Genome-Wide Analysis of Grapevine Ascorbate Oxidase Genes Identifies VaAAO7 in Vitis amurensis as a Positive Regulator of Botrytis cinerea Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Total RNA Extraction, cDNA Synthesis, and qRT-PCR
2.3. VaAAO Gene Family Identification
2.4. Phylogenetic and Synteny Analysis
2.5. Analyses of Gene Structure, Conserved Motif, and Cis-Acting Element
2.6. Genes Cloning and Plasmid Construction
2.7. Subcellular Localization Analysis of VaAAO7 in N. benthamiana Leaves
2.8. Transient Overexpression of VaAAO7 in V. vinifera
2.9. H2O2 Treatment and B. cinerea Inoculation
2.10. Data Analysis
3. Results
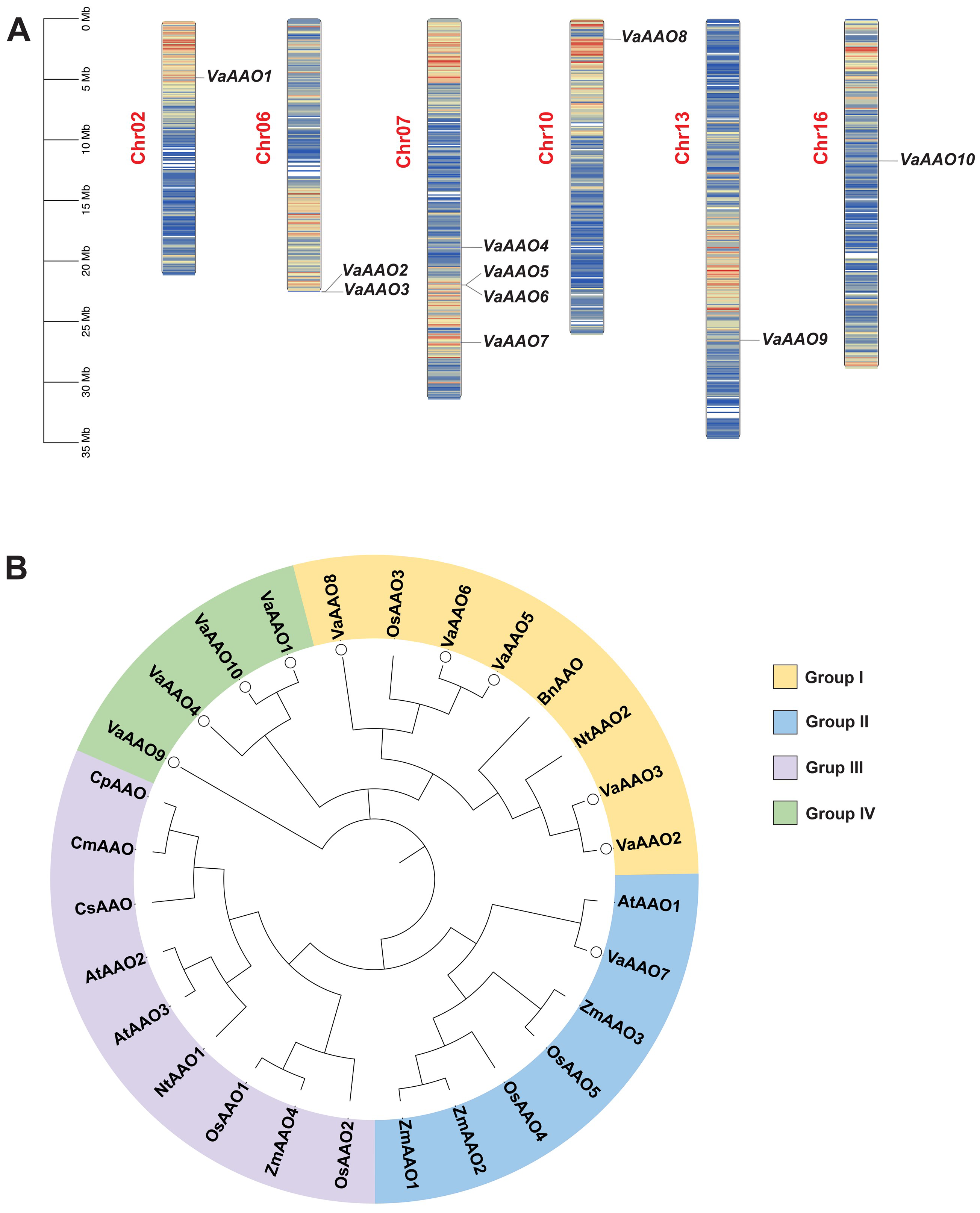
3.1. Genome-Wide Identification and Characterization of the VaAAO Gene Family in V. amurensis
3.2. Collinearity Analysis of VaAAO Family Genes in V. amurensis
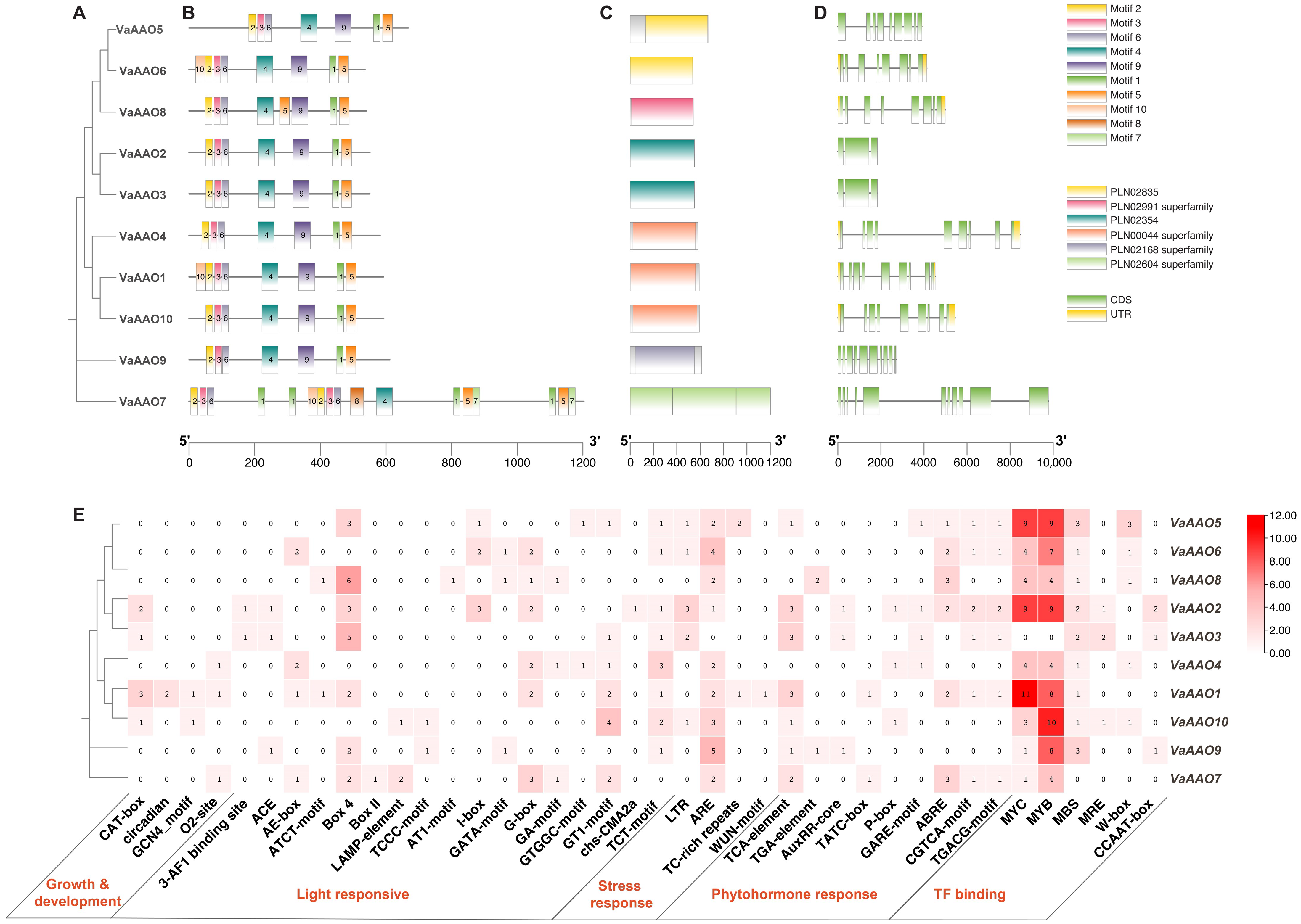
3.3. Motif, Structural and Cis-Regulatory Elements Analysis of VaAAO Family Members in V. amurensis
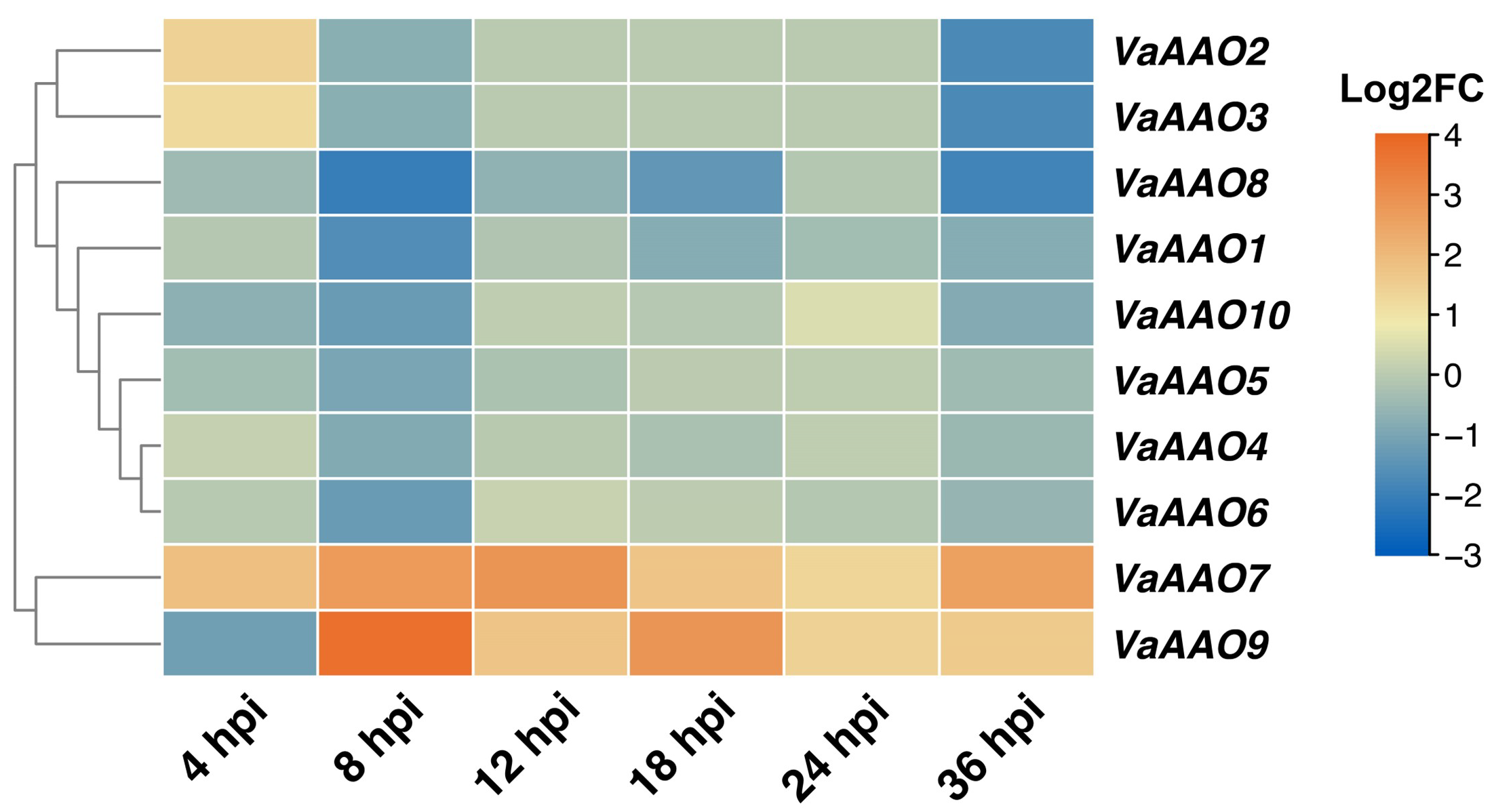
3.4. Expression Patterns of VaAAO Family Genes During B. cinerea Infection
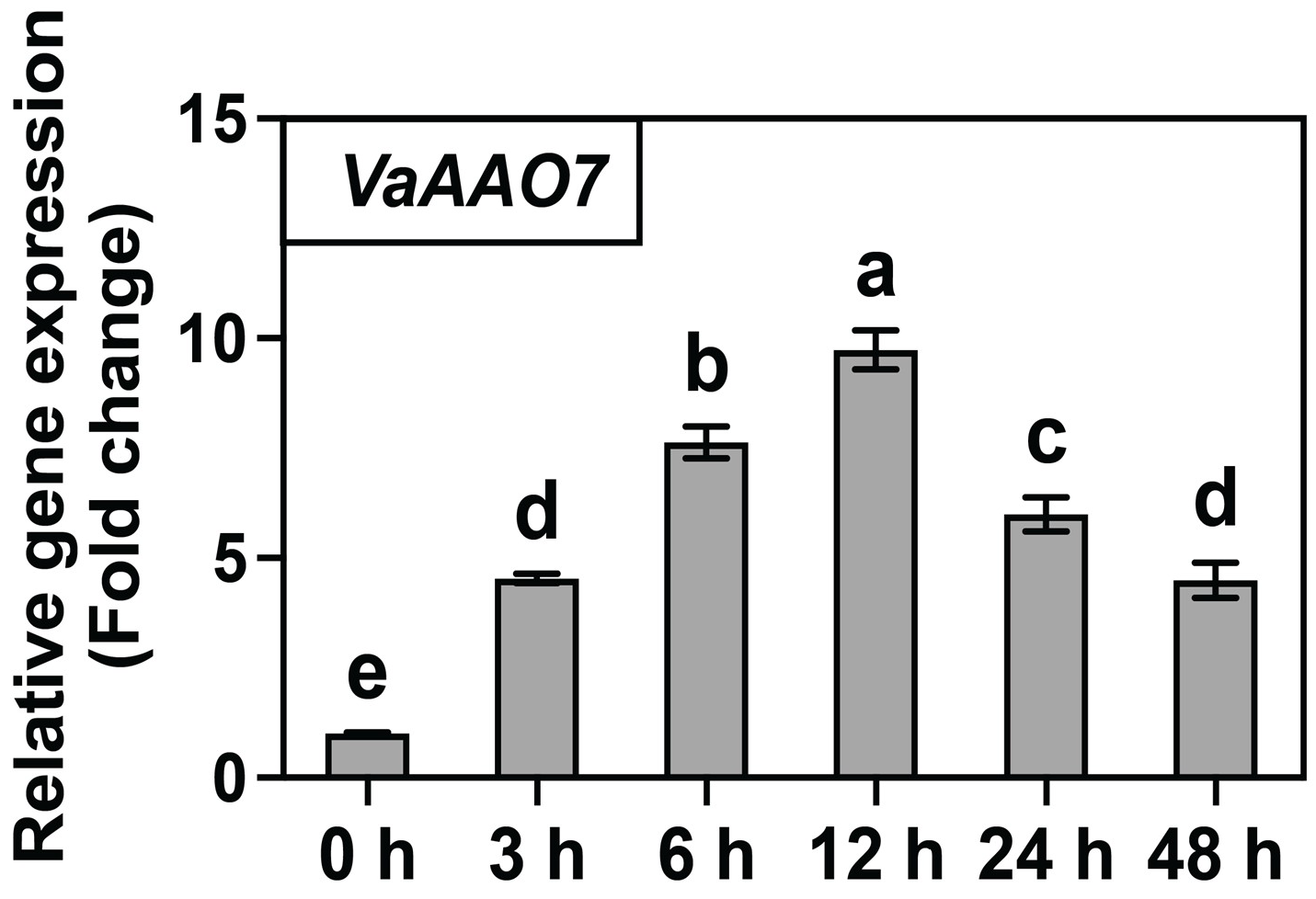
3.5. H2O2 Treatment Triggers Rapid Induction of VaAAO7 in ‘SY’ Leaves
3.6. Transient Overexpression of VaAAO7 Enhanced Resistance to B. cinerea in Susceptible V. vinifera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Bailén, G.; Zapata, P.; Serrano, M.; Castillo, S.; Valero, D. Influence of Carvacrol on Survival of Botrytis cinerea Inoculated in Table Grapes. Int. J. Food Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, G.; Han, R.; Wan, R.; Li, Z.; Wang, X. Ultrastructural Observations of Botrytis cinerea and Physical Changes in Resistant and Susceptible Grapevines. Phytopathology 2022, 112, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretotius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Wan, R.; Hou, X.; Wang, X.; Qu, J.; Singer, S.D.; Wang, Y.; Wang, X. Resistance Evaluation of Chinese Wild Vitis Genotypes against Botrytis cinerea and Different Responses of Resistant and Susceptible Hosts to the Infection. Front. Plant Sci. 2015, 6, 854. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Guo, C.; Hou, X.; Zhu, Y.; Gao, M.; Hu, X.; Zhang, S.; Jiao, C.; Guo, R.; Li, Z.; et al. Comparative Transcriptomic Analysis Highlights Contrasting Levels of Resistance of Vitis vinifera and Vitis amurensis to Botrytis cinerea. Hortic. Res. 2021, 8, 103. [Google Scholar] [CrossRef]
- Dodds, P.N.; Chen, J.; Outram, M.A. Pathogen Perception and Signaling in Plant Immunity. Plant Cell 2024, 36, 1465–1481. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Song, R.; Jia, A.; Xiao, Y.; Sun, Y.; Wang, L.; Mahr, D.; Wu, Z.; Han, Z.; et al. Balanced Plant Helper NLR Activation by a Modified Host Protein Complex. Nature 2025, 639, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tian, X.; Dong, Z.; Li, H.; Chen, X.; Liu, W.; Yin, G.; Ma, S.; Zhang, L.; Cao, A.; et al. An Aegilops Longissima NLR Protein with Integrated CC-BED Module Mediates Resistance to Wheat Powdery Mildew. Nat. Commun. 2024, 15, 8281. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.; He, S.; Xin, X. Pattern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Veloso, J.; van Kan, J.A.L. Many Shades of Grey in Botrytis–Host Plant Interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef]
- Rahman, M.U.; Hanif, M.; Wan, R.; Hou, X.; Ahmad, B.; Wang, X. Screening Vitis Genotypes for Responses to Botrytis cinerea and Evaluation of Antioxidant Enzymes, Reactive Oxygen Species and Jasmonic Acid in Resistant and Susceptible Hosts. Molecules 2019, 24, 5. [Google Scholar] [CrossRef]
- Karpinska, B.; Zhang, K.; Rasool, B.; Pastok, D.; Morris, J.; Verrall, S.R.; Hedley, P.E.; Hancock, R.D.; Foyer, C.H. The Redox State of the Apoplast Influences the Acclimation of Photosynthesis and Leaf Metabolism to Changing Irradiance. Plant Cell Environ. 2018, 41, 1083–1097. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in Plants: Novel Concepts, New Perspectives, and Outstanding Issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Abdelgawad, K.; El-Mogy, M.; Mohamed, M.; Garchery, C.; Stevens, R. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Fotopoulos, V.; Sanmartin, M.; Kanellis, A.K. Effect of Ascorbate Oxidase Over-Expression on Ascorbate Recycling Gene Expression in Response to Agents Imposing Oxidative Stress. J. Exp. Bot. 2006, 57, 3933–3943. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, W.; Li, J.; Pan, X.; Pan, L.; Zhao, J.; Zhang, Y.; Cai, S.; Huang, X.; Wang, A.; et al. The miR528-AO Module Confers Enhanced Salt Tolerance in Rice by Modulating the Ascorbic Acid and Abscisic Acid Metabolism and ROS Scavenging. J. Agric. Food Chem. 2021, 69, 8634–8648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Q.; Liu, F.; Zheng, L.; Bing, J.; Zhou, Y.; Gao, F. Gene Profiling of the Ascorbate Oxidase Family Genes under Osmotic and Cold Stress Reveals the Role of AnAO5 in Cold Adaptation in Ammopiptanthus nanus. Plants 2023, 12, 677. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS Accumulation and Antiviral Defence Control by microRNA528 in Rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef]
- Xu, X.; Miao, X.; Deng, N.; Liang, M.; Wang, L.; Jiang, L.; Zeng, S. Identification of Ascorbate Oxidase Genes and Their Response to Cold Stress in Citrus sinensis. Agriculture 2024, 14, 1643. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, P.; Meng, F.; Yang, Y.; Ding, T.; Liu, H.; Wang, F.; Li, A.; Zhang, Q.; Li, K.; Fan, S.; et al. De Novo Assembling a High-Quality Genome Sequence of Amur Grape (Vitis amurensis Rupr.) Gives Insight into Vitis Divergence and Sex Determination. Hortic. Res. 2024, 11, uhae117. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Andreeva, A.; Blum, M.; Chuguransky, S.R.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Llinares-López, F.; Meng-Papaxanthos, L.; et al. The Pfam Protein Families Database: Embracing AI/ML. Nucleic Acids Res. 2025, 53, D523–D534. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER Web Server: 2018 Update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Glenzel, V.H.; Filgueiras, J.P.C.; Zolet, A.C.T.; Kulcheski, F.R. Evolutionary and Functional Insights into Ascorbate Oxidase Genes in the Fabaceae Plant Family. Plant Gene 2025, 44, 100538. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Turner, D.; Mesirov, J.P. Igv.Js: An Embeddable JavaScript Implementation of the Integrative Genomics Viewer (IGV). Bioinformatics 2023, 39, btac830. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, F.; Su, K.; Lin, H.; Guo, Y. Discovery of Cold-Resistance Genes in Vitis amurensis Using Bud-Based Quantitative Trait Locus Mapping and RNA-Seq. BMC Genom. 2022, 23, 551. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional Classification of Proteins via Subfamily Domain Architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as Designed by Its Users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Withers, J.; Yao, J.; Mecey, C.; Howe, G.A.; Melotto, M.; He, S.Y. Transcription Factor-Dependent Nuclear Localization of a Transcriptional Repressor in Jasmonate Hormone Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 20148–20153. [Google Scholar] [CrossRef]
- Xie, J.; He, C.; Li, Z.; Li, M.; He, S.; Qian, J.; Tan, B.; Zheng, X.; Cheng, J.; Wang, W.; et al. A Rapid and Efficient Agrobacterium-Mediated Transient Transformation System in Grape Berries. Protoplasma 2024, 261, 819–830. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Wang, Z.Q.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.Q.; Li, G.X.; Wu, Y.R.; Yang, J.L.; Ma, J.F.; et al. Transcription Factor WRKY22 Promotes Aluminum Tolerance via Activation of OsFRDL4 Expression and Enhancement of Citrate Secretion in Rice (Oryza Sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Z.; Xiang, J.; Cao, X.; Xu, S.; Zhang, Y.; Zhang, D.; Teng, N. A LlWRKY33-LlHSFA4-LlCAT2 Module Confers Resistance to Botrytis cinerea in Lily. Hortic. Res. 2024, 11, uhad254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, L.; Zhang, L.; Li, J.; Fang, Y.; Zhao, L.; Ren, Y.; Chen, F. Abscisic Acid Enhances Tolerance to Spring Freeze Stress and Regulates the Expression of Ascorbate–Glutathione Biosynthesis-Related Genes and Stress-Responsive Genes in Common Wheat. Mol. Breed. 2020, 40, 108. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Zhang, Y.; Tian, L.; Ma, B.; Chen, Z.; Wei, Z.; Li, A.; Shao, Y.; Cheng, G.; et al. The OsWRKY72–OsAAT30/OsGSTU26 Module Mediates Reactive Oxygen Species Scavenging to Drive Heterosis for Salt Tolerance in Hybrid Rice. J. Integr. Plant Biol. 2024, 66, 709–730. [Google Scholar] [CrossRef]
- Mellidou, I.; Kanellis, A.K. Revisiting the Role of Ascorbate Oxidase in Plant Systems. J. Exp. Bot. 2024, 75, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Dong, L.; Han, X.; Jin, H.; Yin, C.; Han, Y.; Li, B.; Qin, H.; Zhang, J.; Shen, Q.; et al. The TuMYB46L-TuACO3 Module Regulates Ethylene Biosynthesis in Einkorn Wheat Defense to Powdery Mildew. New Phytol. 2020, 225, 2526–2541. [Google Scholar] [CrossRef]
- Li, G.; Liu, J.; Wang, Y.; Han, A.; Liu, H.; Guo, T.; Han, Q.; Kang, G. TaWRKY24-1D, Interacts with TaERFL1a, Regulates DHAR-Mediated ASA-GSH Biosynthesis to Enhance Drought Tolerance in Wheat. Plant Growth Regul. 2024, 104, 713–725. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, P.; Su, H.; Xie, X.; Shao, J.; Ku, L.; Tian, Z.; Deng, D.; Wei, L. Regulatory Mechanisms Used by ZmMYB39 to Enhance Drought Tolerance in Maize (Zea Mays) Seedlings. Plant Physiol. Biochem. 2024, 211, 108696. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Hao, X.; Guo, C.; Wang, X.; Yan, X.; Guo, R. Overexpression of a Grapevine VqWRKY2 Transcription Factor in Arabidopsis Thaliana Increases Resistance to Powdery Mildew. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 157, 16. [Google Scholar] [CrossRef]
- Valeri, M.C.; Novi, G.; Weits, D.A.; Mensuali, A.; Perata, P.; Loreti, E. Botrytis cinerea Induces Local Hypoxia in Arabidopsis Leaves. New Phytol. 2021, 229, 173–185. [Google Scholar] [CrossRef]
- Chen, J.; Clinton, M.; Qi, G.; Wang, D.; Liu, F.; Fu, Z.Q. Reprogramming and Remodeling: Transcriptional and Epigenetic Regulation of Salicylic Acid-Mediated Plant Defense. J. Exp. Bot. 2020, 71, 5256–5268. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Chen, H.; Wang, D.; Zheng, H.; Tang, X.; Guo, Z.; Cheng, J.; Chen, J.; Wang, Y.; Bai, M.; et al. The BZR1-EDS1 Module Regulates Plant Growth-Defense Coordination. Mol. Plant 2021, 14, 2072–2087. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Zhang, D.; Chen, X.; Xia, Z. Transcriptomic and Physiological Analyses Reveal That Jasmonic Acid and Abscisic Acid Coordinately Regulate Cold Stress Response in Myriophyllum aquaticum. Environ. Exp. Bot. 2024, 219, 105645. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Qin, H.; Yang, W.; Liu, Z.; Ouyang, Y.; Wang, X.; Duan, H.; Zhao, B.; Wang, S.; Zhang, J.; Chang, Y.; et al. Mitochondrial VOLTAGE-DEPENDENT ANION CHANNEL 3 Regulates Stomatal Closure by Abscisic Acid Signaling. Plant Physiol. 2024, 194, 1041–1058. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Wei, J.; Liu, L.; Liu, D.; Yan, X.; Yuan, M.; Zhang, L.; Zhang, N.; Ren, Y.; et al. A TaSnRK1α-TaCAT2 Model Mediates Resistance to Fusarium Crown Rot by Scavenging ROS in Common Wheat. Nat. Commun. 2025, 16, 2549. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, M.E.; Arnaiz, A.; Velasco-Arroyo, B.; Grbic, V.; Diaz, I.; Martinez, M. Arabidopsis Response to the Spider Mite Tetranychus Urticae Depends on the Regulation of Reactive Oxygen Species Homeostasis. Sci. Rep. 2018, 8, 9432. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamamoto, S.; Makino, T.; Tobimatsu, Y. Expression of Laccase and Ascorbate Oxidase Affects Lignin Composition in Arabidopsis Thaliana Stems. J. Plant Res. 2024, 137, 1177–1187. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure as Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef]
- Hu, J.; Liu, M.; Zhang, A.; Dai, Y.; Chen, W.; Chen, F.; Wang, W.; Shen, D.; Telebanco-Yanoria, M.J.; Ren, B.; et al. Co-Evolved Plant and Blast Fungus Ascorbate Oxidases Orchestrate the Redox State of Host Apoplast to Modulate Rice Immunity. Mol. Plant 2022, 15, 1347–1366. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A Powerful Tool for Gene Function Study and Crop Improvement. J. Adv. Res. 2021, 29, 207–221. [Google Scholar] [CrossRef]
- Singh, R.R.; Verstraeten, B.; Siddique, S.; Tegene, A.M.; Tenhaken, R.; Frei, M.; Haeck, A.; Demeestere, K.; Pokhare, S.; Gheysen, G.; et al. Ascorbate Oxidation Activates Systemic Defence against Root-Knot Nematode Meloidogyne Graminicola in Rice. J. Exp. Bot. 2020, 71, 4271–4284. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Nobleza, N.; Demeestere, K.; Kyndt, T. Ascorbate Oxidase Induces Systemic Resistance in Sugar Beet Against Cyst Nematode Heterodera Schachtii. Front. Plant Sci. 2020, 11, 591715. [Google Scholar] [CrossRef]


| Gene Name | Sequence ID | Chromosome | Amino Acid No. | Molecular Weight (Kda) | pI | Subcellular Localization | Gene Location |
|---|---|---|---|---|---|---|---|
| VaAAO1 | VAG0101936.1 | Chr02 | 591 | 65.87 | 8.78 | Extr | 4,650,177–4,654,698 |
| VaAAO2 | VAG0108170.1 | Chr06 | 550 | 62.09 | 8.84 | Vacu | 22,520,739–22,522,577 |
| VaAAO3 | VAG0108171.1 | Chr06 | 550 | 62.09 | 8.84 | Vacu | 22,540,213–22,542,051 |
| VaAAO4 | VAG0109268.1 | Chr07 | 581 | 65.14 | 5.8 | Extr | 18,851,122–18,859,588 |
| VaAAO5 | VAG0109429.1 | Chr07 | 667 | 73.81 | 8.89 | Plas | 21,969,204–21,973,103 |
| VaAAO6 | VAG0109430.1 | Chr07 | 535 | 59.98 | 9.23 | Vacu | 21,974,570–21,978,699 |
| VaAAO7 | VAG0109859.1 | Chr07 | 1202 | 134.94 | 6.36 | Cyto | 26,729,891–26,739,673 |
| VaAAO8 | VAG0113138.1 | Chr10 | 540 | 60.80 | 9.46 | Plas | 1,630,999–1,635,987 |
| VaAAO9 | VAG0118612.1 | Chr13 | 611 | 67.47 | 5.29 | Plas | 26,514,513–26,517,220 |
| VaAAO10 | VAG0122405.1 | Chr16 | 592 | 65.70 | 6.42 | Plas | 11,685,114–11,690,564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Yang, Z.; Zheng, L.; Shi, J.; Jiao, J.; Wang, M.; Zhang, K.; Hao, P.; Zhao, Y.; Liu, Y.; et al. Genome-Wide Analysis of Grapevine Ascorbate Oxidase Genes Identifies VaAAO7 in Vitis amurensis as a Positive Regulator of Botrytis cinerea Resistance. Horticulturae 2025, 11, 1211. https://doi.org/10.3390/horticulturae11101211
Shen Y, Yang Z, Zheng L, Shi J, Jiao J, Wang M, Zhang K, Hao P, Zhao Y, Liu Y, et al. Genome-Wide Analysis of Grapevine Ascorbate Oxidase Genes Identifies VaAAO7 in Vitis amurensis as a Positive Regulator of Botrytis cinerea Resistance. Horticulturae. 2025; 11(10):1211. https://doi.org/10.3390/horticulturae11101211
Chicago/Turabian StyleShen, Yawen, Zhenfeng Yang, Liwei Zheng, Jiangli Shi, Jian Jiao, Miaomiao Wang, Kunxi Zhang, Pengbo Hao, Yujie Zhao, Yu Liu, and et al. 2025. "Genome-Wide Analysis of Grapevine Ascorbate Oxidase Genes Identifies VaAAO7 in Vitis amurensis as a Positive Regulator of Botrytis cinerea Resistance" Horticulturae 11, no. 10: 1211. https://doi.org/10.3390/horticulturae11101211
APA StyleShen, Y., Yang, Z., Zheng, L., Shi, J., Jiao, J., Wang, M., Zhang, K., Hao, P., Zhao, Y., Liu, Y., Cong, L., Bai, T., Song, C., Wan, R., & Zheng, X. (2025). Genome-Wide Analysis of Grapevine Ascorbate Oxidase Genes Identifies VaAAO7 in Vitis amurensis as a Positive Regulator of Botrytis cinerea Resistance. Horticulturae, 11(10), 1211. https://doi.org/10.3390/horticulturae11101211






