Banana and Plantain Starches: Exploring Differences and Potential Applications
Abstract
1. Introduction
2. Materials and Methods
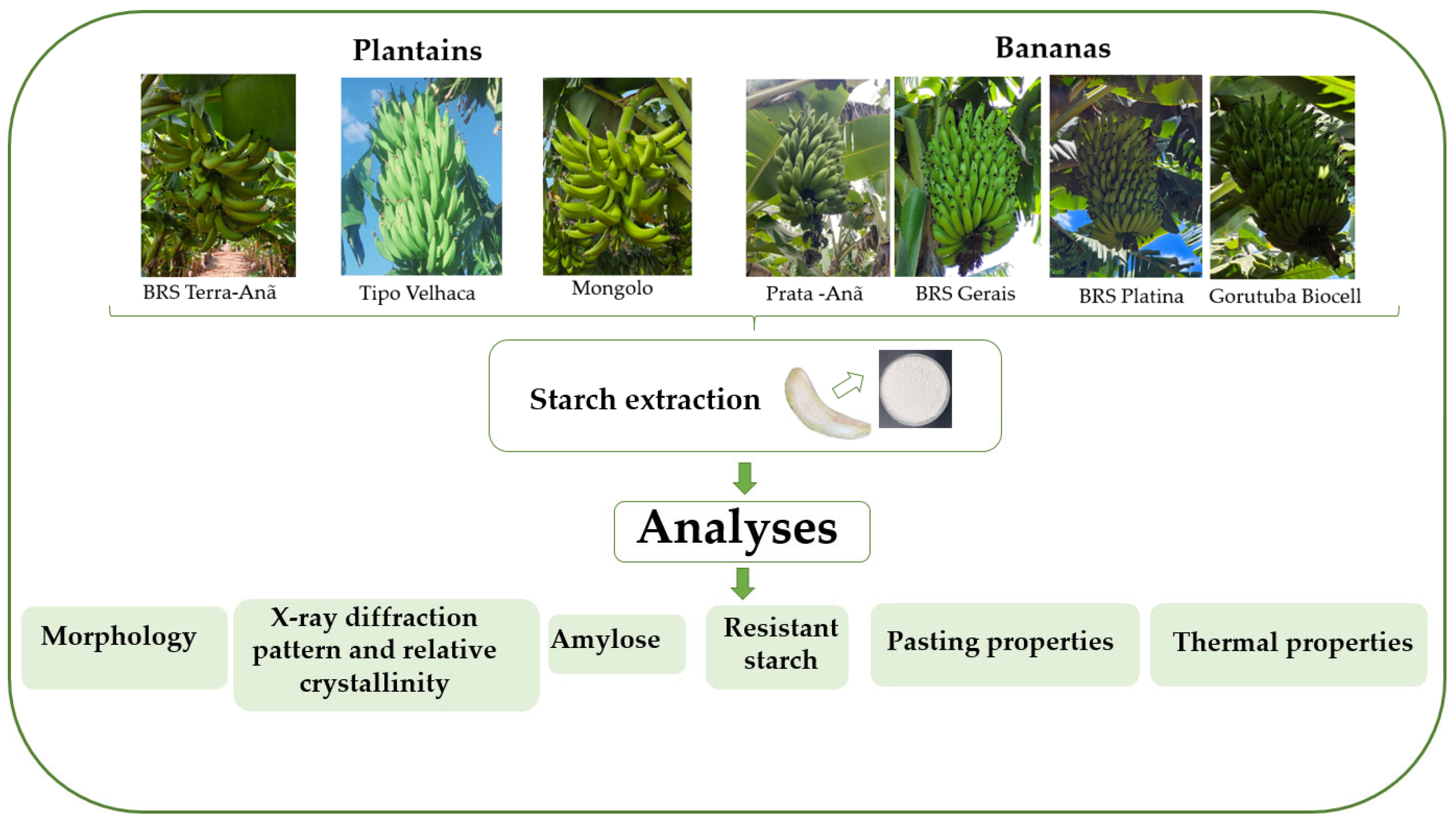
2.1. Banana Cultivars and Location of the Orchard
2.2. Extraction of Starches
2.3. Morphology of Starch Granules
2.4. X-Ray Diffraction Pattern
2.5. Amylose
2.6. Resistant Starch
2.7. Pasting Properties
2.8. Thermal Properties
2.9. Data Analysis
3. Results and Discussion
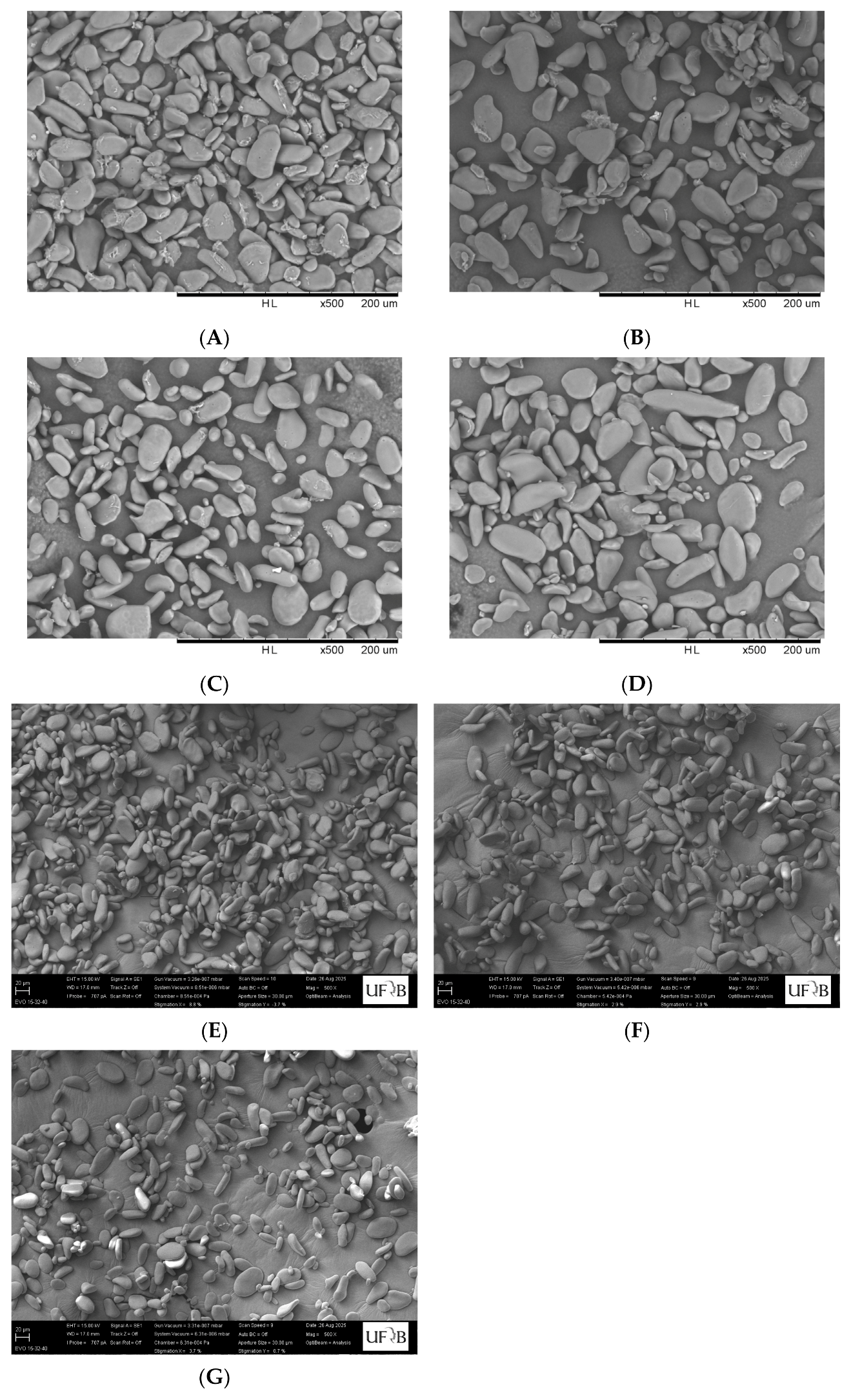
3.1. Morphology
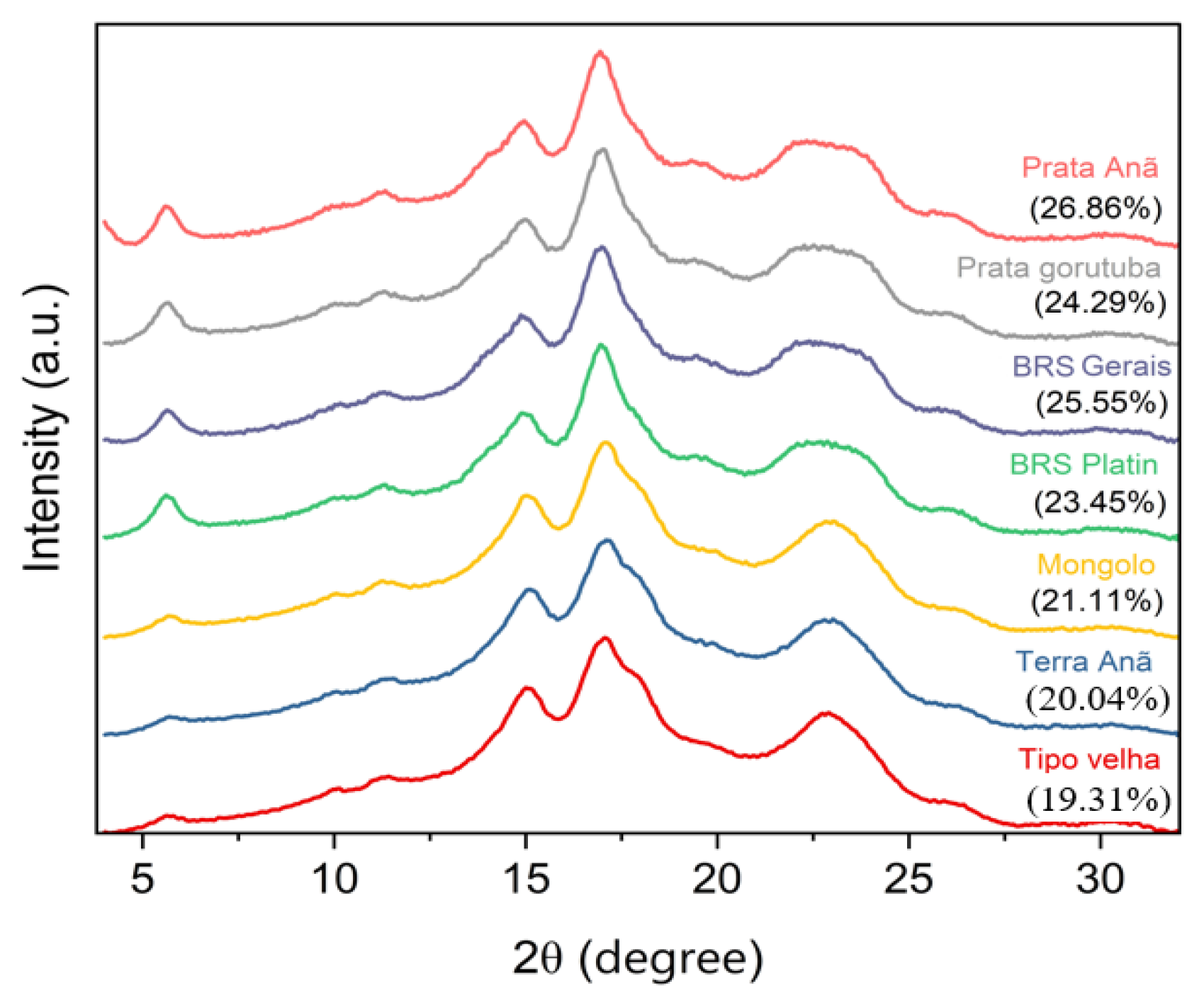
3.2. X-Ray Diffraction Pattern and Relative Crystallinities
3.3. Amylose and Resistant Starch
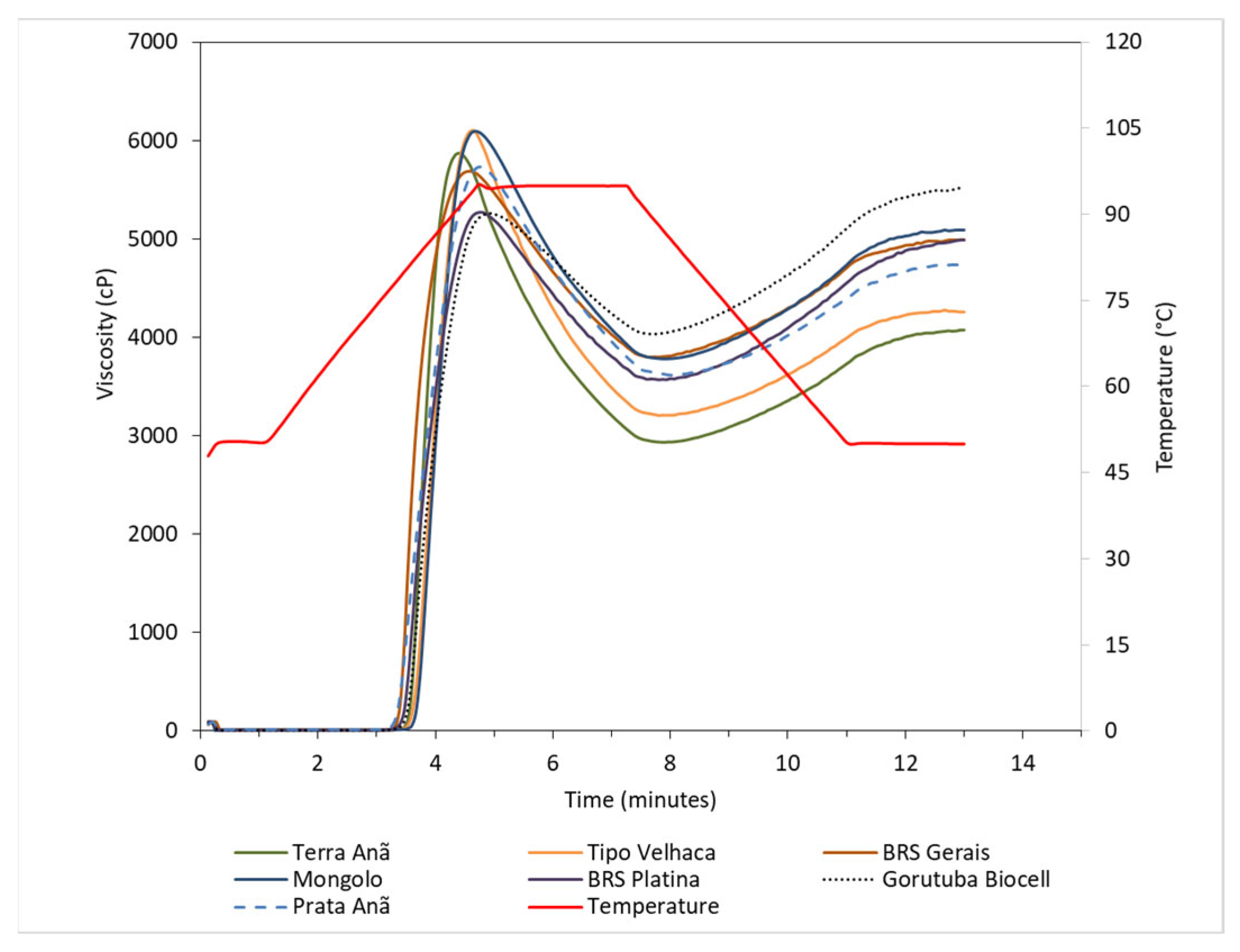
3.4. Pasting and Thermal Properties
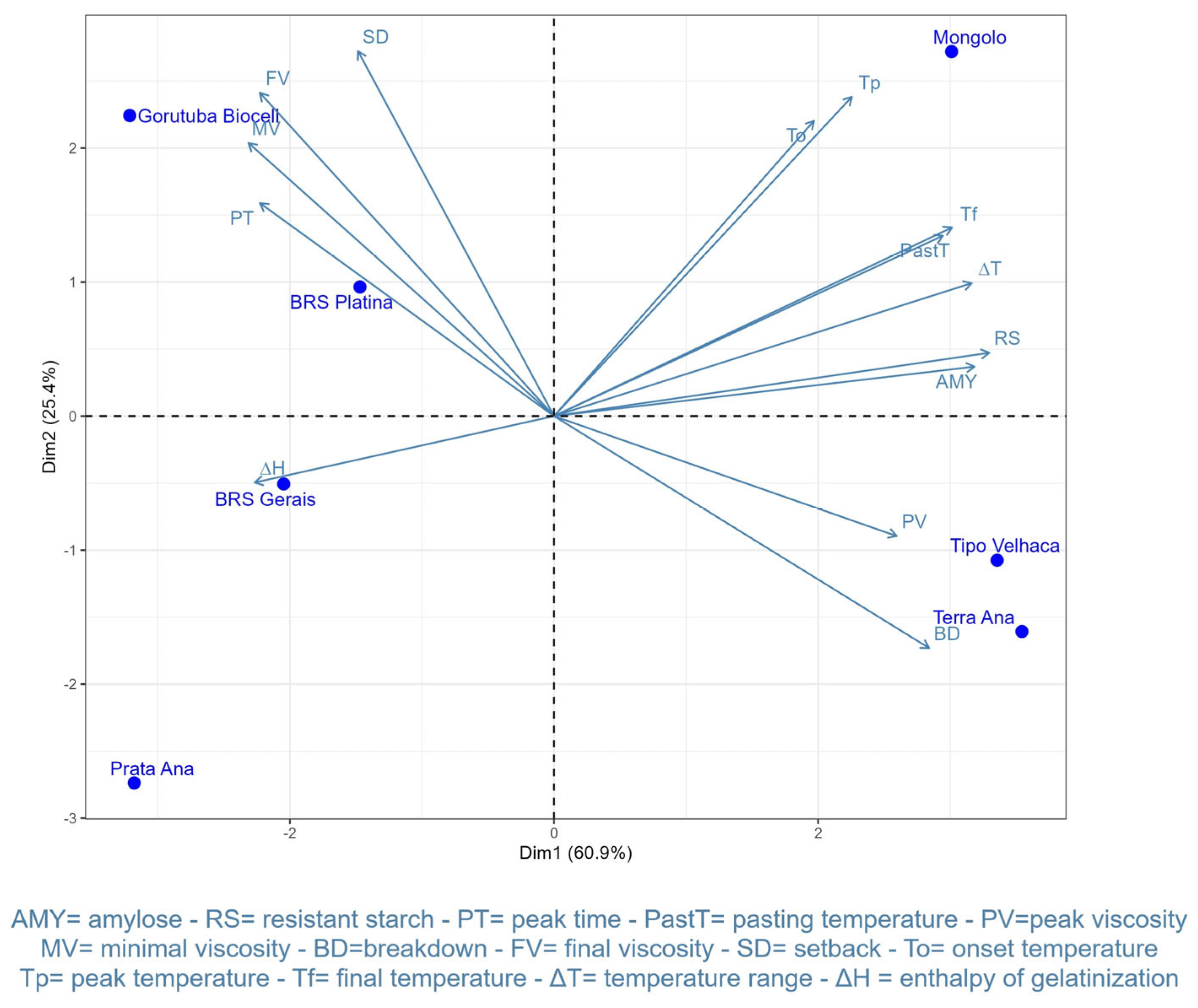
3.5. Clustering and Principal Component Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, B.; Sit, N. Comprehensive review on single and dual modification of starch: Methods, properties and applications. Int. J. Biol. Macromol. 2023, 253, 126952. [Google Scholar] [CrossRef]
- Paramasivam, S.K.; Saravanan, A.; Narayanan, S.; Shiva, K.N.; Ravi, I.; Mayilvaganan, M.; Pushpa, R.; Uma, S. Exploring differences in the physicochemical, functional, structural, and pasting properties of banana starches from dessert, cooking, and plantain cultivars (Musa spp.). Int. J. Biol. Macromol. 2021, 191, 1056–1067. [Google Scholar] [CrossRef]
- Leonel, M.; Bolfarini, A.C.B.; Rodrigues Da Silva, M.J.; Souza, J.M.A.; Leonel, S. Banana fruits with high content of resistant starch: Effect of genotypes and phosphorus fertilization. Int. J. Biol. Macromol. 2020, 150, 1020–1026. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, R.K.; Srivastav, P.P. Exploring novel frontiers of advancements in purple yam (Dioscorea alata L.) starch extraction, modification, characterization, applications in food and other industries. Meas. Food 2024, 15, 100196. [Google Scholar] [CrossRef]
- Lossolli, N.A.B.; Leonel, M.; Leonel, S.; Izidoro, M.; de Paula, G.V.; Santos, T.P.R.; de Oliveira, L.A. Cultivars and fruit part as differentiating factors of physicochemical characteristics of mango starches. Horticulturae 2023, 9, 69. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Faostat. Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 8 June 2025).
- Kumar, P.S.; Saravanan, A.; Sheeba, N.; Uma, S. Structural, functional characterization and physicochemical properties of green banana flour from dessert and plantain bananas (Musa spp.). LWT 2019, 116, 108524. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Hong, Y.; Bi, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Digestibility and changes to structural characteristics of green banana starch during in vitro digestion. Food Hydrocoll. 2015, 49, 192–199. [Google Scholar] [CrossRef]
- Salazar, D.; Arancibia, M.; Lalaleo, D.; Rodríguez-Maecker, R.; López-Caballero, M.E.; Montero, M.P. Physico-chemical properties and filmogenic aptitude for edible packaging of ecuadorian discard green banana flours (Musa acuminanta AAA). Food Hydrocoll. 2022, 122, 107048. [Google Scholar] [CrossRef]
- Yang, M.; Chang, L.; Jiang, F.; Zhao, N.; Zheng, P.; Simbo, J.; Yu, X.; Du, S. Structural, physicochemical and rheological properties of starches isolated from banana varieties (Musa spp.). Food Chem. X 2022, 16, 100473. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Djali, M.; Pramafisi, G. The properties, modification, and application of banana starch. Polymers 2022, 14, 3092. [Google Scholar] [CrossRef]
- Quintero-Castaño, V.D.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Lucas-Aguirre, J.C.; Bello-Pérez, L.A.; Rodríguez-Garcia, M.E. Starch from two unripe plantains and esterified with octenyl succinic anhydride (osa): Partial characterization. Food Chem. 2020, 315, 126241. [Google Scholar] [CrossRef]
- De La Torre-Gutiérrez, L.; Chel-Guerrero, L.A.; Betancur-Ancona, D. Functional properties of square banana (Musa balbisiana) starch. Food Chem. 2008, 106, 1138–1144. [Google Scholar] [CrossRef]
- Zhu, F. Underutilized and unconventional starches: Why should we care? Trends Food Sci. Technol. 2020, 100, 363–373. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-conventional starch sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Leonel, M.; Leonel, S.; Santos, T.P.R.D.; Souza, J.M.A.; Martins, R.C.; Silva, M.S.C.D. Agronomic yield and starch properties of banana cultivars. Pesqui. Agropecuária Bras. 2021, 56, e02491. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Djali, M.; Arcot, J.; Tensiska, T. A Comparative study on the physicochemical and pasting properties of starch and flour from different banana (Musa spp.) cultivars grown in Indonesia. Int. J. Food Prop. 2019, 22, 1562–1575. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Tsai, S.-J.; Sun, N.-N.; Dai, F.-J.; Yu, P.-H.; Chen, Y.-C.; Chau, C.-F. Enhanced thermal stability of green banana starch by heat-moisture treatment and its ability to reduce body fat accumulation and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 160, 915–924. [Google Scholar] [CrossRef]
- Torres-Vargas, O.L.; Gaytan-Martinez, M.; Fernanda, C.-C.; Millán-Malo, B.M.; Rodriguez-Garcia, M.E. Changes in the physicochemical properties of isolated starch and plantain (Musa AAB Simmonds) flours for early maturity stage. Heliyon 2023, 9, e18939. [Google Scholar] [CrossRef]
- Jaiturong, P.; Laosirisathian, N.; Sirithunyalug, B.; Eitssayeam, S.; Sirilun, S.; Chaiyana, W.; Sirithunyalug, J. Physicochemical and prebiotic properties of resistant starch from Musa sapientum Linn., ABB Group, cv. Kluai Namwa Luang. Heliyon 2020, 6, e05789. [Google Scholar] [CrossRef]
- Pereira, L.V.; Narita, N.; Suguino, E.; Takata, W.H.S. Productive behavior of cultivars and banana genotype originating from Prata anã’, irrigated and non-irrigated in the State of Minas Gerais, Brazil. Int. J. Environ. Agric. Res. 2019, 5, 1–7. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 356. [Google Scholar]
- PBMH & PIF—Programa Brasileiro Para a Modernização da Horticultura & Produção Integrada de Frutas. 2006. Available online: https://ceagesp.gov.br/wp-content/uploads/2015/07/banana.pdf (accessed on 7 July 2025).
- Chiang, B.H.; Chu, W.C.; Chu, C.L. A pilot scale study for banana starch production. Starch-Stärke 1987, 39, 5–8. [Google Scholar] [CrossRef]
- Bernardo, C.O.; Ascheri, J.L.R.; Chávez, D.W.H.; Carvalho, C.W.P. Ultrasound assisted extraction of yam (Dioscorea bulbífera) starch: Effect on morphology and functional properties. Starch-Stärke 2018, 70, 1700185. [Google Scholar] [CrossRef]
- ISO 6647:1987; Norme Internationale: Riz-Détermination de la Teneur em Amylose. ISO: Geneva, Switzerland, 1987. Available online: https://www.iso.org/standard/13073.html (accessed on 18 June 2025).
- Goñi, I.; García-Diz, L.; Mañas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 15 July 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Soft. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 15 July 2025).
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; A Series of Books in Biology; W. H. Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. The Comparison of dendrograms by objective methods. TAXON 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Mantel, N. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Mingoti, S.A. Análise de Dados Através de Métodos de Estatística Multivariada: Uma Abordagem Aplicada; Editora UFMG: Belo Horizonte, Brazil, 2005. [Google Scholar]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R package for determining the relevant number of clusters in a data set. J. Stat. Soft. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J.; McCarthy, O.J.; Singh, H. Physico-chemical, rheological and structural properties of fractionated potato starches. J. Food Eng. 2007, 80, 383–394. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, Y.; Chen, X.; Xu, F.; Zhu, K.; Wang, J.; Zhang, Y. Physicochemical, structural, and digestive properties of green banana starch from five Chinese mutant banana species. Foods 2025, 14, 706. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Man, J.; Wang, J.; Zhou, W.; Huai, H.; We, C. Comparison of physicochemical properties of B-type nontraditional starches from different sources. Int. J. Biol. Macromol. 2015, 78, 165–172. [Google Scholar] [CrossRef]
- Mesquita, C.B.; Leonel, M.; Franco, C.M.L.; Leonel, S.; Garcia, E.L.; Santos, T.P.R. Characterization of banana starches obtained from cultivars grown in Brazil. Int. J. Biol. Macromol. 2016, 89, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhang, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Hong, Y. Effect of ripening on in vitro digestibility and structural characteristics of plantain (Musa ABB) starch. Food Hydrocoll. 2019, 93, 235–241. [Google Scholar] [CrossRef]
- Viana, E.B.M.; Oliveira, N.L.; Ribeiro, J.S.; Almeida, M.F.; Souza, C.C.E.; Resende, J.V.; Santos, L.S.; Veloso, C.M. Development of starch-based bioplastics of green plantain banana (Musa paradisiaca L.) modified with heat-moisture treatment (HMT). Food Packag. Shelf Life 2022, 31, 100776. [Google Scholar] [CrossRef]
- Alimi, B.A.; Workneh, T.S.; Oke, M.O. Effect of hydrothermal modifications on the functional, pasting and morphological properties of south african cooking banana and plantain. CyTA-J. Food 2016, 14, 489–495. [Google Scholar] [CrossRef]
- Castañeda-Niño, J.P.; Mina-Hernández, J.H.; Solanilla-Duque, J.F. Extraction and characterization of starches from the pulp and peel of native plantain (Musa AAB Simmonds) from two Colombian departments. Polysaccharides 2025, 6, 34. [Google Scholar] [CrossRef]
- Kaur, L.; Dhull, S.B.; Kumar, P.; Singh, A. Banana starch: Properties, description, and modified variations—A review. Int. J. Biol. Macromol. 2020, 165, 2096–2102. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, C.; Liu, Z.; Xu, H.; Wei, B.; Wang, B.; Sun, Q.; Zhou, C.; Ma, H. Starches modification with rose polyphenols under multi-frequency power ultrasonic fields: Effect on physicochemical properties and digestion behavior. Ultrason. Sonochemistry 2023, 98, 106515. [Google Scholar] [CrossRef]
- Chang, L.; Yang, M.; Zhao, N.; Xie, F.; Zheng, P.; Simbo, J.; Yu, X.; Du, S. Structural, physicochemical, antioxidant and in vitro digestibility properties of banana flours from different banana varieties (Musa spp.). Food Biosci. 2022, 47, 101624. [Google Scholar] [CrossRef]

| Cultivars | Amylose (%) | Resistant Starch (%) |
|---|---|---|
| BRS Terra-Anã | 43.0 ± 0.2 a | 74.4 ± 1.0 a |
| Tipo Velhaca | 42.5 ± 0.3 a | 74.8 ± 1.5 a |
| Mongolo | 42.9 ± 1.2 a | 74.0 ± 1.7 a |
| BRS Gerais | 31.4 ± 0.2 c | 68.5 ± 0.1 b |
| BRS Platina | 35.3 ± 0.2 b | 68.8 ± 1.2 b |
| Gorutuba Biocell | 34.8 ± 1.3 b | 68.1 ± 0.4 b |
| Prata-Anã | 32.7 ± 0.3 c | 66.6 ± 1.3 b |
| Cultivars | TP | PT | PV | MV | BD | FV | SB |
|---|---|---|---|---|---|---|---|
| (°C) | (min.) | cP | |||||
| BRS Terra-Anã | 4.4 ± 0.0 d | 79.5 ± 0.1 b | 5877 ± 264 ab | 2931 ± 148 c | 2945 ± 279 a | 4074 ± 158 d | 1142 ± 36 abc |
| Tipo Velhaca | 4.6 ± 0.0 c | 80.4 ± 0.4 a | 6127 ± 143 a | 3202 ± 116 c | 2925 ± 26 a | 4257 ± 118 cd | 1055 ± 18 c |
| Mongolo | 4.7 ± 0.0 b | 81.0 ± 0.4 a | 6104 ± 200 a | 3778 ± 122 ab | 2327 ± 263 b | 5091 ± 142 ab | 1313 ± 25 abc |
| BRS Gerais | 4.6 ± 0.0 c | 77.3 ± 0.3 de | 5693 ± 108 c | 3787 ± 117 ab | 1906 ± 143 bc | 4992 ± 179 b | 1205 ± 80 abc |
| BRS Platina | 4.8 ± 0.1 b | 78.0 ± 0.2 cd | 5291 ± 299 cd | 3560 ± 222 b | 1732 ± 398 c | 4988 ± 449 b | 1429 ± 410 ab |
| Gorutuba Biocell | 4.9 ± 0.1 a | 78.4 ± 0.6 c | 5273 ± 161 d | 4047 ± 135 a | 1226 ± 37 d | 5517 ± 335 a | 1471 ± 210 a |
| Prata-Anã | 4.7 ± 0.1 b | 76.8 ± 0.5 e | 5752 ± 139 ab | 3611 ± 179 b | 2141 ± 259 bc | 4739 ± 235 bc | 1127 ± 58 bc |
| Cultivars | To (°C) | Tp (°C) | Tf (°C) | ΔT (°C) | ∆Hgel (J g−1) |
|---|---|---|---|---|---|
| BRS Terra-Anã | 70.1 ± 0.2 b | 74.9 ± 0.1 b | 92.7 ± 1.8 a | 22.6 ± 2.0 ab | 17.6 ± 0.7 c |
| Tipo Velhaca | 70.1 ± 0.2 b | 74.9 ± 0.1 b | 92.7 ± 1.8 a | 22.6 ± 2.0 ab | 17.6 ± 0.7 c |
| Mongolo | 71.8 ± 0.2 a | 76.6 ± 0.3 a | 95.5 ± 2.5 a | 23.6 ± 2.6 a | 18.0 ± 1.6 ab |
| BRS Gerais | 70.5 ± 0.3 b | 74.4 ± 0.6 b | 87.8 ± 0.1 bc | 17.3 ± 0.4 c | 21.1 ± 2.4 a |
| BRS Platina | 69.5 ± 1.3 b | 74.8 ± 1.3 b | 89.2 ± 3.8 b | 19.7 ± 3.0 bc | 17.6 ± 1.7 c |
| Gorutuba Biocell | 69.9 ± 1.3 b | 74.6 ± 1.1 b | 87.7 ± 1.7 bc | 17.8 ± 1.0 bc | 19.2 ± 1.6 ab |
| Prata-Anã | 67.9 ± 0.3 c | 72.7± 0.1 c | 83.4 ± 4.4 c | 15.5 ± 4.2 c | 19.6 ± 1.3 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, J.L.d.J.; Leonel, M.; Viana, E.d.S.; Amorim, E.P.; Reis, R.C.; Carvalho, C.W.P.d.; Neta, P.d.J.; Leonel, S. Banana and Plantain Starches: Exploring Differences and Potential Applications. Horticulturae 2025, 11, 1214. https://doi.org/10.3390/horticulturae11101214
Assis JLdJ, Leonel M, Viana EdS, Amorim EP, Reis RC, Carvalho CWPd, Neta PdJ, Leonel S. Banana and Plantain Starches: Exploring Differences and Potential Applications. Horticulturae. 2025; 11(10):1214. https://doi.org/10.3390/horticulturae11101214
Chicago/Turabian StyleAssis, Jaciene Lopes de Jesus, Magali Leonel, Eliseth de Souza Viana, Edson Perito Amorim, Ronielli Cardoso Reis, Carlos Wanderlei Piler de Carvalho, Palmira de Jesus Neta, and Sarita Leonel. 2025. "Banana and Plantain Starches: Exploring Differences and Potential Applications" Horticulturae 11, no. 10: 1214. https://doi.org/10.3390/horticulturae11101214
APA StyleAssis, J. L. d. J., Leonel, M., Viana, E. d. S., Amorim, E. P., Reis, R. C., Carvalho, C. W. P. d., Neta, P. d. J., & Leonel, S. (2025). Banana and Plantain Starches: Exploring Differences and Potential Applications. Horticulturae, 11(10), 1214. https://doi.org/10.3390/horticulturae11101214








