Transcriptomic Analysis Reveals the Impact of Interstock on Vesicle Granulation in ‘Hainan Qingyou’ Pomelo (Citrus maxima) Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fruit Quality Measurement
2.3. Pulp Antioxidant Capacity Assay
2.4. RNA Extraction, cDNA Synthesis, and RT-qPCR Analysis
2.5. Heatmap and Statistical Analysis
3. Results
3.1. Effects of Different Interstocks on Fruit Size and Edible Rate at Maturity
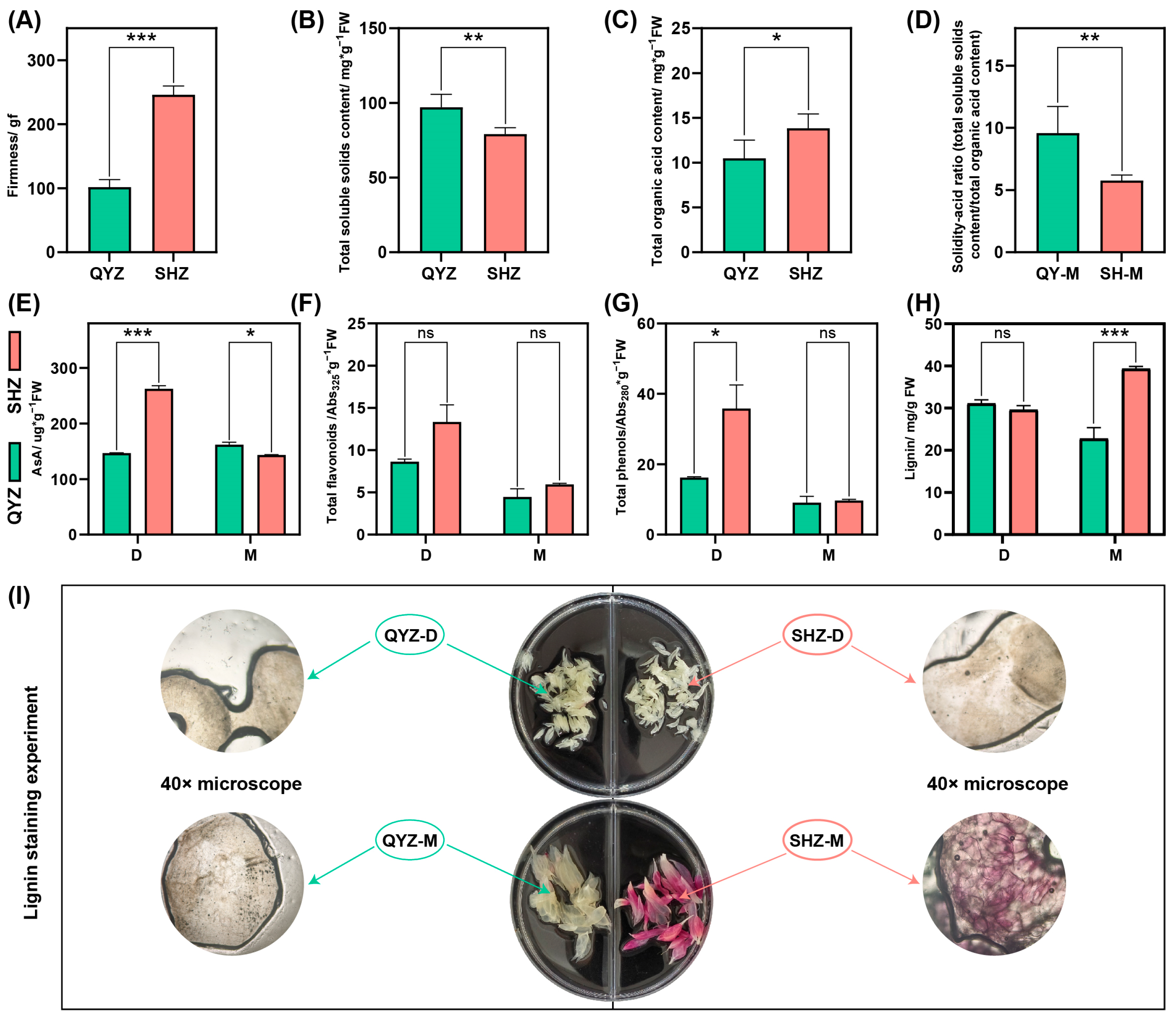
3.2. Effects of Different Interstocks on Internal Fruit Quality
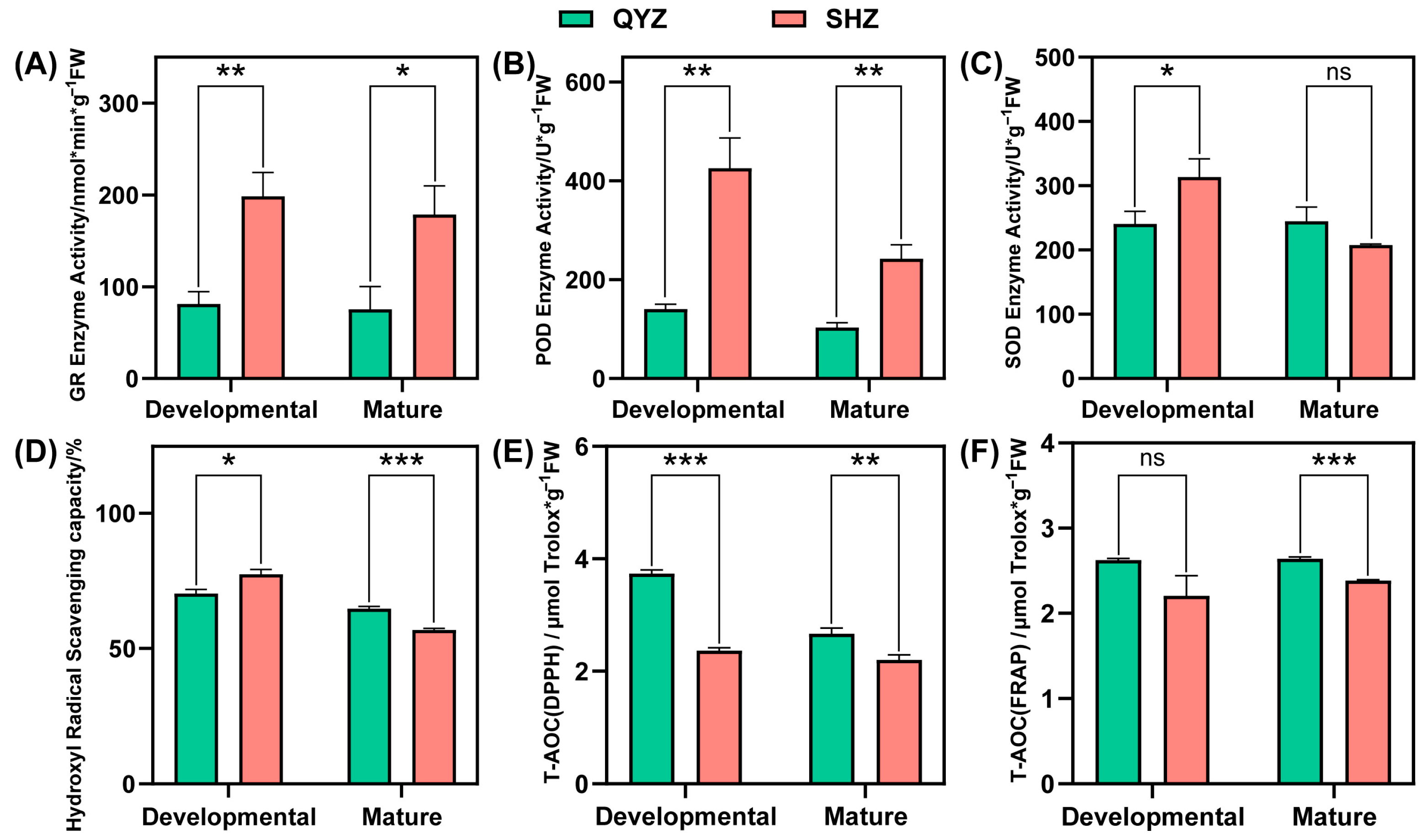
3.3. Effects of Different Interstocks on Pulp Antioxidant Indicators
3.4. Transcriptome Overview
3.5. Differential Gene Expression Analysis
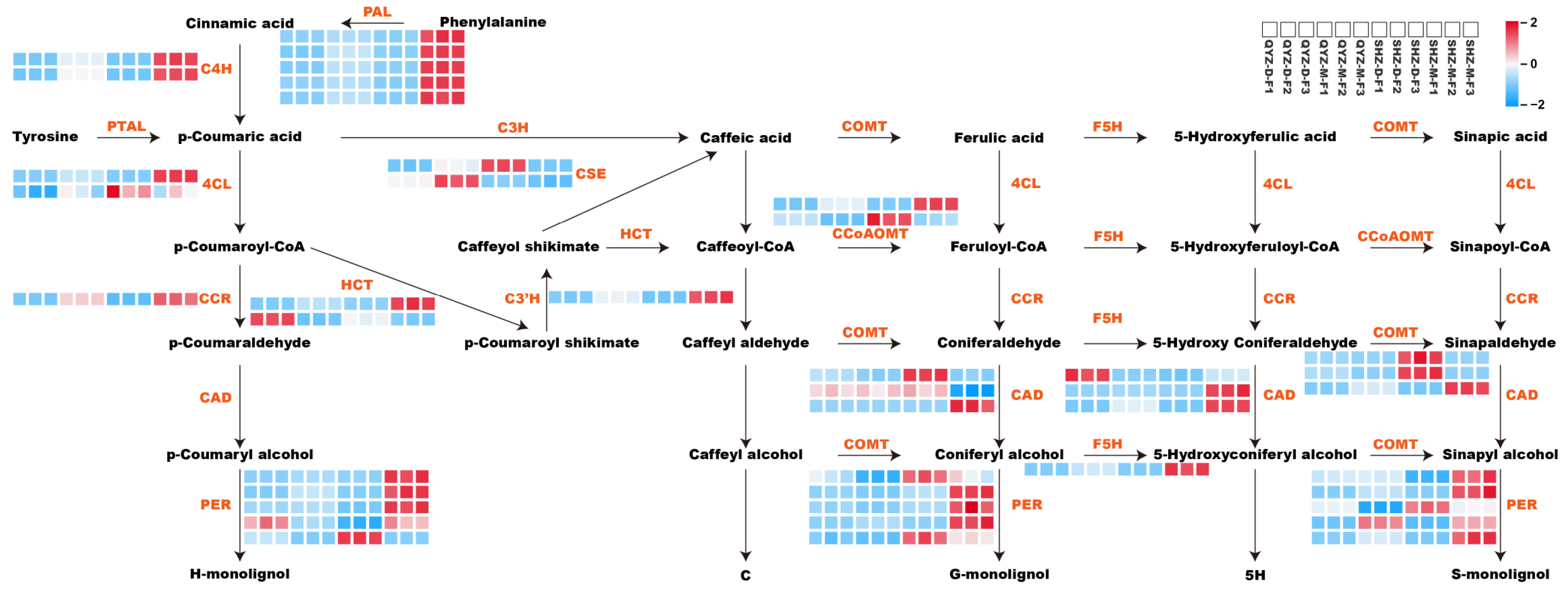
3.6. Expression Profiles of Key Structural Genes in Lignin Biosynthesis
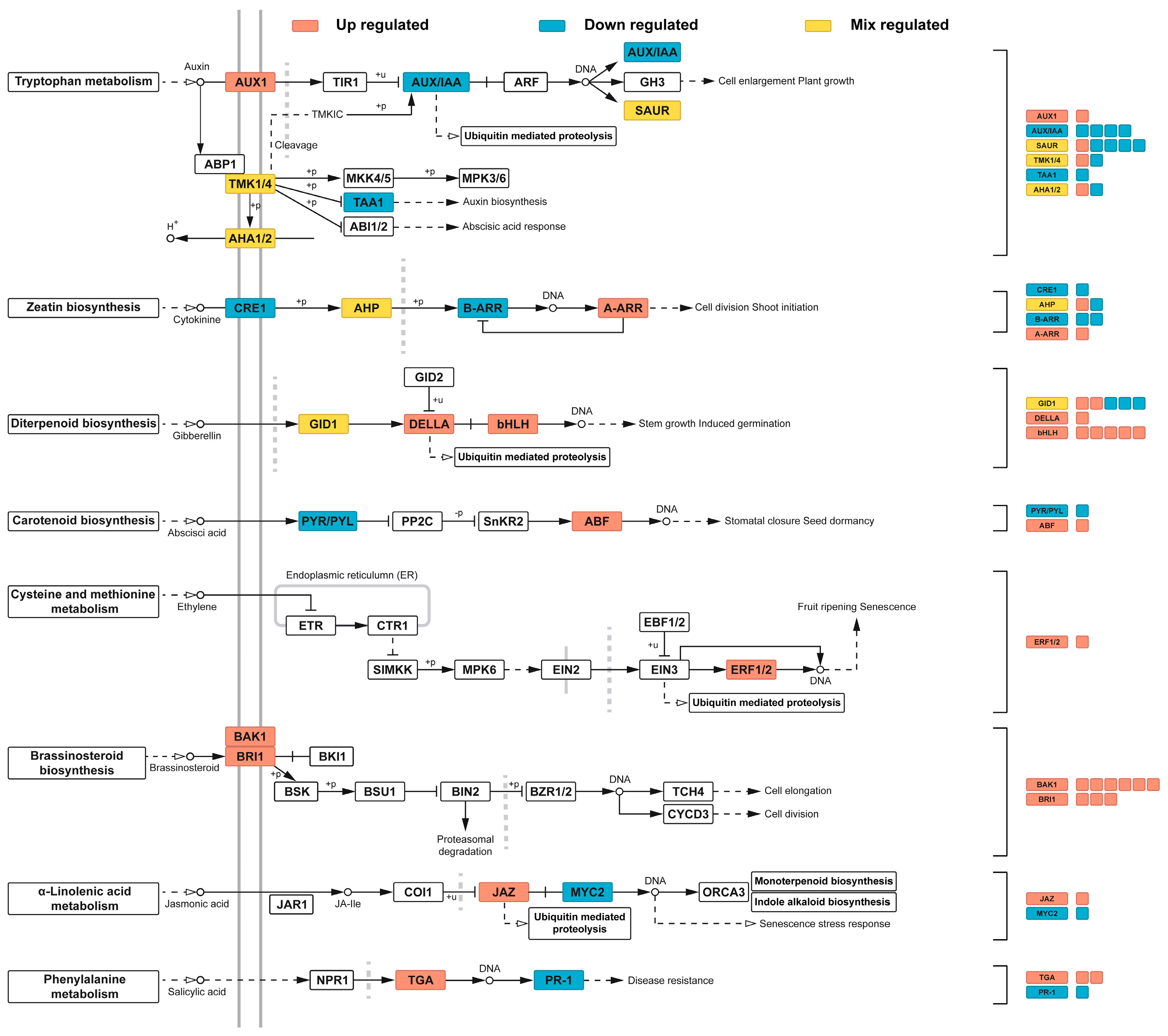
3.7. Expression Profiles of Hormone Signaling Pathway Genes
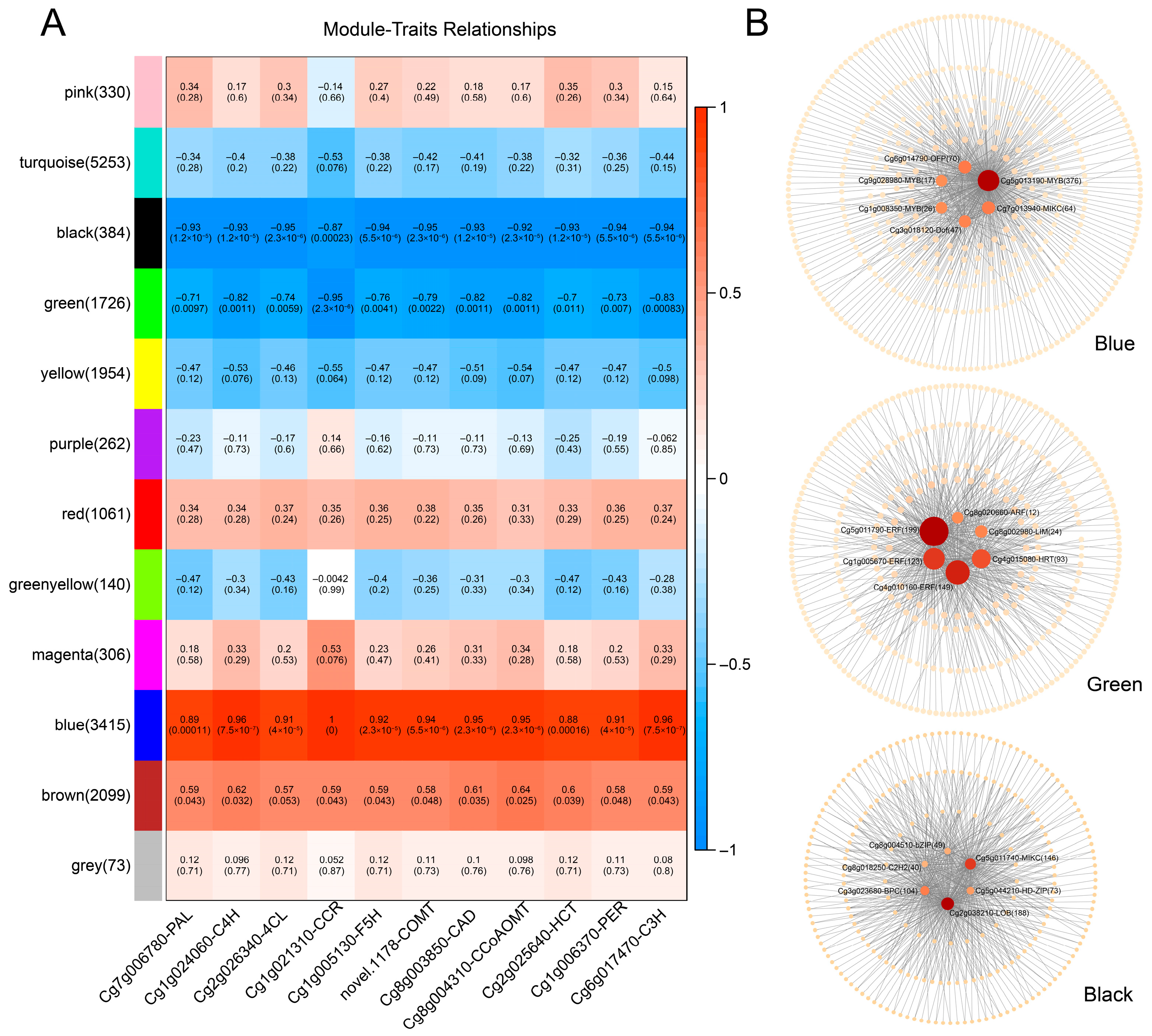
3.8. WGCNA and Key Transcription Factor Screening
3.9. RT-qPCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Opazo, I.; Toro, G.; Salvatierra, A.; Pastenes, C.; Pimentel, P. Rootstocks modulate the physiology and growth responses to water deficit and long-term recovery in grafted stone fruit trees. Agric. Water Manag. 2020, 228, 105897. [Google Scholar] [CrossRef]
- Loupit, G.; Brocard, L.; Ollat, N.; Cookson, S.J. Grafting in plants: Recent discoveries and new applications. J. Exp. Bot. 2023, 74, 2433–2447. [Google Scholar] [CrossRef] [PubMed]
- Chilukamarri, L.; Ashrafzadeh, S.; Leung, D.W.M. In-vitro grafting—Current applications and future prospects. Sci. Hortic. 2021, 280, 109899. [Google Scholar] [CrossRef]
- Gaion, L.A.; Braz, L.; Carvalho, R. Grafting in Vegetable Crops: A Great Technique for Agriculture. Int. J. Veg. Sci. 2017, 24, 85–102. [Google Scholar] [CrossRef]
- Maciá-Vázquez, A.A.; Martínez-Nicolás, J.J.; Núñez-Gómez, D.; Melgarejo, P.; Legua, P. Influence of rootstock on yield, morphological, biochemical and sensory characteristics of ‘Afourer’ variety mandarins. Sci. Hortic. 2024, 325, 112644. [Google Scholar] [CrossRef]
- DuVal, A.E.; Tempeleu, A.; Schmidt, J.E.; Puig, A.; Knollenberg, B.J.; Chaparro, J.X.; Stevens, M.E.; Motamayor, J.C. Evidence of Graft Incompatibility and Rootstock Scion Interactions in Cacao. Horticulturae 2025, 11, 899. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef]
- Huang, C.; Hou, J.; Huang, M.; Hu, M.; Deng, L.; Zeng, K.; Yao, S. A comprehensive review of segment drying (vesicle granulation and collapse) in citrus fruit: Current state and future directions. Sci. Hortic. 2023, 309, 111683. [Google Scholar] [CrossRef]
- Yao, S.; Li, Q.; Cao, Q.; Deng, L.; Zeng, K. Granulation of citrus fruit: Research progress and future directions. Food Ferment. Ind. 2020, 46, 259–263. [Google Scholar]
- Ritenour, M.; Albrigo, G.; Burns, J.K.; Miller, W.M. Granulation in Florida citrus. In Proceedings of the Florida State Horticultural Society, Orlando, FL, USA, 6–8 June 2004; Volume 117, pp. 358–361. [Google Scholar]
- Wang, X.-Y.; Wang, P.; Qi, Y.-P.; Zhou, C.-P.; Yang, L.-T.; Liao, X.-Y.; Wang, L.-Q.; Zhu, D.-H.; Chen, L.-S. Effects of granulation on organic acid metabolism and its relation to mineral elements in Citrus grandis juice sacs. Food Chem. 2014, 145, 984–990. [Google Scholar] [CrossRef]
- Sharma, R.R.; Saxena, S.K. Rootstocks influence granulation in Kinnow mandarin (Citrusnobilis × C.deliciosa). Sci. Hortic. 2004, 101, 235–242. [Google Scholar] [CrossRef]
- Yao, S.; Cao, Q.; Xie, J.; Deng, L.; Zeng, K. Alteration of sugar and organic acid metabolism in postharvest granulation of Ponkan fruit revealed by transcriptome profiling. Postharvest Biol. Technol. 2018, 139, 2–11. [Google Scholar] [CrossRef]
- Yao, S.; Wang, Z.; Cao, Q.; Xie, J.; Wang, X.; Zhang, R.; Deng, L.; Ming, J.; Zeng, K. Molecular basis of postharvest granulation in orange fruit revealed by metabolite, transcriptome and methylome profiling. Postharvest Biol. Technol. 2020, 166, 111205. [Google Scholar] [CrossRef]
- Wu, J.-L.; Pan, T.-F.; Guo, Z.-X.; Pan, D.-M. Specific Lignin Accumulation in Granulated Juice Sacs of Citrus maxima. J. Agric. Food Chem. 2014, 62, 12082–12089. [Google Scholar] [CrossRef]
- Raes, J.; Rohde, A.; Christensen, J.H.; Van de Peer, Y.; Boerjan, W. Genome-Wide Characterization of the Lignification Toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.J.; Su, G.; Zhang, M.; Grierson, D.; Chen, K.S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q. Lignification: Flexibility, Biosynthesis and Regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, F.; Chen, Y.; Hua, M.; Han, J.; Li, X. Introduction Performance and Cultivation Key Points of Malaysian Honey Dew Apples. Trop. Agric. Sci. 2006, 26, 28–29. (In Chinese) [Google Scholar]
- Yang, C.; Wang, X.; Zhu, W.; Weng, Z.; Li, F.; Wu, H.; Zhou, K.; Strid, Å.; Qian, M. Postharvest white light combined with different UV-B doses differently promotes anthocyanin accumulation and antioxidant capacity in mango peel. LWT 2024, 203, 116385. [Google Scholar] [CrossRef]
- Qian, M.; Sun, Y.; Allan, A.C.; Teng, Y.; Zhang, D. The red sport of ‘Zaosu’ pear and its red-striped pigmentation pattern are associated with demethylation of the PyMYB10 promoter. Phytochemistry 2014, 107, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S.J.G.B. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S.J.B.B. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Zhang, H.; Gao, Q.; Song, F.; Cui, X.; Mo, F. Genome-Wide Identification of APX Gene Family in Citrus maxima and Expression Analysis at Different Postharvest Preservation Times. Genes 2024, 15, 911. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Wu, H.; Zhu, W.; Zheng, B.; Wang, S.; Zhou, K.; Qian, M. Genome-Wide Identification and Expression Analysis of WRKY Genes during Anthocyanin Biosynthesis in the Mango (Mangifera indica L.). Agriculture 2022, 12, 821. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Tomić, J.; Glišić, I.; Milošević, N.; Štampar, F.; Mikulič-Petkovšek, M.; Jakopič, J. Determination of fruit chemical contents of two plum cultivars grafted on four rootstocks. J. Food Compos. Anal. 2022, 105, 103944. [Google Scholar] [CrossRef]
- Valverdi, N.; Kalcsits, L. Rootstock Affects Scion Nutrition and Fruit Quality during Establishment and Early Production of ‘Honeycrisp’ Apple. HortScience 2021, 56, 261–269. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, P.-P.; Shen, M.; Zhang, Y.; He, F.; Yang, L.; Gao, X.; Hu, Y.; Xiao, J.-X. Transcriptome and metabolome analyses reveal improvement in blueberry fruit quality by interspecific grafting. Trees 2024, 38, 65–78. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhang, T.; Liu, J.; Sun, X.; Sun, X.; Wang, W.; Zheng, C. Interactions between rootstock and scion during grafting and their molecular regulation mechanism. Sci. Hortic. 2023, 308, 111554. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, P.; Wu, S.; Yang, Q.; He, F.; Gao, X.; Zhang, Y.; Xiao, J. A Better Fruit Quality of Grafted Blueberry Than Own-Rooted Blueberry Is Linked to Its Anatomy. Plants 2024, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, F.; Sun, Y.; Liu, X.; Jin, L. Physiological and Molecular Insights into Citrus Rootstock–Scion Interactions: Compatibility, Signaling, and Impact on Growth, Fruit Quality and Stress Responses. Horticulturae 2025, 11, 1110. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, F.; Yu, H.; Zhang, M.; Jiang, D.; Huang, T.; Xiang, J.; Zhu, S.; Zhao, X. Effects of scion-rootstock interaction on citrus fruit quality related to differentially expressed small RNAs. Sci. Hortic. 2022, 298, 110974. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Rodriguez-Gamir, J.; Martinez-Alcantara, B.; Quiñones, A.; Iglesias, D.J.; Primo-Millo, E.; Forner, J. Performance of Navel orange trees grafted onto two new dwarfing rootstocks (Forner-Alcaide 517 and Forner-Alcaide 418). Sci. Hortic. 2014, 179, 376–387. [Google Scholar] [CrossRef]
- Sau, S.; Ghosh, S.N.; Sarkar, S.; Gantait, S. Effect of rootstocks on growth, yield, quality, and leaf mineral composition of Nagpur mandarin (Citrus reticulata Blanco.), grown in red lateritic soil of West Bengal, India. Sci. Hortic. 2018, 237, 142–147. [Google Scholar] [CrossRef]
- Morales Alfaro, J.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of Rootstock on Citrus Fruit Quality: A Review. Food Rev. Int. 2023, 39, 2835–2853. [Google Scholar] [CrossRef]
- Sharma, R.R.; Awasthi, O.P.; Kumar, K. Pattern of phenolic content, antioxidant activity and senescence-related enzymes in granulated vs non-granulated juice-sacs of ‘Kinnow’ mandarin (Citrus nobilis × C. deliciosa). J. Food Sci. Technol. 2016, 53, 1525–1530. [Google Scholar] [CrossRef]
- Shi, M.; Liu, X.; Zhang, H.; He, Z.; Yang, H.; Chen, J.; Feng, J.; Yang, W.; Jiang, Y.; Yao, J.-L.; et al. The IAA- and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic. Res. 2020, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wu, J.; Xu, Y.; Lu, L.; Yi, H. High-spatiotemporal-resolution transcriptomes provide insights into fruit development and ripening in Citrus sinensis. Plant Biotechnol. J. 2021, 19, 1337–1353. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, R.; Li, X.; Tian, S.; Li, B.; Qin, G. N6-methyladenosine RNA modification regulates strawberry fruit ripening in an ABA-dependent manner. Genome Biol. 2021, 22, 168. [Google Scholar] [CrossRef]
- Hou, J.; Yan, D.; Huang, M.; Zeng, K.; Yao, S. Alteration of pectin metabolism in blood orange fruit (Citrus sinensis cv. Tarocco) in response to vesicle collapse. Food Qual. Saf. 2022, 6, fyac050. [Google Scholar] [CrossRef]
- Wu, H.; He, Q.; He, B.; He, S.; Zeng, L.; Yang, L.; Zhang, H.; Wei, Z.; Hu, X.; Hu, J.; et al. Gibberellin signaling regulates lignin biosynthesis to modulate rice seed shattering. Plant Cell 2023, 35, 4383–4404. [Google Scholar] [CrossRef]
- Jie, H.; Zhao, L.; Ma, Y.; Rasheed, A.; Jie, Y. Integrated Transcriptome and Metabolome Analysis Reveal That Exogenous Gibberellin Application Regulates Lignin Synthesis in Ramie. Agronomy 2023, 13, 1450. [Google Scholar] [CrossRef]
- Ragni, L.; Nieminen, K.; Pacheco-Villalobos, D.; Sibout, R.; Schwechheimer, C.; Hardtke, C.S. Mobile Gibberellin Directly Stimulates Arabidopsis Hypocotyl Xylem Expansion. Plant Cell 2011, 23, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Mäkilä, R.; Wybouw, B.; Smetana, O.; Vainio, L.; Solé-Gil, A.; Lyu, M.; Ye, L.; Wang, X.; Siligato, R.; Jenness, M.K.; et al. Gibberellins promote polar auxin transport to regulate stem cell fate decisions in cambium. Nat. Plants 2023, 9, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Liu, J.; Zhao, J.; Liu, J.; Ren, J.; Li, L.; Li, X.; Yang, D. Uncovering mechanisms governing stem growth in peanut (Arachis hypogaea L.) with varying plant heights through integrated transcriptome and metabolomics analyses. J. Plant Physiol. 2023, 287, 154052. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Liu, J.; Sun, Y.; Tan, P.; Cao, H.; Xie, Y.; Wen, B.; Gu, T.; Liu, J.; Li, M.; et al. Citrus sinensis MYB transcription factors CsMYB330 and CsMYB308 regulate fruit juice sac lignification through fine-tuning expression of the Cs4CL1 gene. Plant Sci. Int. J. Exp. Plant Biol. 2018, 277, 334–343. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.; Tan, P.; Sun, Y.; Lv, Y.; Liu, J.; Sun, J.; Huang, Y.; Lu, J.; Jin, N.; et al. Citrus sinensis MYB Transcription Factor CsMYB85 Induce Fruit Juice Sac Lignification Through Interaction With Other CsMYB Transcription Factors. Front. Plant Sci. 2019, 10, 213. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; She, W.; Guo, Z.; Pan, H.; Yu, Y.; Ye, J.; Pan, D.; Pan, T. Identification and Functional Analysis of the CgNAC043 Gene Involved in Lignin Synthesis from Citrusgrandis “San Hong”. Plants 2022, 11, 403. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Yang, C.; Li, H.; Jiang, C. Transcriptomic Analysis Reveals the Impact of Interstock on Vesicle Granulation in ‘Hainan Qingyou’ Pomelo (Citrus maxima) Fruit. Horticulturae 2025, 11, 1230. https://doi.org/10.3390/horticulturae11101230
Yang C, Yang C, Li H, Jiang C. Transcriptomic Analysis Reveals the Impact of Interstock on Vesicle Granulation in ‘Hainan Qingyou’ Pomelo (Citrus maxima) Fruit. Horticulturae. 2025; 11(10):1230. https://doi.org/10.3390/horticulturae11101230
Chicago/Turabian StyleYang, Chengchao, Chengkun Yang, Haibo Li, and Chengdong Jiang. 2025. "Transcriptomic Analysis Reveals the Impact of Interstock on Vesicle Granulation in ‘Hainan Qingyou’ Pomelo (Citrus maxima) Fruit" Horticulturae 11, no. 10: 1230. https://doi.org/10.3390/horticulturae11101230
APA StyleYang, C., Yang, C., Li, H., & Jiang, C. (2025). Transcriptomic Analysis Reveals the Impact of Interstock on Vesicle Granulation in ‘Hainan Qingyou’ Pomelo (Citrus maxima) Fruit. Horticulturae, 11(10), 1230. https://doi.org/10.3390/horticulturae11101230






