The MADS-Box Transcription Factor BoAGL8 Is Involved in Regulating Flowering in Broccoli
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification and Physicochemical Profiling of the MADS-Box Gene Family in Broccoli
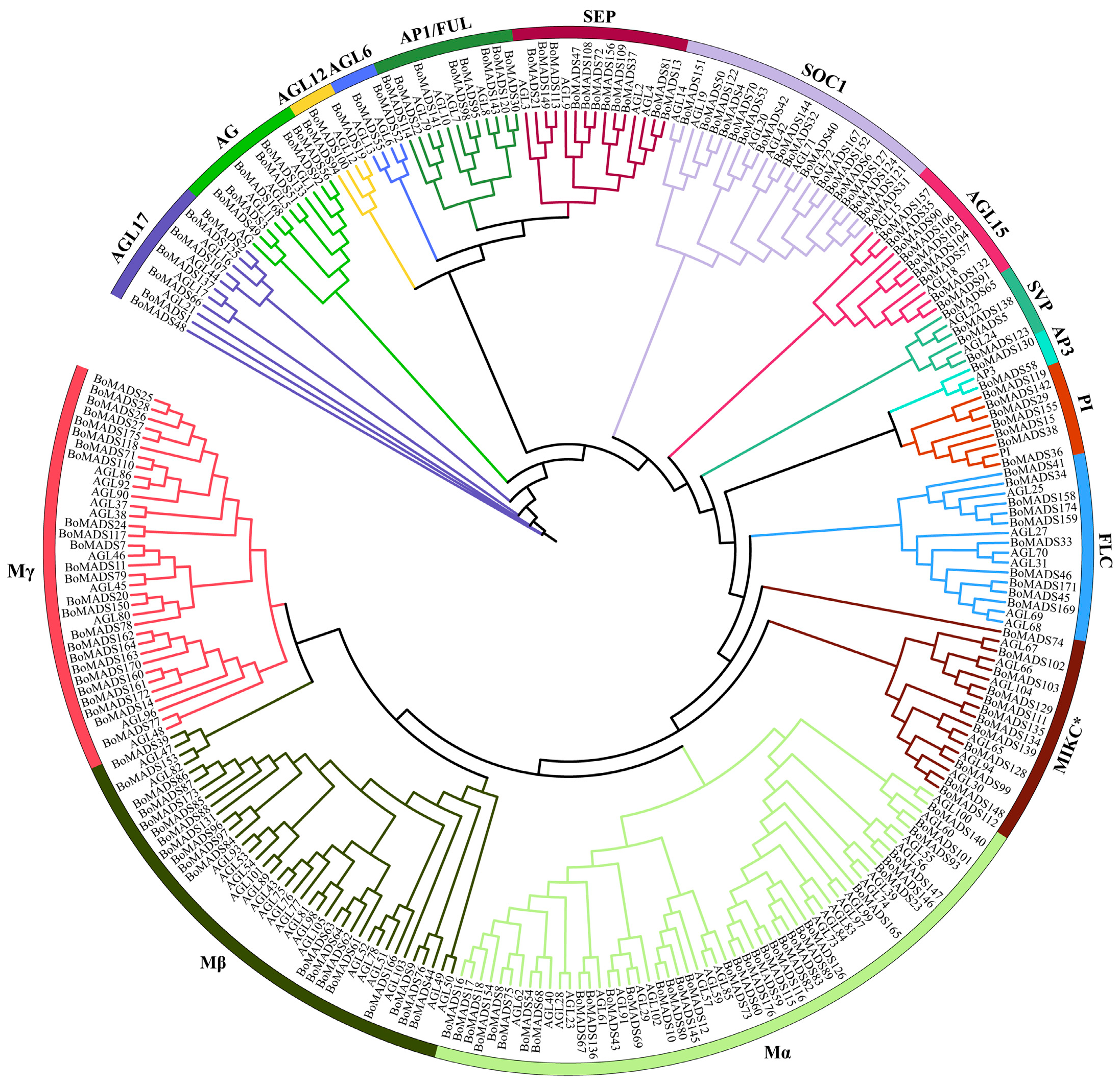
2.3. Phylogenetic Analysis and Classification of MADS-Box Gene Family in Broccoli
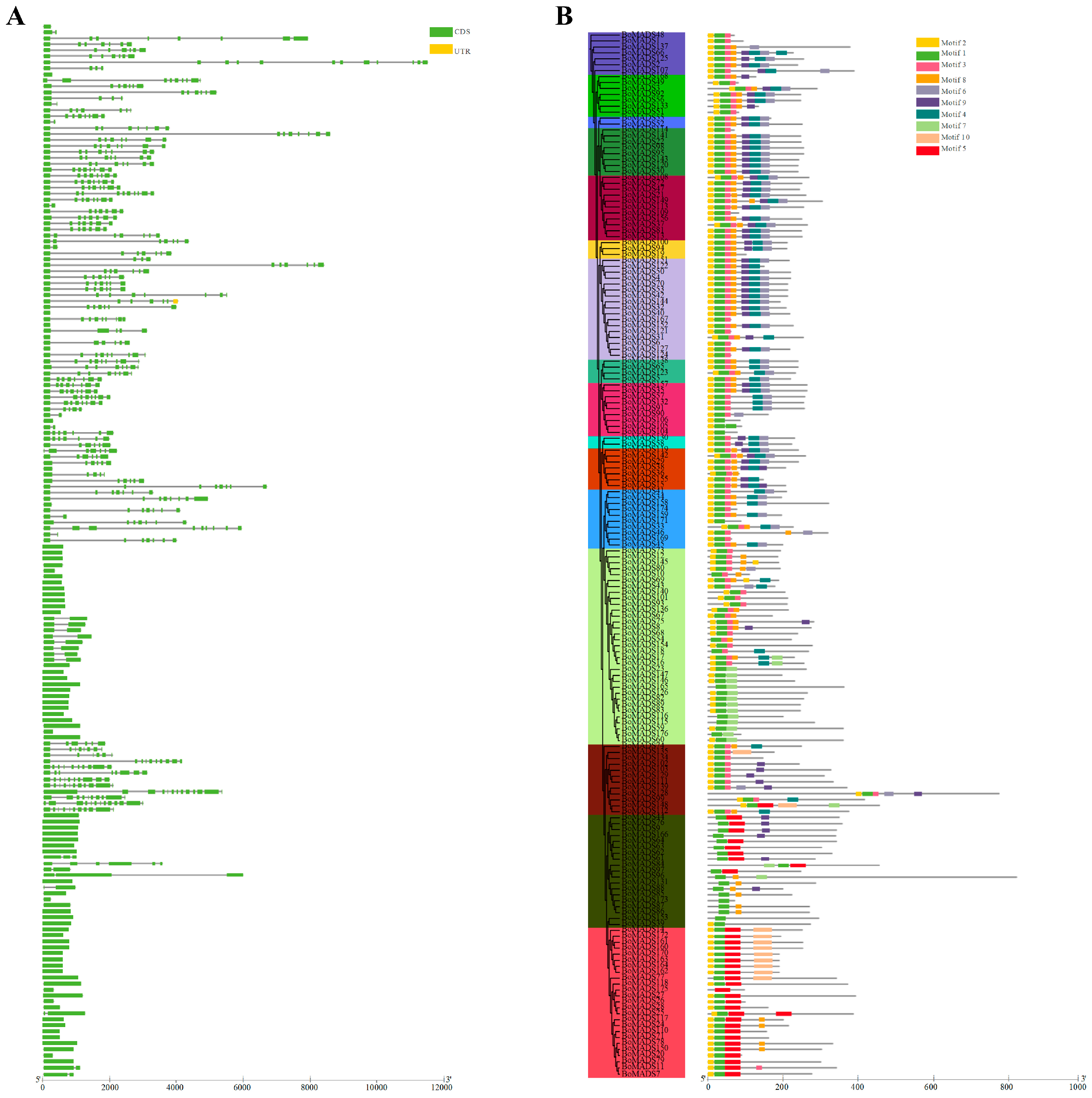
2.4. Comprehensive Analysis of Gene Structure, Conserved Motifs, and Promoter Cis-Acting Elements in Broccoli MADS-Box Gene
2.5. Chromosomal Localization and Collinearity Analysis of the MADS-Box Gene Family in Broccoli
2.6. Expression Analysis of the MADS-Box Gene Family in Broccoli
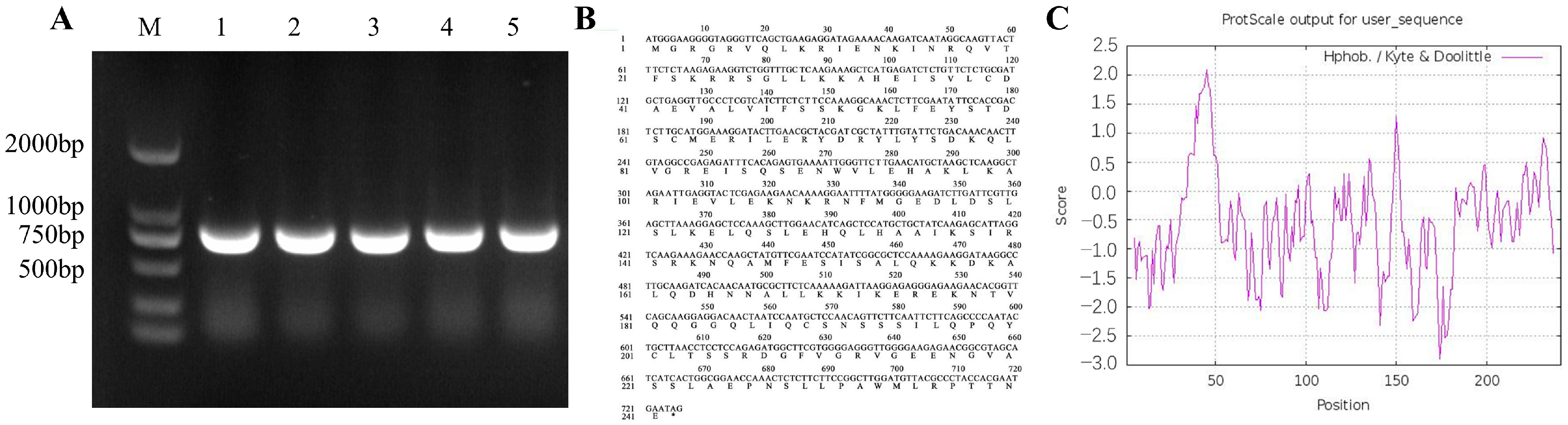
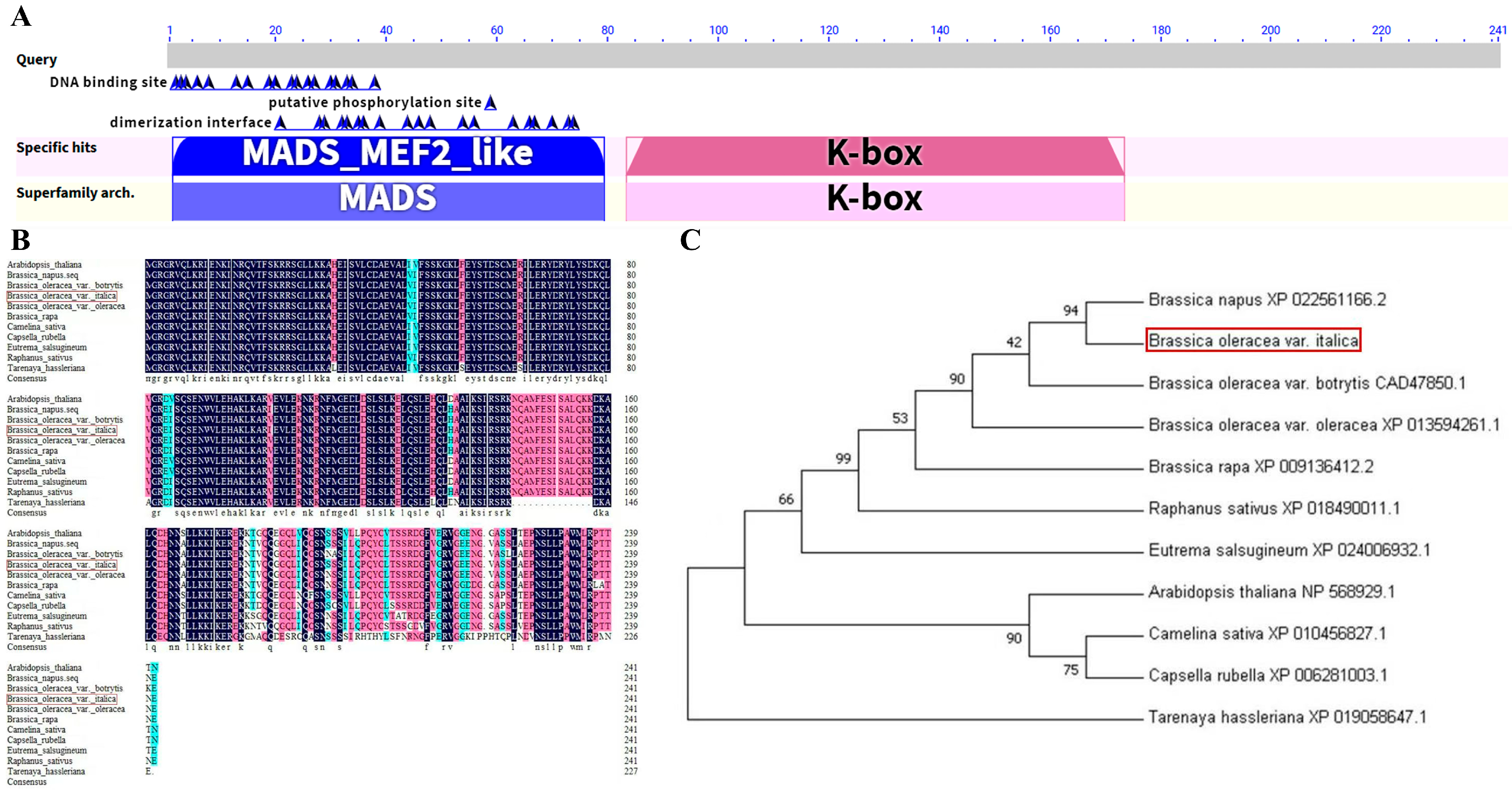
2.7. Cloning and Bioinformatics Analysis of the BoAGL8 Gene in Broccoli
2.8. Tissue-Specific Expression Patterns of BoAGL8 in Broccoli
2.9. Subcellular Localization of Broccoli BoAGL8
2.10. Heterologous Expression of BoAGL8 in Arabidopsis
2.11. Phenotypic Analysis of BoAGL8-Overexpressing Arabidopsis
3. Results
3.1. Identification and Characterization of the MADS-Box Gene Family in Broccoli
3.2. Phylogenetic Analysis and Classification of MADS-Box Gene Family in Broccoli
3.3. Gene Structure, Conserved Motifs, and Promoter Cis-Acting Elements of the MADS-Box Gene Family in Broccoli
3.4. Chromosomal Localization and Collinearity Analysis of MADS-Box Gene Family in Broccoli
3.5. Expression Analysis of the MADS-Box Gene Family in Broccoli
3.6. Sequence Analysis and Physicochemical Properties of the BoAGL8 Gene
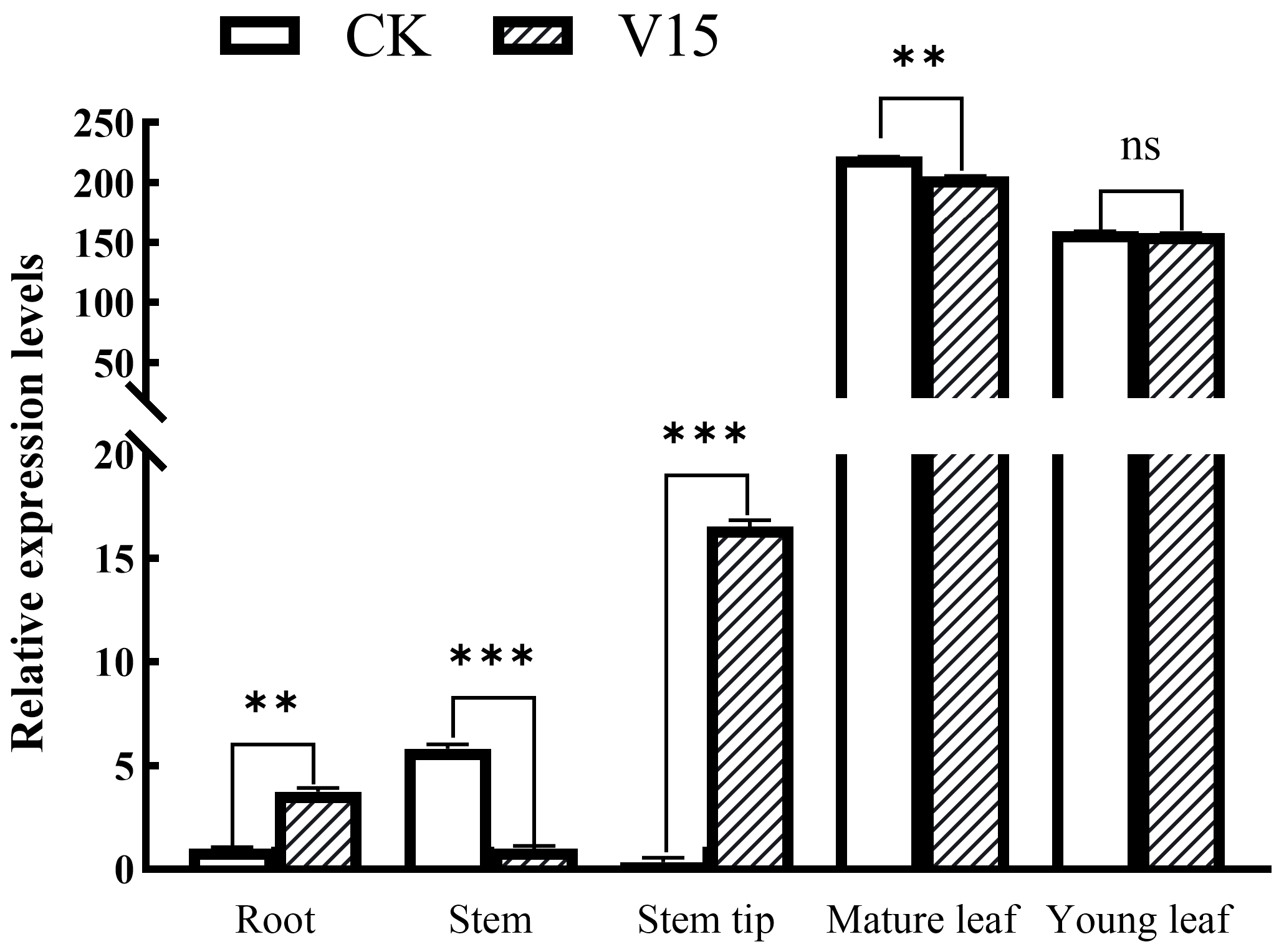
3.7. Expression Pattern of the BoAGL8 Gene in Broccoli
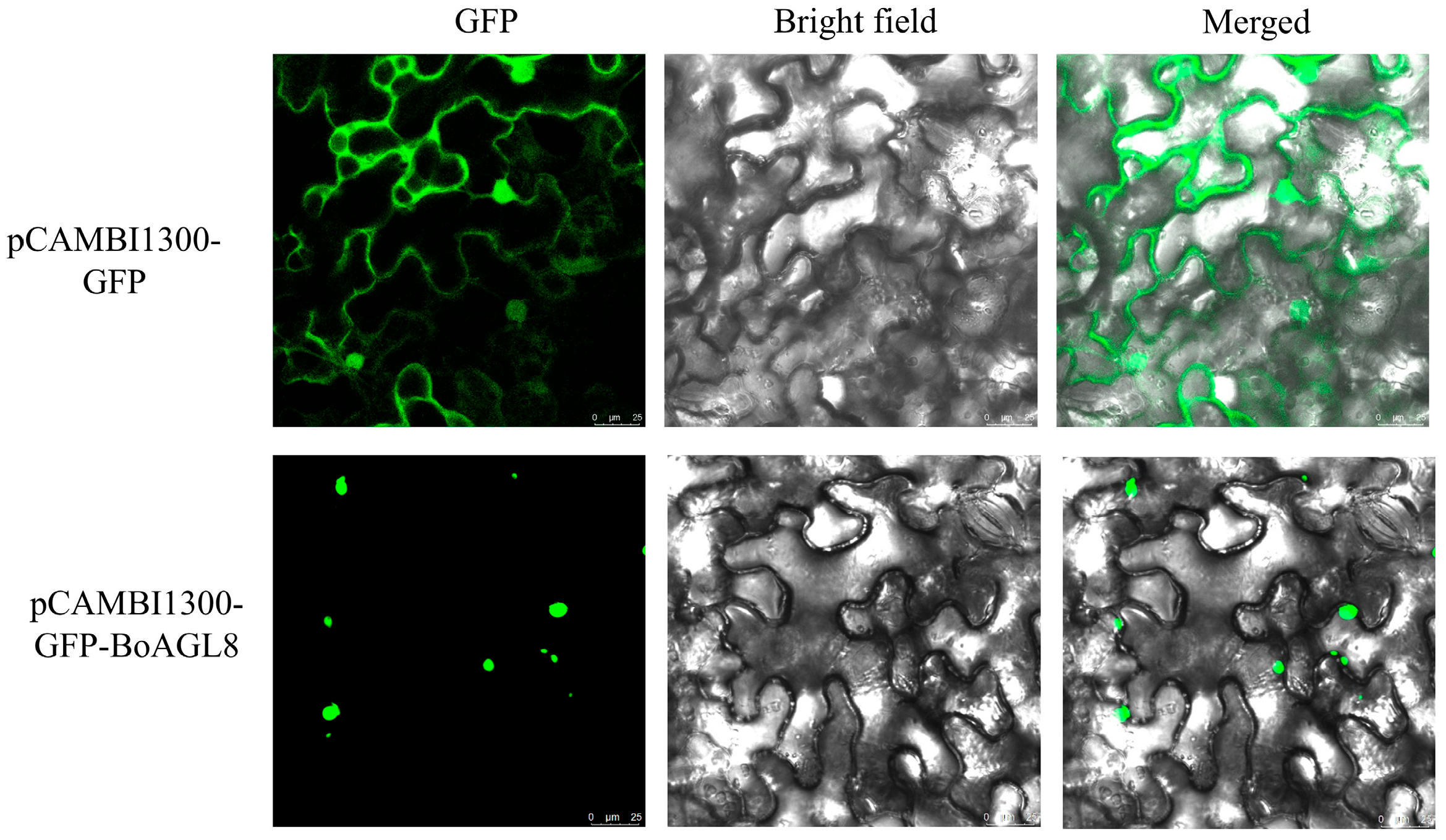
3.8. Subcellular Localization of BoAGL8
3.9. Ectopic Overexpression of BoAGL8 in Arabidopsis Accelerates Flowering
3.10. Expression Analysis of Flowering-Related Genes in Arabidopsis Overexpressing BoAGL8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Bodt, S.; Raes, J.; Van de Peer, Y. And then there were many: MADS goes genomic. Trends Plant Sci. 2003, 8, 475–483. [Google Scholar] [CrossRef]
- Zsuzsanna, S.-S.; Peter, H.; Wolfgang, N.; Heinz, S.; Hans, S. Genetic Control of Flower Development by Homeotic Genes in Antirrhinum majus. Science 1990, 250, 931–993. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yanofsky, M.F.; Meyerowitz, E.M. AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev. 1991, 5, 484–495. [Google Scholar] [CrossRef]
- Smaczniak, R.G.H.; Immink, J.M.; Muiño, R.; Blanvillain, M.; Busscher, J.; Busscher-Lange, Q.D. Characterization of MADS-domain transcription factor complexes in Arabidopsis flower development. Proc. Natl. Acad. Sci. USA 2012, 109, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Song, X.; Liu, X.; Yan, L.; Zhou, Z.; Zhang, X. Genome-wide analysis of CsWOX transcription factor gene family in cucumber (Cucumis sativus L.). Sci. Rep. 2020, 10, 6216. [Google Scholar] [CrossRef]
- Arora, R.; Agarwal, P.; Ray, S. MADS-box gene family in Oryza sativa: Genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genom. 2007, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Chen, W. Whole-genome survey and characterization of MADS-box gene family in Zea mays and sorghum. Plant Cell Tissue Organ Cult. 2011, 105, 159–173. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, S.S.; Bao, M.Z.; Liu, G.F. Advances on molecular mechanism of floral initiation in higher plants. Mol. Plant Breed. 2018, 16, 3681–3692. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Wang, J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014, 65, 4723–4730. [Google Scholar] [CrossRef]
- Kim, D.H. Current understanding of flowering pathways in plants: Focusing on the vernalization pathway in Arabidopsis and several vegetable crop plants. Hortic. Environ. Biotechnol. 2020, 61, 209–227. [Google Scholar] [CrossRef]
- Melzer, S.; Lens, F.; Gennen, J. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 2008, 40, 1489–1492. [Google Scholar] [CrossRef]
- Lars, O.; Sherry, A.; Kempin, D.B.; Harry, J.K.; Martin, F.Y. Pod shatter-resistant Brassica fruit produced byectopic expression of the FRUITFULL gene. Plant Biotechnol. J. 2006, 4, 45–51. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Ma, R. Functional characterization and mapping of two MADS-box genes from peach (Prunus persica). Chin. Sci. Bull. 2008, 53, 853–859. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lu, W.; Liu, S. Preliminary functional characterization of soybean GmAGL8 gene in Arabidopsis thaliana. J. Beijing Univ. Agric. 2020, 35, 20–25. [Google Scholar] [CrossRef]
- Itabashi, E.; Shea, D.; Fukino, N.; Fujimoto, R.; Okazaki, K.; Kakizaki, T.; Ohara, T. Comparison of cold responses fororthologs of cabbage vernalization-related genes. Hortic. J. 2019, 88, 462–470. [Google Scholar] [CrossRef]
- Jeong, H.L.; Soo, H.P.; Jong, S.L.; Ji, H.A. A conserved role of SHORT VEGETATIVE PHASE (SVP) in controlling flowering time of Brassica plants. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2007, 1769, 455–461. [Google Scholar] [CrossRef]
- Li, X.; Shen, C.; Chen, R. Function of BrSOC1b gene in flowering regulation of Chinese cabbage and its protein interaction. Planta 2023, 258, 21. [Google Scholar] [CrossRef]
- Min, J.K.; Hong, S.J.; Yoo, S.N.; Bosl, N. Repression of flowering under a noninductive photoperiod by the HDA9-AGL19-FT module in Arabidopsis. New Phytol. 2014, 206, 281–294. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, X. Effects of different temperatures on flower bud differentiation in broccoli. Plant Physiol. Commun. 2007, 43, 89–92. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E.; Libik, A. Effects of age and cold storage of transplants on the growth and quality of broccoli yield. Veg. Crops Res. Bull. 2007, 66, 31–38. [Google Scholar] [CrossRef]
- Wasserman-Olin, R.; Gómez, M.I.; Björkman, T. Meeting the Expectations of the Customer: Consumer Valuation of Broccoli Produced in the Eastern United States and the Impact of Local Marketing. Sustainability 2023, 15, 7878. [Google Scholar] [CrossRef]
- Chai, W.; He, X.; Wen, B.; Jiang, Y.; Zhang, Z.; Bai, R.; Zhang, X.; Xu, J.; Hou, L.; Li, M. The Molecular Mechanism of Relatively Low-Temperature-Induced Broccoli Flower Bud Differentiation Revealed by Transcriptomic Profiling. Horticulturae 2023, 9, 1353. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time Quantitative PCR and the 2−ΔΔCT Ctmethod. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sparkes, I.; Runions, J.; Kearns, A. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Z.; Li, X. Obtaining Arabidopsis thaliana Transformed with the crtB Gene via Agrobacterium tumefaciens Floral Dip Method. Hubei Agric. Sci. 2013, 52, 200–202. [Google Scholar] [CrossRef]
- Sheng, X.G.; Zhao, Z.Q.; Wang, J.S. Genome wide analysis of MADS-box gene family in Brassica oleracea reveals conservation and variation in flower development. BMC Plant Biol. 2019, 19, 10. [Google Scholar] [CrossRef]
- Duan, W.; Song, X.; Liu, T. Genome-wide analysis of the MADS-box gene family in Brassica rapa (Chinese cabbage). Mol. Genet. Genom. 2015, 290, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Xu, L.; Nie, S.; Chen, Y.; Liang, D.; Sun, X.; Karanja, B.K.; Luo, X.; Liu, L. Genome-wide characterization of the MADS-Box gene family in Radish (Raphanus sativus L.) and assessment of its roles in flowering and floral organogenesis. Front. Plant Sci. 2016, 7, 1390. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Kennedy, A.; Pan, S. Genome-wide analysis of MIKC-type MADS-box genes in wheat: Pervasive duplications, functional conservation and putative neofunctionalization. New Phytol. 2020, 225, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, R.; Berbel, A.; Serrano-Mislata, A. Floral initiation and inflorescence architecture: A comparative view. Ann. Bot. 2007, 100, 659–676. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E.; Kalisz, A.; Wojciechowska, R.; Leja, M.; Sękara, A. Chilling stress applied to broccoli transplants of different age affects yield of the plants cultivated in summer. Hortic. Sci. 2014, 41, 71–79. [Google Scholar] [CrossRef]
- Li, H.G.; Zhang, L.P.; Hu, J.H. Genome-Wide Association Mapping Reveals the Genetic Control Underlying Branch Angle in Rapeseed (Brassica napus L.). Front. Plant Sci. 2017, 8, 1054. [Google Scholar] [CrossRef]
- Miller, C.H.; Konsler, T.R.; Lamont, W. Cold Stress Influence on Premature Flowering of Broccoli. HortScience 1985, 20, 193–195. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Gu, Q.; Martienssen, R. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 2000, 127, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Sather, D.N.; Golenberg, E.M. Duplication of AP1 within the Spinacia oleracea L. AP1/FUL clade is followed by rapid amino acid and regulatory evolution. Planta 2009, 229, 507–521. [Google Scholar] [CrossRef]
- Leseberg, C.H.; Eissler, C.L.; Wang, X. Interaction study of MADS-domain proteins in tomato. J. Exp. Bot. 2008, 59, 2253–2265. [Google Scholar] [CrossRef]
- Wang, J.Y.; Sun, W.X.; Wang, L.; Liu, X.J.; Xu, Y. FRUITFULL is involved in double fruit formation at high temperature in sweet cherry. Environ. Exp. Bot. 2022, 201, 104986. [Google Scholar] [CrossRef]
- Hou, H. Cloning and Functional Characterization of the CpFUL-like Gene from Wintersweet (Chimonanthus praecox). Master’s Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar] [CrossRef]
- Wang, X. Mechanism of GhAGL8 Involved in Flowering Regulation in Upland Cotton (Gossypium hirsutum L.). Ph.D. Thesis, Shandong Normal University, Jinan, China, 2023. [Google Scholar] [CrossRef]
- Johanson, U.; West, J.; Lister, C.; Michaels, S.; Amasino, R.; Dean, C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 2000, 290, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Aete-Loyola, J.; Olivares, F.; Saavedra, G.M.; Zúñiga, T.; Mora, R.; Ríos, I.; Valdovinos, G.; Barrera, M.; Almeida, A.M.; Prieto, H. Artificial Sweet Cherry miRNA 396 Promotes Early Flowering in Vernalization-Dependent Arabidopsis Edi-0 Ecotype. Plants 2025, 14, 899. [Google Scholar] [CrossRef]



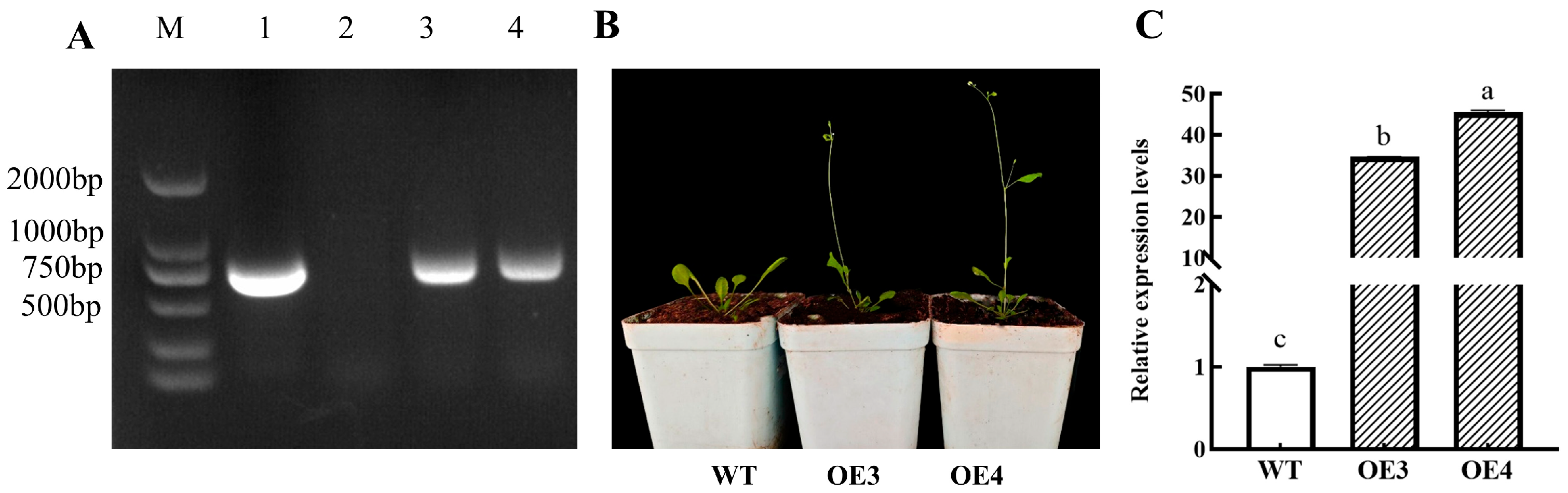

| Plant | Bolting Time (d) | Rosette Leaf Number | Time to First Flowering (d) | Plant Height at First Flowering (cm) | Seed Weight per Plant (mg) |
|---|---|---|---|---|---|
| WT | 29.6 ± 2.1 a | 10.8 ± 1.1 a | 36.7 ± 2.1 a | 9.7 ± 1.2 b | 17.9 ± 5.4 c |
| OE3 | 21.2 ± 0.7 b | 8.7 ± 0.7 b | 31.8 ± 1.0 b | 14.8 ± 1.3 a | 29.1 ± 5.4 b |
| OE4 | 21.8 ± 1.4 b | 8.7 ± 0.5 b | 29.3 ± 1.5 c | 14.2 ± 1.4 a | 36.0 ± 11.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, H.; Jia, P.; Li, Z.; Wang, Y.; Jiang, Y.; He, X.; Wen, B.; Huo, C.; Zhang, W.; et al. The MADS-Box Transcription Factor BoAGL8 Is Involved in Regulating Flowering in Broccoli. Horticulturae 2025, 11, 1227. https://doi.org/10.3390/horticulturae11101227
Li Y, Yang H, Jia P, Li Z, Wang Y, Jiang Y, He X, Wen B, Huo C, Zhang W, et al. The MADS-Box Transcription Factor BoAGL8 Is Involved in Regulating Flowering in Broccoli. Horticulturae. 2025; 11(10):1227. https://doi.org/10.3390/horticulturae11101227
Chicago/Turabian StyleLi, Yuanyuan, Hanbing Yang, Peini Jia, Zairong Li, Yan Wang, Yajie Jiang, Xia He, Boyue Wen, Chensi Huo, Wei Zhang, and et al. 2025. "The MADS-Box Transcription Factor BoAGL8 Is Involved in Regulating Flowering in Broccoli" Horticulturae 11, no. 10: 1227. https://doi.org/10.3390/horticulturae11101227
APA StyleLi, Y., Yang, H., Jia, P., Li, Z., Wang, Y., Jiang, Y., He, X., Wen, B., Huo, C., Zhang, W., Chai, W., Yan, S., & Zhang, J. (2025). The MADS-Box Transcription Factor BoAGL8 Is Involved in Regulating Flowering in Broccoli. Horticulturae, 11(10), 1227. https://doi.org/10.3390/horticulturae11101227





