1. Introduction
Root-knot nematodes (
Meloidogyne spp.) are sedentary endoparasitic nematodes that infect plant roots and have a broad geographic distribution [
1]. They inflict severe economic damage, causing global annual losses estimated at up to USD 100 billion [
2], and have emerged as major phytopathogens worldwide [
3]. Upon penetrating the root cortex, these nematodes establish specialized feeding sites and secrete effector proteins—such as cellulases and pectinases—that induce abnormal cell division, leading to the formation of root galls or tumor-like structures of varying sizes [
4]. Initially, these galls appear white or yellowish white; they later turn brown and decay, causing severe deformities in the root system that impede normal water and nutrient uptake. Although gall formation is often accompanied by an apparent increase in the number of lateral and fine roots, the functional root surface area is reduced, further compromising the plant’s ability to absorb soil resources. These pathological changes ultimately manifest aboveground as stunted growth and leaf chlorosis, and in severe cases, lead to wilting and plant death [
5,
6]. For example, melon plants infected with root-knot nematodes become dwarfed and chlorotic, with poorly developed fruits [
7], while the yields of tomato, cucumber, and other vegetables can decline by more than 50% due to root damage [
8]. In addition to directly consuming plant nutrients, root-knot nematode infection damages root tissues, creating entry points for fungal, bacterial, and viral pathogens, and thereby precipitating secondary diseases such as root rot and wilt [
9,
10].
Over 100 species of root-knot nematodes (RKNs) have been described; the most prevalent include
Meloidogyne incognita,
Meloidogyne hapla,
Meloidogyne arenaria,
Meloidogyne javanica, and
Meloidogyne enterolobii [
11,
12]. Among these,
M. enterolobii (syn.
M. mayaguensis) is considered one of the most virulent RKN species due to its ability to infect and reproduce in host plants that possess resistance against major tropical RKNs [
13]. It was first reported in 1983 on pacara earpod tree (
Enterolobium contortisiliquum) in Danzhou, Hainan Province, China [
14]. Compared with closely related species such as
M. incognita and
M. javanica,
M. enterolobii causes more severe damage. It alone can lead to yield losses of up to 65%, which is higher than those caused by any other root-knot nematode species studied to date [
15]. Zhang et al. confirmed that the minimum infective dose of
M. enterolobii on tomato is 0.08 J2 per cm
3 of soil, compared to 1.0 J2/cm
3 for
M. incognita and 0.64 J2/cm
3 for
M. javanica. Subsequent gall counts, egg mass counts, and reproduction indices were also significantly higher for
M. enterolobii than for other species [
16]. Its distribution is primarily tropical and subtropical but is expanding into higher latitude regions—a trend expected to accelerate under global warming and the increased use of protected agriculture with soil heating [
17]. Moreover,
M. enterolobii can overcome multiple plant resistance genes—such as the
Mi-1 gene in tomato,
Mh in potato,
N in pepper,
Tabasco in sugar beet, and
Mir1 in soybean [
18,
19,
20,
21]—rendering conventional resistant cultivars ineffective, whereas these genes remain effective against other root-knot nematodes. Therefore, further research on
M. enterolobii and the development of effective control strategies are critically important [
22].
Chitin, a linear polymer of β-1,4–linked N-acetylglucosamine, is an abundant structural polysaccharide [
23]. It is primarily found in the cell walls of fungi, the exoskeletons of arthropods (such as insects, crustaceans), and the eggshells and cuticles of nematodes [
24,
25]. In contrast, plants have cell walls composed mainly of cellulose, hemicellulose, and pectin, and they lack the metabolic pathways to synthesize chitin [
26,
27]. Since chitin is an essential component of nematode eggshells and cuticles, disruption of chitin synthesis or hydrolysis leads to embryonic lethality, defective egg-laying, or molting failure in nematodes. Thus, key components of the chitin metabolic pathway are promising targets for the development of anti-nematode agents [
28]. The chitin biosynthesis pathway involves three key steps: substrate generation, chitin polymerization, and modification/deposition. First, glucose undergoes a series of metabolic reactions catalyzed by hexokinase, glucose-6-phosphate isomerase, and other related enzymes to produce UDP-
N-acetylglucosamine (UDP-GlcNAc), the direct substrate for chitin synthesis. Subsequently, chitin synthase, localized on the cell membrane, catalyzes the linkage of
N-acetylglucosamine units via β-1,4-glycosidic bonds to form linear, unbranched chitin polymers. Finally, the synthesized chitin chains undergo further modifications and are deposited in structures such as cell walls, insect exoskeletons, nematode eggshells, and other specialized compartments [
24]. Notably, chitin synthase plays a pivotal role in this process, as its activity and regulation critically determine the quantity and quality of the chitin produced [
29]. Previous studies have shown that silencing chitin synthase genes in plant-parasitic nematodes enhances host resistance [
30,
31]. Although
M. enterolobii also has a triploid genome like its relatives, its unique symmetric differentiation of subgenomes results in greater gene redundancy and higher evolutionary adaptability [
32]. Therefore, the effectiveness of silencing a single gene in
M. enterolobii, such as the chitin synthase gene, remains to be verified. To date, no such study has been reported.
This study aimed to develop a novel control strategy based on host-induced gene silencing (HIGS) by targeting the chitin synthase gene (Me-chs-1) of M. enterolobii. The full-length sequence of this gene was cloned and subjected to bioinformatic analysis. HIGS was employed to knock down the expression of the chitin synthase gene in M. enterolobii, and its control efficacy was validated through inoculation with second-stage juveniles (J2). This research provides important insights into the function and mechanism of the chitin synthase gene, while laying a foundation for breeding M. enterolobii-resistant crop varieties and developing novel environmentally friendly nematicides.
2. Materials and Methods
2.1. Plant and Nematode Materials
Tested tomato variety: The susceptible tomato cultivar Microtom, preserved by the Nematology Laboratory of Yunnan Agricultural University, Kunming, China.
Root-knot nematode source: M. enterolobii was collected, purified, and propagated by the Nematology Laboratory of Yunnan Agricultural University. Live nematodes were maintained in the root systems of the highly susceptible tomato cultivar Zhongshu No. 4 and water spinach (Ipomoea aquatica).
2.2. Total RNA Extraction
Tomato seedlings infected with M. enterolobii were rinsed with distilled water, and root galls containing eggs were collected as extraction samples. Total RNA was isolated using the TransZol Up Plus RNA Kit (TransGen Biotech Co., Ltd., Beijing, China). RNA concentration and purity were measured using a NanoDropTM 2000 spectrophotometer (Thermo Fisher Scientific Inc., Walthem, MA, USA). RNA integrity was further verified via 1% agarose gel electrophoresis. High-quality RNA (A260/A280 = 1.8–2.0, clear ribosomal bands) was reverse-transcribed into cDNA using the PrimeScript™ RT Reagent Kit (Takara Bio Inc., Kusatsu, Shiga, Japan) with gDNA Eraser.
2.3. Cloning of the Full-Length CDS of the Chitin Synthase Gene
Using cDNA as the template, the cloning was performed in two segments, separately obtaining the 5′ and 3′ CDS fragments, which were then assembled into the pUC19 vector using the ClonExpress Ultra One Step Cloning Kit V2 (Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China) to generate the full-length CDS of the chitin synthase gene.
2.4. Sequence Alignment and Functional Analysis
The cDNA sequences were aligned and analyzed using DNAMAN (Version 9), and their nucleotide sequences were translated into amino acid sequences. Conserved domains were identified via the CD-Search tool at the National Center for Biotechnology Information (NCBI) (
http://www.ncbi.nlm.nih.gov/blast/, accessed on 24 September 2024). The amino acid sequences of chitin synthase were retrieved from the NCBI and WormBase (
https://wormbase.org/ accessed on 25 September 2024) databases. Multiple sequence alignment and phylogenetic tree construction were conducted using MEGA (Version 11.0).
2.5. Construction of the Gene Silencing Vector and Agrobacterium-Mediated Transformation of Tomato
An RNAi construct targeting the conserved domain of the
Me-chs-1 gene was generated. Three primer pairs—tatF
+/tatF
−, loop
+/loop
−, and tatR
+/tatR
−—were designed against the target region. The tatF
+/tatF
− and tatR
+/tatR
− fragments were PCR-amplified from a plasmid harboring the
Me-chs-1 CDS, whereas the loop
+/loop
− fragment was amplified from the pCTAG11-GUSPlus vector. (Primer sequences are provided in
Appendix B). PCR products were resolved on a 1% agarose gel, and the bands corresponding to the expected sizes were excised and purified. Purified PCR fragments were then cloned into the pBWA(V)HS vector via homologous recombination to yield recombinant plasmids under the control of the CaMV 35S promoter. Following transformation into
Escherichia coli, positive clones were identified using colony PCR and confirmed via Sanger sequencing; plasmids from verified clones were further validated by using EcoRV restriction analysis. The confirmed RNAi constructs were introduced into
Agrobacterium tumefaciens strain GV3101 via heat shock. Transformants containing the target insert were selected and stored at –80 °C. Finally, tomato cotyledons were transformed using the
Agrobacterium-mediated transformation system. T
0 transgenic plants were screened by PCR to confirm genomic integration of the RNAi cassette, and T
1 seeds were harvested for downstream experiments.
2.6. Inoculation of T1 Generation Seedlings with M. enterolobii and Pathological Analysis
Seeds of T1 transgenic and wild-type tomato lines were surface-sterilized by immersion in 6% sodium hypochlorite for 7 min, followed by thorough rinsing with sterile water. The seeds were germinated on moist filter paper at 25 °C until radicle emergence and then transferred to seedling trays for further growth. When the seedlings developed 4–5 true leaves, they were transplanted into the trays and placed in a solar greenhouse maintained at 25 °C under a 10-h light/14-h dark photoperiod. Four holes, approximately 1–2 cm in depth, were made around the base of each seedling using a 1 mL pipette tip at a distance of about 1 cm from the stem. A suspension containing 500 J2 juveniles of M. enterolobii was inoculated evenly into the root zone through these holes.
Root samples from three biological replicates were collected at 2, 8, 16, 23, and 30 days post-inoculation (dpi) and stained using the sodium hypochlorite–acid fuchsin–glycerol method as described by Byrd et al. [
33],to observe nematode infection and developmental stages. At 45 dpi, the following parameters were recorded for both wild-type and RNAi transgenic lines: number of egg masses, number of galls, number of eggs per mass, and total egg count. Individual galls were excised and treated with 6% sodium hypochlorite for 4–5 min to dissolve the gelatinous matrix; released eggs were counted under a stereomicroscope. The total egg count was extrapolated based on triplicate counts of 100 μL aliquots taken from the nematode egg suspension. The gall formation percentage was calculated and classified according to the grading standards established by Yan et al. [
34].
2.7. qRT-PCR Analysis of Chitin Synthase Gene Expression
At 45 dpi, three biological replicates were collected from both T
1 transgenic tomato plants and wild-type controls. Total RNA of nematodes was extracted following the protocol described in
Section 2.2 and reverse-transcribed into cDNA. The
Actin gene of
M. enterolobii was used as the internal reference, with amplification primers Me-Actin-F/Me-Actin-R, gene-specific qPCR primers Me-QP-F/Me-QP-R were designed based on the CDS of the chitin synthase gene. qRT-PCR was performed to quantify the relative expression of the target gene.
2.8. Effect of Silencing the Chitin Synthase Gene on the M. enterolobii Transcriptome
At 45 dpi with
M. enterolobii, root samples were collected from three biological replicates each of the RNAi line (ZT3) and the wild-type control. Then twenty female adults were manually picked out from the root knots of each root system. Total RNA of nematodes was extracted according to the protocol described in
Section 2.2, and subsequently reverse-transcribed into cDNA. RNA sample concentration and purity were assessed spectrophotometrically, and those samples meeting the quality criteria for high-throughput sequencing were submitted to Shanghai Paiseno Biotechnology Co., Ltd. for transcriptome sequencing. Subsequently, the sequencing reads were compared to the reference genome of the
M. enterolobii (accession number: PRJEB69523) using TopHat2 software (Version 2.1.0), and quantitative gene expression analysis was performed by Cufflinks software (Version 2.2.4) to screen out differentially expressed genes. Then the GO and KEGG enrichment analysis were conducted.
2.9. Statistical Analysis
All experiments in this study included at least three independent biological replicates. Unless otherwise specified, each sample was assayed with three technical replicates. Data are presented as the mean ± standard deviation (Mean ± SD). Parameters related to nematode infection (such as the number of root knots, egg masses, etc.) and gene expression data were visualized using bar graphs, where the bar height represents the mean and the error bars represent the standard deviation.
To evaluate the differences in infection parameters and gene expression levels between wild-type and RNAi transgenic lines, Student’s t-test was used for comparisons between two groups. For comparisons involving more than two groups, one-way analysis of variance (One-way ANOVA) was employed, followed by appropriate post hoc tests (such as Tukey’s HSD test) for multiple comparisons. In all statistical analyses, a p-value less than 0.05 (p < 0.05) was considered statistically significant.
3. Result
3.1. Cloning and Bioinformatics Analysis of the Chitin Synthase Gene
Four putative chitin synthase genes in
M. enterolobii were identified through NCBI database searches: CAD2169478.1, CAD2180532.1, CAK5089395.1, and CAK5088075.1. Sequence alignment revealed that these genes ranged in length from 3684 to 3840 bp (
Figure 1). With high sequence homology (97.69%) among the four genes, primers targeting conserved regions were designed to clone the chitin synthase genes from
M. enterolobii.
The gene was successfully cloned, with a coding sequence (CDS) length of 3788 bp (
Figure 1A). It contains 22 exons and 21 introns, encoding a protein composed of 1261 amino acids. Conserved domain analysis identified seven conserved domains in the gene: Chitin Synthase C, Chitin Synthase 2, CESA-like, CESA-like 1, BcsA, Glyco_trans_2_3, and Gly-co_trans_2_3 (
Figure 1C). Notably, Chitin Synthase C and Chitin Synthase 2 are catalytic domains characteristic of chitin synthase genes, confirming the successful cloning of chitin synthase genes from
M. enterolobii.
A phylogenetic tree was constructed for the proteins encoded by chitin synthase genes from different species. Phylogenetic assessment (
Figure 2) showed that among the four chitin synthase genes of
M. enterolobii, the cloned gene exhibited the highest amino acid sequence homology with the chitin synthase of
M. incognita (Wormbase database accession number: Minc3s00218g07846). In addition, the chitin synthase genes of
M. enterolobii had a relatively close evolutionary relationship with the
chs-1 gene of
C. elegans (GenBank accession number: G5ECD6.1), while the homology with the
chs-2 gene of
C. elegans (GenBank accession number: G5EBQ8.1) was significantly lower. Based on this conserved evolutionary pattern and functional orthology, the cloned chitin synthase gene of
M. enterolobii was systematically designated as
Me-chs-1.
3.2. Construction of Gene Silencing Vectors
Three RNAi silencing vectors—pBWA(V)HS-Chitin Synthase Gene Silencing 1-RNAi (ZT1), pBWA(V)HS-Chitin Synthase Gene Silencing 2-RNAi (ZT2), and pBWA(V)HS-Chitin Synthase Gene Silencing 3-RNAi (ZT3)—were successfully constructed (
Figure 3). These vectors target three distinct conserved domains of the chitin synthase gene: 1497–1698 bp (5′ end of the Chitin synthase_C domain), 1736–1950 bp (3′ end of the Chitin synthase_C domain), and 2207–2408 bp (spanning regions homologous to Chitin Synthase 2, CESA-like, CESA-like 1, BcsA, Glyco_trans_2_3, and Glyco_trans_2_3 domains).
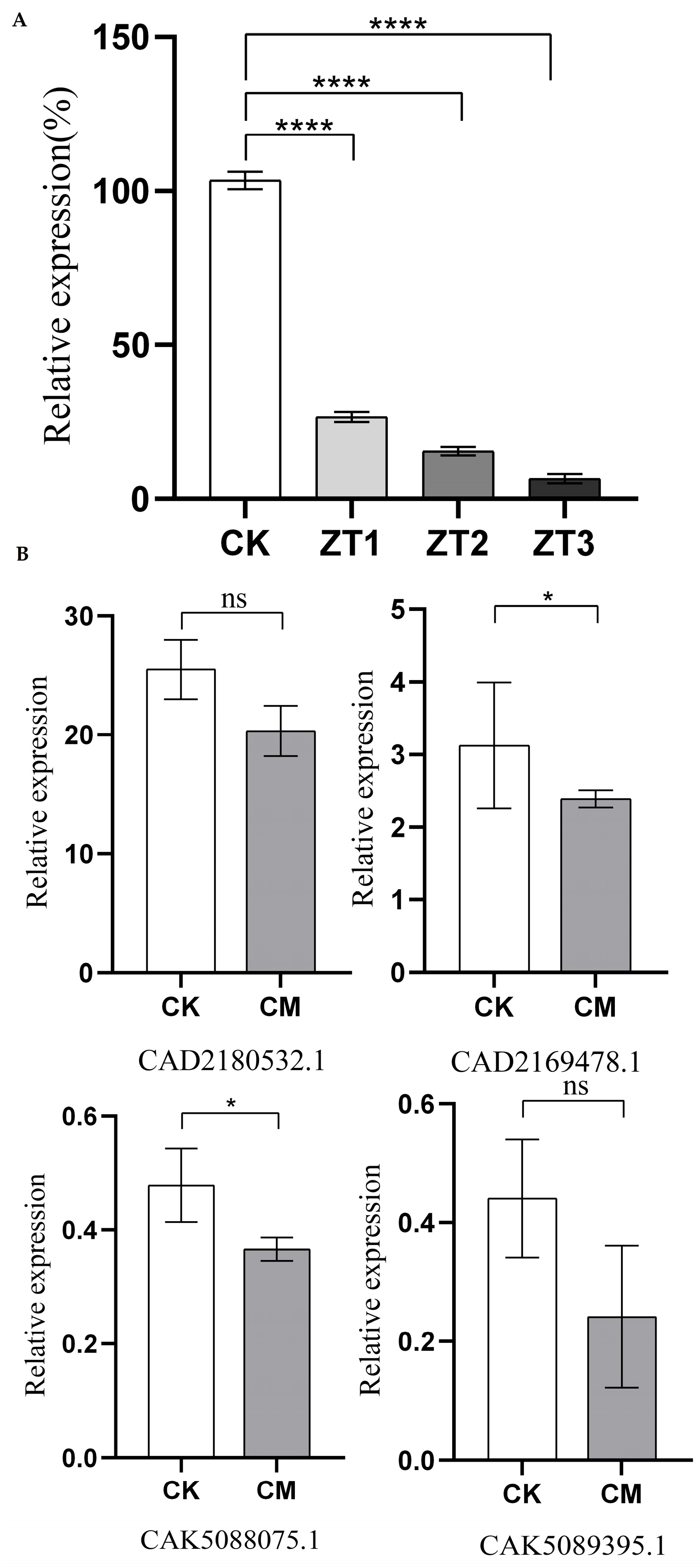
3.3. The Expression Levels of Chitin Synthase Genes After Gene Silencing
The relative expression levels of the
Me-chs-1 gene in wild-type plants (CK) and RNAi lines (ZT1, ZT2, and ZT3) were analyzed via qRT-PCR at 45 days post-inoculation with
M. enterolobii (
Figure 4A). The results revealed that all three RNAi vectors efficiently silenced the gene compared to CK, with expression levels in ZT1, ZT2, and ZT3 reduced to 25.75%, 14.9%, and 6.87% of those in CK, respectively. Silencing efficiency varied among the vectors, with ZT3 exhibiting the strongest suppression of
Me-chs-1. Furthermore, transcriptome sequencing results for the ZT3 strain targeting
M. enterolobii revealed reduced expression levels of four putative chitin synthase genes (CAD2169478.1, CAD2180532.1, CAK5089395.1, and CAK5088075.1) compared to the wild type (
Figure 4B).
Notably, the fold-reduction in CAK5089395.1 and CAK5088075.1 exceeded that observed for CAD2169478.1 and CAD2180532.1. Among the four genes, CAD2180532.1 displayed the highest baseline expression, suggesting that CAD2180532.1 may serve as the predominant chitin synthase gene in M. enterolobii under the current experimental conditions.
3.4. Impact of Me-chs-1 Silencing on Cumulative Infection Rate and Developmental Progression in M. enterolobii
Analysis of cumulative infection rates at 2, 8, 16, 23, and 30 dpi revealed no significant differences between RNAi lines (ZT1-ZT3) and wild-type (CK) controls during the early infection stages (2–16 dpi) (
Figure 5). However, on days 23 and 30, the cumulative penetration rates in the RNAi lines were lower than those in CK, suggesting that silencing of the
Me-chs-1 gene may impair the nematode’s later-stage development and penetration ability, thereby reducing the cumulative invasion rate.
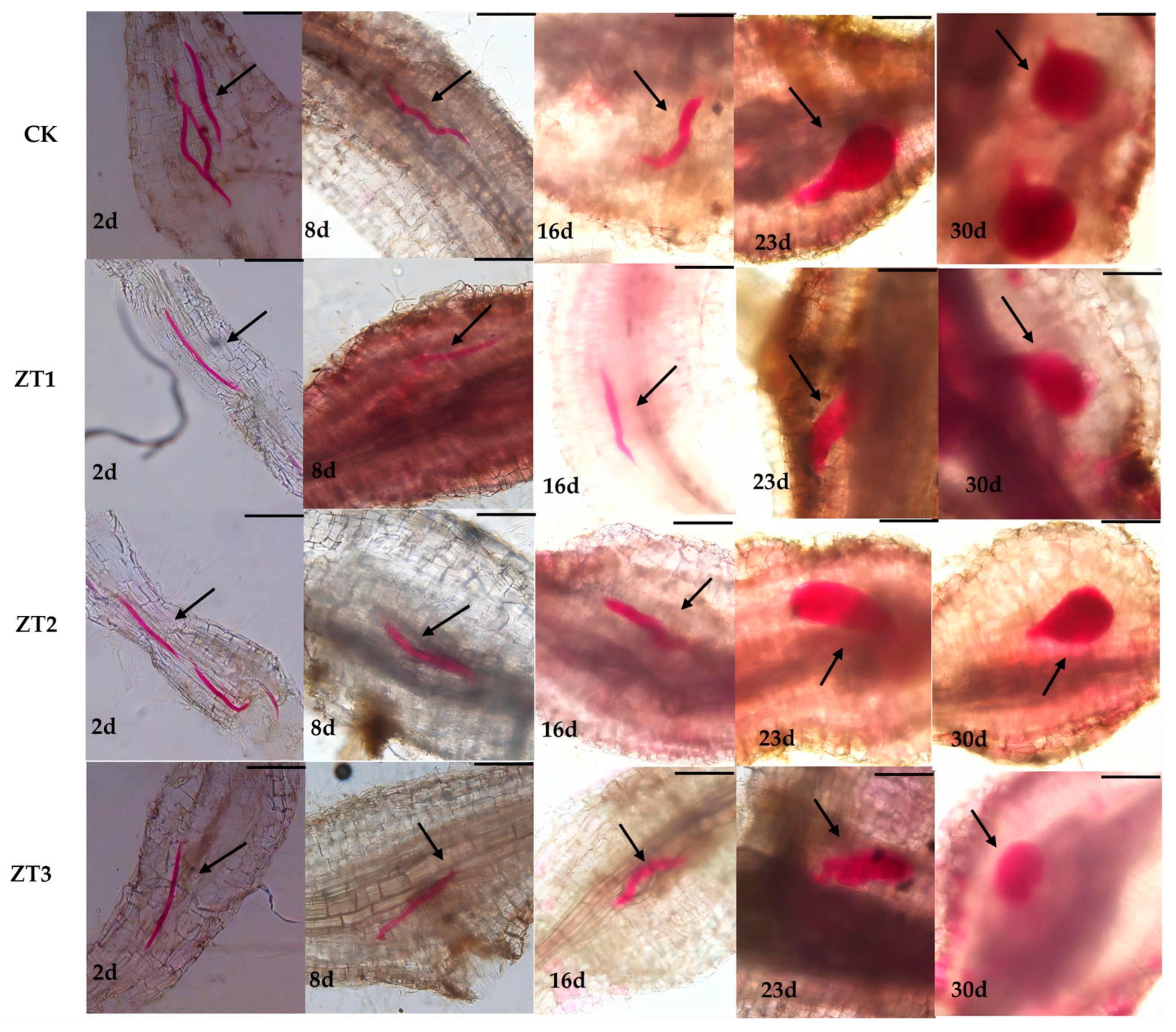
We also evaluated nematode developmental stages in CK and the three RNAi lines (ZT1, ZT2, and ZT3) at the same five time points (
Figure 6). In CK roots, nematode development proceeded normally: by day 16 they had reached the third juvenile stage, by day 23 they exhibited initial female morphology, and by day 30 they were fully developed females. In contrast, although nematode development in ZT1, ZT2, and ZT3 did not differ significantly from CK from day 2 to day 16, between days 23 and 30 development was markedly delayed in all three RNAi lines, with juveniles remaining smaller than those in the wild type. Notably, in ZT3-treated roots, nematodes were not only smaller at days 2 and 30 but also displayed developmental deformities. These results indicate that RNAi-mediated silencing of
Me-chs-1 suppresses middle- and late-stage development of
M. enterolobii, with the strongest effect observed in ZT3.
3.5. Effects of Me-chs-1 Gene Silencing on the Pathogenicity of M. enterolobii
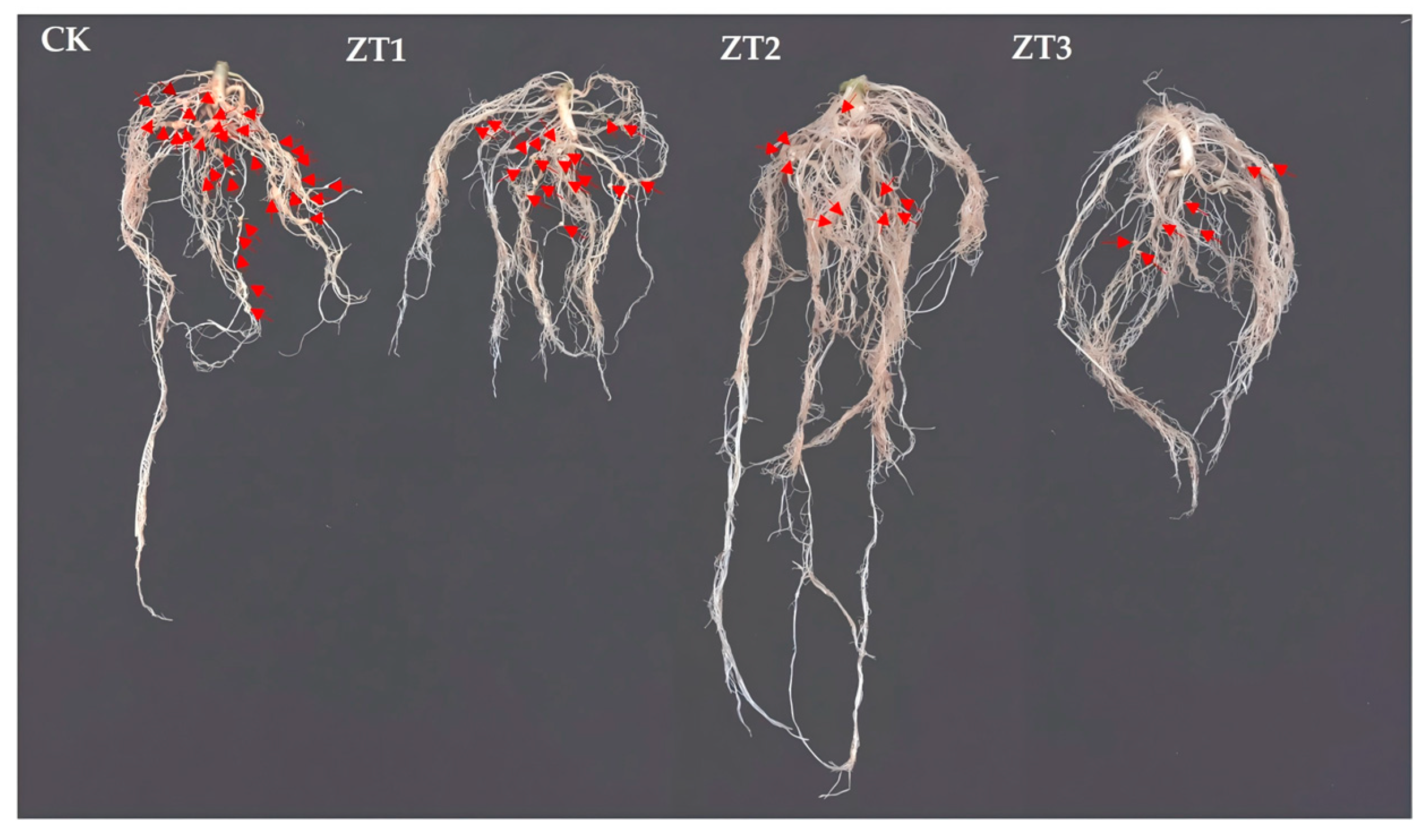
We compared root symptoms between RNAi tomato lines and the wild-type tomato line (CK) following inoculation with
M. enterolobii (
Figure 7). The RNAi lines had significantly fewer root galls and smaller gall diameters than CK did, indicating that silencing
Me-chs-1 impairs nematode establishment and feeding site development.
In addition, we quantified several pathological parameters—root galling percentage, root galling index, gall number, egg mass number, eggs per mass, and total egg count—for CK and the three RNAi lines (ZT1, ZT2, and ZT3) (
Table 1). CK showed a gall incidence of 46.02%, whereas ZT1, ZT2, and ZT3 had values of 35.13%, 34.77%, and 30.72%, representing reductions of approximately 23.7%, 24.5%, and 33.2%, respectively. This demonstrates that
Me-chs-1 silencing lowers the root-knot nematode infection rate.
The average gall count in CK was 67± 7.41, compared with 32 ± 36 (ZT1), 19 ± 4.55 (ZT2), and 16 ± 7.26 (ZT3)—reductions of about 52.2%, 71.6%, and 76.1%, respectively—confirming a significant decrease in gall formation due to RNAi treatment.
Egg mass counts also dropped markedly: CK produced 17 ± 5.31 masses, while ZT1, ZT2, and ZT3 yielded 11 ± 4.19, 8 ± 4.99, and 3 ± 1.25, respectively, respectively, representing reductions of 35.3%, 52.9%, and 82.4% compared to the CK group. Correspondingly, eggs per mass decreased from 402 ± 18.98 in CK to 236 ± 36.5 (ZT1), 209 ± 42.21 (ZT2), and 151 ± 36.55 (ZT3), respectively, with decreases of 41.3%, 48.0%, and 62.4%. Total egg counts fell from 5940 ± 146.97 in CK to 1260 ± 146.97 (ZT1), 1320 ± 224.5 (ZT2), and 1027 ± 224.5 (ZT3), respectively, with decreases as high as 78.8%, 77.8%, and 82.7%. These results clearly indicate that Me-chs-1 silencing substantially reduces the reproductive capacity of M. enterolobii.
3.6. Differential Gene Expression and Functional Annotation
The pathological staining results indicated that chitin synthase gene silencing predominantly impairs the late-stage growth and development of
M. enterolobii; accordingly, samples at 45 days post-inoculation (45 dpi) were selected for transcriptome sequencing. Comparative transcriptome analysis of nematodes in ZT3 RNAi tomato lines (CM) and in wild-type tomato lines (CK) at 45 days post-inoculation (45 dpi) with
M. enterolobii revealed 501 differentially expressed genes (DEGs) (
Figure 8A), of which 230 were upregulated and 271 downregulated in CM relative to CK. Gene Ontology (GO) analysis was performed on the 501 DEGs, yielding annotations for 350 genes. These DEGs were classified into the three main GO categories: molecular function (MF), cellular component (CC), and biological process (BP) (
Figure 8B). In the MF category, the most enriched terms were “structural constituent of cuticle,” “structural molecule activity,” and “L-glutamate transmembrane transporter activity,” with genes associated with cuticle structure markedly more abundant than those for other terms. Under CC, the largest clusters were “eRF1 methyltransferase complex,” “excitatory synapse,” and “acetylcholine-gated channel complex.” For BP, the DEGs were primarily associated with “membrane repolarization during action potential” and general “membrane repolarization.” KEGG pathway enrichment analysis revealed that 128 DEGs were significantly enriched (
Figure 8C). The top enriched pathways included the Wnt signaling pathway, spliceosome, ferroptosis, and ABC transporters, with the Wnt signaling pathway exhibiting the highest number of associated DEGs.
4. Discussion
In 1996, Baulcombe first discovered that double-stranded siRNA could silence genetic material in nematodes [
35].Two years later, Fire and Mello further reported the RNAi phenomenon and revealed the molecular mechanism of double-stranded RNA (dsRNA)-mediated gene silencing [
36]. These two studies laid the theoretical foundation for the application of RNAi technology. In 2002, Urwin and colleagues achieved in vitro RNAi silencing of target genes in infective larvae of
Globodera pallida and
Heterodera glycines for the first time by mediating oral uptake of dsRNA molecules with a neurostimulant [
37], opening the door to the application of this technology in research and control of plant-parasitic nematodes. Studies on the model nematode
C. elegans have shown that RNAi technology can protect crops by targeting essential nematode genes [
38]. Based on this, researchers proposed two core strategies: targeting plant genes involved in the infection process or directly targeting essential nematode genes. The idea of accumulating hpRNA (hairpin RNA) containing key nematode gene sequences in host plants and delivering dsRNA to nematodes via feeding to inactivate their genes has made RNAi the preferred method in plant nematology research due to its high efficiency in inhibiting nematode growth, which has been confirmed effective in gene silencing of root-knot nematodes (
Meloidogyne spp.) and cyst nematodes [
39].Target genes for nematode gene silencing need to meet conditions such as parasitic survival necessity, functional specificity, and RNAi sensitivity, including cell wall degrading enzyme genes, molting and development regulatory genes, etc.
CHS genes, as key genes in the chitin synthesis pathway, are selected as RNAi targets for controlling root-knot nematode diseases. This is because chitin is an important component of nematode eggshells, and chitin is absent in plants, so this strategy not only meets the criteria for gene silencing targets but also has the advantages of high efficiency and safety.
To validate the control potential of chitin synthase genes against nematodes, we used Host-Induced Gene Silencing (HIGS) technology to target the essential gene
Me-chs-1 of
Meloidogyne enterolobii. Results showed that after silencing
Me-chs-1, the number of root knots, egg masses, eggs per egg mass, and total eggs in tomato lines were significantly reduced, consistent with findings in
Meloidogyne incognita and
Heterodera glycines: Mani et al. reduced root knots and egg masses by over 60% and altered female nematode morphology via silencing the chitin synthase gene of
M. incognita [
30]; Kong et al. observed an 83.3% reduction in cyst numbers and 37.5% reduction in eggs per cyst after silencing this gene in
H. glycines [
31]. Notably, this study further revealed that gene silencing decreased nematode invasion rate and caused late-stage developmental disorders: Acid fuchsin staining showed significantly fewer invading nematodes in RNAi-treated roots at 23 and 30 days post-inoculation, with smaller, developmentally delayed larvae and obvious malformations in the ZT3 line. This indicates that
Me-chs-1 not only affects nematode reproduction but also regulates late developmental stages (e.g., molting or cuticle formation), supplementing earlier research with distinct findings.
To explore the mechanism of chitin synthase gene silencing, we performed transcriptome sequencing on the ZT3 line with the strongest silencing effect and wild-type nematodes, finding significant down regulation of four chitin synthase genes in
M. enterolobii and identifying 501 differentially expressed genes (DEGs), of which most of 271 downregulated genes were significantly enriched in “chitin structural component”-related functions by GO analysis. Notably, KEGG annotation showed that DEGs were primarily enriched in the Wnt signaling pathway—a conserved cellular signaling network involved in nematode embryonic development, cell differentiation, and molting [
40,
41]. Although no study has directly confirmed the association between the Wnt pathway and chitin metabolism, combining our data, we propose that members of the Wnt pathway can also serve as potential targets for the management of
M. enterolobii.
Given the remarkable ability of
M. enterolobii to overcome multiple resistance genes and the current scarcity of effective resistance resources, our experimental results demonstrate that chitin synthase gene silencing enhances tomato resistance to
M. enterolobii infection. Considering the absence of chitin synthesis pathways in plants and mammals, thus ensuring minimal impact on non-target organisms,
CHS genes emerge as highly promising targets for combating plant pests through. Disrupting
CHS activity through genetic, chemical, or biological interventions has shown promising results in reducing nematode infestations. RNAi has enabled a pivotal breakthrough in nematode control through targeted silencing of
CHS genes, effectively disrupting chitin synthesis. Key advantages of RNAi include its species-specificity and minimal environmental impact. However, challenges persist, including the effective delivery of dsRNA to nematodes and the risk of off-target effects. Our research revealed that the strategy targeting chitin synthase alone exhibit limited effectiveness on reducing
M. enterolobii infestations, underscoring the necessity for enhanced RNA interference efficiency. Targeting 2–3 unique, pest-specific genes simultaneously (e.g., chitin synthase + a unique digestive enzyme) reduces dependence on a single sequence, thereby minimizing safety risks from off-target effects. Additionally, microbial agents producing chitinolytic enzymes (e.g.,
Streptomyces spp.) degrade nematode eggshells, indirectly affecting CHS activity [
42]. Therefore, future work should prioritize integrating synergistic approaches (e.g., combining RNAi with microbial degradation) alongside developing nematode-resistant crops through gene editing. Additionally, chitin biosynthetic pathway-targeting pesticides offer high efficacy and environmental benefits [
43], commercial chitin-targeting agrochemicals—like nikkomycins against fungi [
44] and benzoylurea insecticides [
45]—remain undeveloped for nematode control. Integrating these chemical approaches with the previously discussed RNAi and microbial biocontrol agents within an integrated pest management (IPM) framework offers the most sustainable and resilient strategy for managing
M. enterolobii and related pests, potentially reducing reliance on broad-spectrum chemicals with greater environmental footprints [
22].
5. Conclusions
M. enterolobii possesses four candidate chitin synthase genes CAD2169478.1, CAD2180532.1, CAK5089395.1, and CAK5088075.1, exhibiting an overall sequence homology of 97.69%. The coding sequence (CDS) of the Me-chs-1 gene spans 3788 base pairs, encoding a protein of 1261 amino acids. This protein shows the highest homology to the chitin synthase protein of M. incognita. Furthermore, the proteins encoded by all four chitin synthase genes display high homology with the chs-1 protein of C. elegans.
Silencing the chitin synthase gene in M. enterolobii effectively reduces its invasion, suppresses its growth and development, and inhibits its reproduction; thereby, tomato resistance to root-knot nematodes was enhanced. Transcriptomic analysis of differentially expressed genes indicates a potential indirect regulatory relationship between the Wnt signaling pathway and chitin metabolism.
This study represents the first systematic validation of the efficacy of HIGS technology targeting the Me-chs-1 gene against M. enterolobii, which not only expands the understanding of chitin synthesis biological functions but also provides a novel target for nematode-resistant crop breeding. Follow-up research should further conduct field efficacy trials and explore multi-target synergistic silencing strategies with other critical genes (such as parasitism essential genes or effector genes) to promote the transition of RNAi technology from laboratory research to field application.