Abstract
Purslane (Portulaca oleracea L.) is a common weed that attracts research and agricultural attention because of its significant nutritional value, and it is used commercially. The present work rated the effect of different nitrogen (N) concentrations, i.e., N50: 50 mg L−1; N100: 100 mg L−1; N200: 200 mg L−1; and N300: 300 mg L−1, on the growth, physiology, antioxidant capacity, and nutrient accumulation in the different plant parts of hydroponically grown purslane. Seedlings were transplanted to a Nutrient Film Technique (NFT) system and exposed to different N levels. The plants grown in high N levels of 300 mg L−1 had decreased biomass production, leaf number, leaf stomatal conductance, and total flavonoid content because of the increased oxidative stress, as shown by the elevated lipid peroxidation levels. Several enzymatic (superoxide dismutase) and non-enzymatic (ascorbic acid, total phenolics) plant antioxidant activities were activated to counteract the oxidative factors. Plants grown in intermediate N levels in the NS (i.e., 100 mg L−1) had decreased oxidative stress as several enzymatic antioxidant activities, i.e., peroxidases and catalases, were increased. Additionally, the water use efficiency and nitrogen uptake, as well as leaf stomatal conductance and leaf chlorophyll fluorescence, were increased in plants grown at a N level of 200 mg L−1. The mineral accumulation in the leaves, stems, and roots fluctuated, with increased calcium, magnesium, and sodium content being observed in the plants grown at high N levels in the nutrient solution. The stems accumulated less N compared with the leaves, but the N content and accumulation rates in purslane leaves were not affected by the N levels in the nutrient solution. Therefore, to increase the yield, nutritional value, and water use efficiency of purslane that is grown hydroponically, a concentration of 200 mg L−1 N in the nutrient solution is recommended.
1. Introduction
Purslane (Portulaca oleracea L.) is a wild herb that has been used as an edible plant and a traditional medicine for centuries. Purslane is appreciated in Mediterranean and Asian cuisines because of its crispy and fresh flavor, and it is known as “the future superfood” because of its great nutritional and pharmacological qualities [1,2]. Purslane consumption has been associated with a number of biological properties, namely, bactericidal, antidiabetic, diuretic, anti-inflammatory, antiseptic, hepatoprotective, nephroprotective, antiulcerogenic, antihyperlipidemic, antiarthritic, and anticancer properties [2,3,4]. A high amount of bioactive components and richness in the nutritional fundamental components of purslane, such as organic acids, ω-3 fatty acids, vitamins, phenolics, and flavonoids, have been reported [5,6]. Purslane is composed of a variety of dietary minerals including potassium (K), magnesium (Mg), calcium (Ca), phosphorus (P), and iron (Fe) [7]. The plant’s succulent stems and leaves taste somewhat acidic and salty, and they are comparable to spinach, providing a unique taste and freshness. Purslane has been reported to grow well in a variety of abiotic stress environments, such as saline, drought, and high-temperature environments, making it an important crop candidate in adverse conditions [8,9,10,11]. Several agronomic studies have been conducted on purslane, referring to its use as a covering crop between rows in vineyards [12], its performance on different growing media in soilless culture [13,14], its heavy metals tolerance [15], and the impacts of nitrogen (N) sources and the levels of ammonium nitrogen when it is grown in a nutrient solution (NS) [16,17] and in soil [18].
The agriculture sector has a high demand for freshwater to meet crop irrigation needs, including the commonly used drip-feeding system, and water is needed to apply appropriate amounts of fertilizers to each crop through the irrigation system. The decrease in available water for crop irrigation, the alternative use of treated wastewater in agriculture, the selection of drought-resistant varieties and species, and inadequate irrigation practices are just some of the critical issues that modern agriculture must address. In addition, rising irrigation costs, rising fertilizer prices, and a number of ecological problems, including pollution from improper N consumption or the improper use of various synthetic fertilizers in agriculture are of serious concern [19]. To address these concerns, soilless culture/hydroponics is seen as a suitable farming system, not solely for growing plants without soil but also for incorporating new/underexplored plant species into intense cultivation systems, enabling precise nutrient concentrations and real crop needs for minerals [20]. Furthermore, the use of a recirculating, commonly named a “closed”, hydroponic system has substantial benefits in terms of avoiding chemical leakage, preserving water resources, and supporting environmentally friendly farming practices via fertilizer and water recycling. These characteristics render a closed hydroponic system a tempting choice for the efficient and sustainable cultivation of crops [21,22].
The research on plant adaptation to minerals focuses primarily on the interactions between fertilizer and water management. Adequate irrigation and nitrogen fertilizer management are essential for plant growth and yield and sustainable agriculture practices [23]. Nitrogen, phosphorous, and potassium have long been known to influence plant development and secondary metabolites. Nitrogen is regarded as an essential plant mineral, being the fourth most prevalent element after carbon, oxygen, and hydrogen and contributing to the synthesis of several organic components such as amino acids, proteins, enzymes, and nucleic acids. Nitrogen is the only mineral that can be taken up by plants in both ionic forms, as a cation of ammonium (NH4+) and as an anion of nitrate (NO3−); hence, the ammonium-to-nitrate ratio affects the total anion-to-cation uptake of nutrients [24]. An elevated N concentration enhances the number and size of leaf cells, resulting in higher leaf area and biomass yield, which may be related to N’s well-known properties in plant life [25]. Moreover, N availability supports root colonization by arbuscular mycorrhizal fungi on strawberry (Fragaria × ananassa Duch.) plants with increased yield and photosynthetic N use efficiency [26]. As a matter of fact, farmers often increase N applications and rates with the expectations of higher yields, albeit without considering the increased cost of resources, the decreases in quality, the safety of the edible products, or the environmental and human constraints of elevated N fertilization in the agriculture sector. Irregular N application in crops will be mirrored in both the roots and the upper parts of the plant based on the understanding of the translocation from the roots to the upper parts of the plant of interest [27]. The right amount of N fertilizer may significantly enhance crop output by increasing nutrient intake, the net assimilation rate, photosynthesis, and the dry matter content [28]. However, excess N application results in nitrate leaching in water bodies, soil, and edible plants. An overabundance of nitrates in water causes eutrophication and lower oxygen levels, which are harmful to ecosystems and human well-being. Although there have been significant efforts to decrease nitrate contaminants in water reservoirs, the excess application of fertilizers and water for irrigation, with their significant risk of nitrate leaching loss, continues to be an issue in many regions of the EU.
As a result, despite purslane’s well-documented quality attributes, there is little information on its performance under an intense cultivation system in hydroponics or on the influence of nitrogen concentrations in an NS. The scope of the present study was to investigate the effects of the N concentration in an NS on purslane’s growth, nutrient accumulation and translocation in different plant organs, antioxidant activity, and water and nutrient use efficacy. The N levels were selected based on the cases that can be used on hydroponic cultivation systems as well as the tailoring of N levels in some species grown in hydroponics [29,30,31].
2. Materials and Methods
2.1. Cropping System, Plant Material, and Growth Conditions
The experiment took place at the greenhouse facilities of the Cyprus University of Technology, Limassol, Cyprus. The greenhouse has an automated climate control system, and the hydroponic installation was constructed according to the principles of NFT (Nutrient Film Technique). After passing through the roots, the drainage NS was recirculated continuously. Twelve individual NFT units were aligned with 12 catchment tanks (60 L). Each unit had a twin plastic channel (4 m long), and it was connected to a water replenishment tank (60 L). The NS that was consumed by the plants was replenished through the automatic refill of water from the replenishment tank and adjusted daily with the concentrate stock NS, as described below. Each hydroponic unit had 10 plants (with 30 plants per treatment), providing a plant density of 25 plants m−2.
The impact of different N concentrations in the NS was tested on purslane (Portulaca oleracea L.) plants. Four N levels (treatments) were used, named N50:50 mg N L−1, N100:100 mg N L−1, N200:200 mg N L−1, and N300:300 mg N L−1. The N concentrations referred to both N forms in the NS, i.e., NO3−-N and NH4+-N, by keeping the ratio of NH4+/total-N constant at 0.05 in all treatments. The concentrations of K and P in the NS were kept constant at 350 mg K L−1 and 70 mg P L−1, respectively, based on preliminary tests and prior results [16]. Three replicate units were used for each of the examined N levels. Greenhouse mean air temperature and humidity were 26.2 °C and 49.3% during daytime for the cultivation period.
Commercial purslane seeds (Geniki Phytotechniki Athinon SA, Athens, Greece) were sown in growing media of peat–perlite at a ratio of 85–15 v/v in seeding trays under nursery conditions. When the first true leaf appeared, the young plants were transplanted into netted pots filled with perlite and were placed in the NFT system. The plants were grown for 1 week in full-strength NS (Starter NS) to enable recovery from transplanting stress. The composition of the Starter NS is presented in Table 1. After 1 week, the plants were subjected to the modified NS with the four N concentrations for 20 days (Table 1). The NSs were monitored daily by measuring the pH and electrical conductivity (EC) levels, and they were adjusted, accordingly, by employing H2SO4 (5% v/v) for pH maintenance (because of the alkaline water used for the NS preparation) and by adding appropriate modified stock NS for the EC balance. The target pH and EC of the NS were 5.7 and 2.2 dS m−1, respectively. Commercial fertilizers and acids were utilized to prepare the various NSs, while all chemical reagents used in the present study were acquired from Sigma Aldrich (Darmstadt, Germany), unless otherwise noted.

Table 1.
Electrical conductivity (EC) and nutrient concentrations in the nutrient solution (NS) supplied to purslane plants grown in a closed hydroponic system.
2.2. Plant Growth and Tissue Analysis
In the present study, 120 purslane plants were used. After 20 days of plant cultivation under the four N levels, six individual plants—from different replication units for each treatment—were selected for the detailed analysis of plant growth features. Plant height (cm), the number of leaves, the fresh weight (g) and dry weight (g) of leaves, stems, roots, and total plants as well as the dry matter content (%) were determined.
2.3. Plant Physiology and Photosynthetic-Related Parameters
Purslane leaf stomatal conductance was determined with a Delta-T AP4 dynamic porometer (Delta-T Devices-Cambridge, UK) and expressed as mmol H2O m−2 s−1. To assess leaf photochemistry, the relative chlorophyll content (optical chlorophyll meter SPAD-502, Minolta, Osaka, Japan) and the leaf chlorophyll fluorescence (Fv/Fm) were measured with an OptiSci OS-30p Chlorophyll Fluorometer (Opti-Sciences, Hudson, NH, USA). Additionally, leaf photosynthetic pigments such as chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (t-Chl), and carotenoids were analyzed. Leaf samples (six replications/treatment, each replication was a pool of leaf tissue from two plants) were used for chlorophyll extraction, by incubation in a heat bath at 65 °C for 30 min in the dark, with 10 mL dimethyl sulfoxide (DMSO). The leaf chlorophyll and carotenoid contents were then calculated (mg g−1 fresh weight) using the corresponding equations [32,33].
To assay electrolyte leakage, the leaf tissue was cut into uniform-sized small pieces by using a round leaf cutter, with a diameter of 10 mm, and placed into a test tube with 20 mL of deionized water. The samples were incubated at 32 °C for 2 h and then autoclaved at 121 °C for 20 min to determine the initial electrolyte conductivity (EC1) and final EC2, as described by Dionisio-Sese and Tobita [34]. Electrolyte leakage (EL) was calculated as follows: EL (%)= × 100.
2.4. Plant Tissue Minerals and Water and Nutrient Use Efficiency
Once the experiment was complete, samples from leaves, stems, and roots were used to determine the mineral content, with four replicates/treatment (three pooled plants per replication). First, leaf, stem, and root tissues were dried to constant weight (at 65 °C for 4 d) and then milled at <0.42 mm. Sub-samples (~0.4 g) were ash-burned in a furnace (Carbolite, AAF 1100, GERO, Lilienthal, Germany) at 450 °C for 6 h and then acid-digested with hydrochloric acid (2 M HCl). The potassium, sodium, and phosphorous contents were determined following Chrysargyris et al. [35], the while magnesium and calcium contents were determined by using an atomic absorption spectrophotometer (PG Instruments AA500FG, Leicestershire, UK). Nitrogen was determined by the Kjeldahl method (BUCHI, Digest automat K-439 and Distillation Kjeldahl K-360, Flawil, Switzerland). Data were expressed in g kg−1 of dry weight.
Water uptake (L plant−1) was computed by recording the total quantity of water utilized by the plants during the cropping period. The total quantity of nutrients utilized by the plants was calculated by combining the inputs from the stock solutions in the NS and subtracting the remaining nutrients in the drainage solution at the end of the cropping period. Nutrient uptake (mL plant−1) was calculated.
The N accumulation rate (AR), the N bioaccumulation coefficient (BAC), the N translocation factor (TF), and the N tolerance index (TI) of purslane were computed with equations from Benimeli et al. [36], Amin et al. [37], and Azooz et al. [38] as follows.
The N accumulation rate was calculated as the sum of N content in the tissue of each plant organ x plant DW, divided by the number of days under N levels by the total plant DW [36]:
The N bioaccumulation coefficient was calculated as the ratio of the N content in the tissue of each plant organ to that of the N concentration in the NS [37]:
The translocation factor was calculated as the ratio of the N content in the tissue of each plant organ (leaves, stems) to that of the N content in the plant roots [37]:
The nitrogen tolerance index was calculated as the quotient of a plant growth-related factor (i.e., total biomass, plant height, leaf number) of the treated plants grown under increased N-treated and lower N conditions, according to the equations by Benimeli et al. [36] and Azooz et al. [38], with the following modifications:
Water use efficiency was determined (indicated as the irrigation water productivity—WPI), and was calculated as the ratio between the marketable yield produced by a crop throughout the growing season and the irrigation water used (IWU) in the same period [39], after modifications:
Nutrient use efficiency (NUE) was calculated as the ratio of the marketable yield produced by a crop to the nutrient supply:
2.5. Total Phenols, Total Flavonoids, Antioxidant Activity, Ascorbic Acid, and Total Soluble Sugars
Polyphenols were extracted from six samples (two individual plants were pooled/sample) for each treatment. Methanolic extracts of the plant tissue (0.7 g) were stored at −20 °C until being used in analysis of total phenolic and flavonoid contents and total antioxidant activity by three assays. The 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) methods were used.
The total phenol content was ascertained using the Folin–Ciocalteu method (at 755 nm; using a microplate spectrophotometer—Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA), as previously mentioned [40]. The results were expressed in gallic acid equivalents (mg GA g−1 FW). The content of total flavonoids was determined by using the aluminum chloride colorimetric method [41], as modified in Chrysargyris et al. [30]. The absorbance was measured at 510 nm. The total flavonoid content was expressed as rutin equivalents (mg rutin g−1 FW).
The free radical scavenging activity was determined as previously described [40]. In brief, the DPPH radical scavenging activity of the leaf extracts was assessed at 517 nm, while the FRAP activity was measured at 593 nm, as described in Chrysargyris et al. [40]. The ABTS assay was implemented according to the methodology described by Woidjylo et al. [42]. The results were expressed as Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) equivalents (mg Trolox g−1 of fresh weight).
Ascorbic acid (AA) was determined by the 2,6-Dichloroindophenol titrimetric method, as described previously [43]. Plant tissue (0.5 g) was extracted in 10 mL oxalic acid 4.0% and titrated by the dye solution until the color changed. Data were expressed as μg of AA per gram of fresh weight.
Total soluble solids (TSS) were determined from the juice derived from plant tissue with a temperature-compensated digital refractometer (model Sper Scientific 300017, Scottsdale, AZ, USA) at 20 °C. The results were expressed in percentage (%).
2.6. Lipid Peroxidation, Hydrogen Peroxide, and Enzyme Antioxidant Activity
The content of hydrogen peroxide (H2O2) was assessed [44]. Six samples (two individual plants were pooled/sample) were used for each treatment. The samples and standards were measured at 390 nm, and the results were expressed as μmol H2O2 g−1 fresh weight.
Lipid peroxidation, in terms of malondialdeyde content (MDA), was assessed [45]. The absorbance of the reaction mixture was recorded at 532 nm and corrected for non-specific absorbance at 600 nm. The MDA content was determined using the extinction coefficient of 155 mM cm−1. The results were expressed as nmol of MDA g−1 fresh weight.
The activity of the antioxidant enzymes for superoxide dismutase (SOD) (EC 1.15.1.1) and for catalase (CAT) (EC 1.11.1.6) was assayed [46], and the absorbance of the reaction mixture was determined at 560 nm for SOD and at 240 nm for CAT. Peroxidase activity (POD) (EC 1.11.1.6) was determined following the increase in absorbance at 430 nm as described previously [47]. The results were expressed as enzyme units per mg of protein. The protein content in leaf tissue was measured using the Bradford method with bovine serum albumin as a standard.
2.7. Statistical Methods
Analysis of variance (ANOVA) was performed on the data by IBM SPSS v.22, and the results were presented as treatment mean ± standard error (SE) of six biological measurements. Duncan’s multiple range tests were performed when AVOVA rendered a significant treatment impact at p < 0.05. The correlation coefficients between N ratios with individual parameters tested as well as the correlation coefficients of nitrogen content in the leaves, stems, and/or roots were determined by Pearson’s correlation test.
3. Results
3.1. Electrical Conductivity and pH of the Drainage Nutrient Solution
The EC of the NS fluctuated from 1.9 to 2.4 dS m−1 among the treatments of different N concentrations in this study (Figure 1). The pH levels of the NS fluctuated in a similar manner for all the examined N concertation treatments, as it was controlled daily.
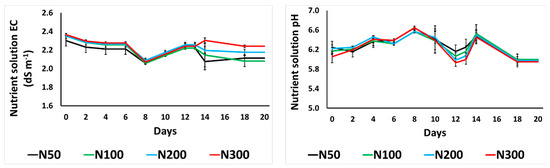
Figure 1.
Effect of increasing nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) on the electrical conductivity (EC) and pH of the drainage nutrient solution from purslane plants grown hydroponically in the NFT system. Error bars show SE (n = 3).
3.2. Growth Parameters
The leaf number was reduced after N application ≥ 200 mg L−1 compared with 50 mg L−1 of N (Table 2). Above-ground plant biomass decreased with 300 mg N L−1 compared with 50 mg L−1 of N, and this was related to the decreased root fresh weight rather than the leaf and stem fresh weights. The stem and root dry weights decreased with the N application of ≥200 mg L−1 and ≥100 mg L−1, respectively, compared with the N application of 50 mg L−1. No significant differences were found in plant height when purslane was grown at different N levels in the NS. The lowest dry matter content was observed in plants grown at 100 mg L−1 of N.

Table 2.
Effect of increasing total nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) on plant height (cm), number of leaves, upper plant (leaf, stem) and root fresh weights (FWs; g) and dry weights (DWs; g), and dry matter content (DM; %) of purslane plants grown hydroponically in the NFT system.
3.3. Physiological Parameters
Different concentrations of N ranging from 50 to 300 mg L−1 resulted in similar SPAD values as well as chlorophyll (Chl a, Chl b, and Total Chls) and carotenoid contents (Table 3). Higher leaf stomatal conductance and leaf chlorophyll fluorescence were found in plants grown at N concentrations of 200 mg L−1. Electrolyte leakage was decreased in plants grown in 300 mg L−1 of N compared with 50 mg L−1 of N.

Table 3.
Effect of increasing the total nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) on the leaf stomatal conductance (mmol m−2 s−1), chlorophyll content (a, b, total; mg g−1 fresh weight), carotenoid content (mg g −1 fresh weight), SPAD value, leaf chlorophyll fluorescence (Fv/Fm), and electrolyte leakage (%) of purslane plants grown hydroponically in the NFT system.
Total phenolic and total flavonoid contents, as well as the antioxidant activity of purslane, were affected by the N concentrations (Table 4). The highest content of total phenols was found in plants grown at 50 and 300 mg L−1 of N. The total flavonoid content increased in plants grown at ≤200 mg N L−1. The antioxidant activity of purslane, as assayed by ABTS, was high in plants subjected to 300 mg N L−1 (including 50–100 mg N L−1 for DPPH and FRAP). The ascorbic acid content increased in plants grown at 300 mg N L−1, while greater TSS was found in the case of the 50 mg L−1 applied N treatment but decreased with the applications of ≥100 mg L−1 compared with 50 mg L−1 of N.

Table 4.
Effect of increasing the total nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) on the total phenols (mg GA g−1 Fw), antioxidant activity [(2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS); mg Trolox g−1 Fw)] and flavonoids (mg rutin g−1 Fw), ascorbic acid (μg g−1 Fw), and total soluble sugars (TSS: %) of purslane plants grown hydroponically in the NFT system.
The different N concentrations affected the tested damage indicators, H2O2 and MDA, and the antioxidant enzyme activities of the treated purslane plants (Table 5). The production of H2O2 in the leaf tissue increased with the 100 mg N L−1 application compared with the 50 mg L−1 and 200 mg N L−1 applications. MDA increased when the plants were grown in 50 or 300 mg N L−1, but it remained at low levels in the case of the 100 mg N L−1 treatment. The SOD activity increased in the plants grown at ≤100 mg N L−1, while the opposite was found for the POD activity, which increased at ≥200 mg N L−1.

Table 5.
Effect of increasing total nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, N300) on the hydrogen peroxide (H2O2; μmol g−1), lipid peroxidation malondialdeyde content (MDA; nmol g−1), and antioxidant enzyme activity of superoxide dismutase (SOD; units mg−1 protein), catalase (CAT; units mg−1 protein), and peroxidase (POD; units mg−1 protein) of purslane plants grown hydroponically in the NFT system.
3.4. Leaf and Root Nutrient Content
In the leaves, the highest K content was found at the N100 and N300 levels, while P accumulated more in the plants grown at N100. The Mg, Na, and Ca accumulations increased with increased N concentrations in the NS (Figure 2). The nitrogen accumulation was not impacted by the N levels in the NS. In the stems, N accumulated more at the high N levels, while Ca, Mg, and Na accumulated more in plants grown at ≥200 mg L−1, and P accumulated less in plants grown at 300 mg L−1. In the roots, the increased N content in the NS appeared to lead to increased accumulation of some minerals, as evidenced by the increased N, K, Mg, and Na contents in the roots.
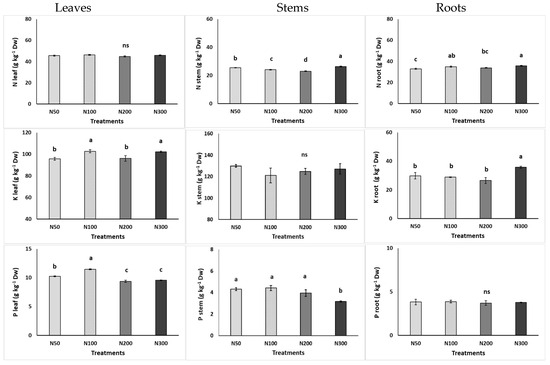
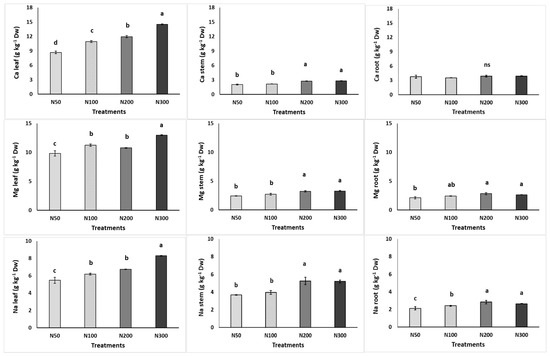
Figure 2.
Effect of increasing nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) in the nutrient solution on the content of macronutrients in the leaves, stems, and roots of purslane plants grown hydroponically in the NFT system. Significant differences (p < 0.05) among N concentrations are indicated by different letters; ns indicates non-significant. Error bars show standard error—SE (n = 4).
3.5. Nitrogen and Water Uptake and Use Efficiency
The purslane plants grown at 200 mg L−1 of N revealed a higher nitrogen uptake compared with the relevant applications of 50 and 300 mg L−1 of N (Table 6). The water uptake and agronomic efficiency of N did not differ among the examined N levels in the NS. The greatest irrigation water productivity was found with the N200 application, while the smallest one was found with the N50 application.

Table 6.
Influence of increasing the total nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) on the nutrient uptake (mL plant−1), water uptake (L plant−1), nutrient use efficiency (NUE; g DW mL−1 NS), and water use efficiency (WUE; g DW L−1 H2O) of purslane plants grown hydroponically in the NFT system.
Considering the different N concentrations, the nitrogen accumulation rate significantly decreased as the N concentration in the NS increased (Table 7). Similarly, the bioaccumulation coefficient in the leaves, stems, and roots decreased as the N concentration in the nutrient solution increased. The highest TF in the leaves was observed with the application of 50 mg L−1 of N compared with 300 mg L−1 of N, while increased TF in the stems was found with the applications of 50 and 300 mg L−1 N. The tolerance index (TI) for plant biomass, leaf number, and stem fresh weight decreased at ≥100 mg L−1 compared with 50 mg L−1 of N.

Table 7.
Influence of increasing the total nitrogen (N) concentration (50, 100, 200, and 300 mg L−1; as N50, N100, N200, and N300) on the nitrogen accumulation rate—AR (g kg−1 Dw day−1), bioaccumulation coefficient—BAC, translocation factor—TF, and tolerance indices—TIs (%) of purslane plants grown hydroponically in the NFT system.
3.6. Correlation of N Concentrations with Individual Parameters
Linear correlation coefficients were calculated and reported in detail in Table S1 to analyze the contribution of the N concentration in the NS for each individual parameter. There was a positive correlation among the N (50–100–200–300 mg L−1) concentrations in the NS and leaf N, leaf K, leaf Ca, stem Na, stem Mg, stem Ca, and stem N, root N, root K, root Ca, and SOD activity. There was a negative correlation among the leaf number, fresh biomass and root fresh weight, dry matter content, leaf P, stem P, total flavonoids, and POD activity.
4. Discussion
Plants need significant quantities of N, P, and K to ensure proper crop growth and development. However, when these elements are insufficient, plants boost the formation of secondary metabolites in anticipation of abiotic stress [48]. In the present study, the effects of the N content in an NS on the growth, nutrient accumulation, and antioxidant capacity, as well as WUE and NUE, of hydroponically grown purslane were investigated. Given that hydroponics offers a method to regulate mineral levels in nutrient solutions and conserves water and fertilizer by reusing drainage solutions, research indicates that this cultivation method can significantly improve crop efficiency, particularly in enhancing vegetable quality and yield [49]. The N concentrations at 300 mg L−1 in the NS decreased purslane total biomass (leaves, stems, and roots), while no significant differences were found in the plants grown at lower N levels. However, the purslane yield was not affected by the different N levels, as the fresh weight of the edible parts of the plant, which are the leaves and stems, were at similar levels. This is probably related to the short cultivation period of 20 days under the N-modified NS, which is of high importance since it is indicated that for short-growing-period plants, low levels of mineral supplements may not significantly affect the commercial yield of the examined crop. However, each species needs to be evaluated individually. In that sense, the similar purslane yield in the current study differs from prior results on lettuce (Lactuca sativa) yield when plants were subjected to different N levels in a deep floating hydroponic system [50,51] and sowthistle (Sonchus oleraceus) in NFT [31]. It has been reported that varying the N concentration from 150 to 250 mg L−1 (N 10.7–17.8 mmol L−1) in an NS had no impact on spearmint (Mentha spicata) yield [29].
Leaf photosynthetic rates are affected by changes in leaf stomatal conductance, the structure of chloroplasts, and chlorophyll content [52]. In the current study, the leaf stomatal conductance increased at 200 mg L−1 of N compared with higher N levels (i.e., 300 mg L−1), indicating partial leaf stomatal closure for the latter, which was possibly due to some oxidative stress as observed with the high MDA content because of lipid peroxidation. The content of chlorophylls was not affected by the different N levels, indicating similar plant photosynthetic rates among the examined N treatments. This result is in agreement with previous reports on sowthistle exposed to different N levels in an NS [31] and different ratios of ammonium to total nitrogen for sowthistle [31] and purslane [16]. The Fv/Fm ratio is an important characteristic of plants since it shows how the light reaction functions appropriately, and it is used to assess any stress in plants. A measured Fv/Fm value between 0.79 and 0.84 is normal for several species [53]. In the present study, the greatest Fv/Fm value was observed in the purslane plants grown at 200 mg L−1 of N in the NS.
Electrolyte leakage is an indicator of plant cell stress responses [54], while increased values are mirrored the induction of oxidative stress in plant tissue. In that sense, the increased electrolyte leakage observed in the plants grown at the lowest tested N levels (i.e., 50 mg L−1) indicated oxidative stress in plant tissue, as mirrored by the increased MDA levels and increased in POD enzymatic activity, as reported in a previous study [55].
Flavonoids, one of the most important secondary metabolites, are widely known for their ability to prevent free radical damage to cell membranes and to treat illnesses, cardiovascular abnormalities, and cognitive problems. Flavonoids also have high antioxidant capacity, and purslane is considered a candidate with increased antioxidant capacity [56]. These components are often produced by photosynthesis in regard to biotic and abiotic challenges, as well as nutritional deficiencies, in order to shield the plant from possible damage caused by free radicals [57]. This has an impact on the phenylpropanoid pathway, which initiates the synthesis of significant chemicals like flavonoids and provides plants with a rich supply of metabolites [58]. Nguyen and Niemeyer [59] demonstrated that modifications to N fertilization throughout the plant growing period had a substantial influence on basil phenolic compounds, specifically rosmarinic acid. Nitrogen deficit leads to a greater accumulation of phenols and flavonoids, while the increased use of N leads to the higher formation of polyunsaturated fatty acids [60]. However, in the present study, plants subjected to low N levels of 50 mg L−1 had a higher content of total phenols and flavonoid. This is most probably related to the short cultivation period of 20 days and/or to the fact that N was similarly accumulated in leaves, whereas the same tissue was used for the phenol and flavonoid determinations. However, in the stems and roots, N was differently accumulated in regard to N levels in the NS. Therefore, plant tissue sampling by using stems might provide more accurate results for the N accumulation in plant tissue compared with leaf sampling, and this can be further explored in future studies. Additionally, total flavonoids were at high levels at ≤200 mg N L−1 but significantly decreased at 300 mg N L−1. Moreover, the purslane grown in ≥100 mg N L−1 had decreased TSS levels, as previously reported when sowthistle plants were subjected to various N concentrations in hydroponics [31]. In general, plants stimulate several components by increasing AA, phenols, and proline to withstand hyperosmotic stress [61].
As stated by Cakmak [62], the use of optimum N concentrations to fertilize plants reduces plant stress responses and, as a consequence, the accumulation of reactive oxygen species (ROS) in plant tissue. Plants trigger detoxification mechanisms to combat ROS production by the capture of free radicals by stimulating enzymatic antioxidant molecules such as SOD, CAT, POD, ascorbate peroxidase (APX), and glutathione reductase (GR) [63] or by triggering non-enzymatic antioxidant systems such as polyphenols, ascorbic acid, proline, and so on. In the present work, both low and high levels of N in the NS stimulated MDA, indicating oxidative stress in the plants grown in those N applications. The plants activated antioxidant enzymes (i.e., SOD and POD) and non-enzymatic reactions, i.e., total phenols, flavonoids, and AA, to detoxify the stress condition by scavenging the molecules that were oxidative stressors.
The increased N levels in the NS impacted macronutrients in the leaves, stems, and roots. In the current study, the content of N in the stems and roots increased at the high N concentrations in the NS. However, the N content in the leaves did not differ among the examined N levels and averaged 45.6 g kg−1 of dry weight. These contents were similar to prior results for purslane under different ammonium levels in an NS [16]. The N content in the leaves (average of 4.5%) and stems (average of 2.5%) of the purslane plants were higher than the relevant N levels (i.e., 1.5%) that are appropriate for plant growth [64], indicating well-maintained cultivation schemes in the hydroponic system and conditions used in the present study. Purslane is rich in dietary minerals, including K, Mg, Ca, and P [7], while the purslane produced in the present study had a high nutritive value; for example, K averaged 335 mg K per 100 g fresh weight in leaves. The nitrogen forms in soil were found to influence the nitrate accumulation in the plant tissue of cultivated purslane, and among the various N forms used, an ammonium sulfate source was suggested [65]. However, in hydroponics, mineral levels can be precisely controlled, and there are several ways to reduce nitrate buildup in plants, such as increasing ammonium levels in an NS [31].
In the present study, the N concentrations in the NS influenced plant growth, plant physiology, N uptake, and WUE. Although the highest N uptake was observed at 200 mg N L−1, N accumulated more in the N50 treatment at a rate of 39.1 g of N per kg of dry tissue of purslane per day. It is noteworthy to mention that NUE was similar to all tested N levels in the NS with no significant differences, even though a higher numerical NUE (0.17 g dry weight per mL of NS) was found at 200 mg N L−1. The highest WUE was found at 200 mg N L−1 and differed significantly from the relevant WUE observed at 50 mg N L−1, which rendered the former a more sustainable cultivation practice compared with the latter. Water savings are fundamental in the agriculture sector, especially in the Mediterranean region where there are great events of water shortage. However, the WUE, NUE, nitrogen uptake, and water uptake, as well as the yield (edible leaves and stems) produced, did not differ in the case of the N100 and N200 applications, indicating that both levels can be considered favorable for purslane cultivation in hydroponics. Because of the increasing constraints caused by the excessive use of chemical fertilizers and water in the agriculture sector, crop management with high WUE and NUE is recommended. In such cases, the biochemical profile of purslane needs to be studied to secure the maintenance of the high nutritive and biocidal value of edible purslane.
The N accumulation rate reduced as the N concentration increased in the NS. Similarly, the bioaccumulation coefficient in the leaves, stems, and roots significantly decreased as the N levels increased in the NS. The reduced translocation of N from the roots to the leaves was found at the N300 application, while the TF from the roots to the stems was decreased at N100 and N200, compared with higher or lower N concentrations in the NS. The TF and BAC indices are important parameters to identify plant responses to phytoremediation and element accumulation in different plant organs. In the case of hyperaccumulating plant species, both TF and BAC are more than one, as this is evidenced by the BAC and TF in the leaves in the present study. However, the stems had TF < 1, which shows that little N was translocated from the roots to the stems and that stems do not accumulate N compared with leaves. Considering the TI, it clearly showed the increased N concertation in the NS. These vital markers provide new insights into nutrient management in closed hydroponic systems, leading to a better knowledge of nitrogen accumulation in plant tissue.
5. Conclusions
The results of this study showed that soilless culture techniques, such as NFT, may be used to cultivate purslane successfully, which is an underutilized crop. Additionally, the current study demonstrated that the N supply concentration affects the growth and nutritional value of purslane cultivated hydroponically. The plants grown in high N levels of 300 mg L−1 had the lowest biomass production and decreased flavonoids because of the increased oxidative stress, as indicated by the high MDA and increased phenols and antioxidants. Increased phenols and MDA levels were also found in the plants grown in 50 mg N L−1. Intermediate N levels in the NS (i.e., 100 mg L−1) caused decreased oxidative stress as the enzymatic antioxidant, i.e., POD activity, increased. Additionally, WUE and nutrient uptake, as well as leaf stomatal conductance and leaf fluorescence, had the highest values in the plants grown at N of 200 mg L−1. Therefore, to increase the yield (leaves and stems that are the edible parts), nutritional value, and water use efficiency of hydroponically grown purslane, a concentration of 200 mg L−1 N in the NS is recommended, but 100 mg L−1 N in the NS is also a valuable solution.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10091007/s1, Table S1: Correlation coefficients and p-values between the N concentrations in the NS and the nitrogen accumulation in plant tissue with the tested parameters.
Author Contributions
Conceptualization, N.T.; methodology, N.T.; software, A.C.; validation, A.C.; formal analysis, P.X. and A.C.; investigation, P.X., G.Z. and A.C.; resources, N.T.; data curation, A.C.; writing—original draft preparation, N.T. and A.C.; writing—review and editing, A.C., G.Z. and N.T.; visualization, A.C. and G.Z.; supervision, N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by PRIMA (grant Number Prima2019-11, PRIMA/0009/2019, P2P/PRIMA/1218/0006, 01DH20006, Prima2019-12, STDF Valuefarm, 18-3-2021, TUBITAK-119N494, 301/October 18th, 2020, PCI2020-112091) a program supported by the European Union with co-funding by the Funding Agencies RIF—Cyprus.
Data Availability Statement
The authors declare data availability only upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chugh, V.; Mishra, V.; Dwivedi, S.V.; Sharma, K.D. Purslane (Portulaca oleracea L.): An underutilized wonder plant with potential pharmacological value. Pharma Innov. J. 2019, 8, 236–246. [Google Scholar]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Napolitano, A.; Heiss, E.H.; Dirsch, V.M.; Piacente, S. Portulaca oleracea, a rich source of polar lipids: Chemical profile by LC-ESI/LTQOrbitrap/MS/MSn and in vitro preliminary anti-inflammatory activity. Food Chem. 2022, 388, 132968. [Google Scholar] [CrossRef] [PubMed]
- Jalali, J.; Rahbardar, M.G. Ameliorative effects of Portulaca oleracea L. (purslane) on the metabolic syndrome: A review. J. Ethnopharmacol. 2022, 299, 115672. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Durante, M.; Gonnella, M.; Renna, M.; Montesano, F.F.; Parente, A.; Mita, G.; Serio, F. Enhancing the nutritional value of Portulaca oleracea L. by using soilless agronomic biofortification with zinc. Food Res. Int. 2022, 155, 111057. [Google Scholar] [CrossRef] [PubMed]
- Montoya-García, C.O.; García-Mateos, R.; Becerra-Martínez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Jesús Magdaleno-Villar, J. Bioactive compounds of purslane (Portulaca oleracea L.) according to the production system: A review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose Benefits of an Underexplored Species Purslane (Portulaca oleracea L.): A Critical Review. Environ. Manage. 2021, 72, 309–320. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- de Lacerda, L.P.; Lange, L.C.; Costa França, M.G.; Zonta, E. Salinity Reduction and Biomass Accumulation in Hydroponic Growth of Purslane (Portulaca oleracea). Int. J. Phytoremediat. 2015, 17, 235–241. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Hydroponic production of purslane as a sodium-removing vegetable in NaCl-rich nutrient solution. HortScience 2014, 49, 201–206. [Google Scholar] [CrossRef]
- Camalle, M.; Standing, D.; Jitan, M.; Muhaisen, R.; Bader, N.; Bsoul, M.; Ventura, Y.; Soltabayeva, A.; Sagi, M. Effect of salinity and nitrogen sources on the leaf quality, biomass, and metabolic responses of two ecotypes of Portulaca oleracea. Agronomy 2020, 10, 656. [Google Scholar] [CrossRef]
- Peng, J.; Wei, W.; Lu, H.C.; Chen, W.; Li, S.D.; Wang, J.; Duan, C.Q.; He, F. Effect of Covering Crops between Rows on the Vineyard Microclimate, Berry Composition and Wine Sensory Attributes of ‘Cabernet Sauvignon’ (Vitis vinifera L. cv.) Grapes in a Semi-Arid Climate of Northwest China. Horticulturae 2022, 8, 518. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Olive-mill and grape-mill waste as a substitute growing media component for unexploded vegetables production. Sustain. Chem. Pharm. 2023, 31, 100940. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Louka, S.; Petropoulos, S.A.; Tzortzakis, N. Soilless Cultivation of Portulaca oleracea Using Medicinal and Aromatic Plant Residues for Partial Peat Replacement. Horticulturae 2023, 9, 474. [Google Scholar] [CrossRef]
- Atrooz, O.M.; Al-Maitah, S.Z. Characterization of the crude extract of Portulaca oleracea and the determination of the polyphenol oxidase kinetics in the presence of Cu and Zn. J. Appl. Biol. Biotechnol. 2022, 10, 28–33. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Ammonium to total nitrogen ratio affects the purslane (Portulaca oleracea L.) growth, nutritional, and antioxidant status. Heliyon 2023, 9, e21644. [Google Scholar] [CrossRef]
- Szalai, G.; Dai, N.; Danin, A.; Dudai, N.; Barazani, O. Effect of nitrogen source in the fertilizing solution on nutritional quality of three members of the Portulaca oleracea aggregate. J. Sci. Food Agric. 2010, 90, 2039–2045. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; Volke-Haller, V.H.; Trinidad-Santos, A.; Villanueva-Verduzco, C. Change in the contents of fatty acids and antioxidant capacity of purslane in relation to fertilization. Sci. Hortic. 2018, 234, 152–159. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Varlagas, H.; Savvas, D.; Mouzakis, G.; Liotsos, C.; Karapanos, I.; Sigrimis, N. Modelling uptake of Na+ and Cl− by tomato in closed-cycle cultivation systems as influenced by irrigation water salinity. Agric. Water Manag. 2010, 97, 1242–1250. [Google Scholar] [CrossRef]
- Savvas, D.; Neocleous, D. Developments in soilless/hydroponic cultivation of vegetables. In Achieving Sustainable Cultivation of Vegetables; Hochmuth, G., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 211–243. [Google Scholar]
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Editorial: Soilless Cultivation Through an Intensive Crop Production Scheme. Management Strategies, Challenges and Future Directions. Front. Plant Sci. 2020, 11, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Fei, L.; Huang, D.; Zeng, J.; Chen, L.; Cai, Y. Coupling effects of irrigation and nitrogen levels on yield, water and nitrogen use efficiency of surge-root irrigated jujube in a semiarid region. Agric. Water Manag. 2019, 213, 146–154. [Google Scholar] [CrossRef]
- Akl, I.A.; Savvas, D.; Papadantonakis, N.; Lydakis-Simantiris, N.; Kefalas, P. Influence of Ammonium to Total Nitrogen Supply Ratio on Growth, Yield and Fruit Quality of Tomato Grown in a Closed Hydroponic System. Eur. J. Hortic. Sci. 2003, 68, 204–211. [Google Scholar]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Castellanos-Morales, V.; Villegas-Moreno, J.; Vierheilig, H.; Cárdenas-Navarro, R. Nitrogen availability drives the effect of Glomus intraradices on the growth of strawberry (Fragaria x ananassa Duch.) plants. J. Sci. Food Agric. 2012, 92, 2260–2264. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Wang, Y.-S.; Wang, X.-C.; Rengel, Z.; Zhang, W.-J.; Shu, L.-Z. Alternate partial root-zone drip irrigation with nitrogen fertigation promoted tomato growth, water and fertilizer-nitrogen use efficiency. Agric. Water Manag. 2020, 233, 106049. [Google Scholar] [CrossRef]
- Mahajan, M.; Pal, P.K. Yield response, accumulation of bioactive ingredient and ion uptake of Stevia rebaudiana to different soil-moisture and nitrogen levels. Agric. Water Manag. 2022, 264, 107511. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crop. Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Optimising fertigation of hydroponically grown sowthistle (Sonchus oleraceus L.): The impact of the nitrogen source and supply concentration. Agric. Water Manag. 2023, 289, 108528. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Wellburn, A.R.A.R. The spectral determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Benimeli, C.S.; Medina, A.; Navarro, C.M.; Medina, R.B.; Amoroso, M.J.; Gómez, M.I. Bioaccumulation of copper by Zea mays: Impact on root, shoot and leaf growth. Water Air Soil Pollut. 2010, 210, 365–370. [Google Scholar] [CrossRef]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, A.R.; Mangi, J.; Abbasi, M.S.; Amin, F. Copper (Cu) tolerance and accumulation potential in four native plant species: A comparative study for effective phytoextraction technique. Geol. Ecol. Landsc. 2021, 5, 53–64. [Google Scholar] [CrossRef]
- Azooz, M.M.; Abou-Elhamd, M.F.; Al-Fredan, M.A. Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Aust. J. Crop. Sci. 2012, 6, 688–694. [Google Scholar]
- Fernández, J.E.; Alcon, F.; Diaz-Espejo, A.; Hernandez-Santana, V.; Cuevas, M.V. Water use indicators and economic analysis for on-farm irrigation decision: A case study of a super high density olive tree orchard. Agric. Water Manag. 2020, 237, 106074. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Kloukina, C.; Vassiliou, R.; Tomou, E.-M.E.-M.; Skaltsa, H.; Tzortzakis, N. Cultivation strategy to improve chemical profile and anti-oxidant activity of Sideritis perfoliata L. subsp. perfoliata. Ind. Crop. Prod. 2019, 140, 111694. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crop. Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Massa, D.; Vandecasteele, B. The Tripartite of Soilless Systems, Growing Media, and Plants through an Intensive Crop Production Scheme. Agronomy 2022, 12, 1896. [Google Scholar] [CrossRef]
- Wenceslau, D.d.S.L.; de Oliveira, D.F.; deO Rabelo, H.; Ferbonink, G.F.; Gomes, L.A.A.; Leonel, É.C.A.; Caione, G. Nitrate concentration and nitrate/ammonium ratio on lettuce grown in hydroponics in Southern Amazon. Afr. J. Agric. Res. 2021, 17, 862–868. [Google Scholar]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol. Environ. Saf. 2010, 73, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Ying, X.; Stien, D. New flavonoids from Portulaca oleracea L. and their activities. Fitoterapia 2018, 127, 257–262. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arab. B. 2011, 9, e0152. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef]
- Verardo, V.; Riciputi, Y.; Sorrenti, G.; Ornaghi, P.; Marangoni, B.; Caboni, M.F. Effect of nitrogen fertilisation rates on the content of fatty acids, sterols, tocopherols and phenolic compounds, and on the oxidative stability of walnuts. LWT 2013, 50, 732–738. [Google Scholar] [CrossRef]
- He, J.; You, X.; Qin, L. High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of C4 Halophyte Portulaca oleracea L. Grown Hydroponically Under LED Lighting. Front. Plant Sci. 2021, 12, 651341. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, H.C. Effect of nitrogen forms on growth, yield and nitrate accumulation of cultivated purslane (Portulaca oleracea L.). Bulg. J. Agric. Sci. 2013, 19, 444–449. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).