Physiological, Productive, and Nutritional Performance of Tomato Plants Treated with Iron and Zinc Nanoparticles via Foliar Application Under Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
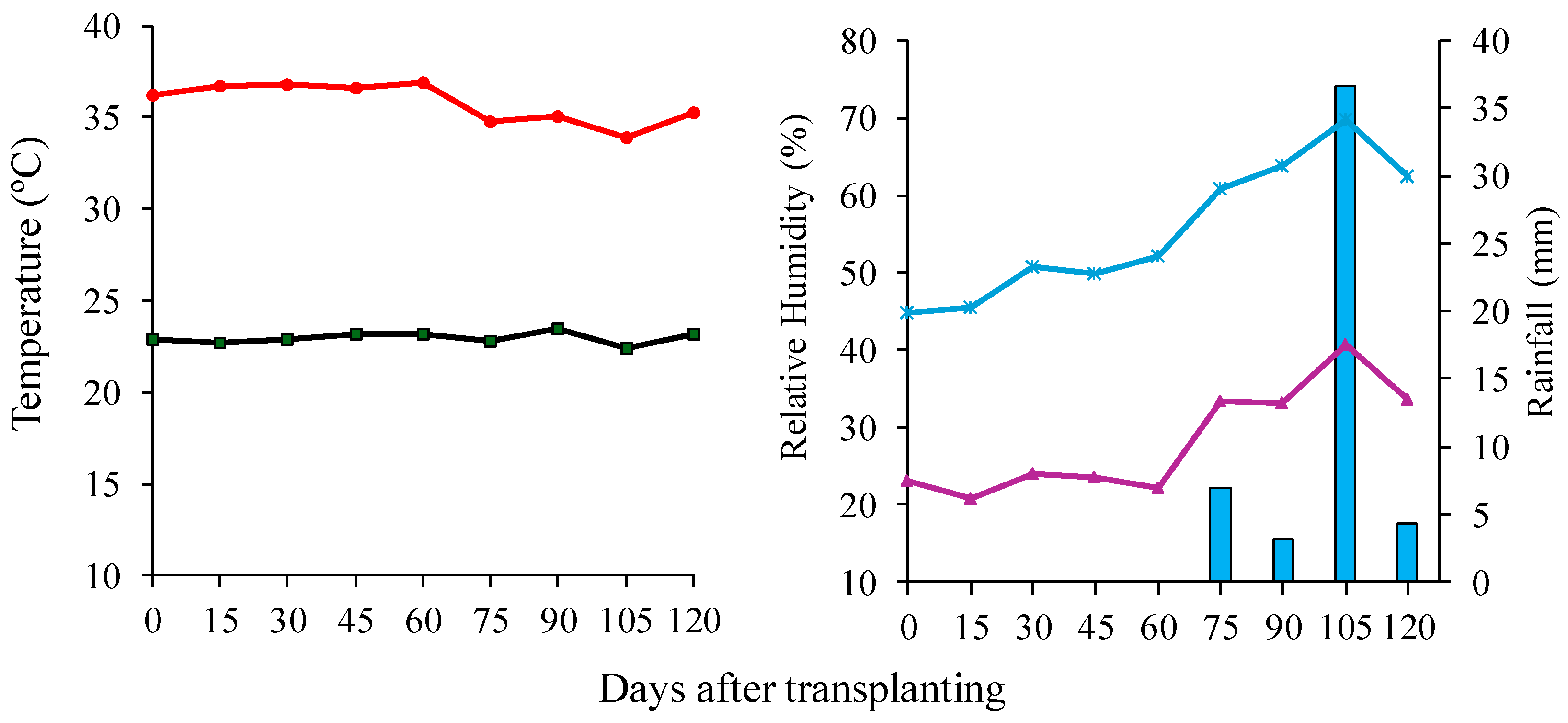
2.1. Characterization of the Experimental Area
2.2. Treatments and Experimental Design
2.3. Seedling Transplanting
2.4. Application of Treatments with Iron and Zinc Sources
2.5. Irrigation Depth Application
2.6. Cultural Practices and Phytosanitary Control
2.7. Evaluated Variables
2.7.1. Plant Growth
2.7.2. Gas Exchange and Photosynthetic Pigments
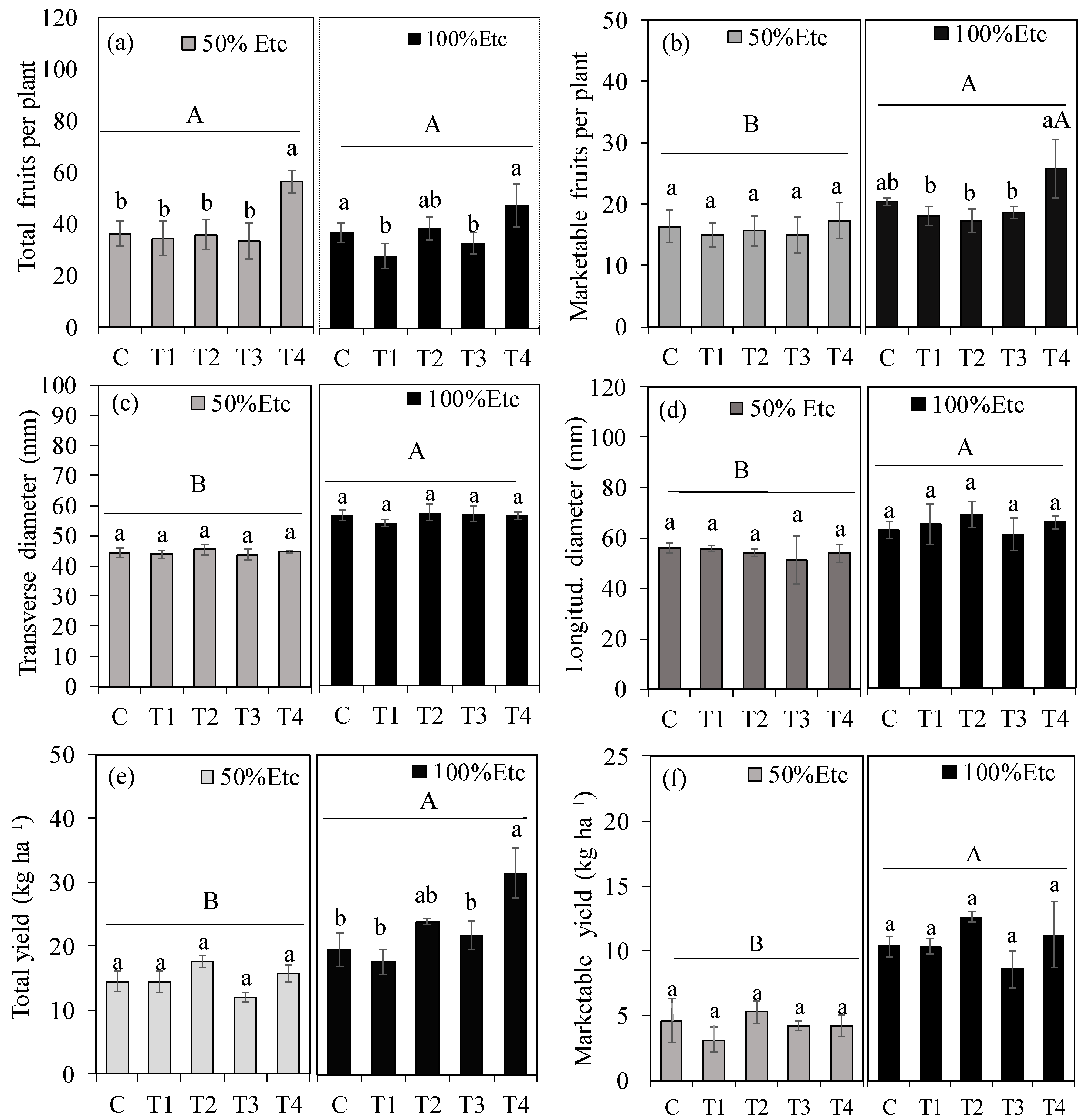
2.7.3. Fruit Yield
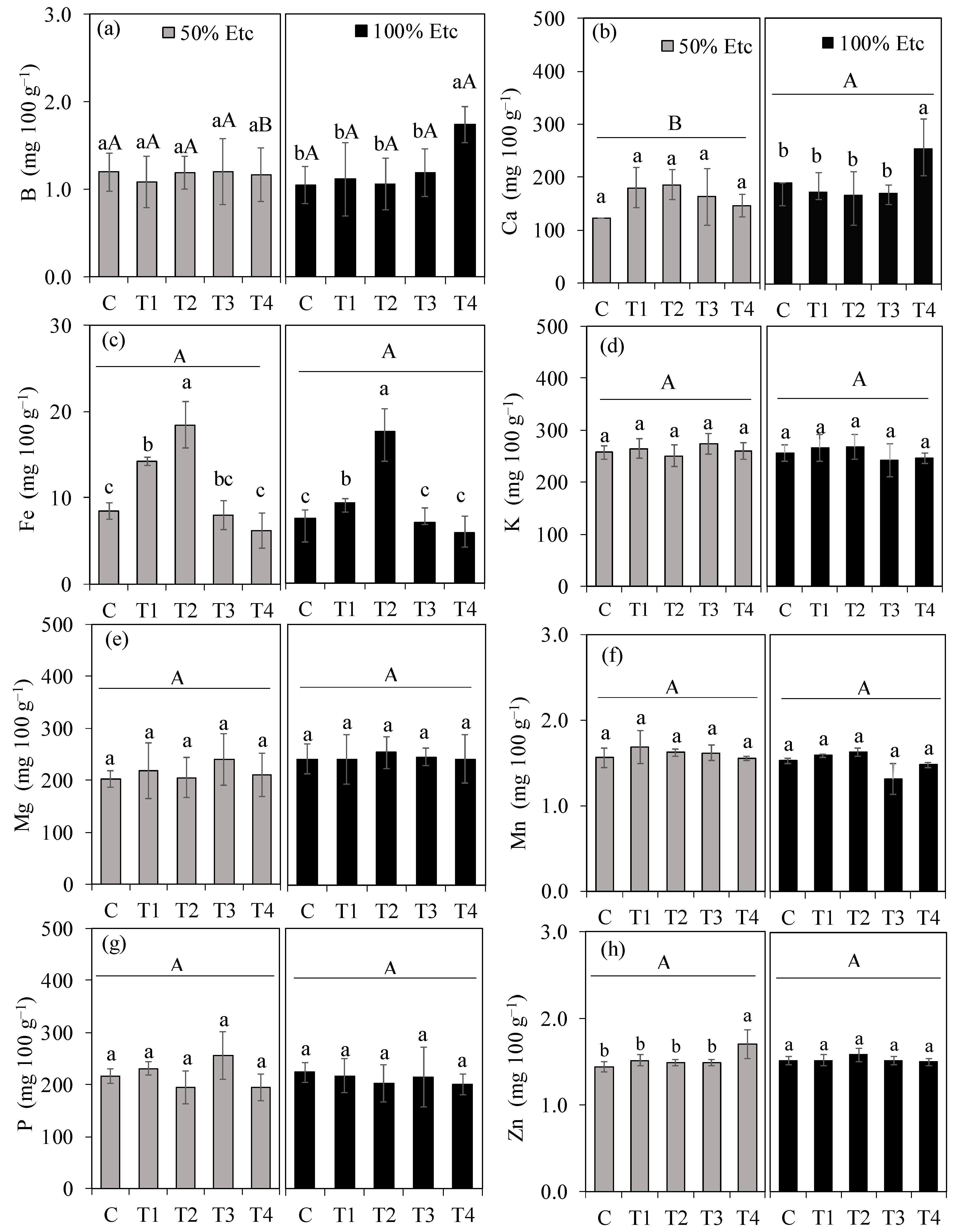
2.7.4. Mineral Nutrient Content in Fruits
2.7.5. Radar Chart
2.8. Statistical Analyses
3. Results
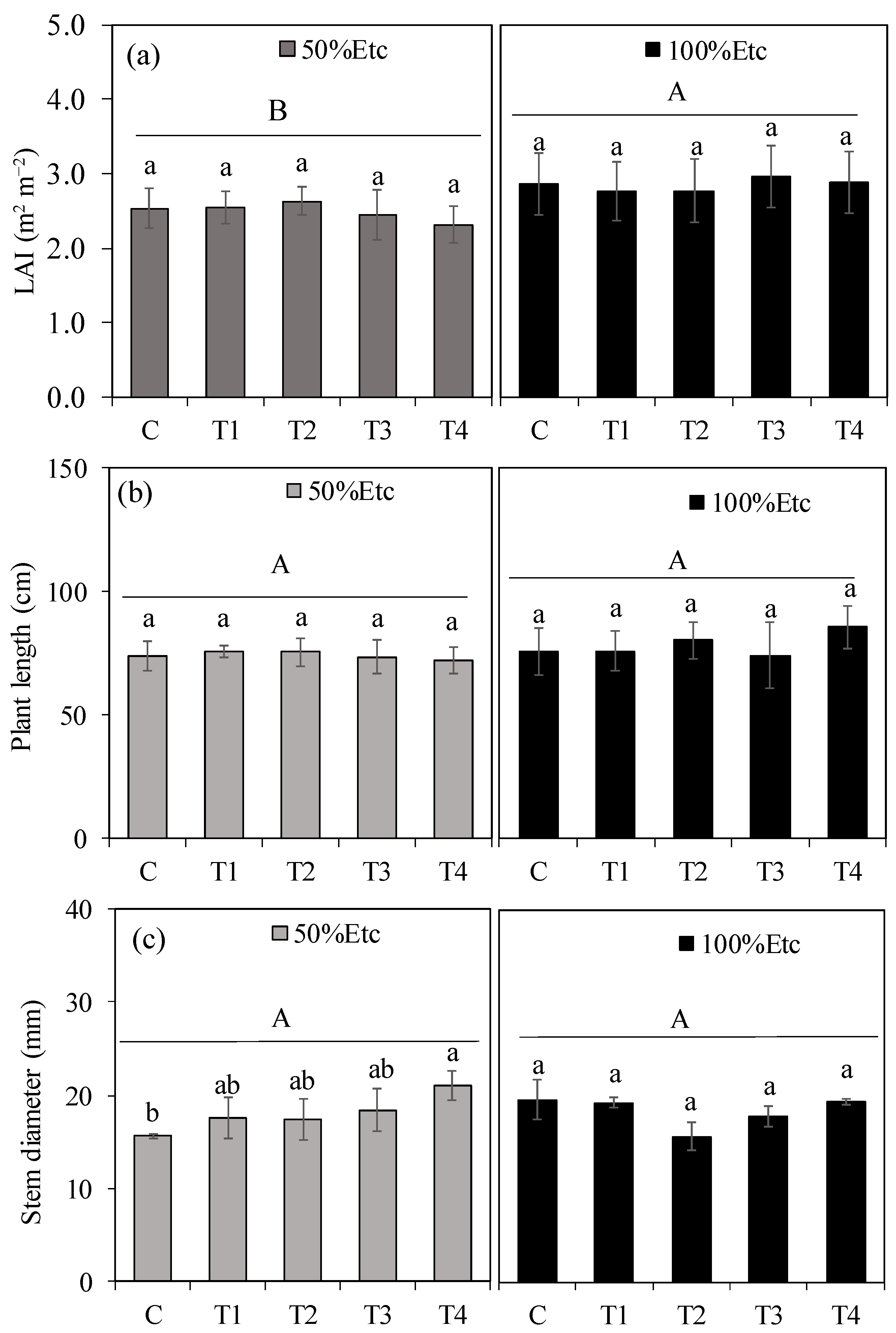
3.1. Plant Growth
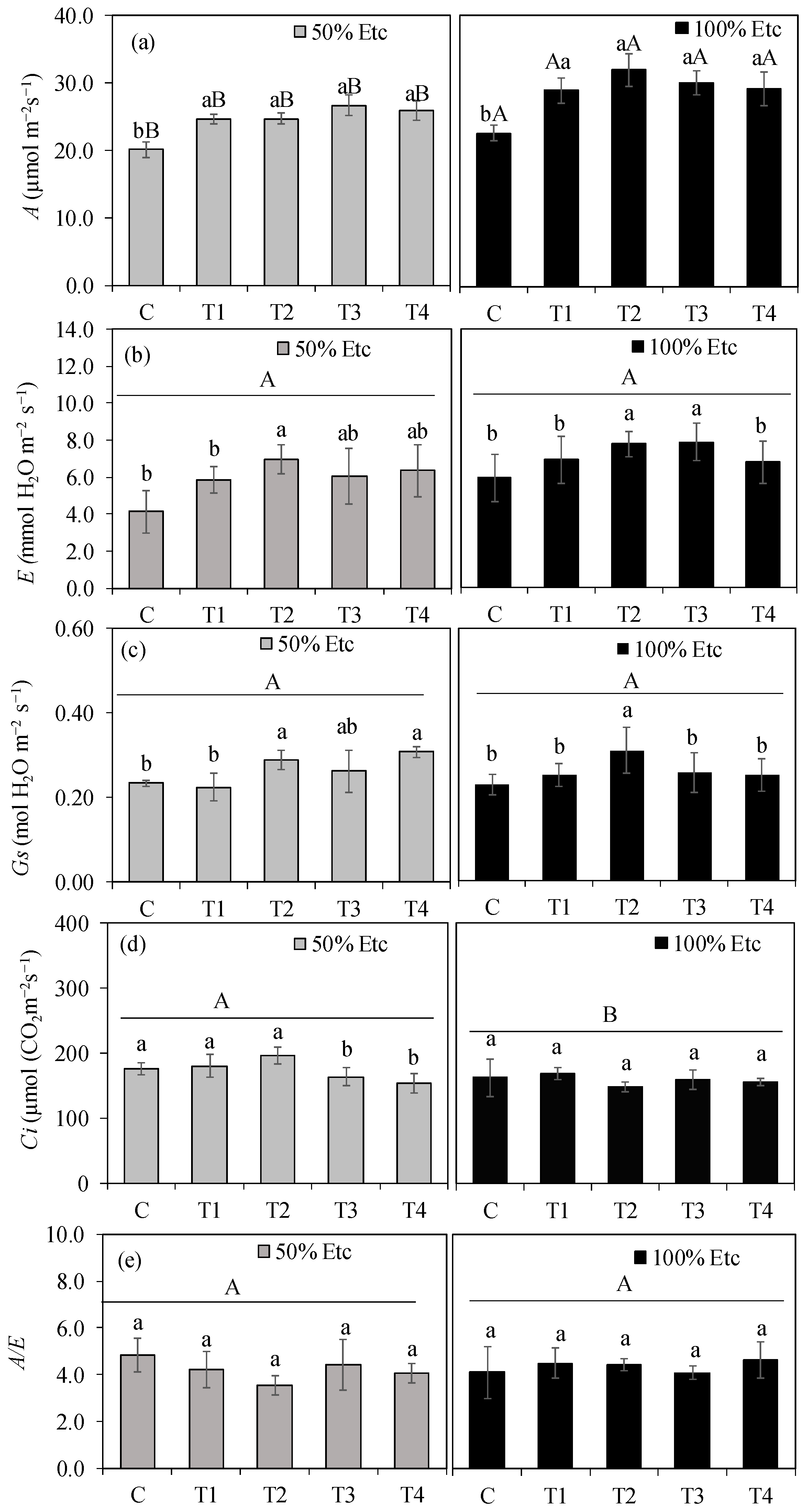
3.2. Gas Exchange
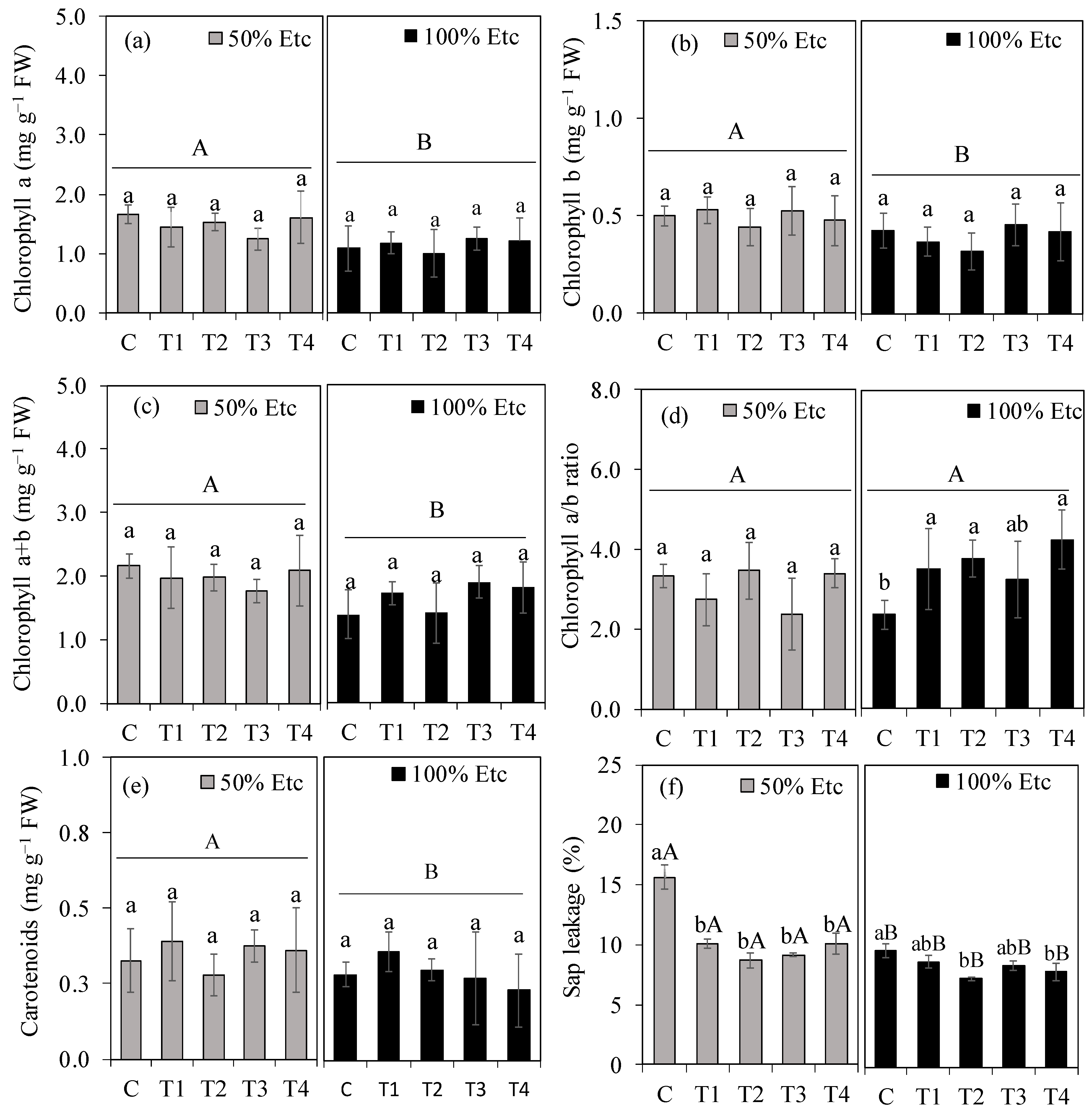
3.3. Photosynthetic Pigments and Electrolyte Leakage
3.4. Fruit Productivity
3.5. Mineral Element Content in Fruits
4. Discussion
4.1. Plant Growth
4.2. Gas Exchange, Photosynthetic Pigments, and Electrolyte Leakage
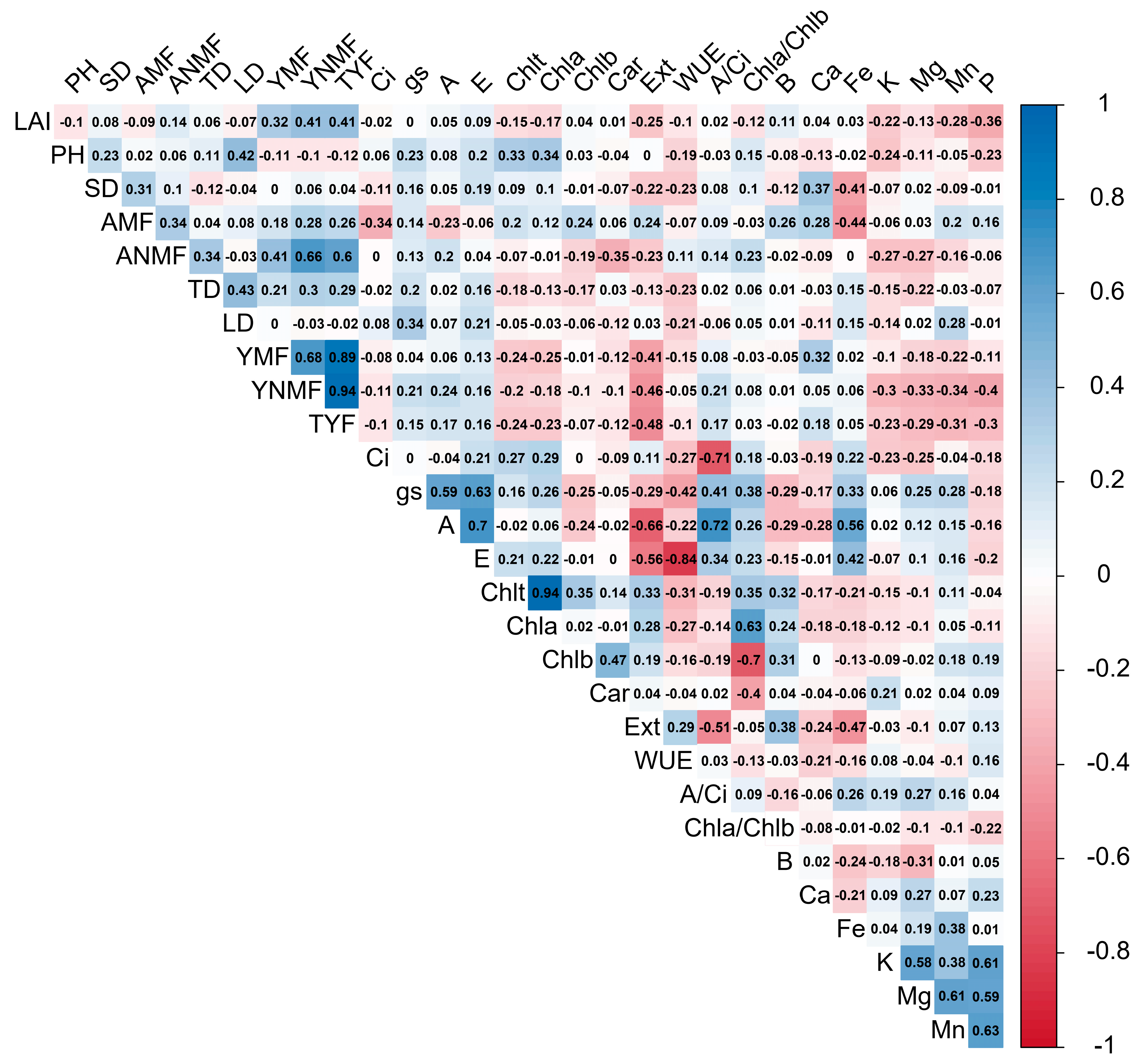
4.3. Yield, Fruit Nutrient Content, and Variable Correlations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Li, M.; Wu, X.; Zhunag, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Caseiro, A.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT Food Sci. Technol. 2020, 127, 109323. [Google Scholar] [CrossRef]
- EMBRAPA. Embrapa Hortaliças. Tomate: Produção e Mercado no Brasil; Documentos 245; Embrapa: Brasília, Brazil, 2022. [Google Scholar]
- Agência Nacional de Águas e Saneamento Básico (ANA). Conjuntura dos Recursos Hídricos no Brasil 2023: Relatório Pleno; ANA: Brasília, Brazil, 2023. Available online: https://www.snirh.gov.br/portal/snirh/centrais-de-conteudos/conjuntura-dos-recursos-hidricos/conjuntura_2023.pdf (accessed on 10 June 2024).
- United Nations. Water (UN-Water). In The United Nations World Water Development Report 2021; Valuing Water; UNESCO: Paris, France, 2021. [Google Scholar]
- Locatelli, S.; Barrera, W.; Verdi, L.; Nicoletto, C.; Marta, A.D.; Mauricieri, C. Modelling the response of tomato on deficit irrigation under greenhouse conditions. Sci. Hortic. 2024, 326, 112770. [Google Scholar] [CrossRef]
- Wadood, A.; Hameed, A.; Akram, S.; Ghaffar, M. Unraveling the impact of water deficit stress on nutritional quality and defense response of tomato genotypes. Front. Plant Sci. 2024, 15, 1403895. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.C.; Carvalho, J.A.; Rezende, F.C.; Freitas, W.A.; Ramos, M.M. Stomatal regulation and photosynthetic performance in tomato under water deficit. Environ. Exp. Botany 2021, 187, 104465. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought tolerance in plants: Physiological and molecular responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Li, J.; Zafar, S.; Javaid, A.; Perveen, S.; Hasnain, Z.; Ihtisham, M.; Abbas, A.; Usman, M.; El-Sappah, A.H.; Abbas, M.; et al. Zinc nanoparticles (ZnNPs): High-fidelity amelioration in turnip (Brassica rapa L.) production under drought stress. Sustainability 2023, 15, 6512. [Google Scholar] [CrossRef]
- Martins, B.L.R.; Ferreira, K.N.; Rocha, J.L.A.; Araujo, R.H.C.R.; Lopes, G.; Santos, L.C.d.; Bezerra Neto, F.; Sá, F.V.d.S.; Silva, T.I.d.; da Silva, W.I.; et al. Nano ZnO and Bioinoculants Mitigate Effects of Deficit Irrigation on Nutritional Quality of Green Peppers. Horticulturae 2024, 10, 969. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Uresti-Porras, J.G.; Cabrera-De La Fuente, M.; Benavidez-Mendoza, A.; Olivares-Sáenz, E.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of graft and nano ZnO on nutraceutical and mineral content in bell pepper. Plants 2021, 10, 2793. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Jafari, A.; Hatami, M. Foliar-applied nanoscale zero-valent iron (nZVI) and iron oxide (Fe3O4) induce differential responses in growth, physiology, antioxidative defense and biochemical indices in Leonurus cardiaca L. Environ. Res. 2022, 215, 114254. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Mfarrej, M.F.B.; Usman, M.; Anayatullah, S.; Rizwan, M.; Alharby, H.F.; Zeid, I.M.A.; Alabdallah, N.M.; Ali, S. Effect of iron nanoparticles and conventional sources of Fe on growth, physiology and nutrient accumulation in wheat plants grown on normal and salt-affected soils. J. Hazard. Mater. 2023, 458, 131861. [Google Scholar] [CrossRef] [PubMed]
- Golshahi, S.; Ahangar, A.G.; Mir, N.; Ghorbani, M. A comparison of the use of different sources of nanoscale iron particles on the concentration of micronutrients and plasma membrane stability in sorghum. J. Soil. Sci. Plant Nutr. 2018, 18, 236–252. [Google Scholar] [CrossRef]
- Yousaf, N.; Ishfaq, M.; Qureshi, H.A.; Saleem, A.; Yang, H.; Sardar, M.F.; Zou, C. Characterization of Root and Foliar-Applied Iron Oxide Nanoparticles (α-Fe2O3, γ-Fe2O3, Fe3O4, and Bulk Fe3O4) in Improving Maize (Zea mays L.) Performance. Nanomaterials 2023, 13, 3036. [Google Scholar] [CrossRef]
- Kurdekar, A.K.; Desai, B.K.; Koppalakar, B.G.; Sannagoudar, M.S.; Rathod, P.S.; Hiregoudar, S.; Rajesh, N.L.; Almutairi, K.; Elansary, H.; Nazim, M. Comparative evaluation of green synthesized and commercial iron and zinc nanoparticles on germination, growth and productivity of pigeonpea. Sci. Rep. 2025, 15, 25956. [Google Scholar] [CrossRef]
- Kumari, A.; Gupta, A.K.; Sharma, S.; Jadon, V.S.; Sharma, V.; Chun, S.C.; Sivanesan, I. Nanoparticles as a Tool for Alleviating Plant Stress: Mechanisms, Implications, and Challenges. Plants 2024, 13, 1528. [Google Scholar] [CrossRef]
- Li, R.; Zhang, R.; Li, Y.; Liu, C.; Wang, P.; Sun, H.; Wang, L. Foliar Uptake and Distribution of Metallic Oxide Nanoparticles in Maize (Zea mays L.) Leaf. Environ. Sci. Technol. 2024, 58, 16994–17003. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Azam, M.; Bhatti, H.N.; Khan, A.K.; Zafar, L.; Iqbal, M. Zinc oxide nano-fertilizer application (foliar and soil) effect on the growth, photosynthetic pigments and antioxidant system of maize cultivar. Biocat Agric. Biotechnol. 2022, 42, 102343. [Google Scholar] [CrossRef]
- Yadav, M. Nanoparticle-facilitated targeted nutrient delivery in plants: Breakthroughs and mechanistic insights. Plant Nano Biol. 2025, 12, 100156. [Google Scholar] [CrossRef]
- Amanda-Bandara, R.M.B.; Dissanayaka, D.M.S.B. Agronomic biofortification of vegetables to achieve iron and zinc nutritional security in food systems. J. Plant Nutr. 2025, 48, 974–994. [Google Scholar] [CrossRef]
- Raiesi-Ardali, T.; Ma’mani, L.; Chorom, M.; Moezzi, A. Improved iron use efficiency in tomato using organically coated iron oxide nanoparticles as efficient bioavailable Fe sources. Chem. Biol. Technol. Agric. 2022, 9, 59. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Kang, Y.; Shi, M.; Yang, X.; Li, H.; Yu, H.; Wang, Y.; Qin, S. Application of different foliar iron fertilizers for improving the photosynthesis and tuber quality of potato (Solanum tuberosum L.) and enhancing iron biofortification. Chem. Biol. Tech. Agric. 2022, 9, 79. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Ybaez, Q.E.; Sanchez, P.B.; Badayos, R.B. Synthesis and characterization of nano zinc oxide foliar fertilizer and its influence on yield and postharvest quality of tomato. Philipp. Agric. Sci. 2020, 103, 55–65. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.; Bozdar, B.; et al. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Nagao, R.; Kato, K.; Hamaguchi, U.; Ueno, Y.; Tsuboshita, N.; Shimizu, S.; Furutani, S.; Ehira, S.; Nakajima, Y.; Kawakami, K.; et al. Structure of a monomeric photosystem I core associated with iron-stress-induced-A proteins from Anabaena sp. PCC 7120. Nat. Commun. 2023, 14, 920. [Google Scholar] [CrossRef]
- Zahedifar, M.; Moosavi, A.A.; Gavili, E.; Ershadi, A. Tomato fruit quality and nutrient dynamics under water deficit conditions: The influence of an organic fertilizer. PLoS ONE 2025, 20, e0310916. [Google Scholar] [CrossRef]
- EMBRAPA. Manual of Soil Analysis Methods, 2nd ed.; Centro Nacional de Pesquisa em Solo: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- AGRITEMPO. Agrometeorological Monitoring System: Meteorological Stations for the State of Paraiba. 2023. Available online: https://www.agritempo.gov.br/br/estado/PB/estacoes/ (accessed on 15 October 2023).
- Cavalcante, F.J.A. Fertilization Recommendations for the State of Pernambuco (Brazil), 2nd ed.; Instituto Agronômico de Pernambuco: Recife, Brazil, 2008. [Google Scholar]
- Christiansen, J.E. Irrigation by Sprinkling; University of California Agricultural Experiment Station: Berkeley, CA, USA, 1943. [Google Scholar]
- Jensen, M.E. Water consumption by agriculture plants. In Water Deficit and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; Volume 2, pp. 1–22. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Campos, P.; Thi, A. Effects of an abscisic acid pretreatment on membrane leakage and lipid composition of Vigna unguiculata leaf discs subjected to osmotic stress. Plant Sci. 1997, 130, 11–18. [Google Scholar] [CrossRef]
- Juzon-Sikora, K.; Lasko’s, K.; Warchoł, M.; Czyczyło-Mysza, I.M.; Dziurka, K.; Grzesiak, M.; Skrzypek, E. Water Relations and Physiological Traits Associated with the Yield Components of Winter Wheat (Triticum aestivum L.). Agriculture 2024, 14, 1887. [Google Scholar] [CrossRef]
- Gayet, J.P.; Bleinroth, E.W.; Matallo, M.; Garcia, E.E.C.; Garcia, A.E.; Ardito, E.F.G.; Bordin, M.R. Tomate para Exportação: Procedimentos de Colheita e Pós-colheita; Série Publicações Técnicas FRUPEX 13; EMBRAPA-SPI: Brasília, Brazil, 1995. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Analysis of Soil, Plants and Other Materials, 2nd ed.; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (Complete Samples). Biometrika 1965, 52, 591–609. [Google Scholar] [CrossRef]
- R Core Team. R, version 4.4.1. R: A Language and Environment for Statistical Computing [Computer Software]. R Foundation for Statistical Computing: Vienna, Austria, 2025. Available online: https://www.R-project.org/ (accessed on 20 March 2024).
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2017. [Google Scholar]
- Suárez-Salazar, J.C.; Guaca-Cruz, L.; Quiceno-Mayo, E.J.; Ortiz-Morea, F.A. Photosynthetic responses and protective mechanisms under prolonged drought stress in cocoa. Pesqui. Agropecuária Bras. 2024, 59, e03543. [Google Scholar] [CrossRef]
- Yan, W.; Lu, Y.; Guo, L.; Liu, Y.; Li, M.; Zhang, B.; Zhang, B.; Zhang, L.; Qin, D.; Huo, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence in blue honeysuckle. Plants 2024, 13, 2115. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.-H.; Penuelas, J.; Sardans, J.; Du, L.; Yuan, Z.; Luo, Y.; Shen, Y.; Tian, R.; Li, N.; et al. Leaf area modulates the chlorophyll fluorescence of Leymus chinensis in response to different drought scenarios. Environ. Exp. Bot. 2025, 237, 106175. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Increase in membrane permeability and exudation in roots of zinc deficient plants. J. Plant Physiol. 1988, 132, 356–361. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and nondestructive evaluation of wheat chlorophyll under drought stress using hyperspectral imaging. Inter. J. Molec. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dash, P.K.; Das, D.; Das, S. Growth, yield and water productivity of tomato as influenced by deficit irrigation water management. Environ. Process. 2023, 10, 10. [Google Scholar] [CrossRef]
- Peroni, E.C.; Messoas, R.S.; Gallo, V.; Bardwosko, J.M.; Souza, E.R.; Avola, L.D.; Bamberg, A.L.; Rambo, C.V. Mineral content and antioxidant compounds in strawberry fruit submitted to drought stress. Food Sci. Technol. 2019, 39, 245–254. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Khalid, M.H.B.; Zhou, X.B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhao, S.; Huang, R.; Geng, Y.; Guo, L. The effects of exogenous iron on the photosynthetic performance and transcriptome of rice under salt-alkali stress. Agronomy 2024, 14, 1253. [Google Scholar] [CrossRef]
- Wei, L.; Liu, J.; Jiang, G. Nanoparticle-specific transformations dictate nanoparticle effects associated with plants and implications for nanotechnology use in agriculture. Nat. Commun. 2024, 15, 7389. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z. Nano-enabled agriculture: How do nanoparticles cross barriers in plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef]
- Alordzinu, K.E.; Appiah, S.A.; AL Aasmi, A.; Darko, R.O.; Li, J.; Lan, Y.; Adjibolosoo, D.; Lian, C.; Wang, H.; Qiao, S. Evaluating the influence of deficit irrigation on fruit yield and quality indices of tomatoes grown in sandy loam and silty loam soils. Water 2022, 14, 1753. [Google Scholar] [CrossRef]
- Oliveira, M.L.J.; Valladares, G.S.; Vieira, J.S.; Coelho, R.M. Availability and spatial variability of copper, iron, manganese and zinc in soils of the State of Ceará, Brazil. Revista Ciência Agronômica 2018, 49, 371–380. [Google Scholar] [CrossRef]
- NIH (National Institute of Health). Iron/Zinc—Health Professional Fact Sheet. 2024. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 16 July 2024).
- Bouhadi, M.; Javed, Q.; Jakubus, M.; Elkouali, M.; Fougrach, H.; Ansar, A.; Ban, S.G.; Ban, D.; Heath, D.; Cerne, M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy 2025, 15, 1131. [Google Scholar] [CrossRef]
- Dokoupil, L.; Mlcek, J. Foliar application of ZnO-NPS influences chlorophyll fluorescence and antioxidants pool in Capsicum annum L. under salinity. Horticulturae 2022, 8, 908. [Google Scholar] [CrossRef]

| Chemical | Value | Physical | Value |
|---|---|---|---|
| pH (CaCl2) | 6.45 | Sand (g kg−1) | 389 |
| OM (g kg−1) | 11.2 | Silt (g kg−1) | 430 |
| P (mg dm−3) | 180.1 | Clay (g kg−1) | 181 |
| K+ (mg dm−3) | 256.8 | BD (g cm−3) | 1.30 |
| Ca++ (cmolc dm−3) | 7.72 | PD (g cm−3) | 2.59 |
| Mg++ (cmolc dm−3) | 2.83 | TP (m3 m−3) | 0.47 |
| Na+ (cmolc dm−3) | 0.06 | FC (%) | 12.87 |
| H + Al+++ (cmolc dm−3) | 1.35 | PWP (%) | 5.29 |
| Fe (mg dm−3) | 18.0 | AWC (%) | 7.58 |
| Zn (mg dm−3) | 1.69 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, E.C.; Pereira, F.H.F.; Ferreira, K.N.; de Sena Rodrigues, A.C.; Araújo, R.H.C.R.; de Souza, J.E.J.; Ramos, C.S.G.; Lopes, G.; dos Santos, L.C.; Neto, F.B.; et al. Physiological, Productive, and Nutritional Performance of Tomato Plants Treated with Iron and Zinc Nanoparticles via Foliar Application Under Deficit Irrigation. Horticulturae 2025, 11, 1228. https://doi.org/10.3390/horticulturae11101228
Almeida EC, Pereira FHF, Ferreira KN, de Sena Rodrigues AC, Araújo RHCR, de Souza JEJ, Ramos CSG, Lopes G, dos Santos LC, Neto FB, et al. Physiological, Productive, and Nutritional Performance of Tomato Plants Treated with Iron and Zinc Nanoparticles via Foliar Application Under Deficit Irrigation. Horticulturae. 2025; 11(10):1228. https://doi.org/10.3390/horticulturae11101228
Chicago/Turabian StyleAlmeida, Erika Caminha, Francisco Hevilásio Freire Pereira, Kaiki Nogueira Ferreira, Antonio Carlos de Sena Rodrigues, Railene Hérica Carlos Rocha Araújo, José Ebson Janoca de Souza, Carlos Sávio Gomes Ramos, Guilherme Lopes, Leônidas Canuto dos Santos, Francisco Bezerra Neto, and et al. 2025. "Physiological, Productive, and Nutritional Performance of Tomato Plants Treated with Iron and Zinc Nanoparticles via Foliar Application Under Deficit Irrigation" Horticulturae 11, no. 10: 1228. https://doi.org/10.3390/horticulturae11101228
APA StyleAlmeida, E. C., Pereira, F. H. F., Ferreira, K. N., de Sena Rodrigues, A. C., Araújo, R. H. C. R., de Souza, J. E. J., Ramos, C. S. G., Lopes, G., dos Santos, L. C., Neto, F. B., Sá, F. V. d. S., Santos, J. Z. L., Nascimento, R. d., & Rocha, J. L. A. (2025). Physiological, Productive, and Nutritional Performance of Tomato Plants Treated with Iron and Zinc Nanoparticles via Foliar Application Under Deficit Irrigation. Horticulturae, 11(10), 1228. https://doi.org/10.3390/horticulturae11101228










