Allaby, R.G.; Stevens, C.J.; Kistler, L.; Fuller, D.Q. Emerging evidence of plant domestication as a landscape-level process. Trends Ecol. Evol. 2022, 37, 268–279. [Google Scholar] [CrossRef]
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic evidence for complex domestication history of the cultivated tomato in Latin America. Mol. Biol. Evol. 2020, 37, 1118–1132. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Olaniyan, B.A.; Fagbohun, I.K.; Muftaudeen, T.K. Genetic diversity and utilization of cultivated eggplant germplasm in varietal improvement. Plants 2021, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Grumet, R.; McCreight, J.D.; McGregor, C.; Weng, Y.; Mazourek, M.; Reitsma, K.; Labate, J.; Davis, A.; Fei, Z. Genetic resources and vulnerabilities of major cucurbit crops. Genes 2021, 12, 1222. [Google Scholar] [CrossRef]
- Maragal, S.; Rao, E.S.; Lakshmana Reddy, D.C. Genetic analysis of fruit quality traits in prebred lines of watermelon derived from a wild accession of Citrullus amarus. Euphytica 2019, 215, 199. [Google Scholar] [CrossRef]
- Maragal, S.; Rao, E.S.; Reddy, D.L. Genetic mapping and meta-analysis identify several candidate genes for watermelon (Citrullus lanatus) fruit quality traits. Sci. Hortic. 2023, 308, 111545. [Google Scholar] [CrossRef]
- Satori, D.; Tovar, C.; Faruk, A.; Hammond Hunt, E.; Muller, G.; Cockel, C.; Kühn, N.; Leitch, I.J.; Lulekal, E.; Pereira, L.; et al. Prioritising crop wild relatives to enhance agricultural resilience in sub-Saharan Africa under climate change. Plants People Planet. 2022, 4, 269–282. [Google Scholar] [CrossRef]
- Wambugu, P.W.; Henry, R. Supporting in situ conservation of the genetic diversity of crop wild relatives using genomic technologies. Mol. Ecol. 2022, 31, 2207–2222. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F. Harnessing crop wild diversity for climate change adaptation. Genes 2021, 12, 783. [Google Scholar] [CrossRef]
- Henry, R.J.; Nevo, E. Exploring natural selection to guide breeding for agriculture. Plant Biotechnol. J. 2014, 12, 655–662. [Google Scholar] [CrossRef]
- Guarino, L.; Lobell, D.B. A walk on the wild side. Nat. Clim. Change 2011, 1, 374–375. [Google Scholar] [CrossRef]
- von Wettberg, E.; Davis, T.M.; Smýkal, P. Wild plants as source of new crops. Front. Plant Sci. 2020, 11, 591554. [Google Scholar] [CrossRef]
- Hufford, M.B.; Martínez-Meyer, E.; Gaut, B.S.; Eguiarte, L.E.; Tenaillon, M.I. Inferences from the historical distribution of wild and domesticated maize provide ecological and evolutionary insight. PLoS ONE 2012, 7, e47659. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Rosenthal, J.P.; Dirzo, R. Effects of life history, domestication and agronomic selection on plant defence against insects: Evidence from maizes and wild relatives. Evol. Ecol. 1997, 11, 337–355. [Google Scholar] [CrossRef]
- Chomicki, G.; Renner, S.S. Watermelon origin solved with molecular phylogenetics including L innaean material: Another example of museomics. New Phytol. 2015, 205, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Assis, J.D.A.; de Queiroz, M.A.; de Araújo, S.C.; Bandel, G.; Martins, P.S. Implications of the introgression between Citrullus colocynthis and C. lanatus characters in the taxonomy, evolutionary dynamics, and breeding of watermelon. Plant Genet. Resour. Newsl. 2000, 121, 15–19. [Google Scholar]
- Levi, A.; Jarret, R.; Kousik, S.; Patrick Wechter, W.; Nimmakayala, P.; Reddy, U.K. Genetic Resources of Watermelon. In Genetics and Genomics of Cucurbitaceae; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–110. [Google Scholar]
- Hashizume, T.; Shimamoto, I.; Hirai, M. Construction of a linkage map and QTL analysis of horticultural traits for watermelon [Citrullus lanatus (THUNB.) MATSUM & NAKAI] using RAPD, RFLP and ISSR markers. Theor. Appl. Genet. 2003, 106, 779–785. [Google Scholar] [CrossRef]
- Levi, A.; Thomas, C.E.; Keinath, A.P.; Wehner, T.C. Genetic diversity among watermelon (Citrullus lanatus and Citrullus colocynthis) accessions. Genet. Resour. Crop Evol. 2001, 48, 559–566. [Google Scholar] [CrossRef]
- McGregor, C.E.; Waters, V. Pollen viability of F1 hybrids between watermelon cultivars and disease-resistant, infraspecific crop wild relatives. HortScience 2013, 48, 1428–1432. [Google Scholar] [CrossRef]
- Verma, K.S.; ul Haq, S.; Kachhwaha, S.; Kothari, S.L. RAPD and ISSR marker assessment of genetic diversity in Citrullus colocynthis (L.) Schrad: A unique source of germplasm highly adapted to drought and high-temperature stress. 3 Biotech 2017, 7, 288. [Google Scholar] [CrossRef]
- Brown, A.C.; Summers, W.L. Carbohydrate accumulation and color development in watermelon. J. Am. Soc. Hortic. Sci. 1985, 110, 683–687. [Google Scholar] [CrossRef]
- Gichimu, B.M.; Owuor, B.O.; Mwai, G.N.; Dida, M.M. Morphological characterization of some wild and cultivated watermelon (Citrullus sp.) accessions in Kenya. ARPN J. Agric. Biol. Sci. 2009, 4, 10–18. [Google Scholar]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of high-intensity pulsed electric field processing conditions on lycopene, vitamin C and antioxidant capacity of watermelon juice. Food Chem. 2009, 115, 1312–1319. [Google Scholar] [CrossRef]
- Gusmini, G.; Schultheis, J.R.; Wehner, T.C. Rind thickness of watermelon cultivars for use in pickle production. HortTechnology 2004, 14, 540–545. [Google Scholar] [CrossRef]
- Fekete, D.; Stéger-Máté, M.; Bőhm, V.; Balázs, G.; Kappel, N. Lycopene and flesh colour differences in grafted and non-grafted watermelon. Acta Univ. Sapientiae Aliment. 2015, 8, 111–117. [Google Scholar] [CrossRef]
- Vinson, E.L.; Woods, F.M.; Kemble, J.M.; Perkins-Veazie, P.; Davis, A.; Kessler, J.R. Use of external indicators to predict maturity of mini-watermelon fruit. HortScience 2010, 45, 1034–1037. [Google Scholar] [CrossRef]
- Levi, A.; Thomas, C.E.; Thies, J.A.; Simmons, A.M.; Ling, K.S.; Harrison, H.F.; Keinath, A.P. Novel Watermelon Breeding Lines Containing Chioroplast and Mitochondrial Genomes derived from the desert species Citrullus colocynthis. HortScience 2006, 41, 463–464. [Google Scholar] [CrossRef]
- Hernandez, J.; Meints, B.; Hayes, P. Introgression breeding in barley: Perspectives and case studies. Front. Plant Sci. 2020, 11, 541942. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Martín-Cardoso, H.; Nogués, S.; Courtois, B.; Grenier, C.; Sacchi, G.A.; San Segundo, B. Integrative approach for precise genotyping and transcriptomics of salt tolerant introgression rice lines. Front. Plant Sci. 2022, 12, 797141. [Google Scholar] [CrossRef]
- Wang, X.; Han, B.; Sun, Y.; Kang, X.; Zhang, M.; Han, H.; Zhou, S.; Liu, W.; Lu, Y.; Yang, X.; et al. Introgression of chromosome 1P from Agropyron cristatum reduces leaf size and plant height to improve the plant architecture of common wheat. Theor. Appl. Genet. 2022, 135, 1951–1963. [Google Scholar] [CrossRef]
- Hwang, J.; Jumsoon, K.A.N.G.; Byeonggu, S.O.N.; Kwanghwan, K.I.M.; Younghoon, P.A.R.K. Genetic diversity in watermelon cultivars and related species based on AFLPs and EST-SSRs. Not. Bot. Horti Agrobot. 2011, 39, 285–292. [Google Scholar] [CrossRef]
- Camus, M.F.; Alexander-Lawrie, B.; Sharbrough, J.; Hurst, G.D. Inheritance through the cytoplasm. Heredity 2022, 129, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Skuza, L.; Szućko, I.; Filip, E.; Strzała, T. Genetic diversity and relationship between cultivated, weedy and wild rye species as revealed by chloroplast and mitochondrial DNA non-coding regions analysis. PLoS ONE 2019, 14, e0213023. [Google Scholar] [CrossRef]
- Bhattarai, G.; Shi, A.; Feng, C.; Mou, B.; Correll, J.C. Genome wide association studies in multiple spinach breeding populations refine downy mildew race 13 resistance genes. Front. Plant Sci. 2020, 11, 563187. [Google Scholar] [CrossRef]
- Guo, S.; Sun, H.; Zhang, H.; Liu, J.; Ren, Y.; Gong, G.; Jiao, C.; Zheng, Y.; Yang, W.; Fei, Z.; et al. Comparative transcriptome analysis of cultivated and wild watermelon during fruit development. PLoS ONE 2015, 10, e0130267. [Google Scholar] [CrossRef]
- Levi, A.; Wechter, P.; Massey, L.; Carter, L.; Hopkins, D. An extended genetic linkage map for watermelon based on a testcross and a BC 2 F 2 population. Am. J. Plant Sci. 2011, 2, 93. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Archer, M.A.; Wayne, R.K. Transgressive segregation, adaptation and speciation. Heredity 1999, 83, 363–372. [Google Scholar] [CrossRef]
- Guindon, M.F.; Martin, E.; Cravero, V.; Cointry, E. Transgressive segregation, heterosis and heritability for yield-related traits in a segregating population of Pisum sativum L. Exp. Agric. 2019, 55, 610–620. [Google Scholar]
- Gupta, P.; Prasad, R.; Sharma, M. Identification and genetic assessment of transgressive segregants for yield and its contributing traits in wheat (Triticum aestivum L.). Environ. Conserv. J. 2023, 24, 160–166. [Google Scholar] [CrossRef]
- Fulks, B.K.; Scheerens, J.C.; Bemis, W.P. Natural hybridization of two Citrullus species. J. Hered. 1979, 70, 214–215. [Google Scholar] [CrossRef]
- Parihar, A.; Vaja, M.B.; Dhruve, J.J.; Rukhsar; Kumar, S. Identification of useful recombinants from interspecific hybrids of Citrullus lanatus and C. colocynthis. Vegetos 2020, 33, 475–482. [Google Scholar] [CrossRef]
- Xu, Y. Quantitative trait loci: Separating, pyramiding, and cloning. Plant Breed. Rev. 1997, 15, 85–140. [Google Scholar]
- Knox, M.R.; Ellis, T. Excess heterozygosity contributes to genetic map expansion in pea recombinant inbred populations. Genetics 2002, 162, 861–873. [Google Scholar] [CrossRef]
- Manjappa, G.; Uday, G.; Hittalmani, S.; Krishnappa, G. Marker assisted selection and segregation distortion studies in rice (Oryza sativa). Indian J. Agric. Sci. 2022, 92, 853–856. [Google Scholar] [CrossRef]
- Sun, X.; Yu, T.; Bin, M.; Hu, C.; Bi, F.; Peng, X.; Yi, G.; Zhang, X. Transcriptomic analysis reveals the defense mechanisms of citrus infested with Diaphorina citri. Hortic. Plant J. 2023, 9, 450–462. [Google Scholar] [CrossRef]
- Levi, A.; Thomas, C.E. Polymorphisms among chloroplast and mitochondrial genomes of Citrullus species and subspecies. Genet. Resour. Crop Evol. 2005, 52, 609–617. [Google Scholar] [CrossRef]
- Navot, N.; Sarfatti, M.; Zamir, D. Linkage relationships of genes affecting bitterness and flesh color in watermelon. J. Hered. 1990, 81, 162–165. [Google Scholar] [CrossRef]
- Kırca, L.; Aygün, A. Determination and comparison of morpho-physiological characteristics of Turkish sweet cherry (Prunus avium L.) grown in Afyonkarahisar: Local cultivars and genotypes. Genet. Resour. Crop Evol. 2024, 71, 4359–4373. [Google Scholar] [CrossRef]
- Kumar, M.; Choudhary, B.R.; Kumar, R. Phenotypic profiling and identification of trait specific genotypes of seed purpose watermelon in Thar desert of India. Sci. Rep. 2025, 15, 29973. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qadumii, L.W.; Sadder, M.T.; Alkharabsheh, B.; Salem, S.Y.; Salem, M.S.; Bani-Yaseen, K. Towards Introgression Between Watermelon (Citrullus lanatus) and Its Wild Relative, Bitter Apple (C. colocynthis). Horticulturae 2025, 11, 1304. https://doi.org/10.3390/horticulturae11111304
Al-Qadumii LW, Sadder MT, Alkharabsheh B, Salem SY, Salem MS, Bani-Yaseen K. Towards Introgression Between Watermelon (Citrullus lanatus) and Its Wild Relative, Bitter Apple (C. colocynthis). Horticulturae. 2025; 11(11):1304. https://doi.org/10.3390/horticulturae11111304
Chicago/Turabian StyleAl-Qadumii, Lana W., Monther T. Sadder, Bayan Alkharabsheh, Samih Y. Salem, Mohammad S. Salem, and Karam Bani-Yaseen. 2025. "Towards Introgression Between Watermelon (Citrullus lanatus) and Its Wild Relative, Bitter Apple (C. colocynthis)" Horticulturae 11, no. 11: 1304. https://doi.org/10.3390/horticulturae11111304
APA StyleAl-Qadumii, L. W., Sadder, M. T., Alkharabsheh, B., Salem, S. Y., Salem, M. S., & Bani-Yaseen, K. (2025). Towards Introgression Between Watermelon (Citrullus lanatus) and Its Wild Relative, Bitter Apple (C. colocynthis). Horticulturae, 11(11), 1304. https://doi.org/10.3390/horticulturae11111304






