1. Introduction
Blueberries (
Vaccinium spp.), as berry crops with high nutritional and economic value, have become a pivotal component of the global agricultural product trade, with their industrial scale and market influence experiencing explosive growth over the past two decades. According to data from the IBO 2024 report (
http://www.THEIBO.org, accessed on 4 December 2024), the global total blueberry yield has exceeded 1.7823 million tonnes, of which 71% is consumed as fresh fruit and 29% for processing—representing a nearly 250% increase in total yield over ten years. In terms of trade flows, the global blueberry trade exhibits the characteristics of ‘Core producing regions leverage climatic differences to achieve staggered maturity periods, enabling nearly year-round supply of blueberries’. Fresh blueberry trade primarily relies on a combination of short-distance air transportation and long-distance maritime shipping, with the United States, the European Union and China as the three major consumer markets. According to Li’s report [
1], China’s fresh blueberry imports increased continuously from 2012, reaching 22,000 tonnes in 2019, followed by a fluctuating upward trend after 2020. After peaking at 42,900 tonnes in 2022 (the highest in recent years), imports began to decline. The primary driver of this decline is the early ripening of fresh blueberries from protected cultivation in Yunnan and solar greenhouses in Liaoning, which fills the gap in China’s fresh fruit market as early as February.
Consumer preference is the core driver of the blueberry industry’s development. Consumer expectations for blueberry shelf life have shifted from ‘basic freshness’ to ‘stable quality + extended shelf life’. In the South Korean market, despite competition from cheaper imported blueberries, local consumers exhibit a stronger preference for fresher domestic blueberries. Both global and domestic industry reports confirm that the returns on high-quality blueberries are substantial, with the high-end market willing to pay a premium for such products. Shelf life refers to the duration during which fruit retain safety, sensory appeal and physicochemical integrity under recommended conditions after harvest or storage [
2]. Postharvest performance directly impacts consumer preference and market competitiveness.
Postharvest research has predominantly focused on preservation techniques involving exogenous treatments [
3]. Existing studies have identified storage temperature, relative humidity and gas atmosphere as core factors affecting blueberry shelf-life quality, and preliminary insights have been gained into the regulatory mechanisms of different storage conditions on fruit physiological metabolism and quality changes. Angeletti [
4] focused on two highbush blueberry cultivars, ‘O’Neal’ and ‘Bluecrop’, investigating the regulatory effect of preharvest calcium application on fruit firmness. The study confirmed that preharvest calcium treatment significantly increased firmness in ‘O’Neal’ compared to the control; in contrast, ‘Bluecrop’ showed no significant firmness difference from the control on the harvest day, but, after 23 days of storage at 2 °C, both cultivars exhibited significantly higher firmness under calcium treatment than the control. The research emphasized the benefits of preharvest calcium fertilization for blueberry storage. Srivastava [
5] noted that storage at −20 °C inhibited the degradation of total polyphenols and total anthocyanins in ‘Tifblue’ and ‘Powderblue’ blueberries; in contrast, significant degradation of these compounds occurred after 15 days of storage at 23 °C and 35 °C. Therefore, refrigeration or freezing minimizes adverse effects on total polyphenols and total anthocyanins in blueberry fruit. Paniagua [
6] explored the correlation between air flow rate, weight loss and firmness changes in postharvest ‘Centurion’ rabbiteye blueberries by modifying storage air flow rates. They found that faster air flow resulted in greater weight loss and more rapid firmness decline. Intriguingly, independent of air flow rate and storage duration, ‘Centurion’ berries exhibited a ‘firming increase phenomenon’ when weight loss was below 1.34%, whereas softening occurred when weight loss exceeded 3.47%. Additionally, magnetic resonance imaging (MRI) revealed that water loss primarily occurred in the epidermal layer at low weight loss; at high weight loss, mesocarp cells shrank due to dehydration, leading to increased intercellular spaces.
Dragisić [
7] investigated mid- and late-season blueberry cultivars ‘Bluecrop’ and ‘Liberty’ under regular atmosphere (RA) and modified-atmosphere packaging (MAP) during 30 d storage at 2 °C, revealing significant varietal divergence in firmness decay, where crisp-fleshed ‘Liberty’ exhibited higher initial firmness (428 g) versus soft-fleshed ‘Bluecrop’ (296 g), with MAP significantly enhancing quality preservation in ‘Bluecrop’ through superior Total Quality Index (TQI) and 13% higher sugar retention compared to RA, whereas conversely inhibiting quality retention in ‘Liberty’, demonstrating that MAP applicability cannot be universally assumed for long-term storage and necessitating precision postharvest technologies aligned with cultivar-specific traits to optimize shelf-life extension and distribution logistics and, similarly, necessitating systematic analysis of phenotypic dynamics to refine postharvest logistics and preservation strategies. Harb [
8] reported that excessively high CO
2 concentrations induce texture deterioration and flavor abnormalities in blueberries, but the cultivar-specific CO
2 tolerance thresholds remain unclear. Precise postharvest treatment technologies tailored to cultivar characteristics are therefore required to extend shelf life, necessitating systematic analysis of phenotypic dynamics to optimize postharvest logistics and preservation strategies.
As consumer demands for commercial traits continue to rise, blueberry shelf-life quality evaluation has evolved from ‘single-index assessment’ to ‘multi-dimensional comprehensive evaluation’. Texture, color and functional components are core evaluation indicators. Accurate assessment of fruit phenotypic traits and postharvest phenotypic changes across blueberry cultivars is of great value to both breeders and consumers.
In terms of texture evaluation, traditional studies have primarily used Texture Analyzers for compression testing. Rivera [
9] used two blueberry cultivars, ‘Nui’ and ‘Rahi’, harvested at three maturity stages, to investigate texture changes under different storage technologies and MAP treatments. They identified hardness slope and skin break slope as indicators capable of distinguishing harvest maturity and storage technology, which could also detect fruit softening and firming during storage. While this study advanced the blueberry texture evaluation system, it did not address the mechanistic link between texture changes and cellular microstructure. Li [
10] employed Optical Coherence Tomography (OCT) to nondestructively assess mesocarp microstructure in stored berries. They found that the thickness of the top two epidermal cell layers increased with weight loss due to moisture depletion, and this thickness exhibited a linear Pearson correlation with TPA parameters of blueberries. However, the high equipment cost of this technology limits its application in industrial practice. The CIE Lab color space is a widely used color evaluation method. Jimenes [
11] found that ‘Snowchaser’ blueberries maintained stable peel color (unchanged L* and b*, minimal a* variation) under 22 °C and 60% relative humidity, with a 6-day maximum shelf life. Cesa [
12] reported that the hue angle (h
ab) in the CIELAB color space is negatively correlated with total anthocyanin content and closely associated with the ratio of different anthocyanins, making it a viable indirect indicator of anthocyanin changes.
The fresh blueberry industry still faces significant challenges in accurately evaluating inter-cultivar differences and optimizing cultivation management and postharvest treatments to maintain quality stability based on inherent cultivar traits. This study focuses on 24 blueberry cultivars, including southern highbush, northern highbush and their hybrids. To eliminate the interference of environmental variables on inter-cultivar quality comparisons, comprehensive quality evaluations were conducted under uniform cultivation and laboratory conditions. During a 15-day shelf life at a consistent refrigeration temperature of 4 °C, postharvest quality indicators related to commercial value—including soluble solids content, color and fruit texture—were compared across cultivars. Dynamic changes in these parameters during the 15-day postharvest period were analyzed. By comparing textural trait differences among multiple cultivars, evaluations of both fresh fruit and shelf-life fruit (in terms of color and texture) were conducted, aiming to provide a reference for commercial value analysis based on phenotypic and textural traits.
5. Conclusions
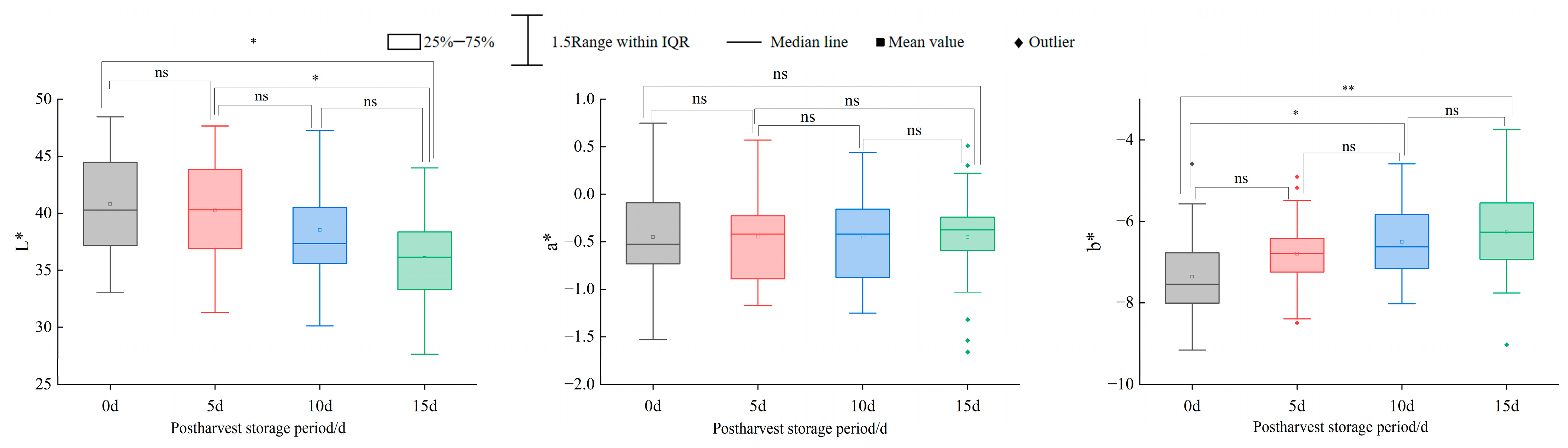
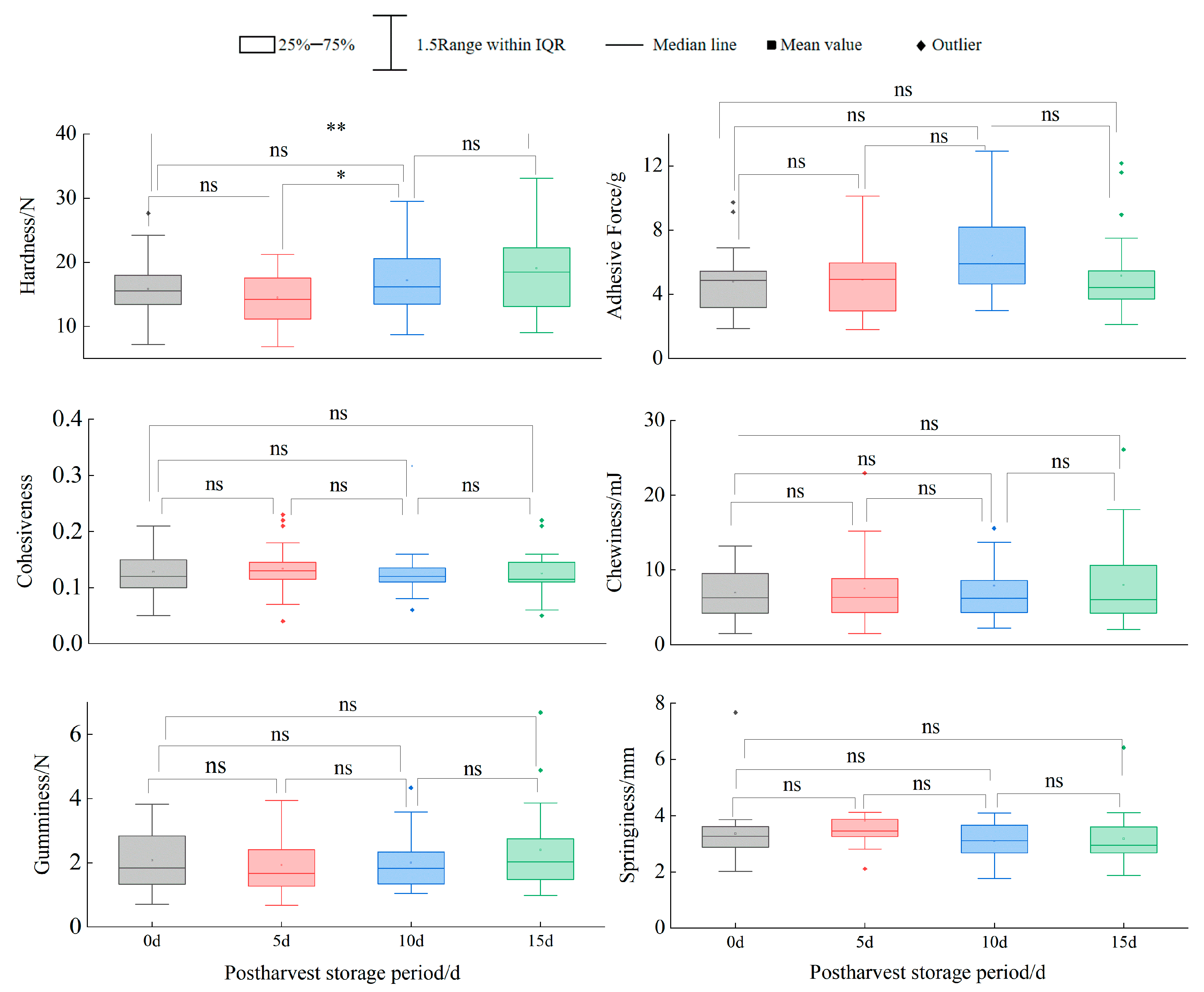
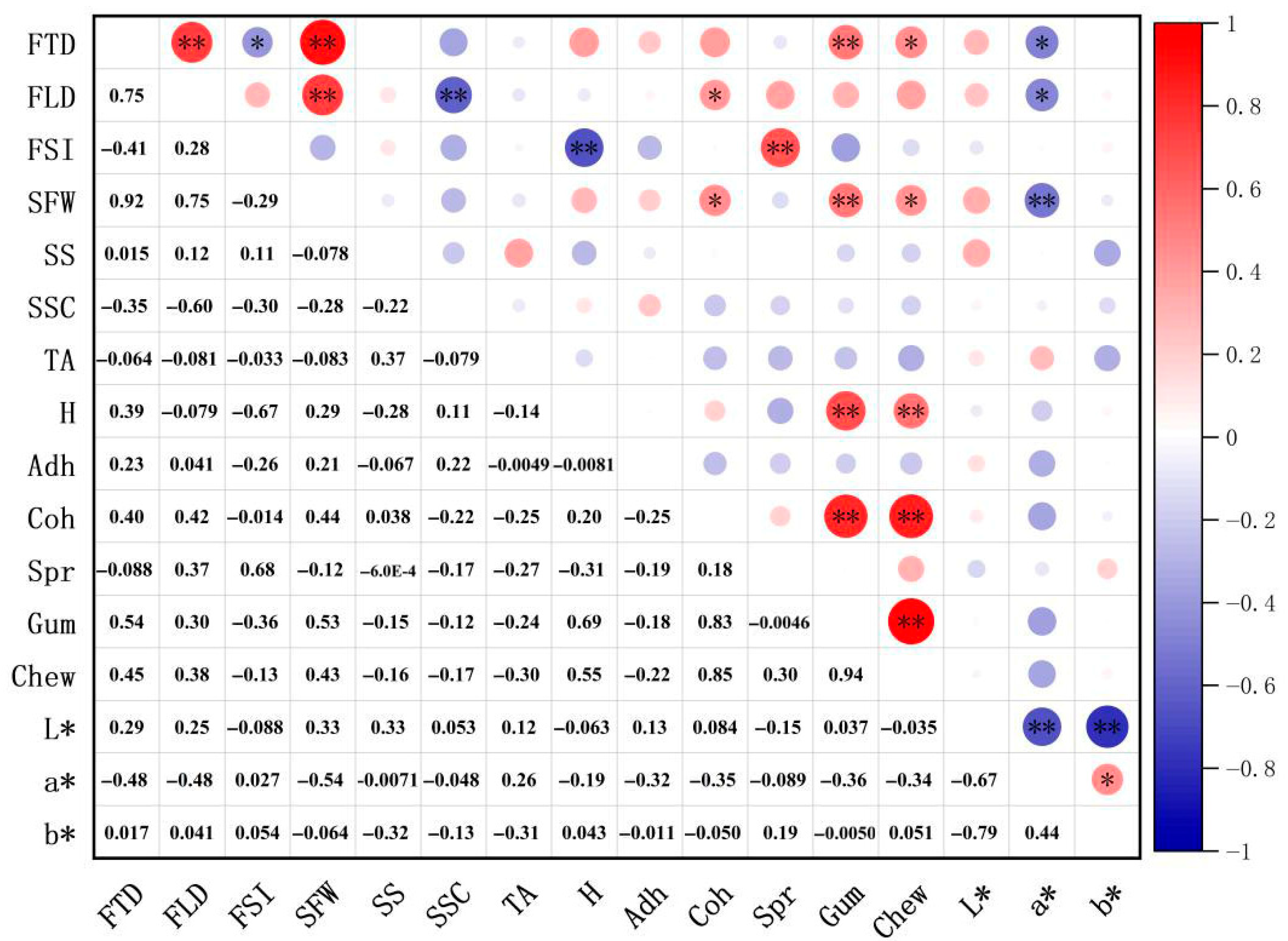
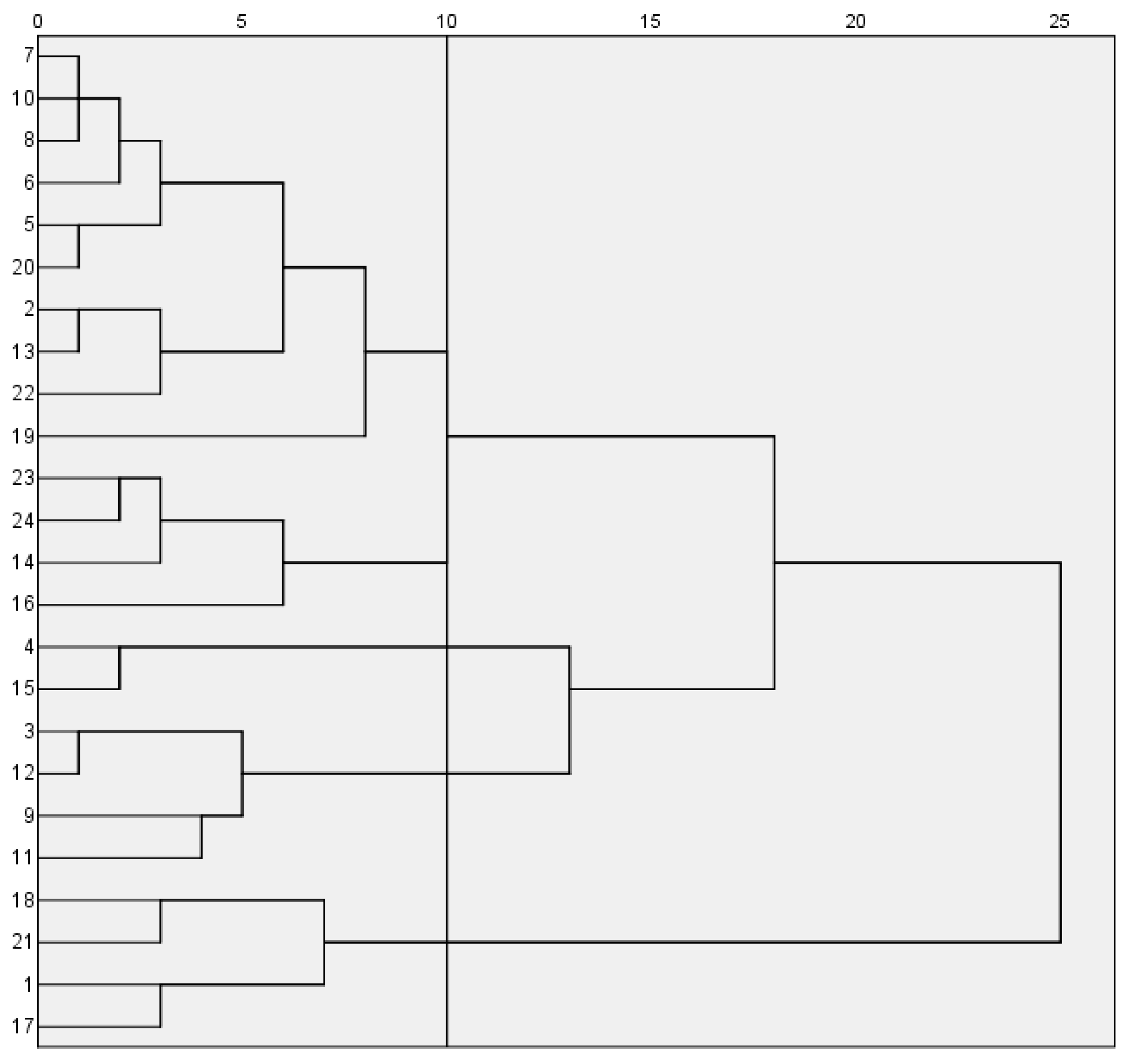
A comprehensive evaluation of 24 blueberry cultivars revealed that southern highbush cultivars ‘EB 9-2’, ‘Meadowlark’, ‘Primadonna’, ‘Eureka’ and ‘Camellia’ exhibited superior fruit size, texture and color, ranking highest in overall quality. Postharvest analysis identified significant hardness differences (p < 0.05) between day 0 and 15 in cultivars ‘Star’, ‘Camellia’, ‘Farthing’, ‘EB 9-2’, ‘Legacy’, ‘Unknown’ and ‘Gulfcoast’, while other cultivars showed no significant temporal variations. Color stability (ΔE < 3.5) was maintained only by ‘Legacy’ throughout the 15 days. Although minimal ΔE shifts occurred in most cultivars during days 0–5 or 0–10, pronounced color changes (ΔE > 3.5) emerged by day 15. Scar size, fruit shape index and springiness were validated as key predictors of shelf-life hardness dynamics, providing actionable metrics for cultivar selection and postharvest management.
The findings of this study can provide scientific references for the development of the entire blueberry industry chain. For future variety breeding, it is recommended to comprehensively consider fruit texture, color stability and flavor quality to cultivate varieties with synergistically excellent ‘fresh-eating palatability and postharvest storability’. The revealed characteristics of inter-varietal quality differences and shelf-life variability can serve as references for growers to select varieties and determine harvest timing, as well as for distributors to optimize the matching of sales cycles based on the storage and transportation characteristics of different varieties, thereby avoiding the risk of quality deterioration caused by immature harvesting.
However, this study only focused on the postharvest performance of highbush blueberries under constant low-temperature conditions and did not include rabbiteye blueberries and other blueberry types, resulting in certain limitations in the generalizability and explanatory depth for complex industrial scenarios. Future research can further expand the sample coverage by including multi-geographical origin blueberry varieties. Simultaneously, it should simulate complex environmental conditions, such as temperature fluctuations and different preservation measures in the supply chain, and construct an integrated comprehensive evaluation system of ‘variety evaluation—storage and transportation optimization—sales adaptation’, so as to provide more systematic scientific support for the high-quality and high-yield development of the blueberry industry.