Abstract
Crop productivity can be affected by biotic and abiotic stressors, and plant growth-promoting bacteria (PGPB) from the genera Bacillus and Burkholderia have the potential to maintain fruit yield and quality, as these bacteria can promote plant growth by solubilizing nutrients, fixing atmospheric nitrogen, producing phytohormones, and exhibiting antagonistic activity against pathogens. This study aimed to evaluate the effects of inoculating plants with Bacillus subtilis and Burkholderia seminalis on their morphological characteristics, fruit technological attributes and yield of common cherry tomatoes (Solanum lycopersicum L.) subjected to induced water deficit. The study was arranged on a split-plot randomized block design, with four water replacement levels (40%, 60%, 80% and 100% of crop evapotranspiration, ETc) and three inoculation treatments (Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation). Data were subjected to analysis of variance using the F-test and compared using Tukey’s test (p < 0.05) and multivariate statistics from principal component analysis. Inoculation with Burkholderia seminalis increased the plant fresh and dry shoot and root mass, as well as root volume. Inoculation with Bacillus subtilis increased carotenoid and chlorophyll b contents. Both inoculations enhanced leaf water content in plants experiencing severe water deficit (40% of ETc). The use of these strains as PGPB increased the fruit soluble solids content. Higher productivity in inoculated plants was achieved through a greater number of fruits per cluster, despite the individual fruits being lighter. Treatments with higher water replacement levels resulted in greater yield. Inoculations showed biotechnological potential in mitigating water deficit in cherry tomatoes.
1. Introduction
Crop productivity can be affected by biotic and abiotic stressors, especially salinity and water deficit in tropical conditions [1,2,3,4]. Some crops are more susceptible to anatomical, physiological and productive changes when subjected to these stressors [5,6,7]. Water stress compromises plant health and productivity by altering its biochemical and molecular functions [8]. This condition, arising from low soil water availability due to high evapotranspiration rates [9,10], manifests in several ways. Key effects include chlorophyll degradation, lowered photosynthetic efficiency, reduced leaf water potential, loss of turgor and consequent stomatal closure, all of which contribute to stunted growth and diminished yields [11,12].
To mitigate the impacts caused by water deficit, the inoculation of plant growth-promoting bacteria (PGPB) can be employed as a sustainable technique, as microorganisms can colonize the rhizosphere and/or plant tissues, conferring beneficial properties that enhance plant performance under environmental adversities [13,14,15,16]. PGPB employs direct and indirect mechanisms to promote plant growth, such as (i) phytostimulation through the synthesis of phytohormones (e.g., auxins, cytokinins, gibberellins) and 1-aminocyclopropane-1-carboxylate (ACC) deaminase, an enzyme that modulates ethylene levels; (ii) biofertilization by making macro- and micronutrients such as nitrogen (N), phosphorus (P) and iron (Fe) more available; and (iii) biological control through competition with phytopathogens for nutrients, induction of systemic resistance in plants and production of secondary metabolites with antimicrobial properties and siderophores [2,12,17,18,19,20,21,22,23].
However, despite the importance of microorganisms in vegetable crops, studies on the use of PGPB to mitigate the effects of water deficit remain limited, with few PGPB strains available for commercialization on a large scale [24]. Further research is needed to assess the potential of microorganisms to promote plant growth and development under abiotic stress conditions, such as water deficit, in tropical conditions [25]. Water deficit occurs when soil water availability decreases, usually resulting from evapotranspiration due to high temperatures [9]. The deficit stresses the plant, alters its biochemical and molecular properties, and can lead to stunted plant growth and low yield [8].
The cherry tomato (Solanum lycopersicum L.) is a key agricultural crop due to its global popularity and widespread consumption [26,27,28]. The crop is widely cultivated, particularly in protected environments, and requires management strategies to mitigate the impacts of abiotic stressors, such as water deficit. Using PGPB in tomato production can enhance crop yield, fruit resistance, antioxidant activity and secondary metabolite concentration [29,30,31], ultimately improving fruit quality [32]. Fruit quality is the primary determinant of consumer purchase preference and is influenced by pre- and post-harvest management, which can affect the fruit’s nutritional composition, size and aroma [32,33]. In this context, Bacillus and Burkholderia have the potential to enhance productivity and fruit quality, as these bacteria can promote plant growth either directly or indirectly [22,34,35,36,37,38,39,40,41,42].
This study aimed to analyze the morphological characteristics, technological attributes and productivity of cherry tomatoes inoculated with Bacillus subtilis strain ATCC 23858 and Burkholderia seminalis strain TC3.4.2R3 under induced water deficit conditions. Additionally, we determined the optimal water replenishment levels using regression analysis and principal component multivariate analysis.
2. Materials and Methods
2.1. Characterization of the Study Area
The experiment was conducted in a greenhouse located in the experimental area of the Goiano Federal Institute of Education, Science and Technology (IF Goiano–Campus Ceres) in the municipality of Ceres, Goiás, Brazil (15°21′01.5″ S latitude, 49°35′55.2″ W longitude, 580 m altitude), from February to July of 2023 (Figure 1).
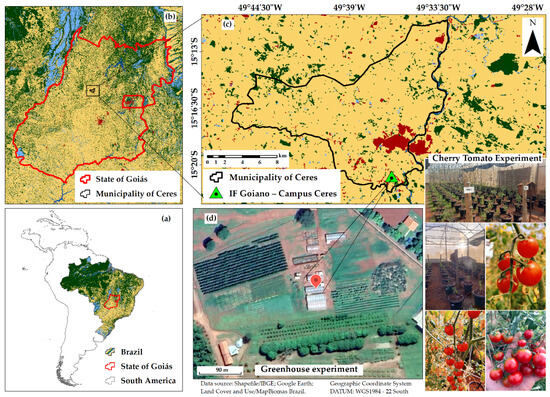
Figure 1.
Spatial location of the study area at continental, regional, and local levels: Brazil (a), state of Goiás (b), municipality of Ceres (c), and the greenhouse (d), with an overview of the tomato (Solanum lycopersicum L.) experiment at the IF Goiano–Campus Ceres.
The region’s climate is classified as Aw, tropical with a dry season during winter according to the Köppen–Geiger climate classification [43]. Temperatures inside the greenhouse were recorded using a weather station (Vantage Pro 2; Davis; Hayward, CA, USA). The recorded air temperature throughout the study ranged from a minimum of 10.5 °C to a maximum of 41.3 °C, with an average of 24.4 °C (Figure 2).
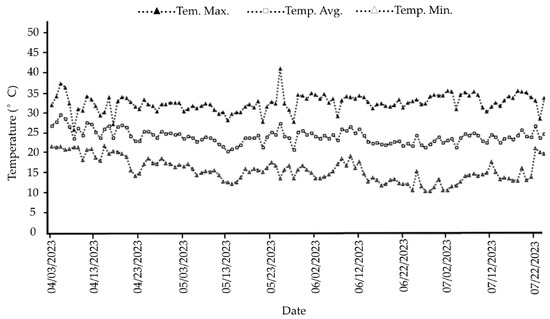
Figure 2.
Maximum (Temp. Max.), minimum (Temp. Min.), and average (Temp. Avg.) daily temperature during the greenhouse experiment with cherry tomato (Solanum lycopersicum L.) at the IF Goiano–Campus Ceres.
2.2. Experimental Design and Layout
The inoculation treatments used in this study were determined using the overlay method [44], which revealed antagonism between the Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 strains. Therefore, the strains were inoculated separately rather than in combination.
The study was arranged in a split-plot randomized block design (RBD) with four blocks. The main plots consisted of four water replacement levels (40%, 60%, 80% and 100% of crop evapotranspiration, ETc), and the subplots comprised three inoculation treatments: (i) inoculation with Bacillus subtilis ATCC 23858, (ii) Burkholderia seminalis TC3.4.2R3 and (iii) non-inoculation (NI). Each experimental plot consisted of four plants, totaling 192 plants.
2.3. Microbiolization
The bacterial strains were cultured in nutrient broth (Kasvi®; Pinhais, PR, Brazil) and incubated at 28 °C in a shaker incubator (SL 222; Solab, Piracicaba, SP, Brazil) at 150 rpm for 24 h. The inoculum was adjusted to an optical density (O.D.) of 1.0 at 600 nm using a spectrophotometer (Dual-Beam UV-Visible Global Analyzer model GTA-101; Calgary, Canada) to obtain a final concentration of 109 colony-forming units (CFU) mL−1.
Cherry tomato seeds (Blueline series; TopSeed Garden, Monte Alto, SP, Brazil) were surface-disinfected by immersion in a 1% sodium hypochlorite (NaOCl) solution for 1 min, followed by immersion in 70% ethanol for 1 min [45]. The seeds were then rinsed with sterile distilled water and placed on sterile filter paper for natural drying in a laminar flow hood. After this process, the seeds were inoculated with bacterial suspensions for 20 min at a ratio of 1 mL of bioinoculant per gram of seed. For the non-inoculated treatment, the seeds were soaked in sterile nutrient broth under the same conditions.
2.4. Seeding, Transplanting and Experimental Management
The inoculated cherry tomato seeds were sown in expanded polystyrene trays with 128 cells (Isoeste, Castanhal, PA, Brazil), containing substrate (Tropstrato HA Hortaliças; Vida Verde, Mogi Mirim, SP, Brazil). This substrate was composed of pine bark, vermiculite, 14-16-18 fertilizer, potassium nitrate, single superphosphate and peat.
At 15 days after sowing (DAS), the seedlings were transferred to 200 cm3 containers filled with the same substrate and re-inoculated with 5 mL of bacterial suspension (109 CFU mL−1) via drenching at the base of the seedling stem. The non-inoculated treatment received 5 mL of sterile nutrient broth. At 30 DAS, the seedlings were transplanted into 16 L commercial flexible pots (23.5 cm height, 27 cm upper width and 23.5 cm base) filled with a substrate composed of soil and sand in a 2:1 ratio. The soil used in the substrate preparation had the following characteristics: 24.2% sand, 8.2% silt and 67.5% clay; pH in H2O of 4.4; organic matter: 3.8 g dm−3; calcium: 0.7 cmolc dm−3; magnesium: 0.6 cmolc dm−3; potassium: 25.9 mg dm−3; phosphorus: 2.6 mg dm−3; base saturation: 35.84%.
Following transplanting into the pots, reinoculation with a bacterial suspension (109 CFU mL−1) was performed by drenching the plant base around the stem with 10 mL of bacterial solution, with additional inoculations conducted biweekly until 90 days after transplanting (DAT). The non-inoculated treatment received sterile nutrient broth under the same conditions.
Before transplanting, the substrate moisture content in the pots was raised to pot capacity (0.37 m3 m−3) [46,47] from transplanting until 105 DAT. A drip irrigation system was used, employing button-type drippers with a flow rate of 2 L h−1 and self-compensating flux. Irrigation was manually managed based on climatic conditions using a Class A mini pan installed at the center of the greenhouse. Daily readings from the pan were taken and the irrigation was applied according to the water replenishment levels (40%, 60%, 80% and 100% of ETc). The water deficit began at 30 DAT and ended at 115 DAT, totaling 85 days. The amount of water deficit was specific to each of the 192 pots. Therefore, we highlight here only calculations of proportional values of the deficit, based on the field capacity adopted in the experiment, with water deficit levels corresponding to 40%: 0.148 (m3 m−3) irrigated/0.222 (m3 m−3) deficit; 60%: 0.222 (m3 m−3) irrigated/0.148 (m3 m−3) deficit; 80%: 0.296 (m3 m−3) irrigated/0.074 (m3 m−3) deficit; 100%: 0.370 (m3 m−3) irrigated/0.000 (m3 m−3) deficit.
The spacing used was 0.7 m between plants, 0.7 m between rows and 1.0 m between double rows, equivalent to a plant density of 20,408 plants per hectare. The plants were trellised using a vertical string system, and preventive disease management was carried out through the application of Bordeaux mixture at 10 DAT and the application of systemic fungicide (Score®; Syngenta, Basel, Switzerland) and the systemic, contact, and ingestion insecticide Platinum Neo (Lagare®; Syngenta, Basel, Switzerland), following label recommendations. Lateral shoot removal (pruning) was performed weekly from 30 DAT. Weed management was conducted manually throughout the experimental period, both in the pots and on the greenhouse floor.
2.5. Experimental Analyses
2.5.1. Plant Morphometric Analyses
Plant morphometric analyses were performed at 105 DAT, assessing root volume (RV), root length (RL, cm), root fresh weight (RFW), root dry weight (RDW), shoot fresh weight (SFW) and shoot dry weight (SDW). Fresh mass was maintained in a forced-air circulation oven for 72 h at 65 °C [48].
2.5.2. Fruit Morphometric Analyses, Productivity and Quality
Fruit harvests were also performed biweekly starting at 60 DAT, totaling four harvests throughout the experimental period. After each harvest, the fruit longitudinal diameter (LD) and equatorial diameter (ED) were measured using a digital caliper (King Tools, Pompéia, SP, Brazil), average fruit weight (FW), number of clusters per plant (NCP), number of fruits per cluster (NFC), number of seeds per fruit (NSF) and total fruit productivity. The tomato fruit quality parameters were assessed through physicochemical analyses of pH, soluble solids content (SSC), water activity (WA) and titratable acidity (TA), using 10 fruits from each treatment per harvest. The evaluations were conducted in triplicate.
The fruit pH was measured directly in the samples by the electrometric method [49]. The total soluble solids content was quantified using a digital refractometer (Brix/RI-Check; Reichert Technologies, Munich, Germany), with results expressed in °Brix [49].
For fruit titratable acidity, 5 g of homogenized tomato juice was used in a 100 mL volumetric flask, with the volume completed with distilled water. A 10 mL aliquot of this solution was transferred to a 50 mL Erlenmeyer flask and 2 to 3 drops of phenolphthalein indicator were added. The sample was titrated with a 0.01 N sodium hydroxide solution until a pink color was reached. WA was measured by direct reading of the sample on a digital device (AquaLab CX-2; Meter Group, Pullman, WA, USA).
The texture of the whole tomato was evaluated using a texture analyzer (TA-XT Plus, Surrey, UK) to assess fruit compression and puncture properties. The pre-test, test and post-test speeds were set at 1, 2 and 10 mm s−1, respectively. The maximum sample height for calibration was 20 mm, with compression P100 and puncture P2. No lubrication was used and the deformation was set to 50%.
2.5.3. Physiological Analyses
For the pigment analysis (chlorophyll a and b and carotenoids), six leaf disks with a 6 mm diameter were placed in a solution of dimethyl sulfoxide (DMSO) saturated with calcium carbonate in a water bath at 65 °C for 24 h before readings were taken on a spectrophotometer at wavelengths of 649, 665 and 480 nm [50].
To determine the relative water content, six leaf disks with a diameter of 1 cm were used. After weighing, the disks were immersed in distilled water and stored in a refrigerator for 6 h. The disks were then weighed again to determine their turgid weight, and subsequently, they were placed in a forced-air circulation oven at 80 °C for 24 h to determine their dry weight. After obtaining the fresh, turgid and dry weights of the leaf disks, the leaf water content (LWC) was determined according to Equation (1) from Smart and Bingham [51].
where FW—Fresh weight of the leaf disks; DW—Dry weight of the leaf disks and TW—Turgid weight of the leaf disks.
2.6. Statistical Analyses
The data were subjected to the Shapiro–Wilk normality test (5% significance) to verify homoscedasticity. Subsequently, they were subjected to analysis of variance (ANOVA) using the F test and significant variables were compared using the Tukey mean comparison test for inoculation types and regression analysis for water replacement levels (WRL) at 5% and 1% significance levels. All analyses were performed using statistical software (R version R-4.2.1 for Windows, R Foundation, Kaysville, UT, USA).
We evaluated the effect of inoculation types (Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculated—control treatment) and water replacement levels on several variables: carotenoid content, chlorophyll a and b, shoot and root fresh weight, shoot and root dry weight, root volume, fruit soluble solids content, total productivity, number of clusters per plant, number of seeds per fruit, number of fruits per cluster, average fruit weight, leaf water content, stem diameter, longitudinal diameter and equatorial diameter. Multivariate statistical analysis was performed using principal component analysis (PCA). To identify the variables that showed correlation, Kaiser’s criterion was applied, based on eigenvalues greater than 1, which generate principal components with a relevant amount of information contained in the original data [52]. Thus, the possible relationships and/or differences among the set of variables were observed through correlation. These analyses and PCA were conducted using statistical software (R version R 3.3.0+ for Windows, R Foundation, Kaysville, UT, USA) [53].
3. Results and Discussion
The results highlight the great biotechnological potential of inoculations, which are promising in mitigating water deficit in cherry tomato cultivation, especially under severe water deficit conditions, such as at a water replacement level of 40% of crop evapotranspiration. The total volume of water applied to cherry tomato plants in both inoculated and non-inoculated treatments was 256.66 mm, 343.06 mm, 429.47 mm, and 515.88 mm at the following water replacement levels of 40%, 60%, 80%, and 100% of ETc, respectively.
3.1. Plant Morphometric Analyses
There was no significant interaction between water replacement levels and inoculations for the variables shoot fresh and dry weight, root fresh and dry weight and root volume. This suggests that the effects of water availability and microbial inoculation can act independently on plant development. Therefore, these factors were analyzed separately. Interestingly, inoculation with Burkholderia seminalis strain TC3.4.2R3 increased the shoot dry and fresh weight and root fresh weight by 135%, 122% and 58%, respectively, compared to non-inoculated plants, whereas inoculation with Bacillus subtilis strain ATCC 23858 did not differ from inoculation with Burkholderia seminalis strain TC3.4.2R3 or the non-inoculated treatment in terms of shoot and root fresh weight (p < 0.05) (Table 1).

Table 1.
Shoot fresh weight (SFW), shoot dry weight (SDW), root fresh weight (RFW), root dry weight (RDW) and root volume (RV) of cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants.
A linear increasing trend was observed for root volume (Figure 3b) and root fresh weight (Figure 3a). Being higher in plants under more severe water deficit or near 100% water replacement (Figure 3). The same was observed in the cultivation of cherry tomato ‘Sweet Heaven’ and ‘Mascot F1’ [54]. However, in this study, root fresh weight was also not affected by the different water replacement levels. Another study reported that the water deficit did not affect the yield parameters of cherry tomato plants [55]. However, the crop responses to the water deficit are closely related to the deficit exposure time [56].
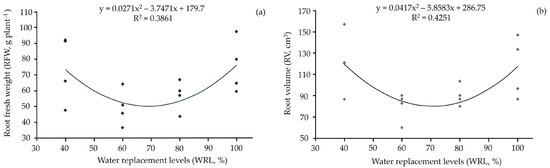
Figure 3.
(a) Root fresh weight and (b) root volume of cherry tomato (Solanum lycopersicum L.) plants subjected to different water replacement levels.
These findings indicate that the plant growth-promoting effect is strain-dependent, reinforcing previous reports that not all beneficial microorganisms exert the same magnitude of response in tomato plants [57,58,59,60,61]. Overall, plants inoculated with either Bacillus subtilis or Burkholderia seminalis exhibited increases in shoot fresh and dry weight, root fresh weight, and root volume, highlighting the potential of the tested strains to enhance tomato growth under varying water regimes.
The positive impact of Burkholderia seminalis on shoot and root weight may be attributed to multiple mechanisms, including the production of phytohormones, enhanced nutrient acquisition, and improved stress tolerance [58]. Several studies have demonstrated that bacterial endophytes can enhance photosynthetic efficiency and stimulate root system architecture, thereby improving water and nutrient uptake [59,60]. Such effects are consistent with the significant improvements in shoot fresh and dry weight observed in inoculated plants. A plant endophytic bacterium, Burkholderia seminalis strain 869T2, promoted plant growth in Arabidopsis thaliana, pak choi (Brassica rapa subsp. chinensis), Chinese amaranth (Amaranthus dubius), lettuce (Lactuca sativa), hot pepper (Capsicum annuum), increasing leaf size, weight, number, and area, in addition to longer and heavier roots [62]. Inoculation with Burkholderia seminalis, either alone or in combination with arbuscular mycorrhizal fungi (Rhizophagus spp.), significantly increased biomass, root and shoot length, as well as chlorophyll content in tomato and bell pepper under drought stress [63].
The inoculation of the tested bacteria did not influence root dry weight, suggesting that the inoculation primarily influenced water-related tissue accumulation rather than structural biomass. Similar results were reported by Ünlü et al. [64] with B. thuringiensis U4-3, B. megaterium strain EU.U21, and Bacillus strain EU.5 in tomato plants.
The root volume was affected by inoculation with the Bacillus subtilis strain ATCC 23858 and the Burkholderia seminalis strain TC3.4.2R3, resulting in increases of 61.61% and 74.10%, respectively, compared to non-inoculated plants (p < 0.05). Inoculation with other strains of the Bacillus genus significantly increased the fresh weight of stems and roots of tomato plants [33,57] and the fresh, dry weight and root volume of ‘Cherry 261’ cherry tomato [58]. Increases in root and aerial part dry matter indices of tomatoes have already been reported in plants inoculated with Burkholderia gladioli and Bacillus subtilis [59]. In another Solanaceae species, Capsicum frutescens, certain Burkholderia strains have shown promise in enhancing plant growth parameters [60].
Inoculation with plant growth-promoting microorganisms, especially from the Bacillus and Burkholderia genus, increases root volume and surface area, as well as the fresh and dry mass production of tomato roots and aerial parts [59,61]. Other research reports that tomato growth may be associated with the ability of Bacillus and Burkholderia bacteria to produce indoleacetic acid, solubilize phosphate and fix nitrogen [19,65].
Taken together, these findings demonstrate that inoculation with selected PGPBs can significantly enhance tomato growth regardless of water availability, probably due to the greater availability of nutrients and phytohormones from the bacteria [66,67,68,69]. These results have practical implications for sustainable tomato production, as microbial inoculation represents a promising strategy to increase crop resilience and productivity under variable irrigation regimes. Future studies should focus on elucidating the physiological and molecular mechanisms involved, as well as evaluating the long-term effects under field conditions.
3.2. Fruit Morphometric Analyses, Productivity and Quality
There was no significant interaction between water replacement levels and inoculations for the variables average fruit weight, soluble solids content, number of seeds per fruit, equatorial and longitudinal diameters. Therefore, these factors were analyzed separately. Plants inoculated with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 exhibited higher soluble solids content but also showed lower average fruit weight and number of seeds per fruit (p < 0.05) (Table 2). Fruits from plants inoculated with the bacterial strains had greater equatorial and longitudinal diameters (p < 0.05). Additionally, under greenhouse conditions, results from Chandrasekaran et al. [33] demonstrated that Bacillus subtilis CBR05 has the potential to enhance plant growth and modify certain quality characteristics of tomato fruit. Studies by Yang et al. [70] using 57 different tomato accessions demonstrated that fruit weight is negatively correlated with soluble solids, possibly due to a dilution effect, where a higher sugar content resulted from increased dry matter content and reduced water content. Similarly, our findings evidenced that, as fruit weight increases, the dilution effect reduces soluble solids content.

Table 2.
Fruit weight (FW), soluble solids content (SSC), number of seeds per fruit (NSF), equatorial diameter (ED) and longitudinal diameter (LD) of cherry tomato (Solanum lycopersicum L.) inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants.
Inoculation with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3, as well as the water replacement levels, did not affect the number of clusters per plant. There was significant interaction between water replacement levels and inoculations for the number of fruits per cluster (p < 0.01) (Table 3). Cherry tomato plants inoculated with Bacillus subtilis ATCC 23858, regardless of water replacement levels, exhibited a similar number of fruits per cluster. In contrast, non-inoculated plants showed a higher number of fruits per cluster only when receiving 100% water replacement (Table 3).

Table 3.
Number of fruits per cluster (NFC) of cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants under different water replacement levels (WRL).
Regarding inoculation with Burkholderia seminalis TC3.4.2R3, the highest number of fruits per cluster was observed at water replacement levels of 80%, 40% and 60%, in that order. The 100% water replacement resulted in the lowest number of fruits per cluster. Additionally, inoculation with Burkholderia seminalis TC3.4.2R3, except at 100% water replacement, increased the number of fruits per cluster compared to plants inoculated with Bacillus subtilis ATCC 23858 and non-inoculated plants. The number of fruits per cluster exhibited a quadratic response to water replacement levels (Figure 4a). Cherry tomato yield also showed a quadratic response to different water replacement levels, with the maximum productivity point corresponding to 92.5% water replacement (Figure 4b).
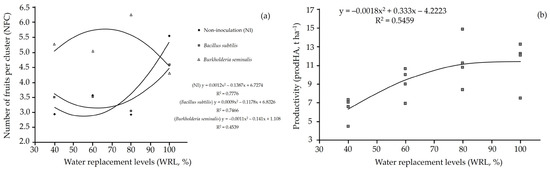
Figure 4.
(a) Number of fruits per cluster and (b) productivity in cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation (NI), subjected to different water replacement levels.
There was no significant interaction between water replacement levels and inoculation regarding fruit quality. Likewise, the physicochemical characteristics of cherry tomatoes, under the conditions of this study, did not differ significantly (Table 4).

Table 4.
Water activity (WA), titratable acidity (TA), pH, puncture (PN) and compression (CP) of cherry tomato (Solanum lycopersicum L.) inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants under different water replacement levels (WRL).
The fruit soluble solids content was not affected by water deficit irrigation, but it was influenced by inoculation with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3. The increase in soluble solids content was higher under water deficit conditions in tomatoes, with increases of 17.9%, 12.2% and 12.4%, respectively, compared to the control treatment [66,71,72]. An increase in soluble solids content in fruits was reported in tomato plants inoculated with PGPB (Azotobacter chroococcum, Azospirillum brasilense, Pseudomonas fluorescens and Bacillus subtilis) [73], which is in agreement with the present work.
The nutritional quality of cherry tomatoes can be affected by changes in the production of amino acids or other secondary metabolites under deficit irrigation or dry conditions, as these pathways are modified under such circumstances [74,75], which may impact the soluble solids content.
Fruit size was not affected by water deficit; however, it was influenced by inoculation with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3. On the other hand, the reduction in fruit size (equatorial diameter and longitudinal diameter) of cherry tomatoes was directly proportional to the water replacement levels, resulting in smaller fruits in situations of induced water deficit [54,66,76]. The average fruit weight of tomato plants that were not inoculated was slightly higher than fruits from plants inoculated with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3. However, similar fruit sizes have been observed in inoculated and non-inoculated tomato plants [77].
Overall, there was an increase in the number of fruits per cluster in cherry tomato plants inoculated with Burkholderia seminalis TC3.4.2R3 compared to the non-inoculated treatment and the inoculated treatment with Bacillus subtilis ATCC 23858. Inoculation with a mix of plant growth-promoting rhizobacteria (PGPR) (Azotobacter chroococcum, Azospirillum brasilense, Pseudomonas fluorescens and Bacillus subtilis) increased the number of clusters per plant, which may be directly related to the number of fruits per cluster [73]. The NC did not differ between inoculated and non-inoculated plants in this study.
The total productivity of cherry tomatoes was not affected by inoculation with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3; however, it was influenced by water replacement levels. Cherry tomatoes under water deficit had reduced productivity. In contrast, the productivity of tomato plants, without water deficit conditions, showed a 36.36% increase in productivity per plant when inoculated with a high concentration of Bacillus velezensis 83 (108 CFU/mL) [78]. A reduction in the productivity of cherry tomatoes from the ‘Sweet Heaven’ and ‘Mascot F1’ cultivars under water deficit conditions was also reported [54].
3.3. Physiological Analyses
The significant interaction between water replacement levels and inoculations with Bacillus subtilis ATCC 23858 for chlorophyll a content stands out (p < 0.05). There was no significant interaction between water replacement levels and inoculations for chlorophyll b and carotenoid content (Table 5). Therefore, these factors were analyzed separately. The chlorophyll b content was higher in plants inoculated with Bacillus subtilis ATCC 23858, while carotenoid content was higher in plants inoculated with Bacillus subtilis ATCC 23858 or in non-inoculated plants (p < 0.05) (Table 5). The study by Chandrasekaran et al. [33] showed that the PGPR strain Bacillus subtilis CBR05 was versatile in improving tomato production, mainly increasing antioxidant activities and carotenoid levels in the fruit.

Table 5.
Chlorophyll a and b and carotenoid contents of cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants under different water replacement levels (WRL).
Plants inoculated with Bacillus subtilis ATCC 23858 showed similar chlorophyll a levels across all water replacement levels, higher than those inoculated with Burkholderia seminalis TC3.4.2R3 and non-inoculated plants (Table 5). The chlorophyll a content exhibited a quadratic response in both inoculated and non-inoculated plants (Figure 5a). The maximum chlorophyll a content in plants inoculated with Bacillus subtilis ATCC 23858 was found at a water replacement of 88.37%. Akila et al. [79] showed a significant increase in Chlorophyll a and b, and carotenoid contents, due to Bacillus subtilis more pronounced inhibition of bacterial spot and tomato leaf spot. The chlorophyll b (Figure 5b) and carotenoid contents (Figure 5c) also showed quadratic behavior, with higher levels observed in water replacement close to 40% or 100%.
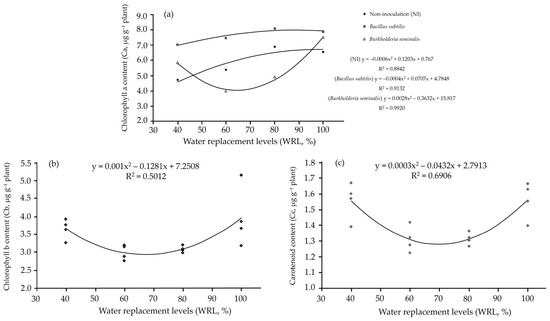
Figure 5.
(a) Chlorophyll a, (b) Chlorophyll b and (c) Carotenoid contents in cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculated plants as a function of water replacement levels.
A significant interaction was observed between water replacement levels and inoculations with Bacillus subtilis ATCC 23858 for leaf water content (p < 0.01, Table 6). Regardless of inoculation, the highest leaf water content was observed in plants that received 100% water replacement. In contrast, plants inoculated with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 that received 40% water replacement showed higher leaf water content than non-inoculated plants (Table 6). The leaf water content exhibited a quadratic response to water replacement levels in plants inoculated with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 and in non-inoculated plants (Figure 6).

Table 6.
Leaf water content (LWC) in cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants under different water replacement levels (WRL).
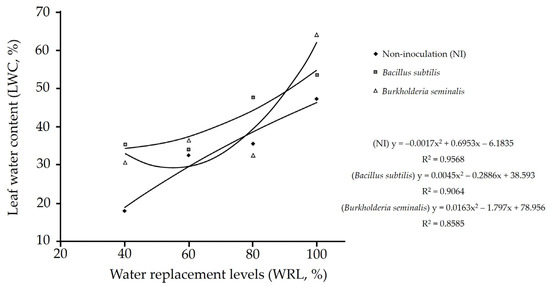
Figure 6.
Leaf water content in cherry tomato (Solanum lycopersicum L.) plants inoculated with Bacillus subtilis ATCC 23858, Burkholderia seminalis TC3.4.2R3 and non-inoculation plants as a function of water replacement levels.
The severe water deficit (40%) did not reduce the levels of chlorophyll a, b and carotenoid content, but lower levels were observed in plants under intermediate water deficit conditions (60% and 80%). An intermediate water deficit in tomatoes resulted in a reduction in these pigments, even under conditions of inoculation with PGPB [80]. Inoculation with Bacillus subtilis ATCC 23858 increased the levels of chlorophyll b and carotenoids, while inoculation with Burkholderia seminalis TC3.4.2R3 increased carotenoid levels compared to non-inoculated plants. However, no differences were reported in the concentration of photosynthetic pigments in cherry tomato cultivars Cherry 261 and Santa Clara 230, whether inoculated or not with Bacillus [58].
The reduction in chlorophyll a, b and carotenoid contents is associated with stomatal closure, the plant’s first response to water stress, to reduce water loss through transpiration. Stomatal closure results in reduced stomatal conductance, which in turn reduces carbon dioxide (CO2) assimilation and, consequently, photosynthesis, leading to smaller plants with lower biomass [81,82].
3.4. Principal Component Analysis (PCA)
The PCA to assess the inoculation effect indicates that the variables under study were concentrated in the bacterial inoculation treatments (Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3), showing greater dissimilarity compared to the non-inoculation treatment (Figure 7a). The sum of the variances of principal components 1 (CP1) and 2 (CP2) accounts for a total variance of 45.81%, meaning that the correlation explanation rate of the information presented corresponds to almost 50% of the total dataset.
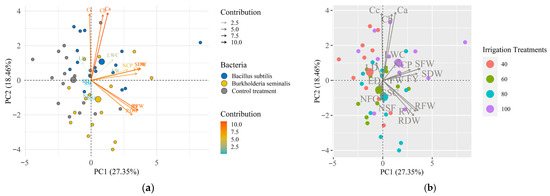
Figure 7.
(a) Principal Component Analysis (PCA) of the variables as a function of the inoculations and (b) irrigation treatments (water replacement levels = 40%, 60%, 80% and 100% of ETc). Legend: shoot fresh weight (SFW), root fresh weight (RFW), shoot dry weight (SDW), root dry weight (RDW), root volume (RV), soluble solids content (SSC), fruit yield (FY), number of clusters per plant (NCP), number of fruits per cluster (NFC), average fruit weight (FW), leaf water content (LWC), chlorophyll a (Ca), chlorophyll b (Cb), carotenoids content (Cc), fruit equatorial diameter (ED), fruit longitudinal diameter (LD) and number of seeds per fruit (NSF).
Figure 7b shows that most variables were concentrated in treatments with 100 and 80% water replacement levels, highlighting a greater dissimilarity between treatments with greater water deficit. According to the PCA biplot, chlorophyll a and b, and carotenoid levels responded to water replacement levels of 40% and 100%, where the increase in the 40% parameters may be associated with positive responses to inoculation with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 (Figure 7).
PCA is of great importance for understanding the morphometric and yield characteristics of the crop, such as shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, root length and root volume, especially when these accumulated values exceed 60% [83,84]. For this type of variable in cherry tomato cultivation, accumulated variances of approximately 70% and 63% have already been found [30,85].
Chandrasekaran et al. [33] highlighted that plant growth-promoting microorganisms, such as Bacillus subtilis, are highly effective microbial competitors, primarily by promoting plant growth through the production of phytohormones and/or increasing nutrient availability via the production of secondary metabolites, or by acting as biocontrol agents to protect plants from phytopathogen infection. The authors specifically emphasize the productive role of Bacillus subtilis CBR05 in significantly increasing the nutritional potential of tomato fruits, total biomass, and root length under greenhouse conditions. Barahona-Pico et al. [86] also observed that the application of vermicompost, either in conjunction with the Bacillus consortium or individually, significantly increases the weight of the fruits, in addition to improving the nutritional value of cherry tomatoes. Xiao-Ying et al. [87] highlights that the microbial effect of Bacillus subtilis is one of the most important in agriculture, acting mainly as a PGPR in greenhouse-grown tomatoes.
4. Conclusions
Inoculation with Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 demonstrated biotechnological potential in mitigating water deficit in cherry tomato cultivation, offering a practical application for more sustainable agriculture.
The use of Bacillus subtilis ATCC 23858 and Burkholderia seminalis TC3.4.2R3 as PGPB did not affect fruit quality under the conditions of this study, except for the soluble solids content. Burkholderia seminalis TC3.4.2R3 promoted an increase in the shoot and root fresh and dry mass, as well as an increase in root volume. On the other hand, Bacillus subtilis ATCC 23858 promoted an increase in carotenoid and chlorophyll b levels.
Both inoculations promoted an increase in the leaf water content of cherry tomato plants under more severe water deficit conditions (40% of crop evapotranspiration). The higher water replacement levels (80% and 100% of crop evapotranspiration) promoted higher fruit productivity, which decreased with deficit irrigation.
Author Contributions
Conceptualization, H.F.E.d.O. and T.D.S.; methodology, T.D.S., J.L.B.d.S., M.V.d.S. and R.S.F.; software, T.D.S., J.A.O.S.S., J.L.B.d.S. and M.V.d.S.; validation, H.F.E.d.O., R.S.F., M.M. and P.J.R.G.S.; formal analysis, M.M., R.S.F., J.A.O.S.S. and P.J.R.G.S.; investigation, T.D.S., J.L.B.d.S., H.F.E.d.O. and M.V.d.S.; resources, H.F.E.d.O., T.D.S. and R.S.F.; data curation, T.D.S., J.L.B.d.S. and M.V.d.S.; writing—original draft preparation, T.D.S., H.F.E.d.O. and R.S.F.; writing—review and editing, J.A.O.S.S., J.L.B.d.S., M.V.d.S., R.S.F., P.J.R.G.S. and M.M.; visualization, J.A.O.S.S., M.M. and P.J.R.G.S.; supervision, H.F.E.d.O.; project administration, H.F.E.d.O.; funding acquisition, H.F.E.d.O. and R.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Council for Scientific and Technological Development (CNPq). The authors also acknowledge the Foundation for Research Support of the State of Goiás (FAPEG) for the financial support provided throughout the second author’s master’s degree (Grant No. 202110267000503).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Cerrado Irrigation Graduate Program, the Laboratory of Irrigation Technologies (Lab.TI) at the Federal Institute of Goiano–Campus Ceres, and the University of Georgia Controlled Environment Agriculture Crop Physiology and Production Laboratory for the technical and technological support provided throughout the development of this research. All individuals acknowledged herein have provided their explicit consent for inclusion in this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could be perceived to influence the work reported in this paper.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Ghaderi, N.; Hatami, M.R.; Mozafari, A.; Siosehmardeh, A. Change in antioxidant enzymes activity and some morpho-physiological characteristics of strawberry under long-term salt stress. Physiol. Mol. Biol. Plants 2018, 24, 833–843. [Google Scholar] [CrossRef]
- Hirst, A.K.; Anee, S.A.; Housley, M.J.; Qin, K.; Ferrarezi, R.S. Selected beneficial microbes alleviate salinity stress in hydroponic lettuce and pak choi. HortTechnology 2024, 34, 345–352. [Google Scholar] [CrossRef]
- Elsharawy, H.; Refat, M. SAL1 gene: A promising target for improving abiotic stress tolerance in plants, a mini review. Physiol. Mol. Biol. Plants 2025, 31, 1–9. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.; Singh, S.; Singh, R.K.; Alexiou, A.; Sousa, J.R.; El-Ramady, H.; Burachevskaya, M.; Rajput, V.D.; Ghazaryan, K. Addressing abiotic stresses and advancing SDGs by biochar for sustainable agriculture and environmental restoration. Egypt. J. Soil Sci. 2025, 65, 463–489. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × Ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Venditti, A.; Zahra, N.; Siddique, K.H.; Farooq, M. Plant adaptation to drought stress: The role of anatomical and morphological characteristics in maintaining the water status. J. Soil Sci. Plant Nutr. 2024, 25, 409–427. [Google Scholar] [CrossRef]
- Machado, J.; Fernandes, A.P.G.; Bokor, B.; Vaculík, M.; Kostoláni, D.; Kokavcová, A.; Heuvelink, E.; Vasconcelos, M.W.; Carvalho, S.M.P. Tomato responses to nitrogen, drought and combined stresses: Shared and specific effects on vascular plant anatomy, nutrient partitioning and amino acids profile. Plant Physiol. Biochem. 2025, 221, 109649. [Google Scholar] [CrossRef]
- Meena, V.S.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Agriculturally important microbes for sustainable agriculture. In Applications in Crop Production and Protection; Springer: Singapore, 2017; Volume 2, pp. 3–23. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Lu, J.; Jia, L.; Menenti, M.; Zheng, C.; Hu, G.; Ji, D. The impacts of drought on water availability: Spatial and temporal analysis in the belt and road region (2001–2020). Int. J. Digit. Earth 2025, 18, 1–26. [Google Scholar] [CrossRef]
- Valença, D.d.C.; de Carvalho, D.F.; Reinert, F.; Azevedo, R.A.; de Pinho, C.F.; Medici, L.O. Automatically controlled deficit irrigation of lettuce in “Organic potponics”. Sci. Agric. 2018, 75, 52–59. [Google Scholar] [CrossRef]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to mitigate the adverse effect of drought stress on crop plants—Influences of soil bacteria: A review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.d.S.; Bastos, A.d.C.; Fernandes, G.C.; de Lima, B.H.; Furlani Junior, E.; de Carvalho, P.H.G.; Galindo, F.S.; Gato, I.M.B.; Teixeira Filho, M.C.M. Nanozinc and plant growth-promoting bacteria improve biochemical and metabolic attributes of maize in tropical cerrado. Front. Plant Sci. 2023, 13, 1046642. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Chakraborty, N.; Halder, S.; Keswani, C.; Vaca, J.; Ortiz, A.; Sansinenea, E. New aspects of the effects of climate change on interactions between plants and microbiomes: A review. J. Basic Microbiol. 2024, 64, e2400345. [Google Scholar] [CrossRef]
- Flores Clavo, R.; Suclupe-Campos, D.O.; Castillo Rivadeneira, L.; Velez Chicoma, R.L.d.J.; Sánchez-Purihuamán, M.; Quispe Choque, K.G.; Casado Peña, F.L.; Binatti Ferreira, M.; Fantinatti Garboggini, F.; Carreño-Farfan, C. Harnessing PGPRs from Asparagus officinalis to increase the growth and yield of Zea mays L. Microb. Ecol. 2024, 87, 174. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Pal, L.; Dwivedi, V.; Dwivedi, V.; Tripathi, D.M. Microbial ACC Deaminase: Stress modulators in plants. In Microbial Enzymes: Production, Purification and Industrial Applications; Wiley: Hoboken, NJ, USA, 2025; Volume 2, pp. 697–720. [Google Scholar] [CrossRef]
- Asari, S.; Tarkowská, D.; Rolčík, J.; Novák, O.; Palmero, D.V.; Bejai, S.; Meijer, J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta 2017, 245, 15–30. [Google Scholar] [CrossRef]
- Gwa, V.I.; Ekefan, E.J. Microbial secondary metabolites and their roles in biocontrol of phytopathogens. In Bioactive Microbial Metabolites; Academic Press: Cambridge, MA, USA, 2024; pp. 1–30. [Google Scholar] [CrossRef]
- Teja, B.S.; Jamwal, G.; Gupta, V.; Verma, M.; Sharma, A.; Sharma, A.; Pandit, V. Biological control of bacterial leaf blight (BLB) in rice–A sustainable approach. Heliyon 2025, 11, e41769. [Google Scholar] [CrossRef]
- Rabbee, F.M.; Baek, K.H. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef]
- Alamoudi, S.A. Using some microorganisms as biocontrol agents to manage phytopathogenic fungi: A comprehensive review. J. Plant Pathol. 2024, 106, 3–21. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, A.; Chen, Y.; Xu, Z.; Liu, Y.; Yao, Y.; Wang, Y.; Jia, B. Beneficial microorganisms: Regulating growth and defense for plant welfare. Plant Biotechnol. J. 2025, 23, 986–998. [Google Scholar] [CrossRef]
- Assouguem, A.; Hamadi, Y.; Amiri, S.; Mokrini, F.; Ennahli, S.; Lahlali, R. Exploring the impact of water stress and PGPR inoculation on morphological, physiological, and biochemical parameters in tomato plants. Atlas J. Plant Biol. 2024, 2024, 106–114. [Google Scholar] [CrossRef]
- Arshad, A.; Cîmpeanu, S.M.; Jerca, I.O.; Sovorn, C.; Ali, B.; Badulescu, L.A.; Drăghici, E.M. Assessing the growth, yield, and biochemical composition of greenhouse cherry tomatoes with special emphasis on the progressive growth report. BMC Plant Biol. 2024, 24, 1002. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Zhou, R.; Hu, E.; Peng, X.; Jiang, F.; Wu, Z. An optimized protocol for comprehensive evaluations of salt tolerance in crop germplasm accessions: A case study of tomato (Solanum lycopersicum L.). Agronomy 2024, 14, 842. [Google Scholar] [CrossRef]
- Vultaggio, L.; Bellitto, P.; Mancuso, F.; Campana, E.; Ciriello, M.; Consentino, B.B.; Rouphael, Y.; Colla, G.; Karavidas, I.; La Bella, S.; et al. Genotype-biostimulant association reveals the guidelines for an improved cherry tomato soilless cultivation. Sci. Hortic. 2025, 343, 114097. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Silva, P.C.; Ferreira, A.F.A.; Araújo, E.S.; Bessa Neto, J.V.; Costa, A.R.D.; Fernandes, L.D.S.; Martins, A.A.S.; Cândido, R.S.; Jardim, A.M.R.F.; Pandorfi, H.; et al. Cherry tomato crop management under irrigation levels: Morphometric characteristics and their relationship with fruit production and quality. Gesunde Pflanzen 2023, 75, 1277–1288. [Google Scholar] [CrossRef]
- Wang, L.; Ju, C.; Han, C.; Yu, Z.; Bai, M.Y.; Wang, C. The interaction of nutrient uptake with biotic and abiotic stresses in plants. J. Integr. Plant Biol. 2025, 67, 455–487. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C.; Oh, J.W.; Paramasivan, M.; Saini, R.K.; Sahayarayan, J.J. Bacillus subtilis CBR05 for tomato (Solanum lycopersicum) fruits in South Korea as a novel plant probiotic bacterium (PPB): Implications from total phenolics, flavonoids, and carotenoids content for fruit quality. Agronomy 2019, 9, 838. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, X.; Wang, C.; Ma, N.; Xie, J.; Zhang, J. Fruit quality analysis and flavor comprehensive evaluation of cherry tomatoes of different colors. Foods 2024, 13, 1898. [Google Scholar] [CrossRef]
- Mellado, J.C.; Lemus, J.O.; Santos, P.E.; Aguilar, L.M. The tomato rhizosphere, an environment rich in nitrogen-fixing Burkholderia species with capabilities of interest for agriculture and bioremediation. Appl. Environ. Microbiol. 2007, 73, 5308–5319. [Google Scholar] [CrossRef]
- Fan, B.; Blom, J.; Klenk, H.P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group b. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Thomas, B.O.; Lechner, S.L.; Ross, H.C.; Joris, B.R.; Glick, B.R.; Stegelmeier, A.A. Friends and foes: Bacteria of the hydroponic plant microbiome. Plants 2024, 13, 3069. [Google Scholar] [CrossRef]
- Singh, S.; Bhoi, T.K.; Vyas, V.; Khan, I.; Rathi, A.; Singh, I. Microbial bioinoculants: Boosting horticultural productivity. In Bio-Inoculants in Horticultural Crops; Woodhead Publishing: Cambridge, UK, 2024; pp. 1–20. [Google Scholar] [CrossRef]
- Sanyal, M.; Chowdhury, D.; Ghosh, A.; Bandyopadhyay, S. Bio-stimulating role of plant growth promoting microorganisms in the sustainable production of micro greens. In Recent Trends and Applications of Leguminous Microgreens as Functional Foods; Springer Nature: Cham, Switzerland, 2025; pp. 315–337. [Google Scholar] [CrossRef]
- Ding, S.; Li, P.; Tang, Y.; He, Z.; She, X. Identification and genomic insights into Bacillus siamensis strains with host colonization potential and activity against tomato bacterial wilt. Pest Manag. Sci. 2024, 81, 1547–1561. [Google Scholar] [CrossRef]
- Vibha, R.; Granada, D.L.; Skariyachan, S.; Ujwal, P.; Sandesh, K. In vitro and in silico investigation deciphering novel antifungal activity of endophyte Bacillus velezensis CBMB205 against Fusarium oxysporum. Sci. Rep. 2025, 15, 684. [Google Scholar] [CrossRef]
- Kaleramana, P.; Sangwan, S.; Swami, P.; Kumar, M.; Singh, S.; Kumar, K. Biosurfactant-mediated synthesis of nanosilver and its antagonistic activity towards microbial phytopathogens of tomato (Solanum lycopersicum L.) Crop. BioNanoScience 2025, 15, 176. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Romero, R.S. Bactérias Fitopatogênicas, 2nd ed.; UFV: Viçosa, Brazil, 2005. [Google Scholar]
- Araújo, W.L.; Maccheroni, W., Jr.; Aguilar-Vildoso, C.I.; Barroso, P.A.V.; Saridakis, H.O.; Azevedo, J.L. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 2001, 47, 229–236. [Google Scholar] [CrossRef]
- Casaroli, D.; Jong van Lier, Q.d. Critérios para determinação da capacidade de vaso. Rev. Bras. Ciência do Solo 2008, 32, 59–66. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Dongli, S.; Zhipeng, L.; Elshaikh, N.A.; Guangcheng, S.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Borges, B.M.M.N.; Lucas, F.T.; Modesto, V.C.; Prado, R.D.M.; Santos, E.; Braos, B.B. Methods of determination dry matter and macronutrient content in lettuce leaves. Rev. Trópica 2011, 5, 12–16. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 30 August 2023).
- Oliveira, H.F.E.; Campos, H.M.; Mesquita, M.; Machado, R.L.; Vale, L.S.R.; Siqueira, A.P.S.; Ferrarezi, R.S. Horticultural performance of greenhouse cherry tomatoes irrigated automatically based on soil moisture sensor readings. Water 2021, 13, 2662. [Google Scholar] [CrossRef]
- Lima, T.P.; Gomes-Filho, R.R.; Cadore, R.; Freitas, D.S.; Carvalho, C.M.; Aguiar-Netto, A.O. Lâminas de irrigação e formas de adubação na produção de tomate de mesa. Agropecuária Técnica 2017, 38, 18–25. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Cui, J.; Wang, X.; Keabetswe, L. Yield, fruit quality and water use efficiency of tomato for processing under regulated deficit irrigation: A meta-analysis. Agric. Water Manag. 2019, 222, 301–312. [Google Scholar] [CrossRef]
- Cendales, T.C.; González, C.A.R.; Cuásquer, C.P.V.; Alzate, O.A.T.; Rodríguez, A.H. Bacillus effect on the germination and growth of tomato seedlings (Solanum lycopersicum L.). Acta Biol. Colomb. 2017, 22, 37–44. [Google Scholar] [CrossRef]
- Zechin, V.J.S.; Ikeda, A.C.; Mógor, Á.F. Alteraciones bioquímicas y de desarrollo de dos cultivares de tomate bajo la inoculación de diferentes dosis de Bacillus spp. Idesia 2022, 40, 59–66. [Google Scholar] [CrossRef]
- Kumar, P.; Aeron, A.; Shaw, N.; Singh, A.; Bajpai, V.K.; Pant, S.; Dubey, R.C. Seed bio-priming with tri-species consortia of phosphate solubilizing rhizobacteria (PSR) and its effect on plant growth promotion. Heliyon 2020, 6, e05701. [Google Scholar] [CrossRef]
- Sabu, R.; Aswani, R.; Nidheesh, K.S.; Ray, J.G.; Remakanthan, A.; Radhakrishnan, E.K. Beneficial changes in Capsicum frutescens due to priming by plant probiotic Burkholderia Spp. Probiotics Antimicrob. Proteins 2019, 11, 519–525. [Google Scholar] [CrossRef]
- Tripti; Kumar, A.; Usmani, Z.; Kumar, V.; Anshumali. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manag. 2017, 190, 20–27. [Google Scholar] [CrossRef]
- Hwang, H.H.; Chien, P.R.; Huang, F.C.; Hung, S.H.; Kuo, C.H.; Deng, W.L.; Chiang, E.I.; Huang, C.C. A plant endophytic bacterium, Burkholderia seminalis Strain 869T2, promotes plant growth in Arabidopsis, pak choi, chinese amaranth, lettuces, and other vegetables. Microorganisms 2021, 9, 1703. [Google Scholar] [CrossRef]
- Tallapragada, P.; Dikshit, R.; Seshagiri, S. Influence of Rhizophagus spp. and Burkholderia seminalis on the growth of tomato (Lycopersicon esculatum) and bell pepper (Capsicum annuum) under drought stress. Commun. Soil Sci. Plant Anal. 2016, 47, 1975–1984. [Google Scholar] [CrossRef]
- Ünlü, E.; Yilmaz, S.; Yetişir, H.; Karim, A.A.; Gün, B.; Idris, A.B. Characterization of multi-trait plant growth-promoting rhizobacteria isolated from alfalfa rhizosphere and evaluation of their efficacy on tomato and watermelon growth. Discov. Agric. 2024, 2, 117. [Google Scholar] [CrossRef]
- Borriss, R. Phytostimulation and biocontrol by the plant-associated Bacillus amyloliquefaciens FZB42: An update. In Bacilli and Agrobiotechnology; Islam, M., Rahman, M., Pandey, P., Jha, C., Aeron, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 163–184. ISBN 978-3-319-44408-6. [Google Scholar] [CrossRef]
- Kumar, P.S.; Singh, Y.; Nangare, D.D.; Bhagat, K.; Kumar, M.; Taware, P.B.; Kumari, A.; Minhas, P.S. Influence of growth stage specific water stress on the yield, physico-chemical quality and functional characteristics of tomato grown in shallow basaltic soils. Sci. Hortic. 2015, 197, 261–271. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.K.; Singh, P.P.; Kumar, A. Interaction of plant growth promoting bacteria with tomato under abiotic stress: A review. Agric. Ecosyst. Environ. 2018, 267, 129–140. [Google Scholar] [CrossRef]
- Muñoz-Torres, P.; Huanca-Mamani, W.; Cárdenas-Ninasivincha, S.; Aguilar, Y.; Quezada, A.; Bugueño, F. Plant growth-promoting and herbicidal bacteria as potential bio-based solutions for agriculture in desertic regions. Plants 2024, 14, 9. [Google Scholar] [CrossRef]
- Taiwo, M.O.; Akintokun, A.K. Plant growth-promoting bacteria delayed wilting and improved tomato yield when grown under water stress condition. J. Plant Nutr. 2025, 48, 1931–1949. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Tang, Y.; Li, Z.; Yang, L.; Gao, J. Comparative evaluation of appearance and nutritional qualities of 57 tomato (Solanum lycopersicum L.) accessions. Horticulturae 2025, 11, 796. [Google Scholar] [CrossRef]
- Lovelli, S.; Potenza, G.; Castronuovo, D.; Perniola, M.; Candido, V. Yield, quality and water use efficiency of processing tomatoes produced under different irrigation regimes in Mediterranean environment. Ital. J. Agron. 2017, 12, 17–24. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, P.; Ren, S.; Jiang, G.; He, X. Effect of regulated deficit irrigation on the plant growth, yield and quality of processing tomato under border irrigation in Hetao Irrigation District. China Agric. Univ. 2016, 21, 83–90. [Google Scholar] [CrossRef]
- Aini, N.; Yamika, W.S.D.; Pahlevi, R.W. The effect of nutrient concentration and inoculation of PGPR and AMF on the yield and fruit quality of hydroponic cherry tomatoes (Lycopersicon esculentum Mill. Var. Cerasiforme). J. Appl. Hortic. 2019, 21, 116–122. [Google Scholar] [CrossRef]
- Klunklin, W.; Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef]
- González-Chavira, M.M.; Herrera-Hernández, M.G.; Guzmán-Maldonado, H.; Pons-Hernández, J.L. Controlled water deficit as abiotic stress factor for enhancing the phytochemical content and adding-value of crops. Sci. Hortic. 2018, 234, 354–360. [Google Scholar] [CrossRef]
- Silva, C.J.; Pontes, N.C.; Golynski, A.; Braga, M.B.; Quezado-Duval, A.M.; Silva, N.E.P. Performance of processing tomatoes under different supply levels of crop evapotranspiration. Hortic. Bras. 2018, 36, 299–305. [Google Scholar] [CrossRef]
- Distefano, M.; Steingass, C.B.; Leonardi, C.; Giuffrida, F.; Schweiggert, R.; Mauro, R.P. Effects of a plant-derived biostimulant application on quality and functional traits of greenhouse cherry tomato cultivars. Food Res. Int. 2022, 157, 111218. [Google Scholar] [CrossRef]
- Balderas-Ruíz, K.A.; Gómez-Guerrero, C.I.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Aranda-Ocampo, S.; Juárez, A.M.; Leyva, E.; Galindo, E.; Serrano-Carreón, L. Bacillus velezensis 83 increases productivity and quality of tomato (Solanum lycopersicum L.): Pre and Postharvest Assessment. Curr. Res. Microb. Sci. 2021, 2, 100076. [Google Scholar] [CrossRef]
- Akila, A.H.; Ali, M.A.S.; Khairy, A.M.; Elnahal, A.S.M.; Alfassam, H.E.; Rudayni, H.A.; Jaber, F.A.; Tohamy, M.R.A. Biological control of tomato bacterial leaf spots and its impact on some antioxidant enzymes, phenolic compounds, and pigment content. Biology 2024, 13, 369. [Google Scholar] [CrossRef]
- Lucas, J.A.; Garcia-villaraco, A.; Montero-palmero, M.B.; Solano, B.R.; Gutierrez-mañero, F.J.; Montalban, B. Physiological and genetic modifications induced by plant-growth-promoting rhizobacteria (PGPR) in tomato plants under moderate water stress. Biology 2023, 12, 901. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Mutari, B.; Sibiya, J.; Matova, P.M.; Gasura, E.; Simango, K. Drought stress impact on agronomic, shoot, physiological, canning and nutritional quality traits of navy beans (Phaseolus vulgaris L.) under field conditions in Zimbabwe. Field Crops Res. 2023, 292, 108826. [Google Scholar] [CrossRef]
- Jardim, A.M.d.R.F.; da Silva, T.G.F.; de Souza, L.S.B.; Souza, M.d.S.; de Morais, J.E.F.; Araújo, G.D.N. Multivariate analysis in the morpho-yield evaluation of forage cactus intercropped with sorghum. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 756–761. [Google Scholar] [CrossRef]
- Jardim, A.M.R.F.; Silva, M.V.; Silva, A.R.; Santos, A.; Pandorfi, H.; Oliveira-Júnior, J.F.; Lima, J.L.M.P.; Souza, L.S.B.; Araújo Júnior, G.N.; Lopes, P.M.O.; et al. Spatiotemporal climatic analysis in Pernambuco State, Northeast Brazil. J. Atmos. Sol.-Terr. Phys. 2021, 223, 105733. [Google Scholar] [CrossRef]
- Rapa, M.; Ciano, S.; Ruggieri, R.; Vinci, G. Bioactive compounds in cherry tomatoes (Solanum lycopersicum Var. Cerasiforme): Cultivation techniques classification by multivariate analysis. Food Chem. 2021, 355, 129630. [Google Scholar] [CrossRef]
- Barahona-Pico, Y.A.; Ortíz-Paz, R.A.; Narváez-Ortiz, I. The effect of Bacillus Spp. and vermicompost on the growth of cherry tomato, Solanum lycopersicum L., Fruits. Rev. Ciencias Agrícolas 2024, 41, e3244. [Google Scholar] [CrossRef]
- Xiao-ying, G.; Chun-e, H.; Tao, L.; Zhu, O. Effect of Bacillus subtilis and Pseudomonas fluorescens on growth of greenhouse tomato and rhizosphere microbial community. J. Northeast. Agric. Univ. (Engl. Ed.) 2015, 22, 32–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).