The Selection of Lettuce Seedlings for Transplanting in a Plant Factory by a Non-Destructive Estimation of Leaf Area and Fresh Weight
Abstract
1. Introduction
2. Materials and Methods
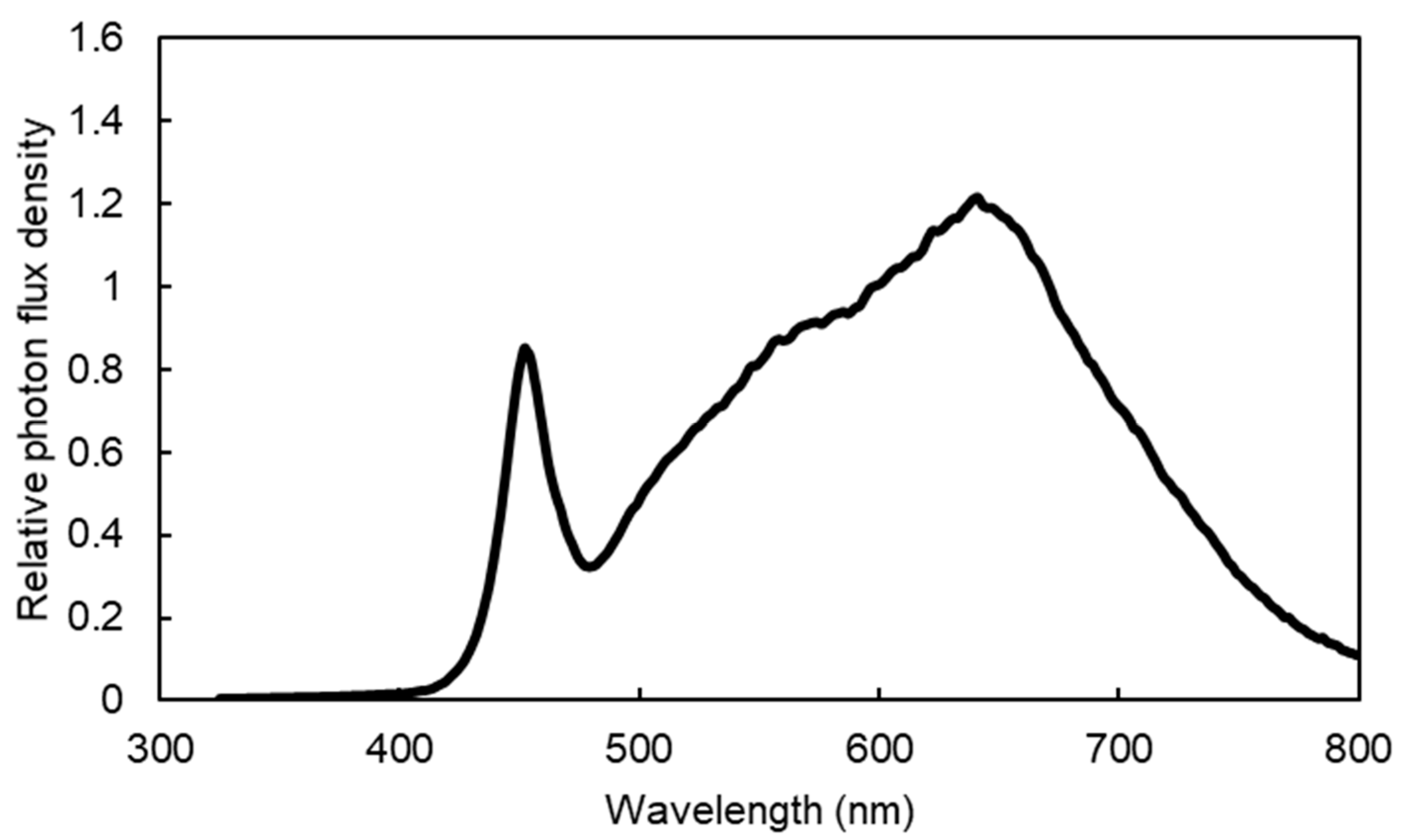
2.1. Plant Materials and Seedling Production
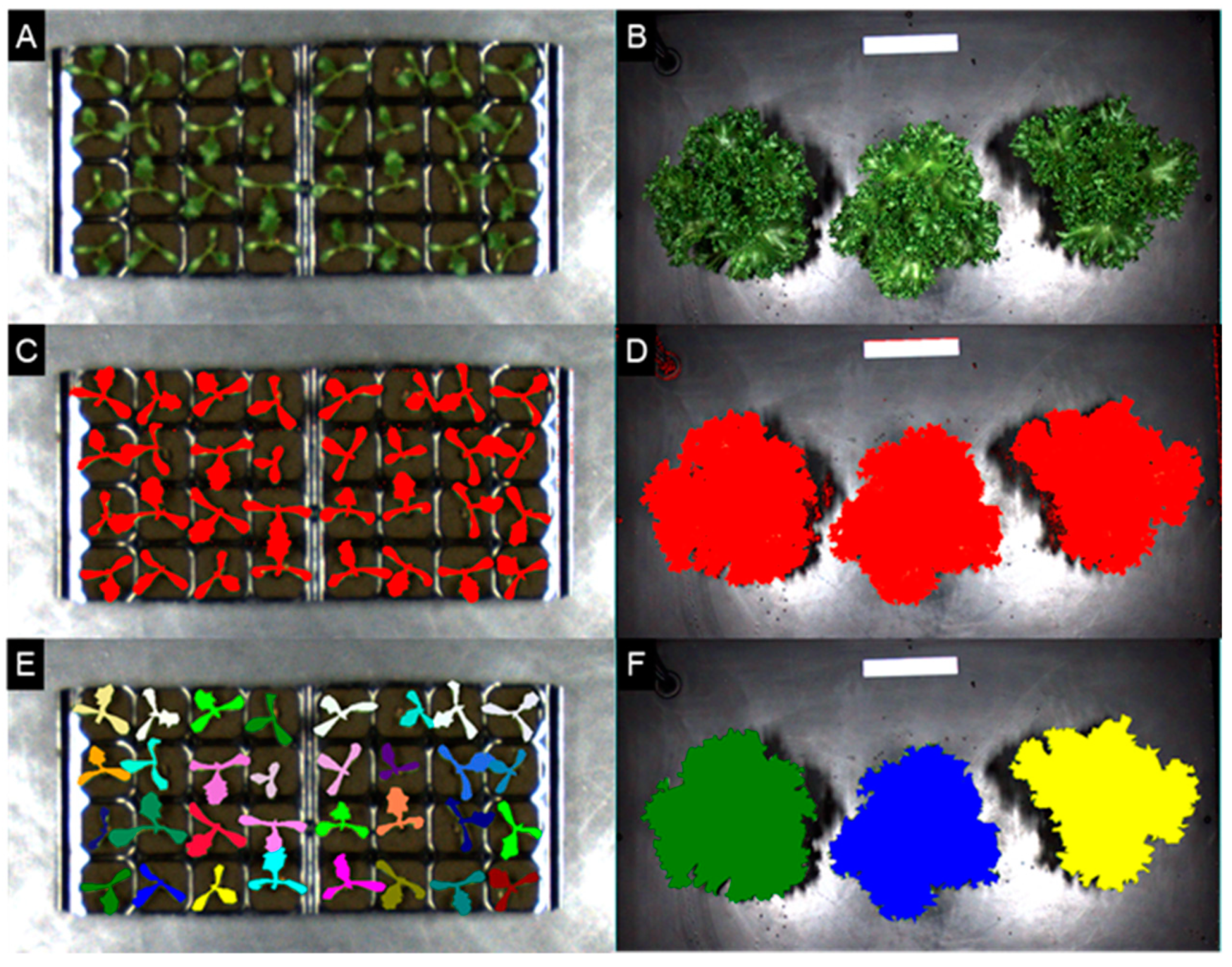
2.2. Growth of Lettuce Crop by Seedling Classification after Transplanting
2.3. Statistical Analysis
3. Results
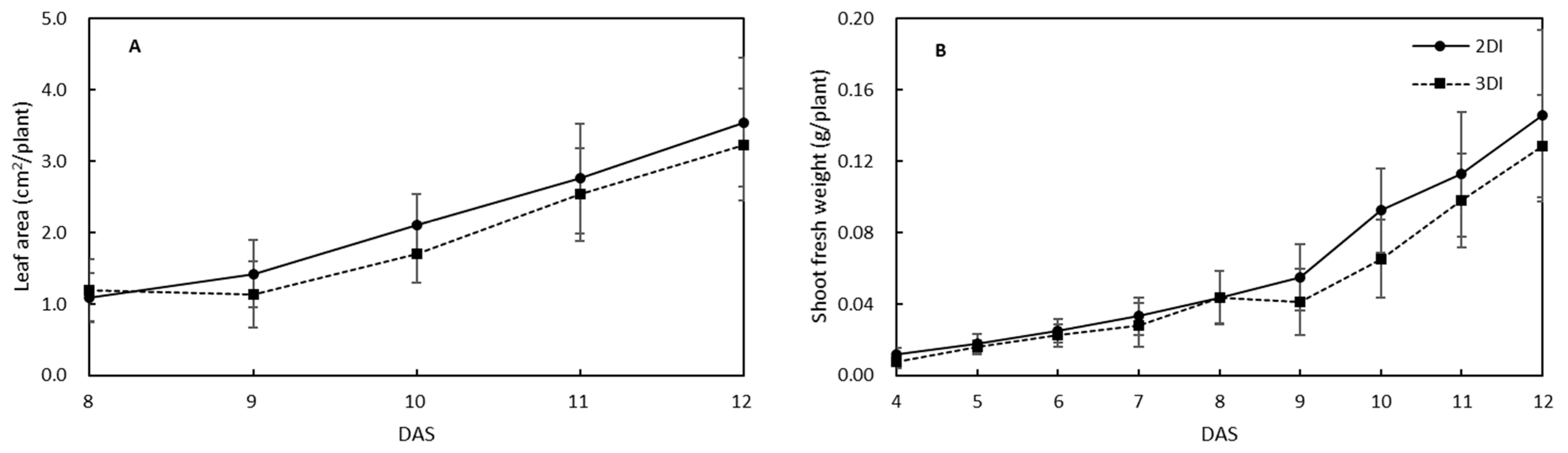
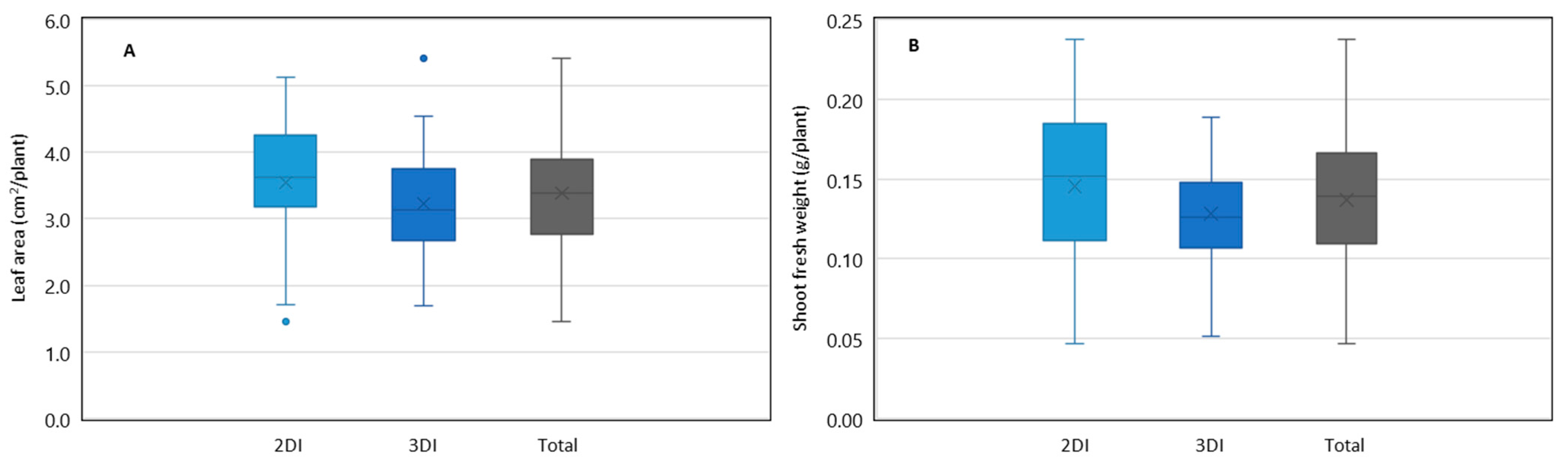
3.1. Differences in Lettuce Seedling Growth between Irrigation Regimes
3.2. Growth Estimation and Grading of Lettuce Seedlings Based on PCS
3.3. Lettuce Growth by Grading after Transplanting
3.4. Growth Estimation of Lettuce after Transplanting Based on PCS
4. Discussion
4.1. Changes in Lettuce Growth by Different Irrigation Regimes during the Period of Seedling Production
4.2. Prediction of Lettuce Growth Based on PCS
4.3. Yield of Lettuce after Transplanting by Seedling Grade Based on PCS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Kim, K.S.; Kim, Y.; Chung, Y.S. A short review: Comparisons of high-throughput phenotyping methods for detecting drought tolerance. Sci. Agric. 2020, 78, e20190300300. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Masabni, J.G. (Eds.) Plant Factory Basics, Applications and Advances; Academic Press: London, UK, 2021; pp. 11–22. [Google Scholar]
- Kozai, T. Smart plant factory. In The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018; Volume 238, 7p. [Google Scholar]
- Lee, J.G.; Choi, C.S.; Jang, Y.A.; Jang, S.W.; Lee, S.G.; Um, Y.C. Effects of air temperature and air flow rate control on the tipburn occurrence of leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 303–310. [Google Scholar] [CrossRef]
- Paim, B.T.; Crizel, R.L.; Tatiane, S.J.; Rodrigues, V.R.; Rombaldi, C.V.; Galli, V. Mild drought stress has potential to improve lettuce yield and quality. Sci. Hortic. 2020, 272, 109578. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of crisphead ‘Iceberg’ and romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Still, D.W. Lettuce. In Vegetables; Springer: Berlin/Heidelberg, Germany, 2007; pp. 127–140. [Google Scholar]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Okayama, T.; Okamura, K.; Park, J.E.; Ushada, M.; Murase, H. A simulation for precision airflow control using multi-fan in a plant factory. Environ. Control Biol. 2008, 46, 183–194. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Nkurunziza, E.; Nyalala, S.; Umuhoza, K.N.J. Effect of seedling quality on growth, yield and quality of tomato (Solanum lycopersicum L.). Int. J. Hortic. Sci. 2022, 28, 64–72. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, L.; Gao, W.; Wang, B.; Hao, X.; Mi, J.; Wang, M. An efficient processing approach for colored point cloud-based high-throughput seedling phenotyping. Remote Sens. 2020, 12, 1540. [Google Scholar] [CrossRef]
- Mishra, K.B.; Mishra, A.; Klem, K.; Govindjee. Plant phenotyping: A perspective. Indian J. Plant Physiol. 2016, 21, 514–527. [Google Scholar] [CrossRef]
- Du, J.; Lu, X.; Fan, J.; Qin, Y.; Yang, X.; Guo, X. Image-based high-throughput detection and phenotype evaluation method for multiple lettuce varieties. Front. Plant Sci. 2020, 11, 563386. [Google Scholar] [CrossRef]
- Jung, D.H.; Park, S.H.; Han, X.Z.; Kim, H.J. Image processing methods for measurement of lettuce fresh weight. J. Biosyst. Eng. 2015, 40, 89–93. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Xiang, L.; Wu, Q.; Jiang, H. Automatic non-destructive growth measurement of leafy vegetables based on kinect. Sensors 2018, 18, 806. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental far-red light stimulates lettuce growth: Disentangling morphological and physiological effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Kim, C.; van Iersel, M.W. Morphological and physiological screening to predict lettuce biomass production in controlled environment agriculture. Remote Sens. 2022, 14, 316. [Google Scholar] [CrossRef]
- Eguchi, M.; Enjoji, A.; Yamaguchi, J.; Iwasaki, Y.; Kitaya, Y. An alternative method for growth analysis of lettuce grown in a plant factory with artificial lighting using projected canopy area and fresh mass. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Advances in Vertical Farming 1369, Angers, France, 14–20 August 2022; pp. 243–248. [Google Scholar]
- Hosoda, Y.; Tada, T.; Goto, H. Lettuce Fresh Weight Prediction in a Plant Factory Using Plant Growth Models. IEEE Access 2024, 12, 97226–97234. [Google Scholar] [CrossRef]
- Mortensen, A.K.; Bender, A.; Whelan, B.; Barbour, M.M.; Sukkarieh, S.; Karstoft, H.; Gislum, R. Segmentation of lettuce in coloured 3D point clouds for fresh weight estimation. Comput. Electron. Agric. 2018, 154, 373–381. [Google Scholar] [CrossRef]
- Xu, D.; Chen, J.; Li, B.; Ma, J. Improving lettuce fresh weight estimation accuracy through RGB-D fusion. Agronomy 2023, 13, 2617. [Google Scholar] [CrossRef]
- Jayalath, T.C.; van Iersel, M.W. Canopy size and light use efficiency explain growth differences between lettuce and mizuna in vertical farms. Plants 2021, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.F.S.; Gomes, É.P.; Sousa, A.D.P.; Glória, M.B.A. Effect of irrigation level on yield and bioactive amine content of American lettuce. J. Sci. Food Agric. 2005, 85, 1026–1032. [Google Scholar] [CrossRef]
- Iradukunda, M.; van Iersel, M.W.; Seymour, L.; Lu, G.; Ferrarezi, R.S. Low-cost imaging to quantify germination rate and seedling vigor across lettuce cultivars. Sensors 2024, 24, 4225. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Hong, I.; Kwack, Y. Prediction of growth and quality of chinese cabbage seedlings cultivated in different plug cell sizes via analysis of image data using multispectral camera. Horticulturae 2023, 9, 1288. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.X.; Lu, J.L.; Li, Y.M.; Yang, Q.C. Lettuce growth, nutritional quality, and energy use efficiency as affected by red–blue light combined with different monochromatic wavelengths. HortScience 2020, 55, 613–620. [Google Scholar] [CrossRef]
- Golzarian, M.R.; Frick, R.A.; Rajendran, K.; Berger, B.; Roy, S.; Tester, M.; Lun, D.S. Accurate inference of shoot biomass from high-throughput images of cereal plants. Plant Methods 2011, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, C.; Paterson, A.H.; Sun, S.; Xu, R.; Robertson, J. Quantitative analysis of cotton canopy size in field conditions using a consumer-grade RGB-D camera. Front. Plant Sci. 2018, 8, 2233. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef]
- Moriyuki, S.; Fukuda, H. High-throughput growth prediction for Lactuca sativa L. seedlings using chlorophyll fluorescence in a plant factory with artificial lighting. Front. Plant Sci. 2016, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Adhikari, R.; Yao, Y.; Miller, A.G.; Kalbaugh, K.; Li, D.; Nemali, K. Measuring plant growth characteristics using smartphone based image analysis technique in controlled environment agriculture. Comput. Electron. Agric. 2020, 168, 105123. [Google Scholar] [CrossRef]
- Zhang, P.; Li, D. YOLO-VOLO-LS: A novel method for variety identification of early lettuce seedlings. Front. Plant Sci. 2022, 13, 806878. [Google Scholar] [CrossRef] [PubMed]

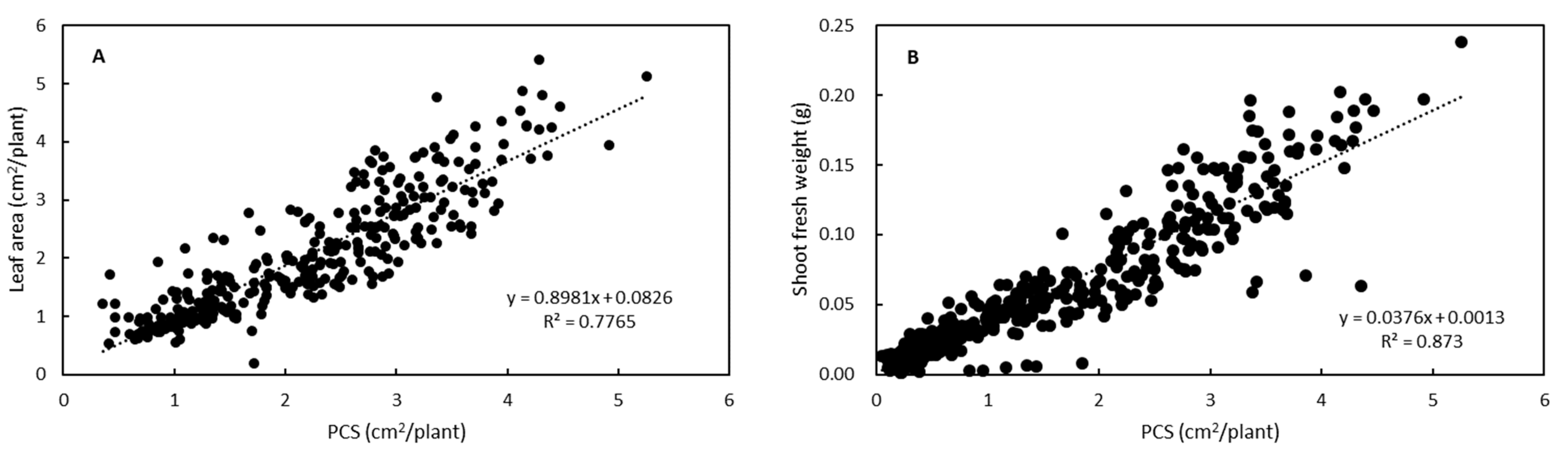


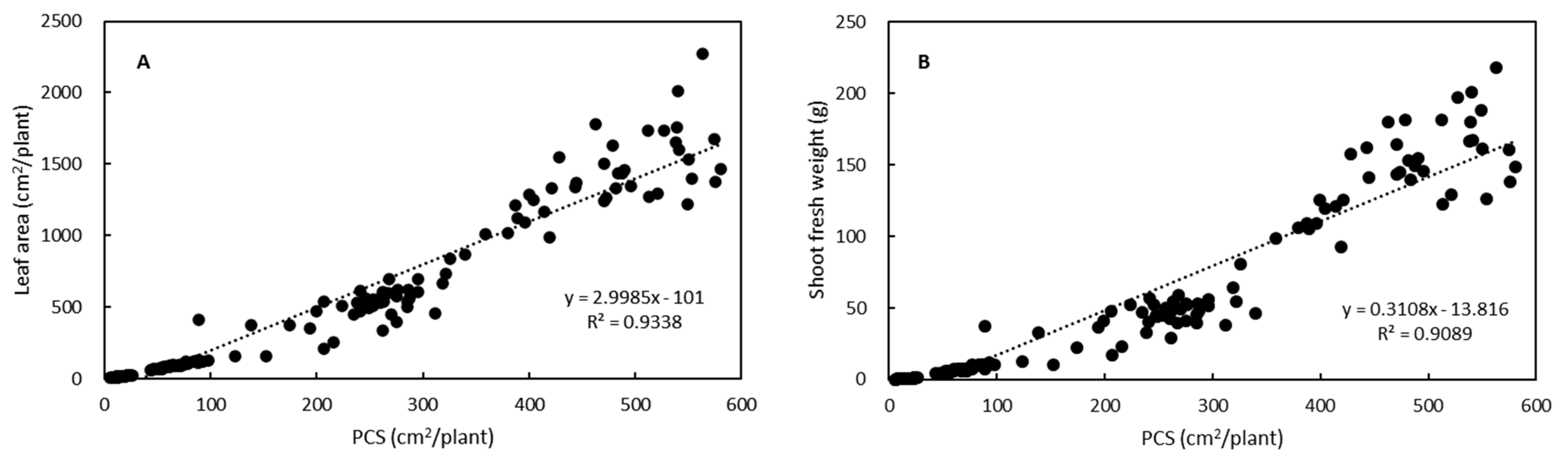
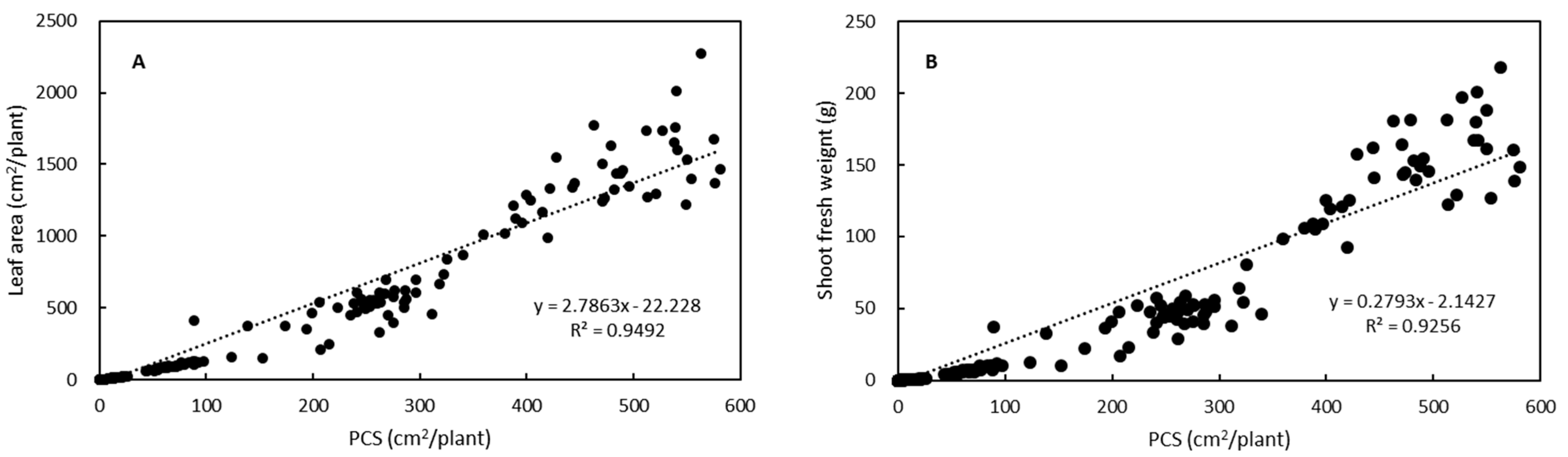
| Grade | PCS (cm2/plant) | Predicted Shoot Fresh Weight z (g/plant) |
|---|---|---|
| A | 5 ≤ X < 7 | 0.21 ≤ Y < 0.32 |
| B | 4 ≤ X < 5 | 0.16 ≤ Y < 0.21 |
| C | 2 ≤ X < 4 | 0.07 ≤ Y < 0.16 |
| D | X < 2 | Y < 0.07 |
| DAT z | Grade | Leaf Length | No. of Leaves | Leaf Area | Shoot Fresh Weight |
|---|---|---|---|---|---|
| (cm) | (/plant) | (cm2/plant) | (g/plant) | ||
| 7 | A | 5.37a y | 6.43a | 21.79a | 1.06a |
| B | 5.47a | 6.29a | 19.54b | 0.94b | |
| C | 4.87b | 6.14a | 12.78c | 0.56c | |
| D | 3.97c | 5.57b | 8.18d | 0.33d | |
| 14 | A | 6.20b | 7.67ab | 88.35b | 6.80b |
| B | 7.45a | 8.23a | 128.33a | 10.67a | |
| C | 6.60b | 7.67a | 93.98b | 7.59b | |
| D | 6.23b | 7.29b | 69.75c | 4.80c | |
| 21 | A | 11.35b | 13.67a | 494.05b | 41.04b |
| B | 12.60a | 13.71a | 663.46a | 55.63a | |
| C | 11.23b | 12.86a | 520.49b | 48.11ab | |
| D | 9.16c | 11.14b | 304.33c | 25.65c | |
| 28 | A | 14.23bc | 18.83a | 1373.08a | 151.80a |
| B | 18.01a | 19.86a | 1629.30a | 175.87a | |
| C | 15.19ab | 19.00a | 1447.08a | 150.88a | |
| D | 13.47c | 16.00b | 1116.58b | 101.70b |
| Irrigation Regimes | Shoot Fresh Weight (g/plant) | |
|---|---|---|
| Grade B | Grade C | |
| 2DI | 175.87 | 133.66 |
| 3DI | 145.88 | 150.88 |
| Significance | NS z | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Ha, Y.; Kwack, Y. The Selection of Lettuce Seedlings for Transplanting in a Plant Factory by a Non-Destructive Estimation of Leaf Area and Fresh Weight. Horticulturae 2024, 10, 919. https://doi.org/10.3390/horticulturae10090919
Jeong J, Ha Y, Kwack Y. The Selection of Lettuce Seedlings for Transplanting in a Plant Factory by a Non-Destructive Estimation of Leaf Area and Fresh Weight. Horticulturae. 2024; 10(9):919. https://doi.org/10.3390/horticulturae10090919
Chicago/Turabian StyleJeong, Jaeho, Yoomin Ha, and Yurina Kwack. 2024. "The Selection of Lettuce Seedlings for Transplanting in a Plant Factory by a Non-Destructive Estimation of Leaf Area and Fresh Weight" Horticulturae 10, no. 9: 919. https://doi.org/10.3390/horticulturae10090919
APA StyleJeong, J., Ha, Y., & Kwack, Y. (2024). The Selection of Lettuce Seedlings for Transplanting in a Plant Factory by a Non-Destructive Estimation of Leaf Area and Fresh Weight. Horticulturae, 10(9), 919. https://doi.org/10.3390/horticulturae10090919







