Drought Tolerance Mechanisms in Grain and Vegetable Amaranthus Species: Physiological, Biochemical and Molecular Insights
Abstract
1. Introduction
2. Amaranth’s Nutritional Value
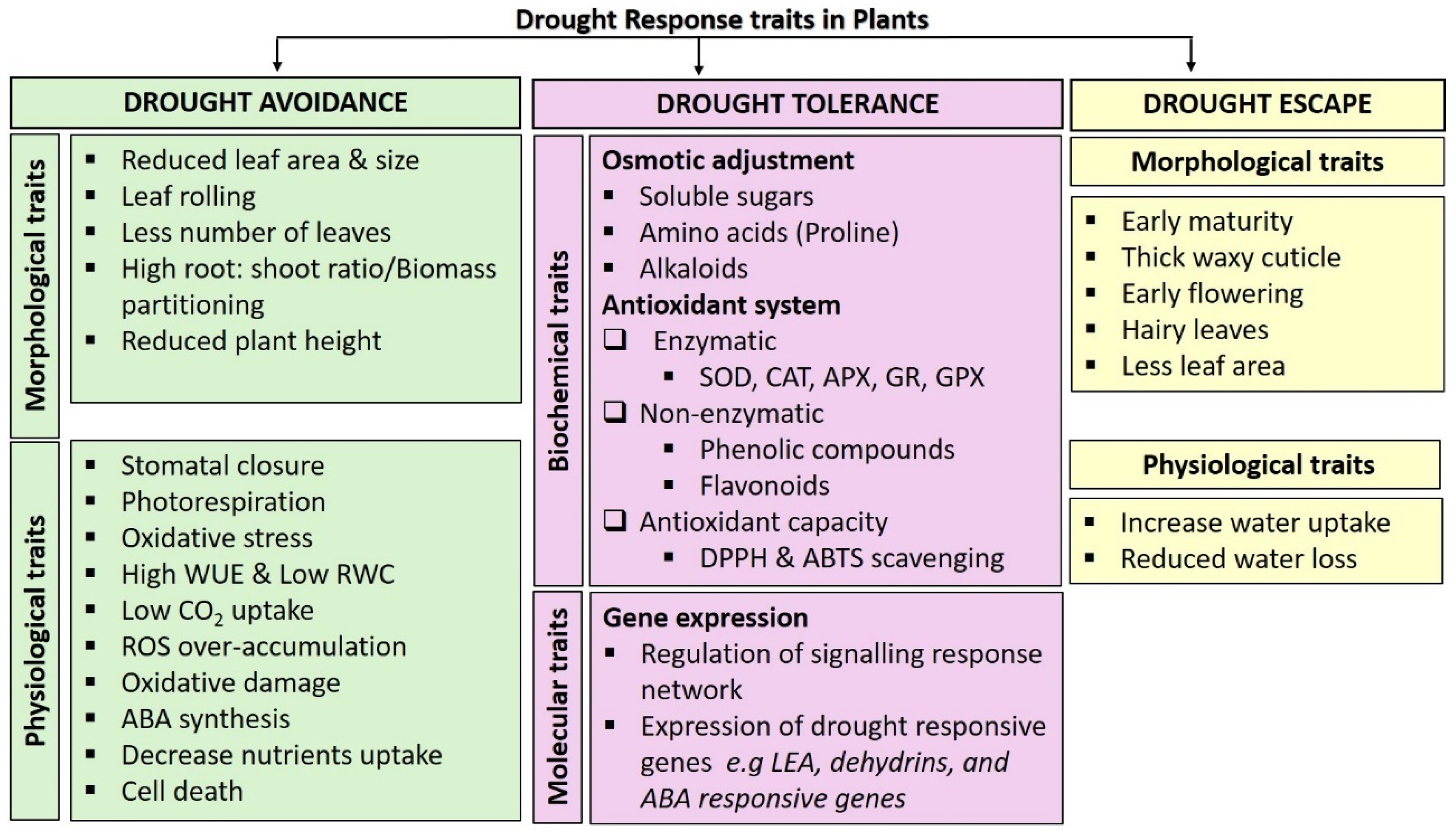
3. The Response of Amaranthus Species to Drought Stress
3.1. Grain Amaranthus Species and Their Response to Drought Stress
3.1.1. Physiological Traits
3.1.2. Biochemical Traits
3.1.3. Molecular Traits
3.2. Leafy Vegetable Amaranth Species and Their Responses to Drought Stress
3.2.1. Morphological Traits
3.2.2. Physiological Traits
3.2.3. Biochemical Traits
3.2.4. Molecular Traits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aryal, J.P.; Manchanda, N.; Sonobe, T. Expectations for household food security in the coming decades: A global scenario. In Future Foods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 107–131. [Google Scholar]
- Grote, U. Can we improve global food security? A socio-economic and political perspective. Food Secur. 2014, 6, 187–200. [Google Scholar] [CrossRef]
- Masipa, T. The impact of climate change on food security in South Africa: Current realities and challenges ahead. Jàmbá J. Disaster Risk Stud. 2017, 9, 411. [Google Scholar] [CrossRef]
- Van der Merwe, J.D.; Cloete, P.C.; Van der Hoeven, M. Promoting food security through indigenous and traditional food crops. Agroecol. Sustain. Food Syst. 2016, 40, 830–847. [Google Scholar] [CrossRef]
- Cechin, I.; da Silva, L.P.; Ferreira, E.T.; Barrochelo, S.C.; de Melo, F.P.d.S.R.; Dokkedal, A.L.; Saldanha, L.L. Physiological responses of Amaranthus cruentus L. to drought stress under sufficient-and deficient-nitrogen conditions. PLoS ONE 2022, 17, e0270849. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. Water Stress Crop Plants Sustain. Approach 2016, 1, 24–40. [Google Scholar]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wędzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Shahid, H.; Ahmad, Z.; Zulfiqar, U.; Mahmood, N.; Al-Ashkar, I.; Ditta, A.; El Sabagh, A. Exogenously Applied Potassium Enhanced Morpho-Physiological Growth and Drought Tolerance of Wheat by Alleviating Osmotic Imbalance and Oxidative Damage. Pol. J. Environ. Stud. 2023, 32, 4447–4459. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.H.; Jenks, M.A. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J. Plant Physiol. 2007, 164, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, T.; Cisneros-Hernández, I.; Acosta Bayona, J.; Ramírez-Chavez, E.; Martínez-Gallardo, N.; Mellado-Mojica, E.; López-Pérez, M.G.; Molina-Torres, J.; Délano-Frier, J. Identification of factors linked to higher water-deficit stress tolerance in Amaranthus hypochondriacus compared to other grain amaranths and A. hybridus, their shared ancestor. Plants 2019, 8, 239. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Lisar, S.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I. Causes, effects and responses. Water Stress 2012, 25, 33. [Google Scholar]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic adjustment and plant adaptation to drought stress. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Springer: Cham, Switezerland, 2016; pp. 105–143. [Google Scholar]
- Pawlak, K.; Kołodziejczak, M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Ebert, A.W. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef]
- Ozeki, K.; Miyazawa, Y.; Sugiura, D. Rapid stomatal closure contributes to higher water use efficiency in major C4 compared to C3 Poaceae crops. Plant Physiol. 2022, 189, 188–203. [Google Scholar] [CrossRef]
- Sage, R. C3 versus C4 photosynthesis in rice: Ecophysiological perspectives. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 13–35. [Google Scholar]
- Achigan-Dako, E.G.; Sogbohossou, O.E.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Kunyanga, C.N. Development of a Supplementary Food from Selected Local Food Ingredients to Optimize Nutritional and Sensory Qualities. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2011. [Google Scholar]
- Phiarais, B.P.N.; Arendt, E.K. Malting and brewing with gluten-free cereals. In Gluten-Free Cereal Products and Beverages; Elsevier: Amsterdam, The Netherlands, 2008; pp. 347–372. [Google Scholar]
- Ruth, O.N.; Unathi, K.; Nomali, N.; Chinsamy, M. Underutilization versus nutritional-nutraceutical potential of the Amaranthus food plant: A mini-review. Appl. Sci. 2021, 11, 6879. [Google Scholar] [CrossRef]
- Grubben, G.; Denton, O.A. Plant Resources of Tropical Africa 2. Vegetables. 2004. Available online: https://portals.iucn.org/library/node/28236 (accessed on 30 May 2024).
- Assad, R.; Reshi, Z.A.; Jan, S.; Rashid, I. Biology of amaranths. Bot. Rev. 2017, 83, 382–436. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Van Der Kooy, F.; Oladimeji, O.; Gouws, C. The importance and use of Amaranthus for crop diversification in the SADC region. S. Afr. J. Bot. 2023, 152, 192–202. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N. Grains–a major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Písaříková, B.; Kráčmar, S.; Herzig, I. Amino acid contents and biological value of protein in various amaranth species. Czech J. Anim. Sci. 2005, 50, 169–174. [Google Scholar] [CrossRef]
- Nyonje, W.A. Nutrients, Anti-Nutrients and Phytochemical Evaluation of Ten Vegetable Amaranth (Amaranthus spp.) Varieties at Two Stages of Growth. 2015. Available online: http://ir.jkuat.ac.ke/handle/123456789/1591 (accessed on 15 March 2025).
- Muriuki, E.N. Nutritional Diversity of Leafy Amaranth (Amaranthus) Species Grown in Kenya. 2015. Available online: http://ir.jkuat.ac.ke/handle/123456789/1592 (accessed on 15 March 2025).
- Rangarajan, A.; Kelly, J.F. 218A further studies exploring iron nutritional quality from amaranthus species. HortScience 1994, 29, 459–467. [Google Scholar] [CrossRef]
- Vidal Torres, E.; Valencia, E.; Simsek, S.; Ramírez, A.M.L. Amaranth: Multipurpose agroindustrial crop. Agronomy 2024, 14, 2323. [Google Scholar] [CrossRef]
- de la Rosa, A.B.; Fomsgaard, I.S.; Laursen, B.; Mortensen, A.G.; Olvera-Martínez, L.; Silva-Sánchez, C.; Mendoza-Herrera, A.; González-Castañeda, J.; De León-Rodríguez, A. Amaranth (Amaranthus hypochondriacus) as an alternative crop for sustainable food production: Phenolic acids and flavonoids with potential impact on its nutraceutical quality. J. Cereal Sci. 2009, 49, 117–121. [Google Scholar] [CrossRef]
- Tetyannikov, N.V.; Motyleva, S.M.; Gins, M.S.; Kozak, N.V.; Panischeva, D.V.; Mertvischeva, M.E.; Kabashnikova, L.F.; Domanskaya, I.N.; Pilipovich, T.S. Drought Effects on Mineral Composition of the Leaves and Seeds of Amaranthus tricolor and Amaranthus cruentus. SABRAO J. Breed. Genet. 2022, 54, 426–436. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Jimoh, M.; Afolayan, A.; Lewu, F. Therapeutic Uses of Amaranthus caudatus L. Trop. Biomed. 2019, 36, 1038–1053. [Google Scholar]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Aderibigbe, O.; Ezekiel, O.; Owolade, S.; Korese, J.; Sturm, B.; Hensel, O. Exploring the potentials of underutilized grain amaranth (Amaranthus spp.) along the value chain for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 656–669. [Google Scholar] [CrossRef]
- Mekonnen, G.; Woldesenbet, M.; Teshale, T.; Biru, T. Amaranthus caudatus production and nutrition contents for food security and healthy living in Menit Shasha, Menit Goldya and Maji Districts of Bench Maji Zone, South Western Ethiopia. Nutr. Food Sci. Int. J. 2018, 7, 23–30. [Google Scholar]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Pandey, A.C.; Mishra, B.K. Diversity in phenotypic and nutritional traits in vegetable amaranth (Amaranthus tricolor), a nutritionally underutilised crop. J. Sci. Food Agric. 2010, 90, 139–144. [Google Scholar] [CrossRef]
- Das, S.; Das, S. Amaranths: The crop of great prospect. In Amaranthus: A Promising Crop of Future; Springer: Dordrecht, The Netherlands, 2016; pp. 13–48. [Google Scholar]
- Jamalluddin, N.; Massawe, F.J.; Mayes, S.; Ho, W.K.; Singh, A.; Symonds, R.C. Physiological Screening for Drought Tolerance Traits in Vegetable Amaranth (Amaranthus tricolor) Germplasm. Agriculture 2021, 11, 994. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Amaranth production and consumption in South Africa: The challenges of sustainability for food and nutrition security. Int. J. Agric. Sustain. 2022, 20, 449–460. [Google Scholar] [CrossRef]
- Van Rensburg, W.J.; Van Averbeke, W.; Slabbert, R.; Faber, M.; Van Jaarsveld, P.; Van Heerden, I.; Wenhold, F.; Oelofse, A. African leafy vegetables in South Africa. Water SA 2007, 33, 317–326. [Google Scholar] [CrossRef]
- Liu, F.; Stützel, H. Leaf water relations of vegetable amaranth (Amaranthus spp.) in response to soil drying. Eur. J. Agron. 2002, 16, 137–150. [Google Scholar] [CrossRef]
- Bang, J.-H.; Lee, K.J.; Jeong, W.T.; Han, S.; Jo, I.-H.; Choi, S.H.; Cho, H.; Hyun, T.K.; Sung, J.; Lee, J. Antioxidant activity and phytochemical content of nine Amaranthus species. Agronomy 2021, 11, 1032. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Gamboa, A.; Valenzuela, E.; Murillo, E. Biochemical changes due to water loss in leaves of Amaranthus hypochondriacus L. J. Plant Physiol. 1991, 137, 586–590. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.A.; Briones-Cerecero, E.P.; Mendoza-Hernandez, G.; De Leon-Rodriguez, A.; Barba de la Rosa, A.P. Proteomic analysis of amaranth (Amaranthus hypochondriacus L.) leaves under drought stress. Int. J. Plant Sci. 2009, 170, 990–998. [Google Scholar] [CrossRef]
- Gimplinger, D.; Dobos, G.; Kaul, H.-P. Optimum crop densities for potential yield and harvestable yield of grain amaranth are conflicting. Eur. J. Agron. 2008, 28, 119–125. [Google Scholar] [CrossRef]
- Pulvento, C.; Sellami, M.H.; Lavini, A. Yield and quality of Amaranthus hypochondriacus grain amaranth under drought and salinity at various phenological stages in southern Italy. J. Sci. Food Agric. 2022, 102, 5022–5033. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; d’Andria, R.; Ragab, R. Parameterization and field validation of SALTMED Model for grain amaranth tested in South Italy. Irrig. Drain. 2015, 64, 59–68. [Google Scholar] [CrossRef]
- Valdayskikh, V.; Voronin, P.Y.; Artemyeva, E.; Rymar, V. Amaranth responses to experimental soil drought. AIP Conf. Proc. 2019, 2063, 030023. [Google Scholar] [CrossRef]
- Motyleva, S.; Gins, M.; Kabashnikova, L.; Kozak, N.; Tetyannikov, N.; Panischeva, D.; Mertvischeva, M.; Domanskaya, I.; Pilipovich, T. Drought effects on the physiological and biochemical parameters of amaranth (C-3) and actinidia (C-4) plants. SABRAO J. Breed. Genet. 2021, 53, 248–262. [Google Scholar]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.J.; Manzanares, M.a.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef]
- Farshbaf-Jafari, S.; Pirzad, A.; Tajbakhsh, M.; Ghassemi-Golezani, K. Effects of water supply and plant density on leaf characteristics of Amaranth (Amaranthus caudatus L.). In Proceedings of the 2nd International Conference on Sustainable Environment and Agriculture IPCBEE, San Diego, CA, USA, 29–30 October 2014. [Google Scholar]
- Huerta-Ocampo, J.; León-Galván, M.; Ortega-Cruz, L.; Barrera-Pacheco, A.; De León-Rodríguez, A.; Mendoza-Hernández, G.; Barba De La Rosa, A. Water stress induces up-regulation of DOF1 and MIF1 transcription factors and down-regulation of proteins involved in secondary metabolism in amaranth roots (Amaranthus hypochondriacus L.). Plant Biol. 2011, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, X.; Jin, Z.; Tian, Y.; Song, H. Free radical and reactive oxygen species scavenging activities of peanut skins extract. Food Chem. 2007, 104, 242–250. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus. Can. J. Plant Sci. 2013, 93, 47–58. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Genotypic diversity in vegetable amaranth for antioxidant, nutrient and agronomic traits. Indian J. Genet. Plant Breed. 2017, 77, 173–176. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jena, P.K.; Tripathy, P.K. Study of wild edible plants among tribal groups of Simlipal Biosphere Reserve Forest, Odisha, India; with special reference to Dioscorea species. Int. J. Biol. Technol. 2012, 3, 11–19. [Google Scholar]
- Netshimbupfe, M.H.; Berner, J.; Gouws, C. The interactive effects of drought and heat stress on photosynthetic efficiency and biochemical defense mechanisms of Amaranthus species. Plant Environ. Interact. 2022, 3, 212–225. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant activity and phenolic composition of amaranth (Amaranthus caudatus) during plant growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef]
- López-Mejía, O.A.; López-Malo, A.; Palou, E. Antioxidant capacity of extracts from amaranth (Amaranthus hypochondriacus L.) seeds or leaves. Ind. Crops Prod. 2014, 53, 55–59. [Google Scholar] [CrossRef]
- Kersey, P.J.; Collemare, J.; Cockel, C.; Das, D.; Dulloo, E.M.; Kelly, L.J.; Lettice, E.; Malécot, V.; Maxted, N.; Metheringham, C. Selecting for useful properties of plants and fungi–Novel approaches, opportunities, and challenges. Plants People Planet. 2020, 2, 409–420. [Google Scholar] [CrossRef]
- Sunil, M.; Hariharan, A.K.; Nayak, S.; Gupta, S.; Nambisan, S.R.; Gupta, R.P.; Panda, B.; Choudhary, B.; Srinivasan, S. The draft genome and transcriptome of Amaranthus hypochondriacus: A C4 dicot producing high-lysine edible pseudo-cereal. DNA Res. 2014, 21, 585–602. [Google Scholar] [CrossRef]
- Ma, X.; Vaistij, F.E.; Li, Y.; Jansen van Rensburg, W.S.; Harvey, S.; Bairu, M.W.; Venter, S.L.; Mavengahama, S.; Ning, Z.; Graham, I.A. A chromosome-level Amaranthus cruentus genome assembly highlights gene family evolution and biosynthetic gene clusters that may underpin the nutritional value of this traditional crop. Plant J. 2021, 107, 613–628. [Google Scholar] [CrossRef]
- Clerico, E.M.; Meng, W.; Pozhidaeva, A.; Bhasne, K.; Petridis, C.; Gierasch, L.M. Hsp70 molecular chaperones: Multifunctional allosteric holding and unfolding machines. Biochem. J. 2019, 476, 1653–1677. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef] [PubMed]
- Casique-Arroyo, G.; Martinez-Gallardo, N.; Gonzalez de la Vara, L.; Delano-Frier, J.P. Betacyanin biosynthetic genes and enzymes are differentially induced by (a) biotic stress in Amaranthus hypochondriacus. PLoS ONE 2014, 9, e99012. [Google Scholar] [CrossRef]
- Palmeros-Suarez, P.A.; Massange-Sanchez, J.A.; Martinez-Gallardo, N.A.; Montero-Vargas, J.M.; Gomez-Leyva, J.F.; Delano-Frier, J.P. The overexpression of an Amaranthus hypochondriacus NF-YC gene modifies growth and confers water deficit stress resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Srivastava, A.; Singh, S. Genotypic variability in vegetable amaranth (Amaranthus tricolor L for foliage yield and its contributing traits over successive cuttings and years. Euphytica 2006, 151, 103–110. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017, 58, 33–39. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Van Staden, J. Aqueous smoke and karrikin influence seed germination of Amaranthus dubius in varying light, temperature and osmotic stress conditions. S. Afr. J. Bot. 2022, 148, 704–709. [Google Scholar] [CrossRef]
- Ribeiro, J.E.M.M.; Pieterse, P.J.; Famba, S.I. Vegetative growth of Amaranthus hybridus and Amaranthus tricolor under different watering regimes in different seasons in southern Mozambique. S. Afr. J. Plant Soil. 2017, 34, 201–210. [Google Scholar] [CrossRef]
- Amma, C.S.; Rajalakshmi, R. Morphological and Physiological Responses in Red Amaranthus (Amaranthus tricolor L.) and Green Amaranthus (Amaranthus dubius Mart. ex Thell.) Under Progressive Water Stress. Asian J. Biol. Life Sci. 2023, 12, 61. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Manyatsi, A.M.; Masarirambi, M.T.; Hachigonta, S. Southern African Agriculture and Climate Change: A Comprehensive Analysis-Swaziland. 2012. Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/93f8681a-a04b-4387-a2fe-2b1b39c45355/content (accessed on 8 June 2024).
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Bai, X.; Ye, X.; Luo, Y.; Liu, C.; Wu, Q. Characterization of the first complete chloroplast genome of Amaranthus hybridus (Caryophyllales: Amaranthaceae) with phylogenetic implications. Mitochondrial DNA B Resour. 2021, 6, 3306–3308. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ortiz, E.; Ramírez-Tobias, H.M.; González-Escobar, J.L.; Gutierrez-Garcia, A.K.; Bojorquez-Velazquez, E.; Espitia-Rangel, E.; de la Rosa, A.P.B. Biomass, chlorophyll fluorescence, and osmoregulation traits let differentiation of wild and cultivated Amaranthus under water stress. J. Photochem. Photobiol. B Biol. 2021, 220, 112210. [Google Scholar] [CrossRef]
- Ferrarotto, S. Proline accumulation in pigweed plants (Amaranthus dubius Mart, and Amaranthus cruentus L.) growing under water stress conditions. Rev. Fac. Agron. 2003, 20, 453–460. [Google Scholar]
- Asadollahzadeh, M.; Rezvani, M.; Zaefarian, F.; Mobasser, H. Physiological Modifications and Enzymatic Defense System of Amaranthus blitoides S. Watson and Amaranthus hybridus L. in Response to Water Deficit Stress. Russ. J. Plant Physiol. 2023, 70, 106. [Google Scholar] [CrossRef]
- Chatti, D. Physiological and Molecular Analyses of Drought Tolerance Responses in Amaranthus (Amaranthus tricolor L.) under Elevated Carbon Dioxide Environments. Chem. Sci. Rev. Lett. 2017, 6, 2025–2031. [Google Scholar]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yan, J.; Li, H.R.; Feng, Y.Q.; Qi, Z.C.; Yan, X.L. The complete chloroplast genome sequence of spleen amaranth (Amaranthus dubius Mart. ex Thell., Amaranthaceae). Mitochondrial DNA B Resour. 2021, 6, 3267–3268. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, E.; Odeny, D.A.; Coetzee, M.P.; Berger, D.K.; Rees, D.J. Application of chloroplast phylogenomics to resolve species relationships within the plant genus Amaranthus. J. Mol. Evol. 2018, 86, 216–239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Tran, D.S.; Tran, T.T.H.; Ohsawa, R.; Yoshioka, Y. Genetic diversity of leafy amaranth (Amaranthus tricolor L.) resources in Vietnam. Breed. Sci. 2019, 69, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-R.; Hong, G.-Y.; Dixit, A.; Chung, J.-W.; Ma, K.-H.; Lee, J.-H.; Kang, H.-K.; Cho, Y.-H.; Gwag, J.-G.; Park, Y.-J. Characterization of microsatellite loci developed for Amaranthus hypochondriacus and their cross-amplifications in wild species. Conserv. Genet. 2008, 9, 243–246. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Lin, Y.-P.; Schafleitner, R.; Shirasawa, K.; Isobe, S.; Nguyen, D.C.; Ohsawa, R.; Yoshioka, Y. Genetic diversity analysis and core collection construction for Amaranthus tricolor germplasm based on genome-wide single-nucleotide polymorphisms. Sci. Hortic. 2023, 307, 111428. [Google Scholar] [CrossRef]


| Species | Morphological and Physiological | Biochemical | Molecular |
|---|---|---|---|
| Grain amaranth | |||
| A. hypochondriacus | Decrease in total performance index, RWC and EL [49,50,66] | Accumulation of osmolytes: 1. Proline. 2. Total soluble sugars, soluble non-structural carbohydrates and raffinose-family oligosaccharides [12,66,69]. 3. Increase in non-enzymatic antioxidants content (i.e., TAC, TPC and TFC) [69]. | Upregulation of stress-responsive genes: 1. Chaperonin 60 kDa (Cpn 60α and Cpn 60β) and heat shock protein 70 (Hsp70) [66]. 2. Cell growth genes. 3. ROS-scavenging proteins. 4. RNA-binding proteins [12] DODA-1, B5-GT, cDOPA5-GT and B5-GI [75]. 5. ABA signalling genes (DREB2A (transcription factor-encoding gene), ABI5 (bZIP transcription factor) and AhRAB18 (ABA-responsive gene) and stress adaptation genes [60]. 6. Upregulation of RFO biosynthesis genes; Gol Synthase 1 (GolS1), genes involved in trehalose synthesis and degradation (TPS11), drought-responsive genes; LEA14 [12]. |
| A. cruentus | Improved leaf water potential, RWC, gas exchange, WUE, and leaf nitrate levels [49]. Increase in photosynthetic pigments such as chlorophyll and carotenoid content. | Increase in proline, soluble carbohydrates, starch, and sucrose synthase activity. Induction of non-enzymatic antioxidants, DPPH scavenging capacity, and polyphenols. | Expression of RFO biosynthesis genes, Rafs synthase and Staquiose synthase genes in the leaves and in the roots. Upregulation of drought-responsive genes (GolS1, LEA14) [49]. |
| A. caudatus | Increase in stomatal conductance and carotenoids. | Increase in soluble carbohydrates and proline content. Increase in alpha-amylase activities, verbascose and starch content [49]. Induction of non-enzymatic antioxidants and total antioxidant capacity (DPPH). | Expression of RFO biosynthesis genes in both leaves and roots; upregulation of drought-responsive genes (GolS1, LEA14) and genes involved in trehalose synthesis and degradation (TPS11) [49]. |
| Vegetable amaranth | |||
| A. tricolor | Reduced internodes, leaf size, leaf area, total plant biomass, chlorophyll fluorescence and leaf gas exchange. | Increased accumulation of oxidative stress markers (MDA and H2O2), EL, proline, non-enzymatic antioxidants such as polyphenols, carotenoids, ascorbic acid, flavonoids and DPPH scavenging capacity [65,69,88]. | N/A |
| A. hybridus | Reduced plant height, leaf number, leaf area [79], RWC [64], chlorophyll fluorescence and electron transport [42,64]. | Increased accumulation of osmolytes (proline and non-structural carbohydrates (glucose, fructose and sucrose). Improved enzymatic activity, antioxidants, and amylase [81]. | Upregulation of drought-responsive genes (GolS1, LEA14) and genes involved in trehalose synthesis and degradation (TPS11) |
| A. dubius | Reduced germination, growth rate, and chlorophyll stability index. High RWC and cell membrane stability. | Increased proline content [80]. | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkuna, M.; Gavhi, P.; Kanyerere, A.M.; Ikebudu, V.C.; Ndou, N.; Faro, A.; Doumbia, I.Z.; Ajayi, R.F.; Mulidzi, A.R.; Lewu, N.; et al. Drought Tolerance Mechanisms in Grain and Vegetable Amaranthus Species: Physiological, Biochemical and Molecular Insights. Horticulturae 2025, 11, 1226. https://doi.org/10.3390/horticulturae11101226
Nkuna M, Gavhi P, Kanyerere AM, Ikebudu VC, Ndou N, Faro A, Doumbia IZ, Ajayi RF, Mulidzi AR, Lewu N, et al. Drought Tolerance Mechanisms in Grain and Vegetable Amaranthus Species: Physiological, Biochemical and Molecular Insights. Horticulturae. 2025; 11(10):1226. https://doi.org/10.3390/horticulturae11101226
Chicago/Turabian StyleNkuna, Mulisa, Pfunzo Gavhi, Alice Mwanjiwa Kanyerere, Vivian Chigozie Ikebudu, Nzumbululo Ndou, Andrew Faro, Ibrahima Zan Doumbia, Rachel Fanelwa Ajayi, Azwimbavhi Reckson Mulidzi, Nike Lewu, and et al. 2025. "Drought Tolerance Mechanisms in Grain and Vegetable Amaranthus Species: Physiological, Biochemical and Molecular Insights" Horticulturae 11, no. 10: 1226. https://doi.org/10.3390/horticulturae11101226
APA StyleNkuna, M., Gavhi, P., Kanyerere, A. M., Ikebudu, V. C., Ndou, N., Faro, A., Doumbia, I. Z., Ajayi, R. F., Mulidzi, A. R., Lewu, N., & Mulaudzi, T. (2025). Drought Tolerance Mechanisms in Grain and Vegetable Amaranthus Species: Physiological, Biochemical and Molecular Insights. Horticulturae, 11(10), 1226. https://doi.org/10.3390/horticulturae11101226










