Influence of Combined Supplemental Lighting and Nutrient Solution Concentration on Fruit Production and Quality of Cherry Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Treatments
2.3. Plant Morphology and Fruit Production
2.4. Photosynthetic Characteristics
2.5. Mineral Element Accumulation
2.6. Fruit Quality
2.7. Contents of Soluble Sugar and Organic Acid
2.8. Statistical Analysis
3. Results
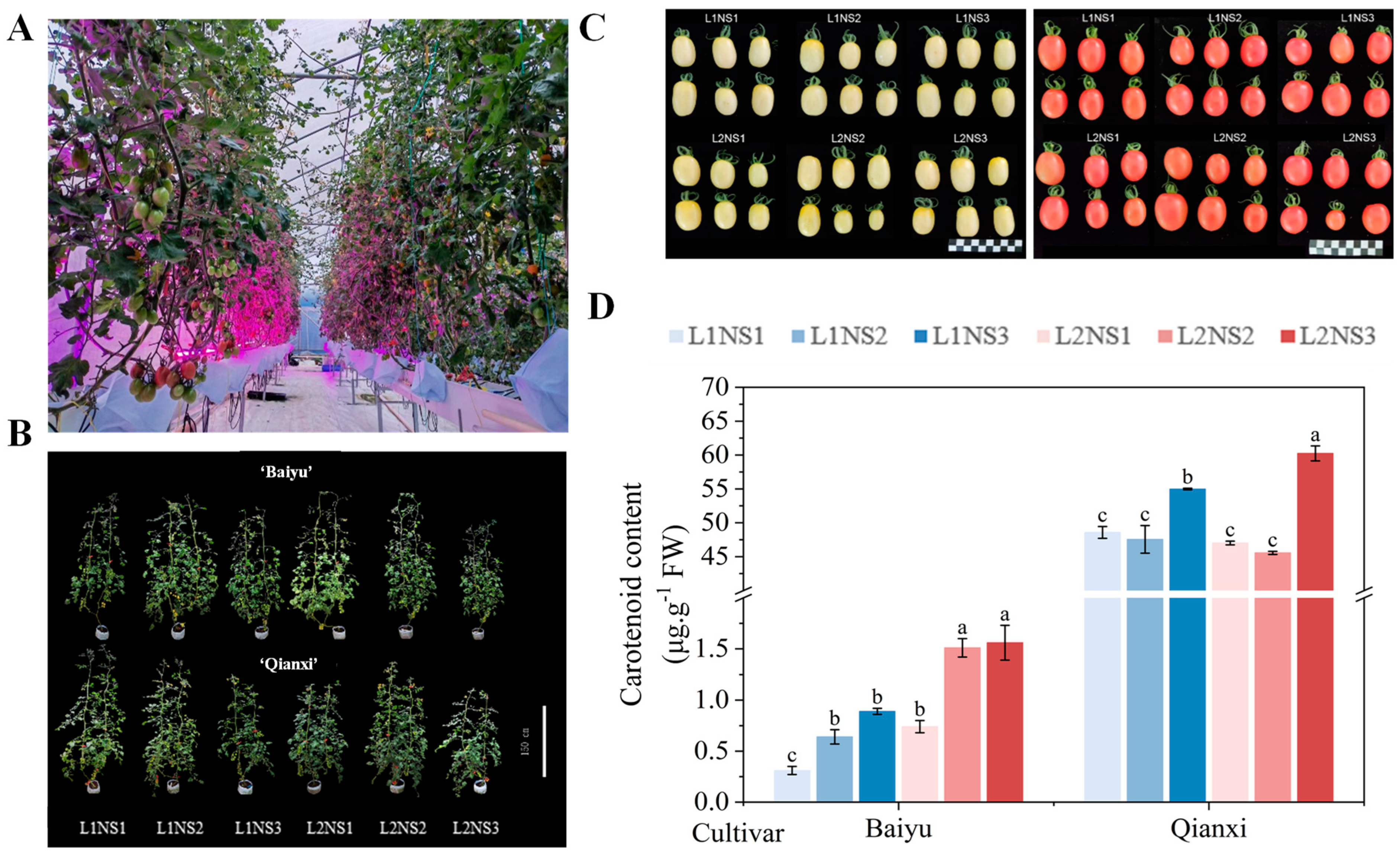
3.1. Plant Morphology
3.2. Photosynthesis Capacity
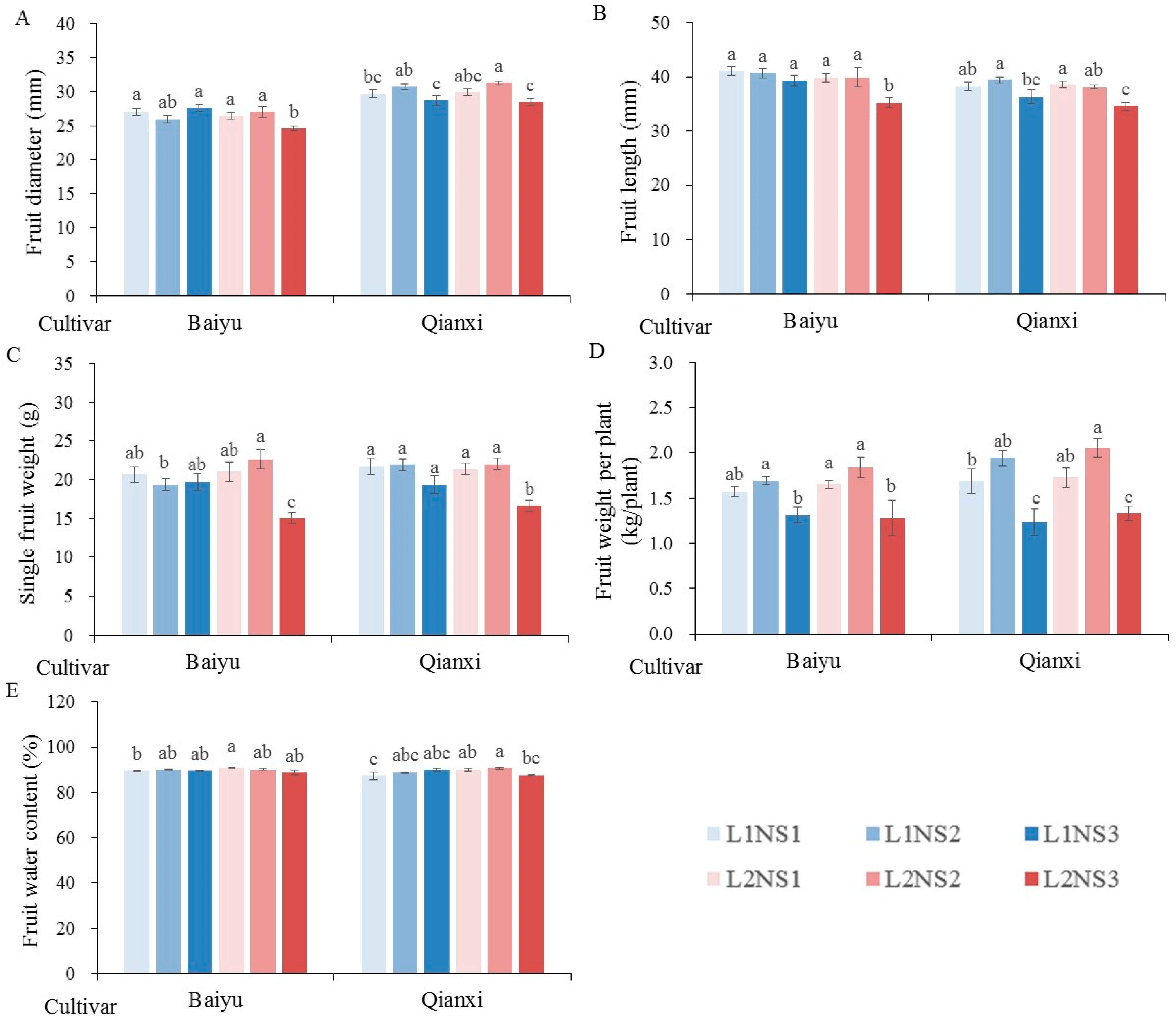
3.3. Fruit Production
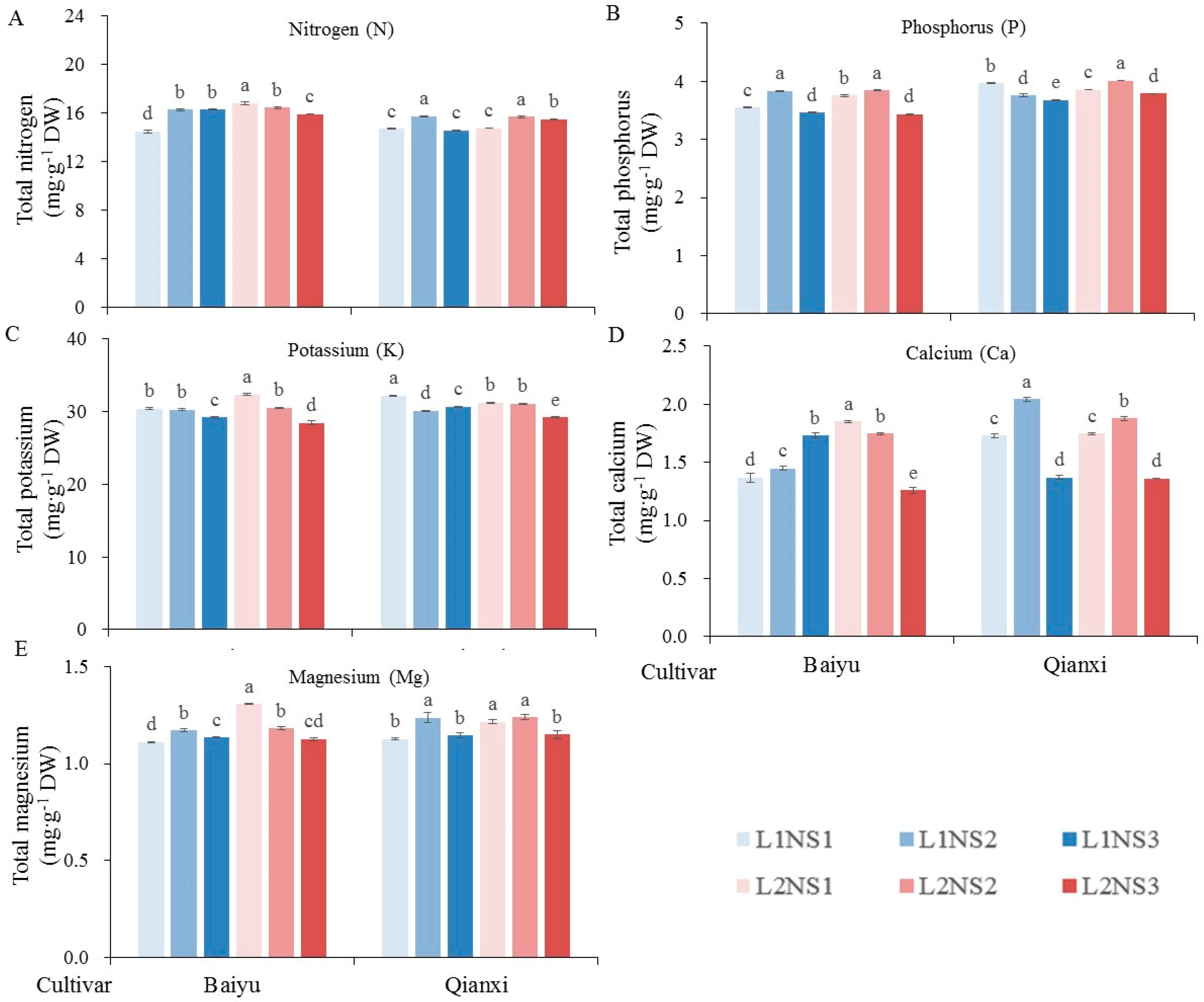
3.4. Mineral Element Content
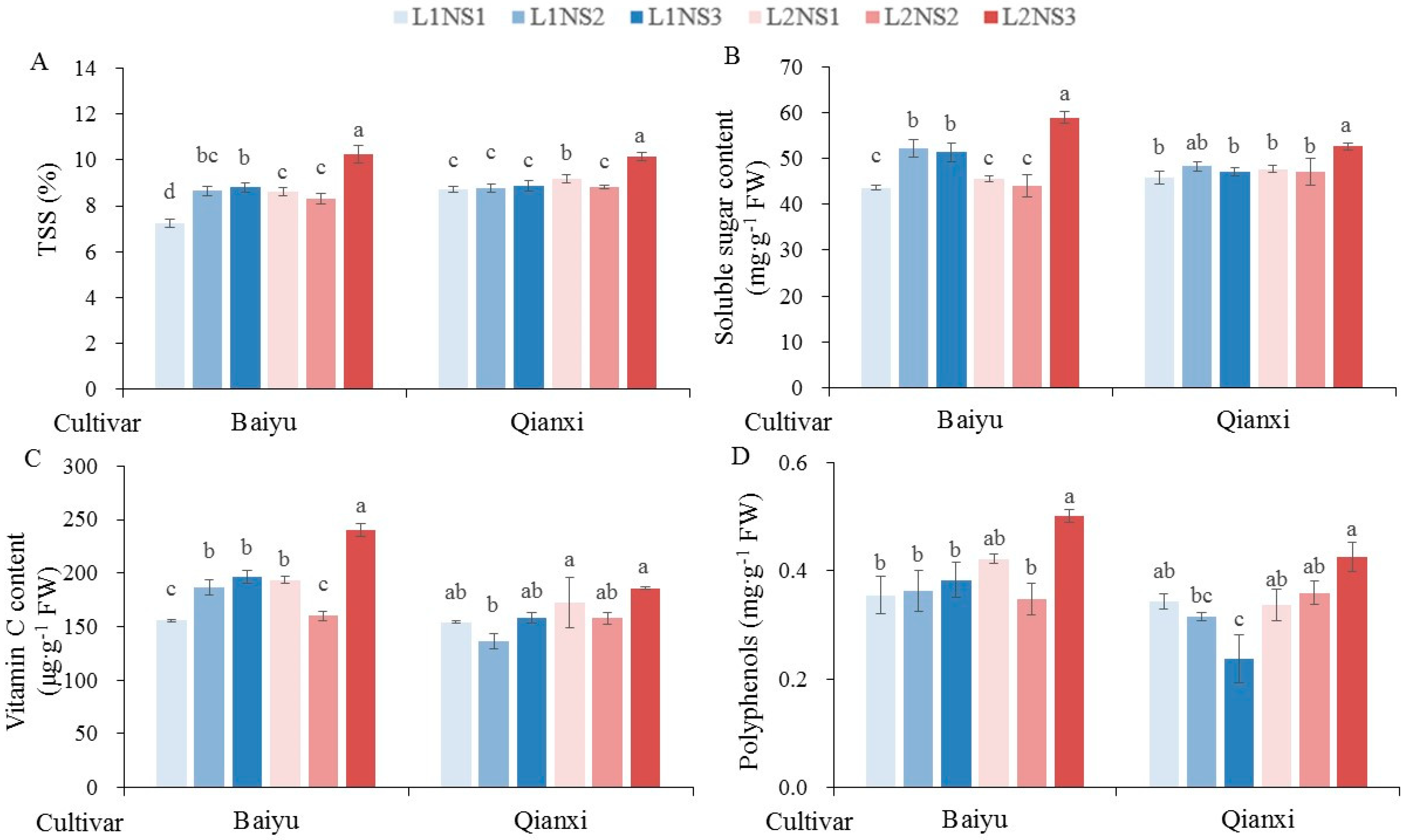
3.5. Fruits Quality
3.6. Contents of Soluble Sugars and Organic Acids
3.7. Interactions, PCA, and Heat Map Analysis
4. Discussion
4.1. Combination Effects of Supplemental Lighting and Nutrient Solution Concentration on Growth and Photosynthesis of Cherry Tomato Plants
4.2. Combination Effects of Supplemental Lighting and Nutrient Solution Concentration on Fruit Production
4.3. Combination Effects of Supplemental Lighting and Nutrient Solution Concentration on Fruit Mineral Content
4.4. Combination Effects of Supplemental Lighting and Nutrient Solution Concentration on Fruit Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Figás, M.; Prohens, J.; Raigón, M.; Fita, A.; García-Martínez, M.; Casanova, C.; Soler, S. Characterization of composition traits related to organoleptic and funtional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Benítez-González Ana, M.; Stinco Carla, M.; Meléndez-Martínez Antonio, J. Antioxidants (carotenoids and phenolics) profile of cherry tomatoes as influenced by deficit irrigation, ripening and cluster. Food Chem. 2018, 240, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Rapa, M.; Ciano, S.; Ruggieri, R.; Vinci, G. Bioactive compounds in cherry tomatoes (Solanum Lycopersicum var. Cerasiforme): Cultivation techniques classification by multivariate analysis. Food Chem. 2021, 355, e129630. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Verheul, M.J. Seasonal variations in the level of plant constituents in greenhouse production of cherry tomatoes. J. Agric. Food Chem. 2005, 53, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poёssel, J.L.; Caris-Veyrat, C.; Genard, M. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Shaji, S.; Beena, R.; Sarada, S.; Rani, T.S.; Stephen, R.; Manju, R.V.; Viji, M.M. High temperature induced changes in quality and yield parameters of tomato (Solanum lycopersicum L.) and similarity coefficients among genotypes using SSR markers. Heliyon 2021, 7, e05988. [Google Scholar] [CrossRef]
- Alsina, I.; Erdberga, I.; Duma, M.; Alksnis, R.; Dubova, L. Changes in greenhouse grown tomatoes metabolite content depending on supplemental light quality. Front. Nutr. 2022, 9, 830186. [Google Scholar] [CrossRef]
- Maeda, K.; Masuda, E.; Tamashiro, T.; Maharjan, G.; Maruo, T. Comparison of supplemental LED top- and interlighting for year-round production of cherry tomato. Agronomy 2022, 12, 1878. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.; Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Rodríguez, F.; Pedreschi, R.; Fuentealba, C.; Kartzow, A.; Olaeta Jose, A.; Alvaro Juan, E. The increase in electrical conductivity of nutrient solution enhances compositional and sensory properties of tomato fruit cv. Patrón. Sci. Hortic. 2019, 244, 388–398. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, H. The regulation of nutrient and flavor metabolism in tomato fruit. Veg. Res. 2022, 2, 5. [Google Scholar] [CrossRef]
- Iglesias, M.J.; García-López, J.; Collados-Luján, J.F.; López-Ortiz, F.; Díaz, M.; Toresano, F.; Camacho, F. Differential response to environmental and nutritional factors of high-quality tomato varieties. Food Chem. 2015, 176, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Nagatsuka, A.; Yoshitomi, A.; Nakagawa, T.; Maruo, T.; Tsukagoshi, S.; Hohjo, M.; Lu, N.; Nakaminami, A.; Tsuchiya, K.; et al. Effect of moderate salinity stress on the sugar concentration and fruit yield in single-truss, high-density tomato production system. J. Jpn. Soc. Hort. Sci. 2014, 83, 229–234. [Google Scholar] [CrossRef]
- Zhang, Y.; Kiriiwa, Y.; Nukaya, A. Effects of lower nitrogen concentration of nutrient solution combined with K supplementation and changing the concentration on growth, yield, and yellow-shoulder disorder for tomatoes grown in extremely low-volume substrate. Hortic. J. 2015, 84, 46–51. [Google Scholar] [CrossRef]
- Liu, A.; Liu, D.; Yin, D.; Lian, H.; Zhang, Y.; Chen, R. Effects of minimal drainage and nutrient concentration regulation on tomato fruit quality. China Veg. 2021, 10, 66–78, (In Chinese with English abstract). [Google Scholar]
- Ou, X.; Liu, D.; Liu, A.; Liu, H.; Chen, R.; Zhang, Y. Effects of nutrient solution management modes on fruit production and quality of tomatoes grown in extremely root restriction. Sci. Hortic. 2023, 321, 112366. [Google Scholar] [CrossRef]
- Itoh, M.; Goto, C.; Iwasaki, Y.; Sugeno, W.; Ahn, D.; Higashide, T. Production of high soluble solids fruits without reducing dry matter production in tomato plants grown in salinized nutrient solution controlled by electrical conductivity. Hortic. J. 2020, 89, 403–409. [Google Scholar] [CrossRef]
- Xie, B.; Wei, J.; Zhang, Y.; Song, S.; Su, W.; Sun, G. Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 2019, 22, 2687. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Qin, M.; Xie, Z.; Chen, R.; Zhang, Y. Effects of supplemental lighting on potassium transport and fruit coloring of tomatoes grown in hydroponics. Int. J. Mol. Sci. 2021, 22, 2687. [Google Scholar] [CrossRef]
- He, R.; Wei, J.; Zhang, J.; Tan, X.; Li, Y.; Gao, M.; Liu, H. Supplemental blue light frequencies improve ripening and nutritional qualities of tomato fruits. Front. Plant Sci. 2022, 13, 888976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Suzuki, K.; Liu, H.; Nukaya, A.; Kiriiwa, Y. Fruit yellow-shoulder disorder as related to mineral element uptake of tomatoes grown in high temperature. Sci. Hortic. 2018, 242, 25–29. [Google Scholar] [CrossRef]
- Xie, B.; Liu, H.; Song, S.; Sun, G.; Chen, R. Effects of light quality on the quality formation of tomato fruits. Adv. Bio. Sci. Res. 2016, 3, 11–15. [Google Scholar]
- Sakuraba, Y.; Kanno, S.; Mabuchi, A.; Monda, K.; Iba, K.; Yanagisawa, S. A phytochrome-B-mediated regulatory mechanism of phosphorus acquisition. Nativ. Plants 2018, 4, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.; Chen, Y.; Liu, C.; Yang, Y. Light regulation of potassium in plants. Plant Physiol. Biochem. 2022, 170, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Hao, Y.; Yang, X.; Chen, R.; Zhang, Y. Effects of extreme root restriction on the nutritional and flavor quality, and sucrose metabolism of tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 813. [Google Scholar] [CrossRef]
- Ojeda, G.; Alcañiz, J.M.; Le Bissonnais, Y. Differences in aggregate stability due to various sewage sludge treatments on a Mediterranean calcareous soil. Agric. Ecosyst. Environ. 2008, 125, 48–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, A.; Hao, Y.; Su, W.; Sun, G.; Song, S.; Liu, H.; Chen, R. Nitric oxide is essential for melatonin to enhance nitrate tolerance of cucumber seedlings. Molecules 2022, 27, 5806. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Huang, X.; Hu, Z. Sugar and acid compositions in the arils of Litchi chinensis Sonn.: Cultivar differences and evidence for the absence of succinic acid. J. Hortic. Sci. Biotechnol. 2006, 81, 57–62. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol inhibition of lipid peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, T.; Zhang, Y.; Bao, B.; Huang, X. Effect of antisense expression of ethylene receptor gene on fruit ripening in tomato. J. Hortic. Sci. 2006, 33, 518–522. [Google Scholar]
- Zhai, Y.; Yang, Q.; Hou, M. The effects of saline water drip irrigation on tomato yield, quality, and blossom-end rot incidence-A 3a case study in the south of China. PLoS ONE 2015, 10, e0142204. [Google Scholar] [CrossRef]
- Madugundu, R.; Al-Gaadi, K.; Tola, E.; Patil, V.; Sigrimis, N. The impact of salinity and nutrient regimes on the agro-morphological traits and water use efficiency of tomato under hydroponic conditions. Appl. Sci. 2023, 13, 9564. [Google Scholar] [CrossRef]
- Zhang, Y.; Kiriiwa, Y.; Nukaya, A. Influence of nutrient solution concentration and composition on the growth, uptake patterns of nutrient elements and fruit coloring disorder for tomatoes grown in extremely low-volume substrate. Hortic. J. 2015, 84, 37–45. [Google Scholar] [CrossRef]
- Liu, H.; Son, J.; Niu, G.; Li, Q. Editorial: Growth and quality formation regulated by light in horticulture plants. Front. Plant Sci. 2024, 15, 1414970. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.; Paponov, I. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, H.; Zhang, X.; Liu, J.; Li, Y.; Song, Y.; Wang, J.; Zheng, Y. Integrating omics reveals insights into tomato abaxial/adaxial leafy supplemental lighting. Front. Plant Sci. 2023, 14, 1118895. [Google Scholar] [CrossRef]
- Saito, T.; Fukuda, N.; Matsukura, C.; Nishimura, S. Effects of salinity on distribution of photosynthates and carbohydrate metabolism in tomato grown using nutrient film technique. J. Jpn. Soc. Hortic. Sci. 2009, 78, 90–96. [Google Scholar] [CrossRef]
- Paponov, M.; Verheul, M.; Dobrev, P.; Paponov, I. Additive effects of light and branching on fruit size and chemical fruit quality of greenhouse tomatoes. Front. Plant Sci. 2023, 14, 1221163. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F. Electrical conductivity of nutrient Solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in ahydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, H.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Effects of photoperiod interacted with nutrient solution concentration on nutritional quality and antioxidant and mineral content in lettuce. Agronomy 2020, 10, 920. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of the combination of ethylene and red LED light irradiation On carotenoid accumulation and carotenogenic gene expression In the flavedo of citrus fruit. Postharvest Biol. Technol. 2015, 99, 99–104. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Yanagasawa, S. Light signalling-induced regulation of nutrient acquisition and utilisation in plants. Semin. Cell Dev. Biol. 2018, 83, 123–132. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, T.; Choi, S.; Wang, Y.J.; Lin, M.Y.; Liceaga, A.M. Supplemental intracanopy far-red radiation to red LED light improves fruit quality attributes of greenhouse tomatoes. Sci. Hortic. 2020, 261, 108985. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C. Effect of salt stress on the growth and fruit quality of tomato plants. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–16. [Google Scholar]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.; Urrestarazu, M.; Álvaro, J. Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless Culture Tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J. Jpn. Soc. Hortic. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef]
- Yin, Y.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruit in an ABA- and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1700–1741. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.; Papadopoulos, A. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Marín, A.; José, S.; Vicente, M.; MaríaI, G. Antioxidant compoundsin green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Sci. Food Agric. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. Supplementary red light results in the earlier ripening of tomato fruit depending on ethylene production. Environ. Exp. Bot. 2020, 175, 104044. [Google Scholar] [CrossRef]
- Tewolde, F.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental LED inter-lighting compensates for a shortage of light for plant growth and yield under the lack of sunshine. PLoS ONE 2018, 13, e0206592. [Google Scholar] [CrossRef]
- Kim, Y.X.; Son, S.; Lee, S.; Jung, E.; Lee, Y.; Sung, J.; Lee, C. Combined effects of nutrients × water × light on metabolite composition in tomato fruits (Solanum lycopersicum L.). Plants 2021, 10, 1437. [Google Scholar] [CrossRef]


| Nutrient Solution Concentration (NS) | EC (dS/m) | Mineral Elements (mM/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Vegetative Stage (17 DPA) | Fruiting Stage (24 DPA) | NO3-N | PO4-P | K | Mg+ | Ca | NH4-N | |
| NS1 | 1.6 | 3.2 (2.1 + P + K) | 14.0 | 3.6 | 13.5 | 1.8 | 3.5 | 1.2 |
| NS2 | 1.6 | 3.7 (2.4 + P + K) | 16.0 | 4.1 | 15.4 | 2.0 | 4.0 | 1.4 |
| NS3 | 1.6 | 4.2 (2.7 + P + K) | 18.0 | 4.6 | 17.4 | 2.3 | 4.5 | 1.6 |
| Treatments | Plant Height (cm) | Stem Diameter (mm) | Leaf Number Per Plant | Leaf Area (dm2 Plant−1) | SPAD | ||
|---|---|---|---|---|---|---|---|
| Cultivar (C) | Light Conditions (L) | Nutrient Solution Concentration (NS) | |||||
| ‘Baiyu’ | L1 | NS1 | 133.25 ± 4.56 ab | 14.93 ± 1.29 c | 33.00 ± 0.63 ab | 8.41 ± 1.07 a | 48.18 ± 1.26 ab |
| NS2 | 135.13 ± 2.78 a | 16.99 ± 0.34 abc | 31.67 ± 0.33 b | 8.67 ± 0.21 a | 48.05 ± 1.11 ab | ||
| NS3 | 136.78 ± 0.94 a | 15.90 ± 0.44 bc | 32.83 ± 0.6 ab | 8.29 ± 0.41 a | 47.13 ± 0.95 ab | ||
| L2 | NS1 | 137.17 ± 1.14 a | 17.76 ± 0.86 ab | 31.67 ± 0.42 b | 8.52 ± 0.45 a | 50.42 ± 1.34 a | |
| NS2 | 136.26 ± 2.41 a | 18.78 ± 0.83 a | 33.83 ± 0.6 a | 8.33 ± 0.65 a | 46.94 ± 1.19 ab | ||
| NS3 | 126.35± 3.18 b | 15.15 ± 0.79 c | 32.67 ± 0.49 ab | 7.38 ± 0.51 a | 45.55 ± 1.23 b | ||
| ‘Qianxi’ | L1 | NS1 | 104.14 ± 1.03 ab | 17.05 ± 0.39 ab | 32.17 ± 0.48 abc | 5.71 ± 0.27 a | 56.98 ± 1.65 a |
| NS2 | 108.42 ± 4.50 a | 17.78 ± 0.32 a | 34.00 ± 0.68 a | 5.12 ± 0.2 ab | 54.55 ± 2.19 a | ||
| NS3 | 96.66 ± 0.70 bc | 15.59 ± 0.59 b | 32.00 ± 0.77 bc | 4.54 ± 0.32 b | 52.00 ± 2.03 a | ||
| L2 | NS1 | 97.60± 0.70 bc | 17.93 ± 0.62 a | 31.33 ± 0.67 bc | 5.54 ± 0.13 a | 56.25 ± 1.97 a | |
| NS2 | 99.24 ± 1.81 bc | 17.79 ± 0.45 a | 33.00 ± 0.68 ab | 5.98 ± 0.61 a | 56.60 ± 3.32 a | ||
| NS3 | 92.68 ± 1.86 c | 16.14 ± 0.53 b | 31.00 ± 0.26 c | 4.56 ± 0.14 b | 52.93 ± 2.18 a | ||
| ANOVA significance | C | ** | NS | NS | ** | ** | |
| L | ** | * | NS | NS | NS | ||
| NS | ** | ** | * | * | * | ||
| C × L | NS | NS | NS | NS | NS | ||
| C × NS | NS | NS | * | NS | NS | ||
| L × NS | NS | NS | NS | NS | NS | ||
| C × L × NS | * | NS | NS | NS | NS | ||
| Treatments | Pn (μmol m−2 s−1) | Ci (μmol mol−1) | Gs (mol m−2 s−1) | Tr (mmol m−2 s−1) | ||
|---|---|---|---|---|---|---|
| Cultivar (C) | Light (L) | Nutrient Solution (NS) | ||||
| ‘Baiyu’ | L1 | NS1 | 13.53 ± 0.18 bc | 264.00 ± 4.04 a | 0.19 ± 0.01 b | 1.15 ± 0.15 ab |
| NS2 | 15.17 ± 0.62 a | 264.00 ± 1.68 a | 0.24 ± 0.00 a | 1.31 ± 0.05 a | ||
| NS3 | 10.67 ± 0.47 d | 272.00 ± 2.08 a | 0.16 ± 0.01 bc | 1.14 ± 0.04 ab | ||
| L2 | NS1 | 12.83 ± 0.35 c | 236.33 ± 5.36 c | 0.15 ± 0.02 c | 0.99 ± 0.08 b | |
| NS2 | 14.47 ± 0.58 ab | 233.00 ± 2.08 c | 0.14 ± 0.01 cd | 1.15 ± 0.04 ab | ||
| NS3 | 12.27 ± 0.45 c | 251.67 ± 6.17 b | 0.13 ± 0.01 d | 1.02 ± 0.01 ab | ||
| ‘Qianxi’ | L1 | NS1 | 20.80 ± 1.42 ab | 282.33 ± 1.45 a | 0.19 ± 0.00 b | 1.36 ± 0.06 b |
| NS2 | 21.63 ± 0.47 a | 275.33 ± 2.33 a | 0.27 ± 0.01 a | 1.15 ± 0.00 c | ||
| NS3 | 14.73 ± 1.03 cd | 253.33 ± 12.25 a | 0.18 ± 0.02 b | 1.28 ± 0.07 bc | ||
| L2 | NS1 | 17.63 ± 0.87 bc | 272.33 ± 7.97 a | 0.19 ± 0.00 ab | 1.57 ± 0.03 a | |
| NS2 | 21.87 ± 1.45 a | 215.00 ± 15.59 b | 0.15 ± 0.01 b | 1.11 ± 0.05 c | ||
| NS3 | 13.73 ± 1.45 d | 278.00 ± 3.06 a | 0.17 ± 0.04 b | 1.18 ± 0.08 c | ||
| Significance | C | ** | ** | * | ** | |
| L | NS | ** | ** | NS | ||
| NS | ** | ** | ** | NS | ||
| C × L | NS | ** | NS | * | ||
| C × NS | ** | ** | NS | ** | ||
| L × NS | NS | ** | ** | NS | ||
| C × L × NS | NS | ** | NS | NS | ||
| Significance | C | L | NS | C × L | C × NS | L × NS | C × L × NS |
|---|---|---|---|---|---|---|---|
| Single fruit weight | NS | NS | ** | NS | NS | ** | NS |
| Fruit weight per plant | NS | NS | ** | NS | NS | NS | NS |
| Fruit diameter | ** | NS | ** | NS | * | ** | NS |
| Fruit length | ** | ** | ** | NS | NS | NS | NS |
| Fruit water content | NS | NS | NS | NS | NS | ** | NS |
| N content | ** | ** | ** | ** | ** | ** | ** |
| P content | ** | ** | ** | NS | ** | ** | ** |
| K content | ** | NS | ** | ** | ** | ** | ** |
| Ca content | ** | * | ** | ** | ** | ** | ** |
| Mg content | NS | ** | ** | * | ** | ** | ** |
| Total soluble solid | ** | ** | ** | NS | ** | ** | * |
| Soluble sugar | NS | NS | ** | NS | ** | ** | NS |
| Vitamin C | ** | ** | ** | NS | * | ** | * |
| Polyphenols | ** | ** | NS | NS | NS | ** | NS |
| Carotenoid | ** | * | ** | ** | ** | ** | ** |
| Sucrose | ** | ** | ** | ** | ** | ** | ** |
| Glucose | NS | * | ** | NS | ** | ** | NS |
| Fructose | ** | * | ** | NS | ** | ** | NS |
| Malic acid | NS | NS | ** | NS | ** | NS | NS |
| Citric acid | NS | * | ** | NS | NS | * | NS |
| Tartaric acid | NS | * | ** | NS | NS | ** | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Chen, J.; Liu, H.; Chen, R.; Yang, X.; Song, S.; Zhang, Y. Influence of Combined Supplemental Lighting and Nutrient Solution Concentration on Fruit Production and Quality of Cherry Tomato. Horticulturae 2024, 10, 990. https://doi.org/10.3390/horticulturae10090990
Xie Z, Chen J, Liu H, Chen R, Yang X, Song S, Zhang Y. Influence of Combined Supplemental Lighting and Nutrient Solution Concentration on Fruit Production and Quality of Cherry Tomato. Horticulturae. 2024; 10(9):990. https://doi.org/10.3390/horticulturae10090990
Chicago/Turabian StyleXie, Zhenbin, Jinxiang Chen, Houcheng Liu, Riyuan Chen, Xiaolong Yang, Shiwei Song, and Yiting Zhang. 2024. "Influence of Combined Supplemental Lighting and Nutrient Solution Concentration on Fruit Production and Quality of Cherry Tomato" Horticulturae 10, no. 9: 990. https://doi.org/10.3390/horticulturae10090990
APA StyleXie, Z., Chen, J., Liu, H., Chen, R., Yang, X., Song, S., & Zhang, Y. (2024). Influence of Combined Supplemental Lighting and Nutrient Solution Concentration on Fruit Production and Quality of Cherry Tomato. Horticulturae, 10(9), 990. https://doi.org/10.3390/horticulturae10090990








