Comparative Evaluation of Quality Traits and Bioactive Compounds in Acca sellowiana (Berg) Peel and Pulp: Effects of Genotype, Harvest Time and Tissue Type
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Design
2.3. Physical-Chemical Analysis of Fruits
2.4. Phenolic Compound Extraction
2.5. UHPLC Q-Orbitrap HRMS Analysis
2.6. Antioxidant Activity Assays
2.7. Vitamin C Determination
2.8. Determination of Iodine Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Physical Quality Traits of the Fruit
3.2. Phenolic Compounds: Analysis by UHPLC-Q-Orbitrap HRMS
3.3. Antioxidant Activity
3.4. Vitamin C Determination
3.5. Determination of Iodine Content
3.6. Comparative Analysis of Bioactive Compounds and Iodine in Feijoa Tissues Using Heatmap Visualization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fischer, G.; Parra-Coronado, A. Influence of Some Environmental Factors on the Feijoa (Acca sellowiana [Berg] Burret): A Review. Agron. Colomb. 2020, 38, 388–397. [Google Scholar] [CrossRef]
- Monforte, M.T.; Lanuzza, F.; Mondello, F.; Naccari, C.; Pergolizzi, S.; Galati, E.M. Phytochemical Composition and Gastroprotective Effect of Feijoa sellowiana Berg Fruits from Sicily. J. Coast. Life Med. 2014, 2, 14–21. [Google Scholar]
- Vatrano, T.; Amenta, M.; Copetta, A.; Guardo, M.; Nunziata, A.; Strano, M.C.; Petriccione, M. Multifunctional Role of Acca sellowiana from Farm Management to Postharvest Life: A Review. Agronomy 2022, 12, 1802. [Google Scholar] [CrossRef]
- Romero-Rodriguez, M.A.; Vazquez-Oderiz, M.L.; Lopez-Hernandez, J.; Simal-Lozano, J. Composition of Babaco, Feijoa, Passionfruit and Tamarillo Produced in Galicia (North-West Spain). Food Chem. 1994, 49, 23–27. [Google Scholar] [CrossRef]
- Beyhan, Ö.; Elmasta, M. Total Phenolic Compounds and Antioxidant Capacity of Leaf, Dry Fruit and Fresh Fruit of Feijoa (Acca sellowiana, Myrtaceae). J. Med. Plants Res. 2010, 4, 1065–1072. [Google Scholar]
- Weston, R.J. Bioactive Products from Fruit of the Feijoa (Feijoa sellowiana, Myrtaceae): A Review. Food Chem. 2010, 121, 923–926. [Google Scholar] [CrossRef]
- Lapčík, O.; Klejdus, B.; Kokoška, L.; Davidová, M.; Afandi, K.; Kubáň, V.; Hampl, R. Identification of Isoflavones in Acca sellowiana and Two Psidium Species (Myrtaceae). Biochem. Syst. Ecol. 2005, 33, 983–992. [Google Scholar] [CrossRef]
- Binder, R.G.; Flath, R.A. Volatile Components of Pineapple Guava. J. Agric. Food Chem. 1989, 37, 734–736. [Google Scholar] [CrossRef]
- Shaw, G.J.; Allen, J.M.; Yates, M.K. Volatile flavour constituents in the skin oil from Feijoa sellowiana. Phytochemistry 1989, 28, 1529–1530. [Google Scholar] [CrossRef]
- Ruggiero, C.; Rotundo, A.; Di Vaio, C.; Iannini, B. Un triennio di prove sull’esigenze idriche della Feijoa (Acca sellowiana Berg) nell’ambiente mediterraneo. Frutticoltura 1990, 7, 51–56. [Google Scholar]
- Graziani, G.; Gaspari, A.; Di Vaio, C.; Cirillo, A.; Ronca, C.L.; Grosso, M.; Ritieni, A. Assessment of In Vitro Bioaccessibility of Phenolic compounds from Annurca, Limoncella, Red Delicious, and Golden Delicious Apples Using a Sequential Enzymatic Digestion Model. Antioxidants 2021, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and Antioxidant Capacity of Bioactive Compounds From Various Typologies of Canned Tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rajurkar, N.; Hande, S. Estimation of Phytochemical Content and Antioxidant Activity of Some Selected Traditional Indian Medicinal Plants. Indian J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef]
- Vahid, B. Titrimetric determination of ascorbic acid contents in plant samples by 2, 6-dichlorophenolindophenol method. J. Chem. Soc. Pak. 2012, 34, 1510–1512. [Google Scholar]
- Istituto Superiore di Sanità. Rapporti ISTISAN 96/34; Istituto Superiore di Sanità: Rome, Italy, 1996. [Google Scholar]
- Pasquariello, M.S.; Mastrobuoni, F.; Di Patre, D.; Zampella, L.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Agronomic, Nutraceutical and Molecular Variability of Feijoa (Acca sellowiana (O. Berg) Burret) Germplasm. Sci. Hortic. 2015, 191, 1–9. [Google Scholar] [CrossRef]
- Ducroquet, J.-P.H.J.; Nunes Eda, C.; Guerra, M.P.; Nodari, R.O. Novas cultivares brasileiras de goiabeira serrana: SCS 414-Mattos e SCS 415-Nonante. Agropecuária Catarin. 2008, 21, 73–76. [Google Scholar] [CrossRef]
- Martínez-Vega, R.R.; Fischer, G.; Herrera, A.; Chaves, B.; Quintero, O.C. Características físico-químicas de frutos de feijoa influenciadas por la posición en el canopi. Rev. Colomb. Cienc. Hortícolas 2011, 2, 21–32. [Google Scholar] [CrossRef]
- Borsuk, L.J.; Saifert, L.; Villamil, J.M.O.; Mora, F.D.S.; Nodari, R.O. Phenotypic variability in feijoa fruits [Acca sellowiana (O. Berg.) Burret] on indigenous lands, quilombolas communities and protected areas in the south of Brazil. Rev. Bras. Frutic. 2017, 39, e-699. [Google Scholar] [CrossRef]
- Rana, M.K. Ripening changes in fruits and vegetables: A review. Haryana J. Hortic. Sci. 2006, 35, 271–279. [Google Scholar]
- Puppo, M.; Rivas, M.; Franco, J.; Barbieri, R.L. Propuesta de descriptores para Acca sellowiana (Berg.) Burret. Rev. Bras. De Frutic. 2014, 36, 957–970. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture Is a Sensory Property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell Wall Disassembly in Ripening Fruit. Funct. Plant Biol. 2006, 33, 103. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D.; Thorp, T.G. Feijoas: Post-Harvest Handling and Storage of Fruit. N. Z. J. Exp. Agric. 1987, 15, 217–221. [Google Scholar] [CrossRef]
- Schotsmans, W.C.; East, A.; Thorp, G.; Woolf, A.B. Feijoa (Acca sellowiana [Berg] Burret). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 115–133, 115e–135e. ISBN 978-1-84569-735-8. [Google Scholar]
- Kader, A.A. Flavor Quality of Fruits and Vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer Acceptance of ‘Brooks’ and ‘Bing’ Cherries Is Mainly Dependent on Fruit SSC and Visual Skin Color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Harman, J.E. Feijoa Fruit: Growth and Chemical Composition during Development. N. Z. J. Exp. Agric. 1987, 15, 209–215. [Google Scholar] [CrossRef]
- Sánchez-Mora, F.D.; Saifert, L.; Ciotta, M.N.; Ribeiro, H.N.; Petry, V.S.; Rojas-Molina, A.M.; Lopes, M.E.; Lombardi, G.G.; Dos Santos, K.L.; Ducroquet, J.P.H.J.; et al. Characterization of Phenotypic Diversity of Feijoa Fruits of Germplasm Accessions in Brazil. Agrosyst. Geosci. Environ. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Amarante, C.V.T.D.; Souza, A.G.D.; Benincá, T.D.T.; Steffens, C.A. Fruit Quality of Brazilian Genotypes of Feijoa at Harvest and after Storage. Pesqui. Agropecuária Bras. 2017, 52, 734–742. [Google Scholar] [CrossRef]
- Parra C, A.; Fischer, G. Maduración y comportamiento poscosecha de la feijoa (Acca sellowiana (O. Berg) Burret). Una revisión. Rev. Colomb. Cienc. Hortícolas 2013, 7, 98–110. [Google Scholar] [CrossRef]
- Rodríguez, M.; Arjona, H.E. Caracterización fisicoquímica del crecimiento y desarrollo de los frutos de feijoa (Acca sellowiana Berg) en los clones 41 (Quimba) y 8-4. Agronomía Colomb. 2006, 24, 54–61. [Google Scholar]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Extraction Optimization, Antioxidant Capacity and Phenolic Profiling of Extracts from Flesh, Peel and Whole Fruit of New Zealand Grown Feijoa Cultivars. Antioxidants 2019, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Schmidt, H.; Rockett, F.C.; Klen, A.V.B.; Schmidt, L.; Rodrigues, E.; Tischer, B.; Augusti, P.R.; De Oliveira, V.R.; Da Silva, V.L.; Flôres, S.H.; et al. New Insights into the Phenolic Compounds and Antioxidant Capacity of Feijoa and Cherry Fruits Cultivated in Brazil. Food Res. Int. 2020, 136, 109564. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Yılmaz, N. Optimizing the extraction of phenolics and antioxidants from feijoa (Feijoa sellowiana, Myrtaceae). J. Food Sci. Technol. 2015, 52, 141–150. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, L.; Deng, W.; Yi, K.; Li, Y. Polyphenol Composition, Antioxidant Capacity and Xanthine Oxidase Inhibition Mechanism of Furong Plum Fruits at Different Maturity Stages. Foods 2023, 12, 4253. [Google Scholar] [CrossRef]
- Vuotto, M.L.; Basile, A.; Moscatiello, V.; De Sole, P.; Castaldo-Cobianchi, R.; Laghi, E.; Ielpo, M.T.L. Antimicrobial and Antioxidant Activities of Feijoa sellowiana Fruit. Int. J. Antimicrob. Agents 2000, 13, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Hosseinimehr, S.J.; Hamidinia, A.; Jafari, M. Antioxidant and free radical scavenging activity of Feijoa sellowiana fruits peel and leaves. Pharmacologyonline 2008, 1, 7–14. [Google Scholar]
- Phan, A.D.T.; Chaliha, M.; Sultanbawa, Y.; Netzel, M.E. Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana). Foods 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical and Biological Properties of Feijoa (Acca sellowiana). Trends Food Sci. Technol. 2018, 81, 121–131. [Google Scholar] [CrossRef]
- Castro, A.M.; Díaz, L.E.; Quintanilla-Carvajal, M.X.; Mayorga, E.Y.; Moreno, F.L. Convective Drying of Feijoa (Acca sellowiana Berg): A Study on Bioactivity, Quality, and Drying Parameters. LWT 2023, 186, 115209. [Google Scholar] [CrossRef]
- Budke, C.; Dierend, W.; Schön, H.-G.; Hora, K.; Mühling, K.H.; Daum, D. Iodine Biofortification of Apples and Pears in an Orchard Using Foliar Sprays of Different Composition. Front. Plant Sci. 2021, 12, 638671. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.; Montesano, D. Caratteristiche nutrizionali del frutto di Feijoa sellowiana. Il contenuto di iodio. LA Riv. DI Sci. Dell’Aliment. 2001, 4, 353–356. [Google Scholar]

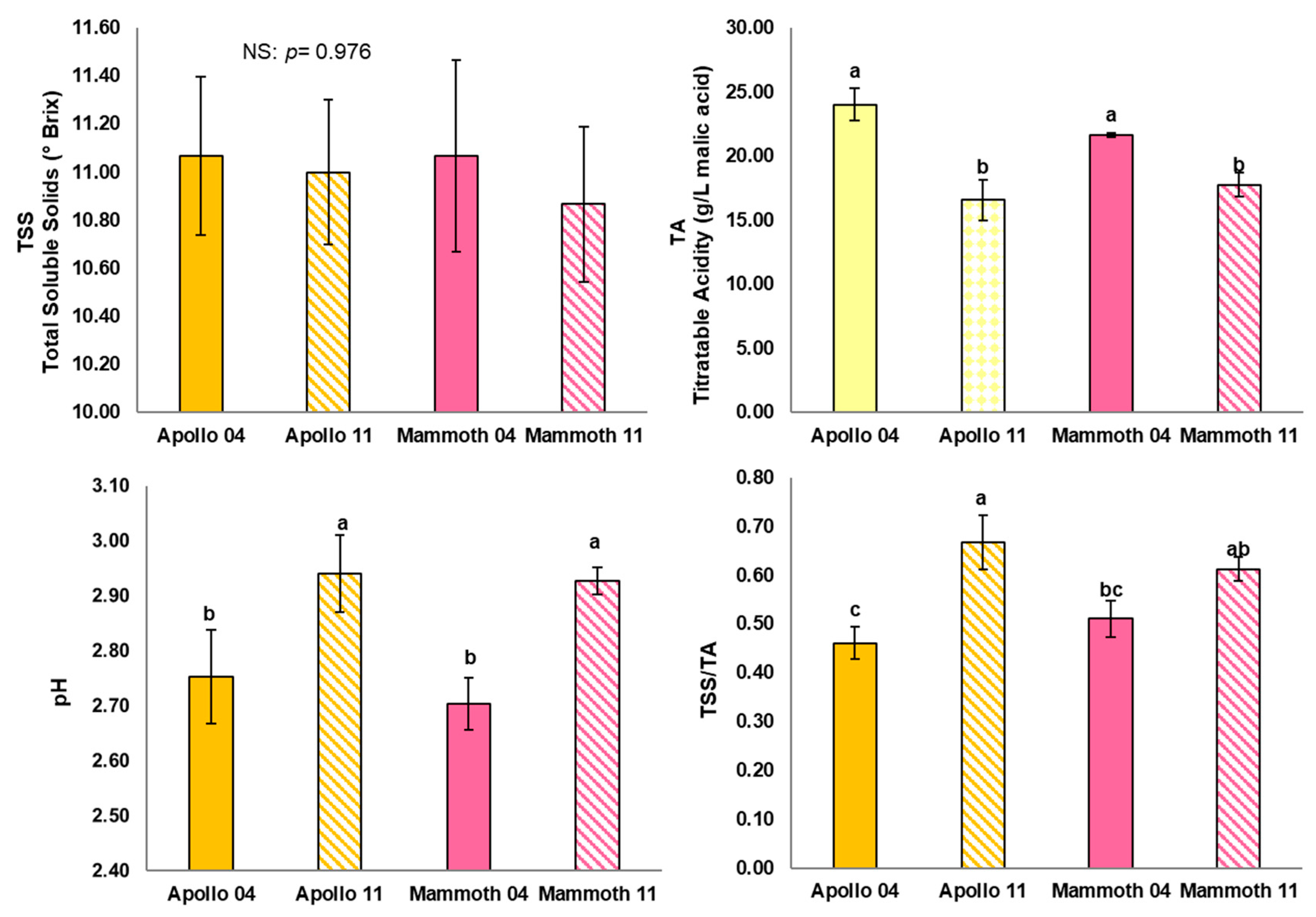
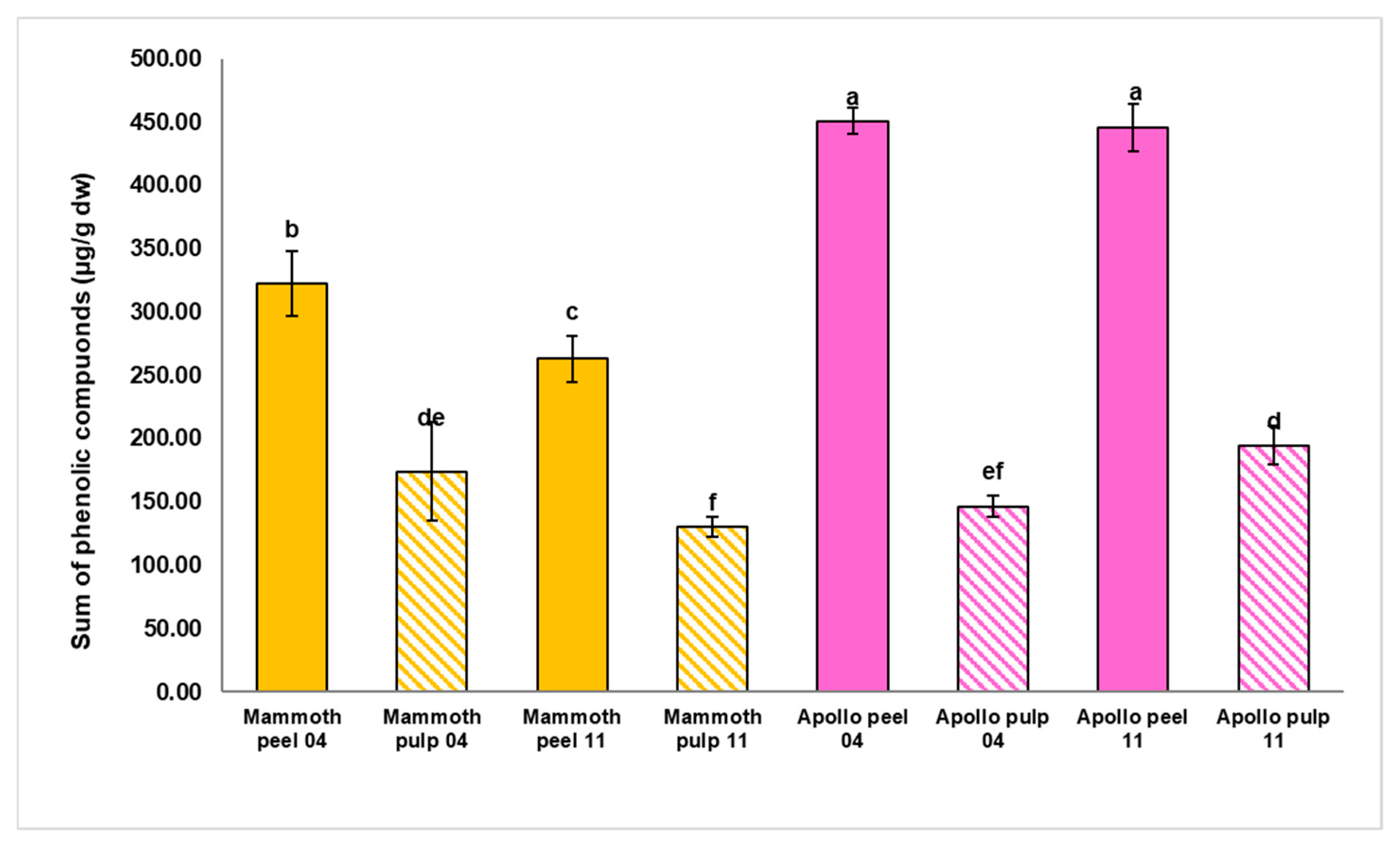

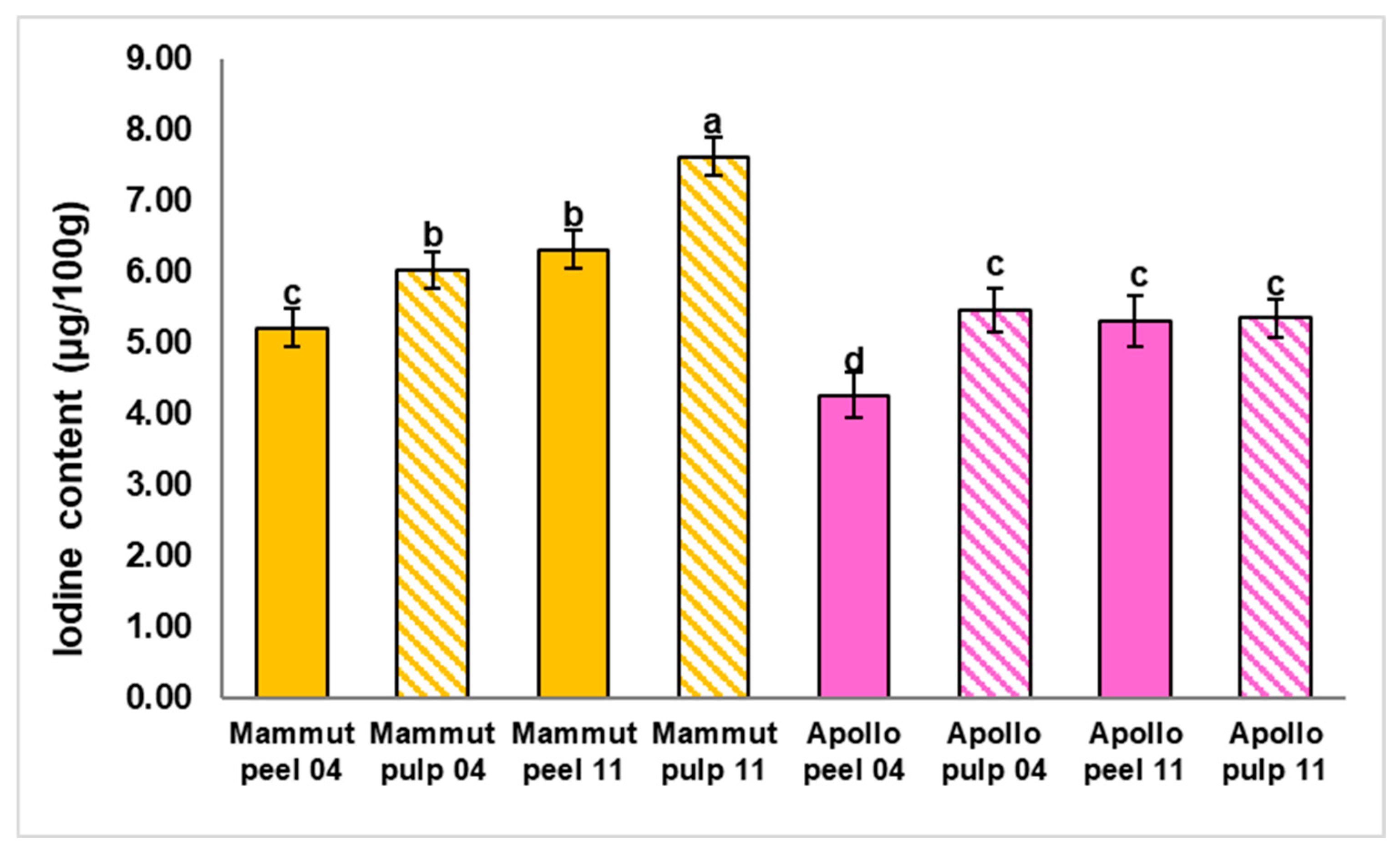
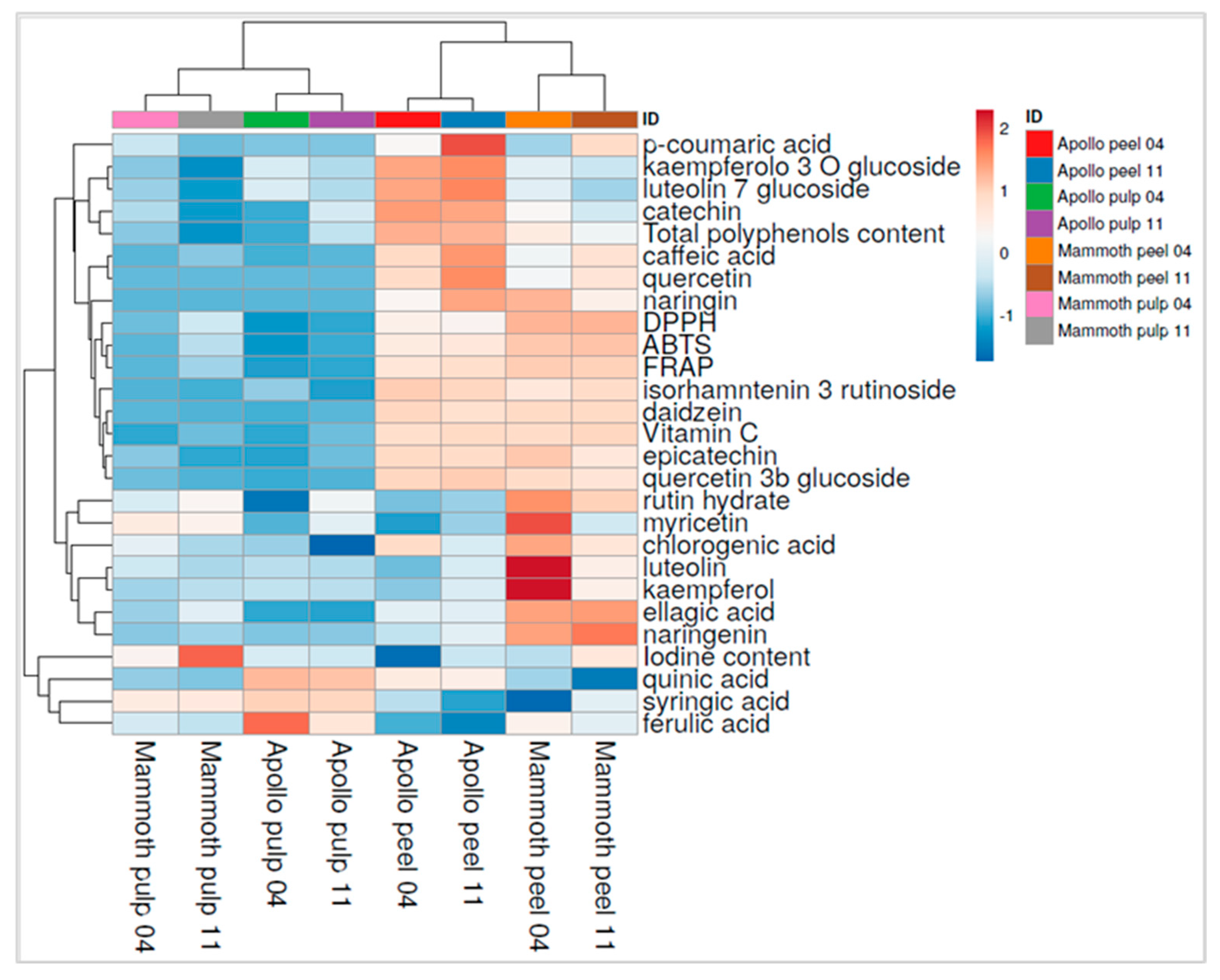
| Fruit Weight (g) | Polar Diameter (mm) | Equatorial Diameter (mm) | Firmness (kg × 0.5 cm−2) | |
|---|---|---|---|---|
| Apollo 04 | 105.64 ± 12.46 a | 60.36 ± 2.95 b | 56.68 ± 2.77 a | 8.02 ± 3.36 a |
| Apollo 11 | 80.63 ± 13.03 b | 54.60 ± 3.88 c | 51.28 ± 2.79 ab | 6.29 ± 2.55 b |
| Mammoth 04 | 73.67 ± 8.49 b | 64.52 ± 4.40 a | 47.98 ± 2.05 b | 3.95 ± 2.27 c |
| Mammoth 11 | 53.46 ± 6.28 c | 59.41 ± 3.57 b | 37.96 ± 10.04 c | 2.50 ± 1.94 d |
| Significance | *** | *** | *** | *** |
| L* | a* | b* | |
|---|---|---|---|
| Apollo 04 | 43.92 ± 7.5 b | −30.25 ± 14.79 | 32.14 ± 7.39 b |
| Apollo 11 | 47.21 ± 3.5 ab | −25.89 ± 15.60 | 37.72 ± 4.39 a |
| Mammut 04 | 50.08 ± 0.5 a | −32.13 ± 13.01 | 37.91 ± 1.39 a |
| Mammut 11 | 49.99 ± 1.5 a | 31.19 ± 11.21 | 40.62 ± 3.39 a |
| Significance | *** | ns | *** |
| Mammoth Peel 04 | Mammoth Pulp 04 | Mammoth Peel 11 | Mammoth Pulp 11 | Apollo Peel 04 | Apollo Pulp 04 | Apollo Peel 11 | Apollo Pulp 11 | Significance | |
|---|---|---|---|---|---|---|---|---|---|
| quinic acid | 8.28 ± 0.29 ba | 8.16 ± 0.89 c | 6.38 ± 1.54 c | 7.99 ± 0.69 c | 11.93 ± 2.07 a | 14.75 ± 4.07 a | 11.63 ± 0.82 ab | 14.03 ± 1.28 a | *** |
| chlorogenic acid | 0.78 ± 0.05 a | 0.59 ± 0.05 bc | 0.68 ± 0.08 abc | 0.53 ± 0.12 cd | 0.71 ± 0.15 ab | 0.52 ± 0.14 cd | 0.57 ± 0.09 bcd | 0.39 ± 0.05 d | *** |
| caffeic acid | 2.42 ± 0.61 c | 0.23 ± 0.12 d | 4.85 ± 0.62 b | 0.47 ± 0.28 d | 6.05 ± 2.30 b | 0.12 ± 0.05 d | 10.64 ± 1.50 a | 0.19 ± 0.06 d | *** |
| catechin | 194.64 ± 15.69 b | 135.39 ± 39.53 c | 147.62 ± 7.90 c | 92.7 ± 4.67 d | 344.58 ±9.03 a | 101.48 ±3.82 d | 332.99 ± 17.73 a | 152.71 ±15.34 c | *** |
| epicatechin | 39.41 ± 5.1 a | 5.4 ± 1.1 d | 25.0 ± 1.6 c | 3.4± 0.2 d | 34.± 2.9 b | 3.3 ± 0.8 d | 32.8 ± 3.2 b | 4.7 ± 0.3 d | *** |
| syringic acid | 3.97 ± 1.12 b | 7.35 ± 1.00 ab | 6.42 ± 1.84 ab | 7.60 ± 2.35 ab | 5.85 ± 2.54 ab | 8.59 ± 2.88 a | 5.32 ± 3.25 ab | 8.13 ± 0.70 a | * |
| p-coumaric acid | 0.25 ± 0.03 cd | 0.28 ± 0.07 cd | 0.56 ± 0.17 b | 0.20 ± 0.05 d | 0.42 ± 0.05 bc | 0.21 ± 0.04 d | 0.82 ± 0.20 a | 0.21 ± 0.06 d | *** |
| ellagic acid | 39.69 ± 5.00 a | 6.03 ± 3.54 b | 42.35 ± 8.35 a | 9.07 ± 0.57 b | 9.54 ± 1.46 b | 2.88 ± 0.10 b | 9.22 ± 0.97 b | 2.75 ± 0.25 b | *** |
| rutin hydrate | 0.06 ± 0.04 a | 0.03 ± 0.02 ab | 0.05 ± 0.03 a | 0.04 ± 0.03 ab | 0.02 ± 0.01 ab | 0.01 ± 0.01 b | 0.02 ± 0.00 ab | 0.04 ± 0.03 ab | ** |
| quercetin 3b glucoside | 21.13 ± 4.44 bc | 2.63 ± 0.30 d | 17.86 ± 2.84 c | 2.13 ± 0.56 d | 22.40 ± 1.16 ab | 1.89 ± 0.07 d | 25.14 ± 2.06 a | 2.11 ± 0.08 d | *** |
| ferulic acid | 4.50 ± 0.70 bc | 3.51 ± 1.18 bc | 4.12 ± 2.21 bc | 3.21 ± 1.02 bc | 2.99 ± 2.17 bc | 7.68 ± 0.81 a | 2.02 ± 0.75 c | 5.18 ± 1.89 ab | *** |
| kaempferolo 3 O glucoside | 1.60 ± 0.21 b | 1.13 ± 0.67 bc | 1.29 ± 0.33 b | 0.65 ± 0.13 c | 3.30 ± 0.38 a | 1.47 ± 0.27 b | 3.60 ± 0.26 a | 1.21 ± 0.27 bc | *** |
| luteolin 7 glucoside | 3.63 ± 0.21 b | 3.02 ± 0.84 bc | 3.05 ± 0.78 bc | 2.37 ± 0.20 c | 5.94 ± 0.42 a | 3.48 ± 0.29 b | 6.35 ± 0.34 a | 3.08 ± 0.33 bc | *** |
| isorhamntenin 3 rutinoside | 0.39 ± 0.12 a | 0.10 ± 0.10 b | 0.44 ± 0.16 a | 0.09 ± 0.05 b | 0.47 ± 0.13 a | 0.15 ± 0.07 b | 0.46 ± 0.09 a | 0.07 ± 0.03 b | *** |
| quercetin | 0.50 ± 0.55 c | 0.00 ± 0.00 c | 1.33 ± 0.52 b | 0.00 ± 0.00 c | 1.67 ± 0.52 b | 0.00 ± 0.00 c | 3.33 ± 0.52 a | 0.00 ± 0.00 c | *** |
| naringin | 0.05 ± 0.02 ab | 0.00 ± 0.00 d | 0.03 ± 0.01 bc | 0.00 ± 0.00 d | 0.03 ± 0.01 c | 0.00 ± 0.00 d | 0.06 ± 0.02 a | 0.00 ± 0.00 d | *** |
| luteolin | 0.09 ± 0.03 a | 0.03 ± 0.01 ab | 0.05 ± 0.01 b | 0.02 ± 0.01 ab | 0.01 ± 0.01 c | 0.02 ± 0.01 ab | 0.03 ± 0.01 ab | 0.02 ± 0.02 ab | *** |
| myricetin | 0.19 ± 0.12 a | 0.11 ± 0.10 ab | 0.07 ± 0.04 ab | 0.10 ± 0.06 ab | 0.03 ± 0.01 b | 0.04 ± 0.02 b | 0.05 ± 0.03 b | 0.08 ± 0.05 ab | *** |
| daidzein | 0.23 ± 0.03 a | 0.01 ± 0.00 b | 0.23 ± 0.07 a | 0.01 ± 0.00 b | 0.23 ± 0.06 a | 0.00 ± 0.00 b | 0.22 ± 0.05 a | 0.01 ± 0.00 b | * |
| kaempferol | 0.09 ± 0.03 a | 0.02 ± 0.01 ab | 0.04 ± 0.01 b | 0.02 ± 0.01 ab | 0.01 ± 0.01 c | 0.02 ± 0.01 ab | 0.03 ± 0.01 ab | 0.02 ± 0.02 ab | *** |
| naringenin | 0.12 ± 0.02 a | 0.01 ± 0.00 b | 0.13 ± 0.10 a | 0.01 ± 0.01 b | 0.02 ± 0.02 b | 0.01 ± 0.01 b | 0.04 ± 0.02 b | 0.01 ± 0.00 b | *** |
| ABTS | DPPH | FRAP | |
|---|---|---|---|
| mmol Trolox/Kg | |||
| Mammoth peel 4 | 339.86 ± 6.75 a | 239.35 ± 32.64 a | 662.70 ± 27.66 a |
| Mammoth pulp 4 | 57.45 ± 20.44 c | 44.15 ± 14.37 c | 106.51 ± 18.89 d |
| Mammoth peel 11 | 357.55 ± 41.42 a | 242.22 ± 30.56 a | 643.75 ± 75.03 a |
| Mammoth pulp 11 | 80.71 ± 5.88 c | 64.56 ± 4.71 c | 141.03 ± 8.45 d |
| Apollo peel 4 | 212.41 ± 21.03 b | 122.80 ± 9.61 b | 475.44 ± 21.13 c |
| Apollo pulp 4 | 39.17 ± 1.23 c | 30.70 ± 3.21 c | 86.06 ± 0.27 d |
| Apollo peel 11 | 233.05 ± 49.43 b | 118.46 ± 28.03 b | 544.31 ± 38.51 b |
| Apollo pulp 11 | 49.53 ± 7.53 c | 34.38 ± 2.52 c | 91.93 ± 6.69 d |
| Significance | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vaio, C.; Cirillo, A.; Ramondini, M.; Cinosi, N.; Di Matteo, A.; Ciampaglia, R.; Izzo, L.; Grosso, M. Comparative Evaluation of Quality Traits and Bioactive Compounds in Acca sellowiana (Berg) Peel and Pulp: Effects of Genotype, Harvest Time and Tissue Type. Horticulturae 2025, 11, 1305. https://doi.org/10.3390/horticulturae11111305
Di Vaio C, Cirillo A, Ramondini M, Cinosi N, Di Matteo A, Ciampaglia R, Izzo L, Grosso M. Comparative Evaluation of Quality Traits and Bioactive Compounds in Acca sellowiana (Berg) Peel and Pulp: Effects of Genotype, Harvest Time and Tissue Type. Horticulturae. 2025; 11(11):1305. https://doi.org/10.3390/horticulturae11111305
Chicago/Turabian StyleDi Vaio, Claudio, Aurora Cirillo, Mariachiara Ramondini, Nicola Cinosi, Angela Di Matteo, Roberto Ciampaglia, Luana Izzo, and Michela Grosso. 2025. "Comparative Evaluation of Quality Traits and Bioactive Compounds in Acca sellowiana (Berg) Peel and Pulp: Effects of Genotype, Harvest Time and Tissue Type" Horticulturae 11, no. 11: 1305. https://doi.org/10.3390/horticulturae11111305
APA StyleDi Vaio, C., Cirillo, A., Ramondini, M., Cinosi, N., Di Matteo, A., Ciampaglia, R., Izzo, L., & Grosso, M. (2025). Comparative Evaluation of Quality Traits and Bioactive Compounds in Acca sellowiana (Berg) Peel and Pulp: Effects of Genotype, Harvest Time and Tissue Type. Horticulturae, 11(11), 1305. https://doi.org/10.3390/horticulturae11111305









