Responses of Growth and Secondary Metabolites in Fish Mint (Houttuynia cordata Thunb.) Cuttings to Far-Red Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Cultural Practices, and Light Treatments
2.2. Determination of Photosynthetic Pigments
2.3. Determination of Secondary Metabolite Content
2.4. Statistical Analysis
3. Results
3.1. Plant Growth Status
3.2. Photosynthetic Pigment Content
3.3. Secondary Metabolite Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An ethnopharmacological review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef]
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A review of phytochemistry and pharmacology and quality control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Pradhan, S.; Rituparna, S.; Dehury, H.; Dhall, M.; Singh, Y.D. Nutritional profile and pharmacological aspect of Houttuynia cordata Thunb. and their therapeutic applications. Pharmacol. Res. Mod. Chin. Med. 2023, 29, 100311. [Google Scholar] [CrossRef]
- Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review. Pharmaceuticals 2022, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Tsai, K.-C.; Huang, Y.-C.; Liaw, C.-C.; Tsai, C.-I.; Chiou, C.-T.; Lin, C.-J.; Wei, W.-C.; Lin, S.J.-S.; Tseng, Y.-H.; Yeh, K.-M.; et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: A bedside-to-bench study. Biomed. Pharmacother. 2021, 133, 111037. [Google Scholar] [CrossRef]
- Wei, W.-C.; Liaw, C.-C.; Tsai, K.-C.; Chiou, C.-T.; Tseng, Y.-H.; Chiou, W.-F.; Lin, Y.-C.; Tsai, C.-I.; Lin, C.-S.; Lin, C.-S.; et al. Targeting spike protein-induced TLR/NET axis by Covid-19 therapeutic NRICM102 ameliorates pulmonary embolism and fibrosis. Pharmacol. Res. 2022, 2, 106424. [Google Scholar] [CrossRef] [PubMed]
- Committee on Chinese Medicine and Pharmacy. Taiwan Herbal Pharmacopeia English Version, 4th ed.; Ministry of Health and Welfare: Taipei, Taiwan, 2022; p. 203.
- Department of Health of Hong Kong. Hong Kong Chinese Materia Medica Standards; Department of Health: Hong Kong, China, 2012; Volume 4, p. 191.
- Loo, Y.-C.; Tsai, Y.-H.; Chen, H.; Hsieh, H.-P.; Chen, Y.-C.; Chen, H.-E.; Lin, Z.-H.; Huang, H.-T.; Liu, J.-M.; Liaw, C.-C.; et al. Quality and production enhancement of fish mint, Houttuynia cordata Thunb., cultivated in a hydroponic planting system with designed plant growth-promoting additives. Heliyon 2024, 10, e25875. [Google Scholar] [CrossRef]
- Li, Y.S.; Lin, K.H.; Wu, C.W.; Chang, Y.S. Effects of temperatures on growth, physiological, and antioxidant characteristics in Houttuynia cordata. Not. Bot. Horti Agrobot. 2021, 49, 12536. [Google Scholar] [CrossRef]
- Boonchaisri, S.; Mokoed, C.; Phuangchaona, P.; Treenate, E. Metabolomic and growth responses of Houttyunia cordata to varying light intensities and subsequent effects on fermented products. Trends Sci. 2024, 21, 7373. [Google Scholar] [CrossRef]
- Druege, U.; Hilo, A.; Perez-Perez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Smith, H. Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, J.; Yu, B.; Yang, L.; Sun, Y. LED light spectrum affects the photosynthetic performance of Houttuynia cordata seedlings. Am. J. Opt. Photonics 2015, 3, 38–42. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Tian, J.Y.; Yang, L. Effects of LED light spectra on active oxygen metabolism and expression of antioxidant isozymes in Houttuynia cordata Thunb. seedlings. Int. J. Environ. Agric. Res. 2015, 1, 28–34. [Google Scholar]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Casal, J.J.; Candia, A.N.; Sellaro, R. Light perception and signalling by phytochrome A. J. Exp. Bot. 2014, 65, 2835–2845. [Google Scholar] [CrossRef]
- Sheerin, D.J.; Hiltbrunner, A. Molecular mechanisms and ecological function of far-red light signalling. Plant Cell Environ. 2017, 40, 2509–2529. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some factors influencing the long-wave limit of photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.; Rabinowitch, E. Red drop and role of auxiliary pigments in photosynthesis. Plant Physiol. 1960, 35, 477. [Google Scholar] [CrossRef] [PubMed]
- Legendre, R.; van Iersel, M.W. Supplemental far-red light stimulates lettuce growth: Disentangling morphological and physiological effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Dale, M.P.; Causton, D.R. Use of the chlorophyll a/b ratio as a bioassay for the light environment of a plant. Funct. Ecol. 1992, 6, 190–196. [Google Scholar] [CrossRef]
- Chen, C.-L.; Yang, J.-P.; Huang, W.-D.; Chen, C.-C. The effect of Far-red light and nutrient level on the growth and secondary metabolites of the in vitro culture of Prunella vulgaris. Agronomy 2023, 13, 2250. [Google Scholar] [CrossRef]
- Kim, Y.L.; Sim, H.-S.; Jang, S.-N.; Lee, J.-H.; Son, K.-H. Changes in the growth and Lancemaside A content of Codonopsis lanceolata (deodeok) sprouts under LED-based lighting at different red/far-red ratios. Front. Plant Sci. 2025, 16, 1548781. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Prokopiuk, B.; Dziurka, K.; Pawłowska, B.; Ekiert, H.; Szopa, A. The influence of different wavelengths of LED light on the production of glucosinolates and phenolic compounds and the antioxidant potential in in vitro cultures of Nasturtium officinale (Watercress). Plant Cell Tissue Organ Cult. 2022, 149, 113–122. [Google Scholar] [CrossRef]
- Park, J.U.; An, S.K.; Kim, J. Far-red light affects stomatal opening and evapotranspiration of sweet basil. Horticulturae 2023, 9, 1095. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Woltering, E.J.; Cheng, R.; Li, T. Far-red radiation during indoor cultivation reduces lettuce nutraceutical quality and shortens the shelf-life when stored at supra optimal temperatures. Postharvest Biol. Technol. 2023, 198, 112269. [Google Scholar] [CrossRef]
- Karamat, U.; Guo, J.; Jiang, S.; Khan, I.; Lu, M.; Fu, M.; Li, G. Comprehensive, genome-wide identification and expression analyses of phenylalanine ammonia-lyase family under abiotic stresses in Brassica oleracea. Int. J. Mol. Sci. 2024, 25, 10276. [Google Scholar] [CrossRef]
- Li, H.S.; Ye, W.; Wang, Y.; Chen, X.H. RNA sequencing-based exploration of the effects of far-red light on lncRNAs involved in the shade-avoidance response of D. officinale. PeerJ 2021, 9, e10769. [Google Scholar] [CrossRef]
- Mohanan, P.; Yang, T.-J.; Song, Y.H. Effect of far-red light on the production and diversity of ginsenosides in leaves of Panax ginseng Meyer. Appl. Biol. Chem. 2023, 66, 16. [Google Scholar] [CrossRef]
- Yoneda, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K.; Shimizu, H. Impact of blue, red, and far-red light treatments on gene expression and steviol glycoside accumulation in Stevia rebaudiana. Phytochemistry 2017, 137, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Miranda Sotelo, M.M.; Tu, Y.-K.; Chang, P.P.-C.; Fang, W.; Chung, H.-Y. Effects of low green light combined with different red and far-red light ratios on the growth and secondary metabolites of cilantro (Coriandrum sativum L.). Agronomy 2025, 15, 1363. [Google Scholar] [CrossRef]
- Yang, C.-M.; Chang, K.-W.; Yin, M.-H.; Huang, H.-M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Ghosh, A.; Ghosh, B.; Parihar, N.; Ilaweibaphyrnai, M.; Panda, S.R.; Alexander, A.; Chella, N.; Murty, U.S.N.; Naidu, V.G.M.; Jagadeesh, K.G.; et al. Nutraceutical Prospects of Houttuynia cordata against the infectious viruses. Food Biosci. 2022, 50, 101977. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and shade-avoidance responses in plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Ali Raza, M.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.W.; Ma, J.J.; Liu, H.M.; Han, F.X.; Li, Y.; Niu, S.H. Gibberellin signaling is required for far-red light-induced shoot elongation in Pinus tabuliformis seedlings. Plant Physiol. 2020, 182, 658–668. [Google Scholar] [CrossRef]
- Tan, X.; Sha, L.; Tian, B.; Yu, M.; Xie, Z.; Chen, W.; Huangfu, Y.; Guo, J.; Liu, J.; Deng, C.; et al. Synergistic integration of light signal, hormone dynamics, and carbohydrate metabolism orchestrates rhizome bud development in Arundo donax. Planta 2025, 262, 85. [Google Scholar] [CrossRef]
- Croce, R.; van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, L.; Sayre, R.T.; Lee, C.-H. Identification of the optimal light harvesting antenna size for high-light stress mitigation in plants. Front. Plant Sci. 2020, 11, 505. [Google Scholar] [CrossRef]
- Jia, H.; Liggins, J.R.; Chow, W.S. Acclimation of leaves to low light produces large grana: The origin of the predominant attractive force at work. Philos. Trans. 2012, 367, 3494–3502. [Google Scholar] [CrossRef]
- Minagawa, J. State transitions—The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 897–905. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, Y.; Fan, P.; Wang, Y.; Xu, X.; Wang, L.; Li, S.; Duan, W.; Liang, Z.; Dai, Z. Far-red light modulates grapevine growth by increasing leaf photosynthesis efficiency and triggering organ-specific transcriptome remodelling. BMC Plant Biol. 2024, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- D’Orso, F.; Hill, L.; Appelhagen, I.; Lawrenson, T.; Possenti, M.; Li, J.; Harwood, W.; Morelli, G.; Martin, C. Exploring the metabolic and physiological roles of hqt in S. lycopersicum by gene editing. Front. Plant Sci. 2023, 14, 1124959. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, D.J.; Menon, C.; Oven-Krockhaus, S.Z.; Enderle, B.; Zhu, L.; Johnen, P.; Schleifenbaum, F.; Stierhof, Y.D.; Huq, E.; Hiltbrunner, A. Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 2015, 27, 189–201. [Google Scholar] [CrossRef]
- Liu, Y.; Singh, S.K.; Pattanaik, S.; Wang, H.; Yuan, L. Light Regulation of the biosynthesis of phenolics, terpenoids, and alkaloids in plants. Commun. Biol. 2023, 6, 1055. [Google Scholar] [CrossRef]
- Bhatia, C.; Gaddam, S.R.; Pandey, A.; Trivedi, P.K. COP1 mediates light-dependent regulation of flavonol biosynthesis through HY5 in Arabidopsis. Plant Sci. 2021, 303, 110760. [Google Scholar] [CrossRef]
- Zeng, Y.; Tian, G.; Liu, X.; Chen, L.; Zhu, J.; He, F.; Li, K.; Yu, H.; Tang, M.; Yang, Z. Integrated metabolomic and transcriptomic analysis of polysaccharides and flavonoids in different tissues of Houttuynia cordata. Sci. Hortic. 2024, 338, 113729. [Google Scholar] [CrossRef]
- Yin, N.; Luo, J.; Wang, C.; Xiong, Y.; Sun, Y.; Yuan, E.; Zhang, H. Comprehensive evaluation of the effects of hot air drying temperature on the chemical composition, flavor characteristics and biological activity of Houttuynia cordata Thunb. Foods 2025, 14, 1962. [Google Scholar] [CrossRef] [PubMed]
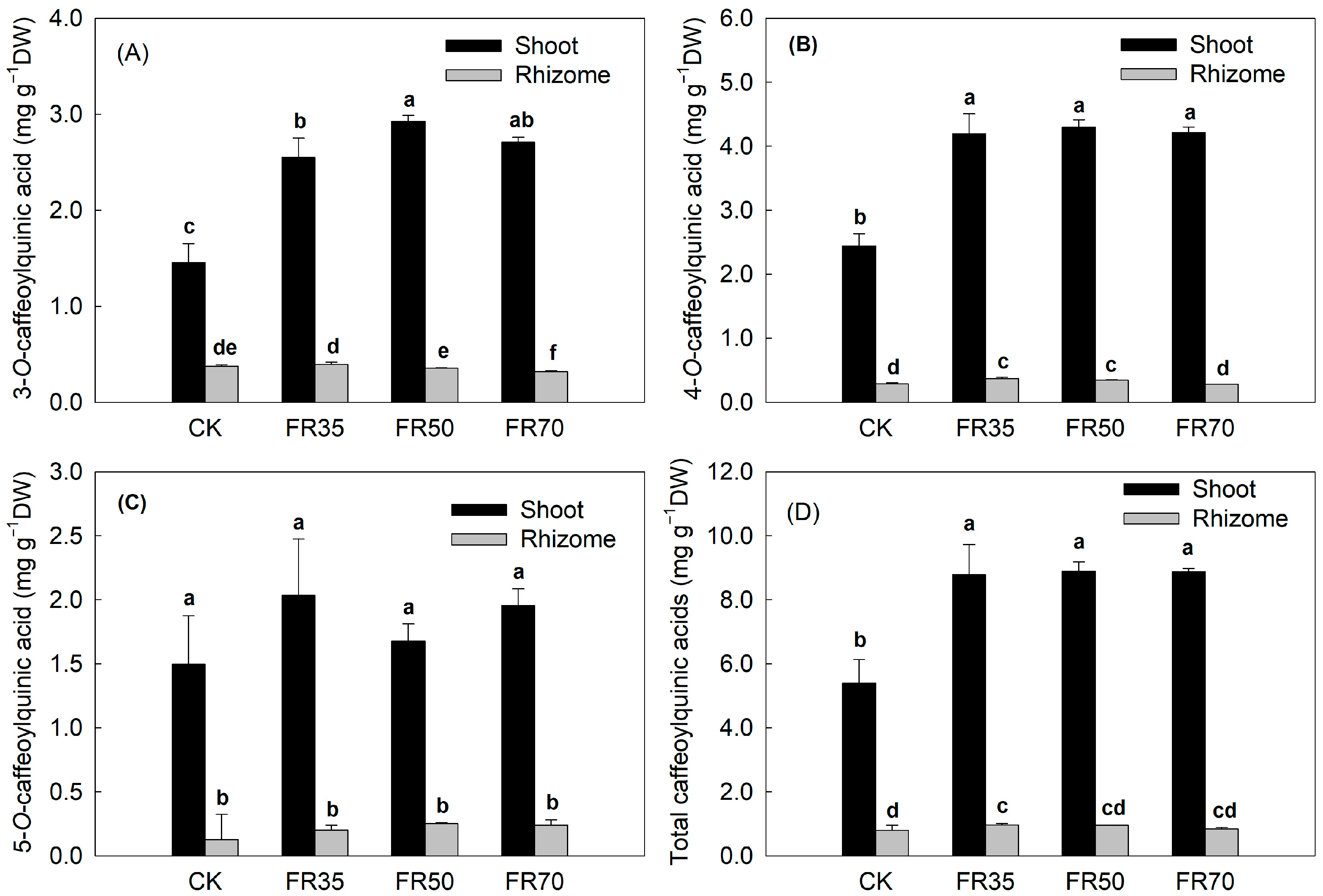
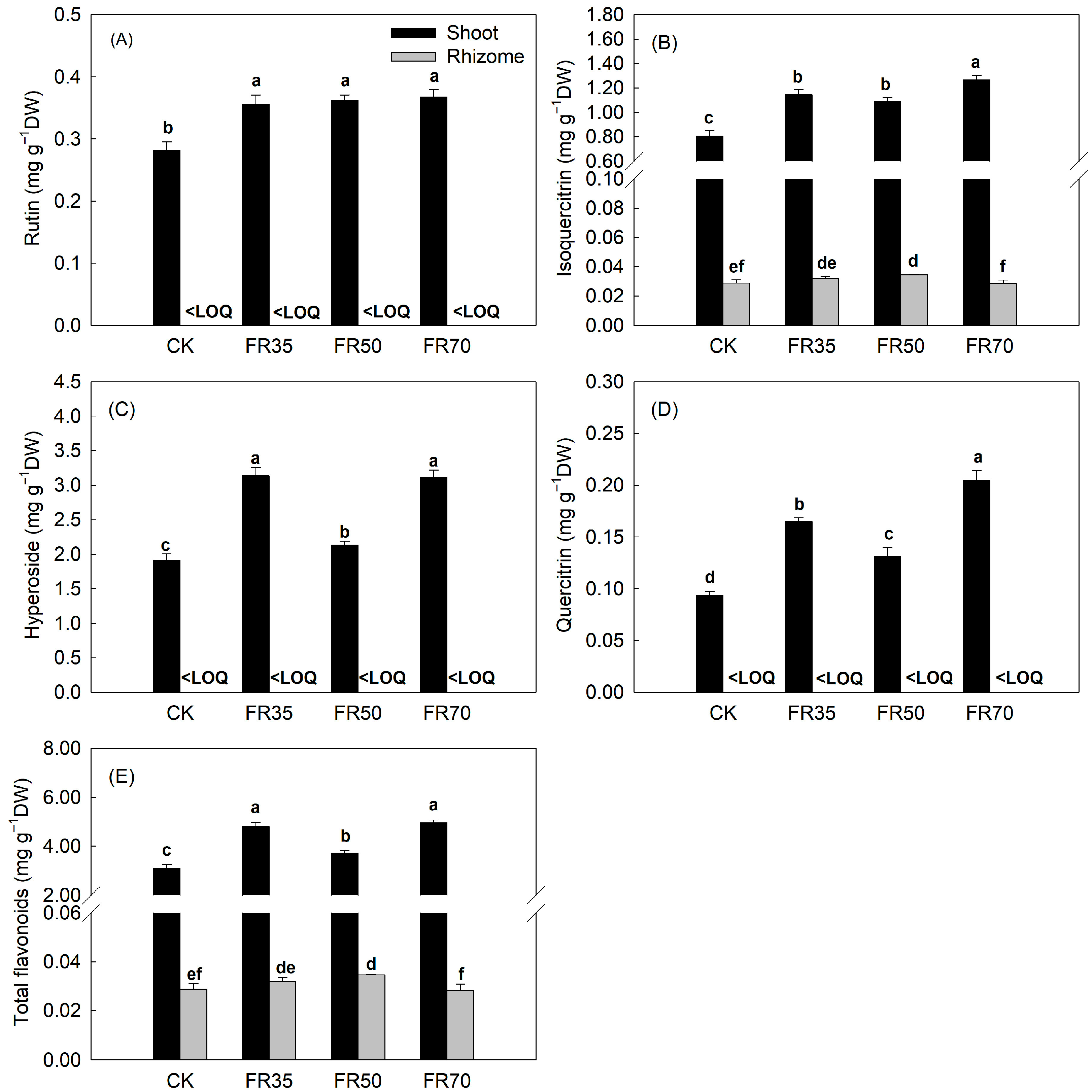
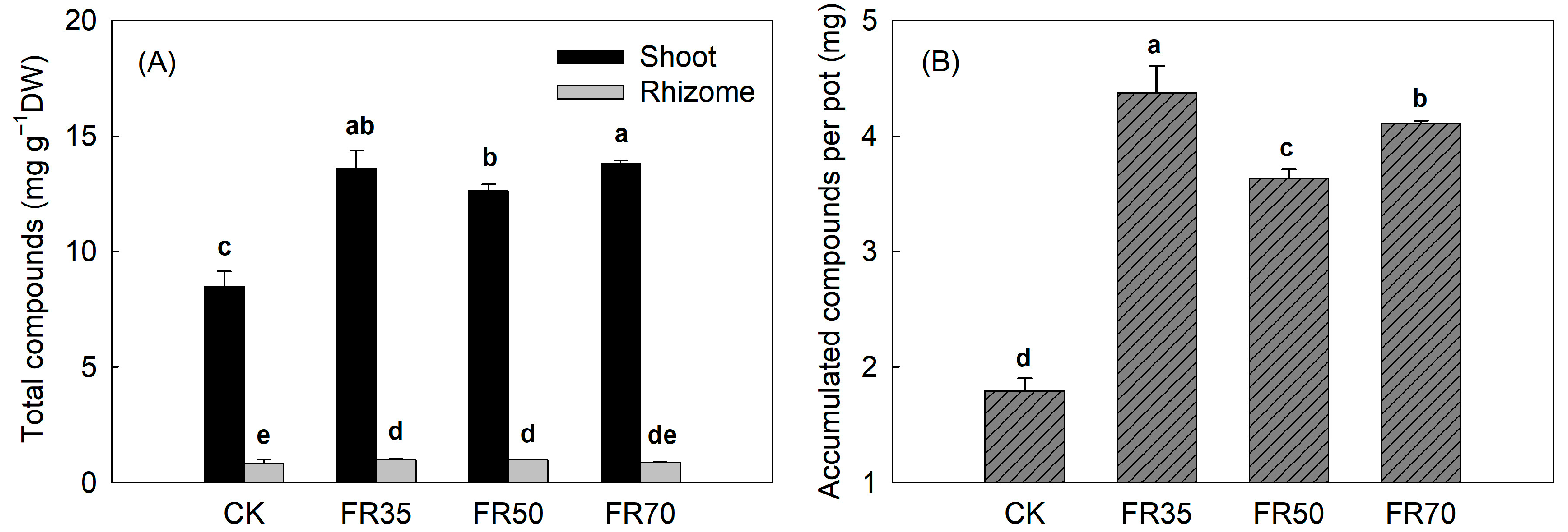
| Treatments | Height Increment (cm) | Total Dry Weight (g per pot) | Shoot/Rhizome | Moisture Content (%) |
|---|---|---|---|---|
| CK | 0.46 ± 0.50 b | 0.35 ± 0.09 b | 1.25 ± 0.39 b | 91.09 ± 1.16 a |
| FR35 | 0.85 ± 0.44 ab | 0.47 ± 0.15 ab | 2.07 ± 0.44 a | 90.48 ± 1.14 ab |
| FR50 | 1.23 ± 1.37 ab | 0.53 ± 0.16 ab | 1.06 ± 0.24 b | 89.06 ± 1.03 b |
| FR70 | 2.20 ± 1.91 a | 0.62 ± 0.21 a | 0.90 ± 0.28 b | 89.55 ± 1.52 b |
| Treatments | Chl a (mg g−1 DW) | Chl b (mg g−1 DW) | Total Chl (mg g−1 DW) | Chl a/b | Car (mg g−1 DW) |
|---|---|---|---|---|---|
| CK | 3.26 ± 0.73 a | 1.37 ± 0.30 a | 4.63 ± 1.03 a | 2.37 ± 0.02 a | 2.07 ± 0.43 a |
| FR35 | 3.19 ± 0.50 a | 1.39 ± 0.23 a | 4.58 ± 0.72 a | 2.30 ± 0.02 a | 2.16 ± 0.32 a |
| FR50 | 3.48 ± 0.31 a | 1.58 ± 0.16 a | 5.06 ± 0.47 a | 2.20 ± 0.07 b | 2.25 ± 0.19 a |
| FR70 | 3.71 ± 0.28 a | 1.72 ± 0.13 a | 5.44 ± 0.41 a | 2.15 ± 0.01 b | 2.50 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-Y.; Lin, K.-H.; Yin, Y.-C.; Chen, C.-C. Responses of Growth and Secondary Metabolites in Fish Mint (Houttuynia cordata Thunb.) Cuttings to Far-Red Light. Horticulturae 2025, 11, 1237. https://doi.org/10.3390/horticulturae11101237
Wang Z-Y, Lin K-H, Yin Y-C, Chen C-C. Responses of Growth and Secondary Metabolites in Fish Mint (Houttuynia cordata Thunb.) Cuttings to Far-Red Light. Horticulturae. 2025; 11(10):1237. https://doi.org/10.3390/horticulturae11101237
Chicago/Turabian StyleWang, Zi-Yi, Kuan-Hung Lin, Yen-Chi Yin, and Chang-Chang Chen. 2025. "Responses of Growth and Secondary Metabolites in Fish Mint (Houttuynia cordata Thunb.) Cuttings to Far-Red Light" Horticulturae 11, no. 10: 1237. https://doi.org/10.3390/horticulturae11101237
APA StyleWang, Z.-Y., Lin, K.-H., Yin, Y.-C., & Chen, C.-C. (2025). Responses of Growth and Secondary Metabolites in Fish Mint (Houttuynia cordata Thunb.) Cuttings to Far-Red Light. Horticulturae, 11(10), 1237. https://doi.org/10.3390/horticulturae11101237








