Application Methods of Zinc Sulphate Increased Safflower Seed Yield and Quality under End-Season Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Application of Treatments
2.2. Field Preparation, Plant Material, and Seed Planting
2.3. Measured Traits
2.3.1. Chlorophyll Content Index (CCI) and Leaf Area Index (LAI)
2.3.2. Relative Water Content (RWC)
2.3.3. Plant Height, Stem Diameter and Yield Components
2.3.4. Biological Yield (BY), Seed Yield (SY), and Harvest Index (HI)
2.3.5. Seed Oil Content (SOC), Oil Yield (OY), and Oil Harvest Index (OHY)
2.3.6. The Seed Nitrogen, Phosphorus, Potassium, Zinc, and Iron Element Measurement
2.4. Statistical Analysis
3. Results
3.1. Chlorophyll Content Index (CCI)
3.2. Leaf Area Index (LAI)
3.3. Plant Height and Stem Diameter
3.4. Diameter of the Capitol
3.5. Number of Capitols per Plant
3.6. The Number of Seeds per Capitol
3.7. Seeds Weight
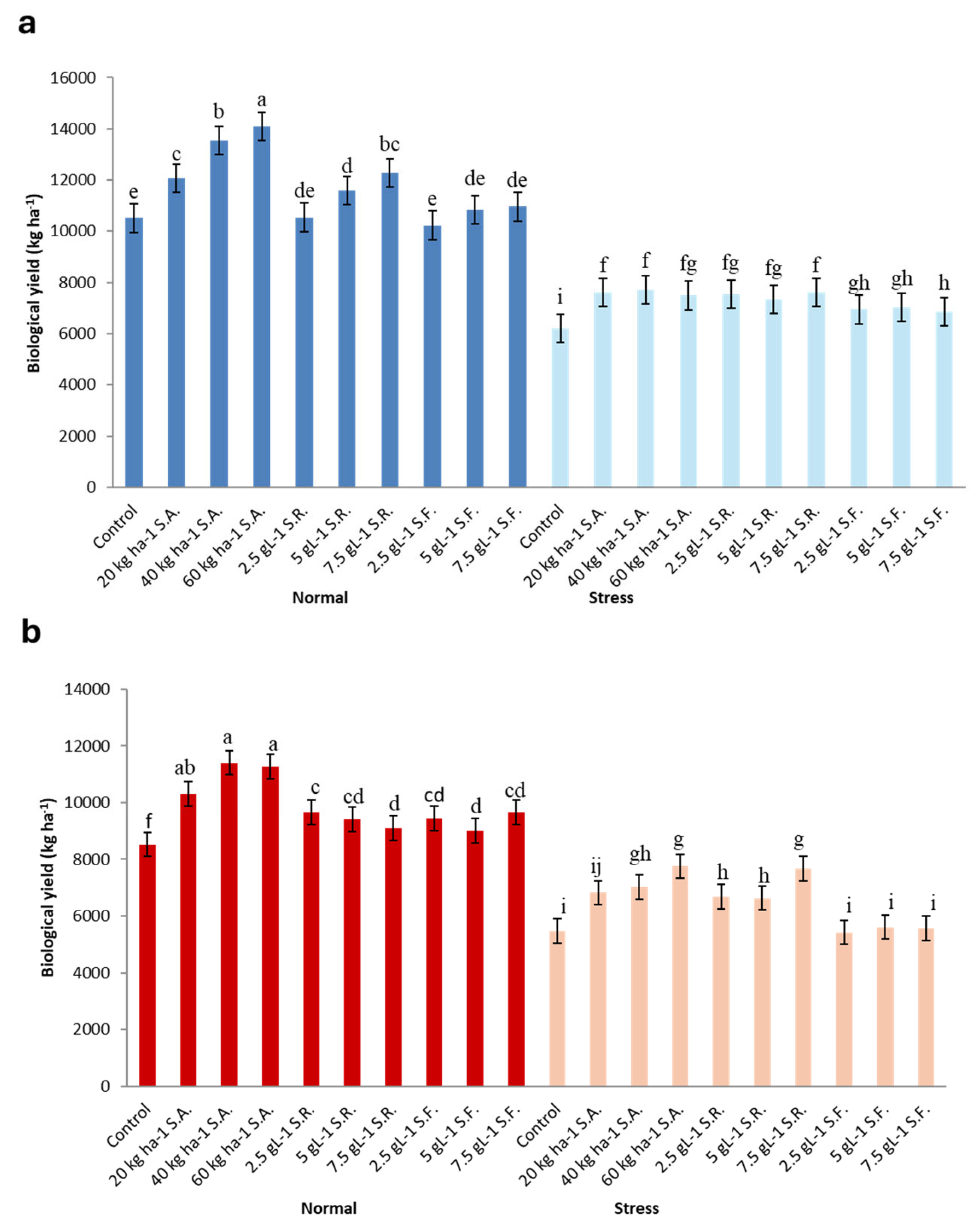
3.8. Biological Yield (BY)
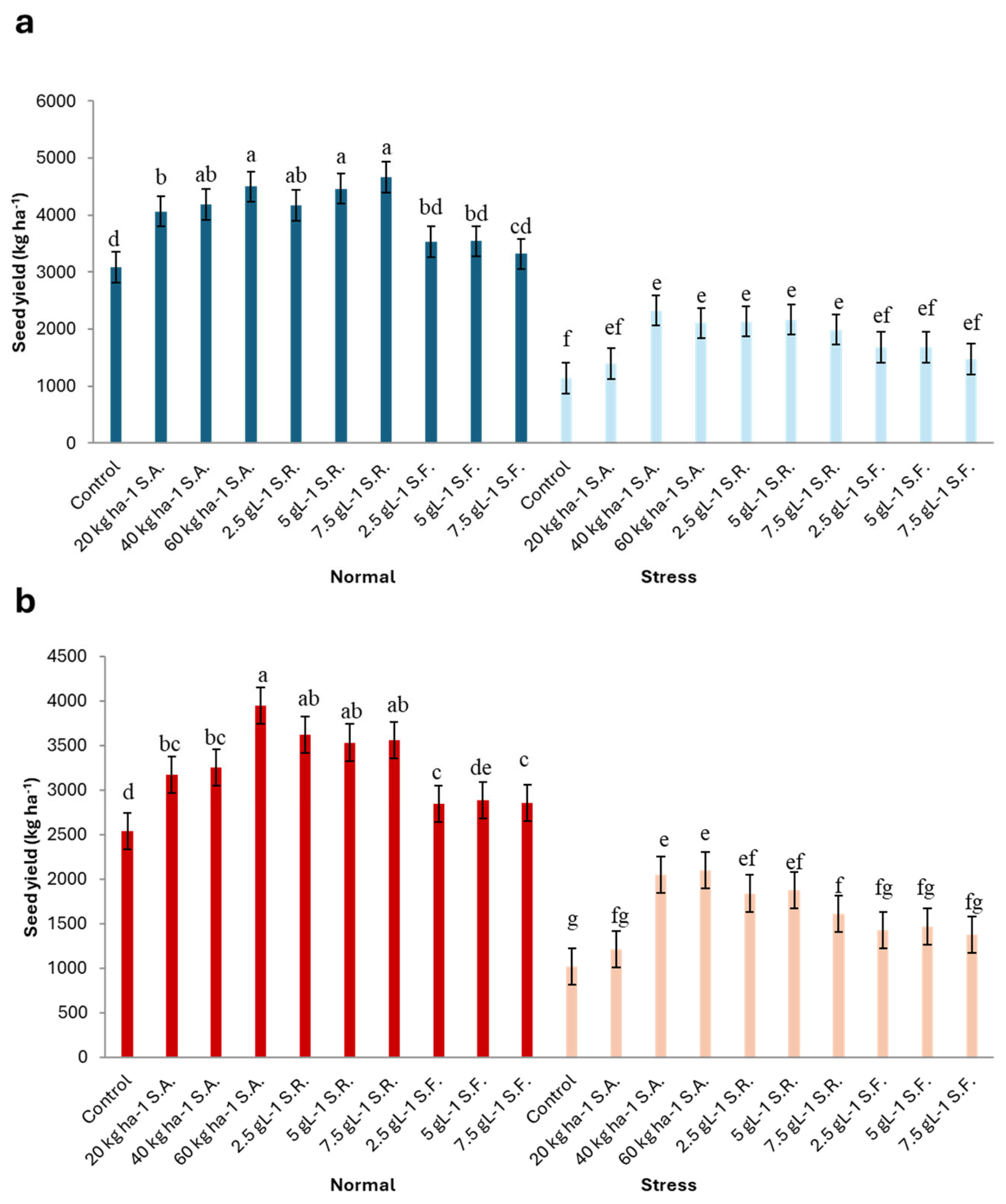
3.9. Seed Yield (SY)
3.10. Harvest Index (HI)
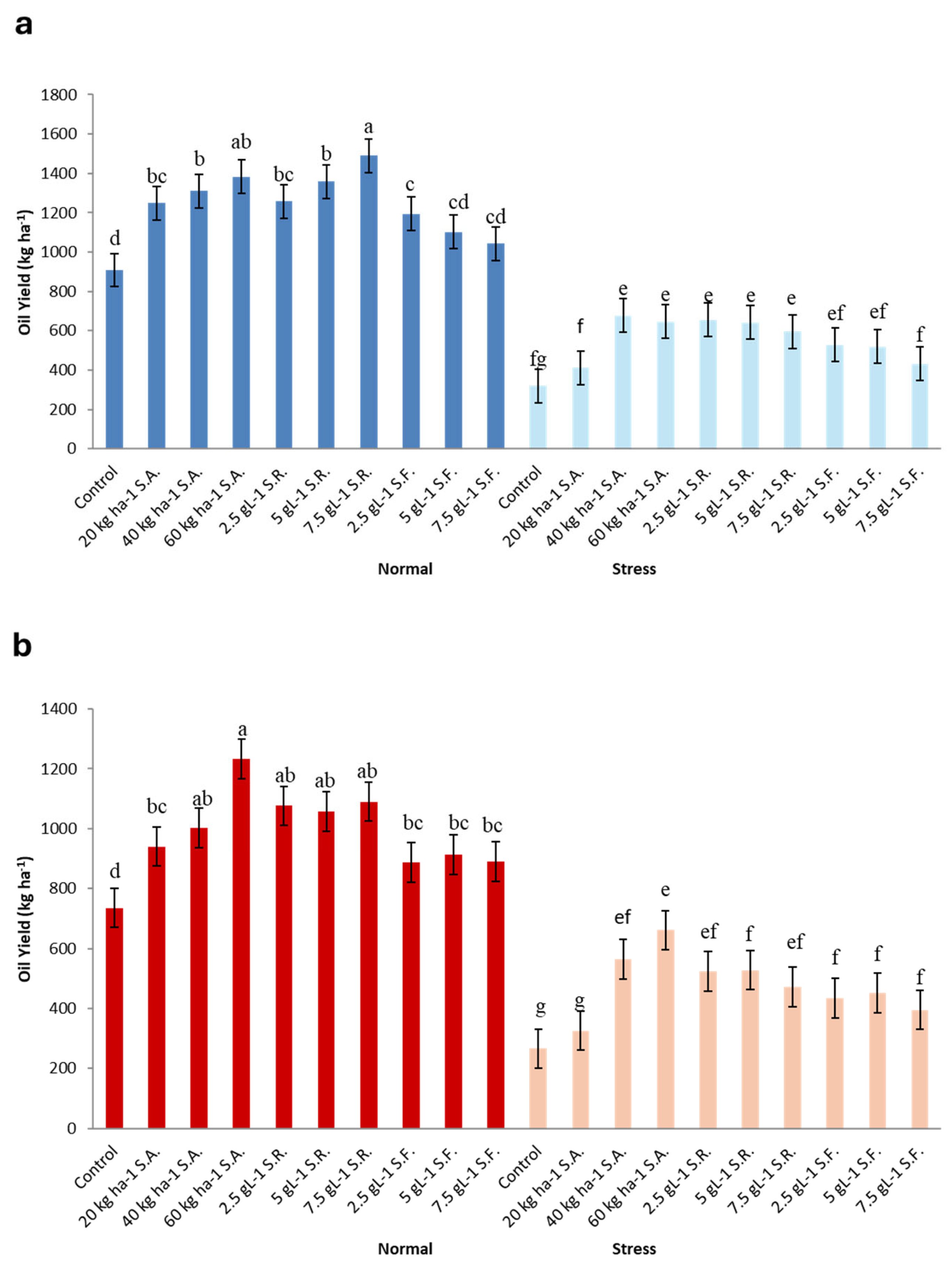
3.11. Seed Oil Content (SOC), Oil Yield (OY), and Oil Harvest Index (OHI)
3.12. The Seed Nitrogen, Phosphorus, Potassium, Zinc, and Iron Element Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vazayefi, M.; Shekari, F.; Zangani, E.; Dolatabadian, A.; Janda, T.; Mastinu, A. Seed treatment with chlormequat chloride improves the physiological and biochemical characteristics of Brassica napus L. under salt stress. Plant Stress 2023, 9, 100175. [Google Scholar] [CrossRef]
- Fukase, E.; Martin, W. Economic growth, convergence, and world food demand and supply. World Dev. 2020, 132, 104954. [Google Scholar] [CrossRef]
- Zafar, S.; Li, Y.-L.; Li, N.-N.; Zhu, K.-M.; Tan, X.-L. Recent advances in enhancement of oil content in oilseed crops. J. Biotechnol. 2019, 301, 35–44. [Google Scholar] [CrossRef]
- Jam, B.J.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Najafi, J.; Uberti, D.; Mastinu, A. Impact of Silicon Foliar Application on the Growth and Physiological Traits of Carthamus tinctorius L. Exposed to Salt Stress. Silicon 2022, 15, 1235–1245. [Google Scholar] [CrossRef]
- Jamshidi Jam, B.; Shekari, F.; Andalibi, B.; Fotovat, R.; Jafarian, V.; Dolatabadian, A. The Effects of Salicylic Acid and Silicon on Safflower Seed Yield, Oil Content, and Fatty Acids Composition under Salinity Stress. Silicon 2023, 15, 4081–4094. [Google Scholar] [CrossRef]
- de Oliveira Neto, S.S.; Zeffa, D.M.; Freiria, G.H.; Zoz, T.; da Silva, C.J.; Zanotto, M.D.; Sobrinho, R.L.; Alamri, S.A.; Okla, M.K.; AbdElgawad, H. Adaptability and Stability of Safflower Genotypes for Oil Production. Plants 2022, 11, 708. [Google Scholar] [CrossRef]
- Tabari, H.; Somee, B.S.; Zadeh, M.R. Testing for long-term trends in climatic variables in Iran. Atmos. Res. 2011, 100, 132–140. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar] [CrossRef]
- de Almeida Silva, M.; Santos, H.L.; de Sousa Ferreira, L.; Silva, D.M.R.; dos Santos, J.C.C.; de Almeida Prado Bortolheiro, F.P. Physiological Changes and Yield Components of Safflower (Carthamus tinctorius L.) Lines as a Function of Water Deficit and Recovery in the Flowering Phase. Agriculture 2023, 13, 558. [Google Scholar] [CrossRef]
- Zafari, M.; Ebadi, A.; Jahanbakhsh, S.; Sedghi, M. Safflower (Carthamus tinctorius) Biochemical Properties, Yield, and Oil Content Affected by 24-Epibrassinosteroid and Genotype under Drought Stress. J. Agric. Food Chem. 2020, 68, 6040–6047. [Google Scholar] [CrossRef]
- Kirkby, E.A. Marschner’s Mineral Nutrition of Higher Plants Third Edition Foreword. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Qamari, P.; Shekari, F.; Afsahi, K.; Tavakoli, A.; Samimifard, R.; Shekari, K.; Mastinu, A. Response of wheat cultivars to zinc application for seed yield and quality improvement. J. Agric. Sci. 2023, 161, 549–562. [Google Scholar] [CrossRef]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An Indispensable Micronutrient. Physiol. Mol. Biol. Plants 2012, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Manvelian, J.; Weisany, W.; Tahir, N.A.-r.; Jabbari, H.; Diyanat, M. Physiological and biochemical response of safflower (Carthamus tinctorius L.) cultivars to zinc application under drought stress. Ind. Crops Prod. 2021, 172, 114069. [Google Scholar] [CrossRef]
- Lazzari, P.; Pau, A.; Tambaro, S.; Asproni, B.; Ruiu, S.; Pinna, G.; Mastinu, A.; Curzu, M.M.; Reali, R.; Bottazzi, M.E.; et al. Synthesis and pharmacological evaluation of novel 4-alkyl-5-thien-2′-yl pyrazole carboxamides. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 254–276. [Google Scholar] [CrossRef] [PubMed]
- Anik, T.R.; Mostofa, M.G.; Rahman, M.M.; Khan, M.A.R.; Ghosh, P.K.; Sultana, S.; Das, A.K.; Hossain, M.S.; Keya, S.S.; Rahman, M.A.; et al. Zn Supplementation Mitigates Drought Effects on Cotton by Improving Photosynthetic Performance and Antioxidant Defense Mechanisms. Antioxidants 2023, 12, 854. [Google Scholar] [CrossRef]
- Aytac, Z.; Gulmezoglu, N.; Sirel, Z.; Tolay, I.; Alkan Torun, A. The Effect of Zinc on Yield, Yield Components and Micronutrient Concentrations in the Seeds of Safflower Genotypes (Carthamus tinctorius L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 202–208. [Google Scholar] [CrossRef]
- Flemmer, A.C.; Franchini, M.C.; Lindström, L.I. Description of safflower (Carthamus tinctorius) phenological growth stages according to the extended BBCH scale. Ann. Appl. Biol. 2015, 166, 331–339. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and Experimental Approaches for the Measurement of Plant Water Status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Li, J.; Li, W.L.; Ye, Z.H.; Sun, Y.W.; Gong, W.J.; Wang, Y.M.; Bi, Y.L. Determination of hydroxyl value of vegetable oils-based polyols through ring-preopening, solvent-extraction, and acetylation method. J. Am. Oil Chem. Soc. 2024. early access. [Google Scholar] [CrossRef]
- Mastinu, A.; Pira, M.; Pani, L.; Pinna, G.A.; Lazzari, P. NESS038C6, a novel selective CB1 antagonist agent with anti-obesity activity and improved molecular profile. Behav. Brain Res. 2012, 234, 192–204. [Google Scholar] [CrossRef]
- Andrews, R.E.; Newman, E.I. The Influence of Root Pruning on the Growth and Transpiration of Wheat under Different Soil Moisture Conditions. New Phytol. 2006, 67, 617–630. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Cenini, G.; Mastinu, A.; Sylvester, M.; Wilkening, A.; Abate, G.; Bonini, S.A.; Aria, F.; Marziano, M.; Maccarinelli, G.; et al. γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients 2019, 11, 753. [Google Scholar] [CrossRef]
- Walinga, I.; Van Der Lee, J.J.; Houba, V.J.G.; Van Vark, W.; Novozamsky, I. Digestion in tubes with H2SO4-salicylic acid- H2O2 and selenium and determination of Ca, K, Mg, N, Na, P, Zn. In Plant Analysis Manual; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Ambrus, A.; Visi, E.; Zakar, F.; Hargitai, E.; Szabo, L.; Papa, A. General-Method for Determination of Pesticide-Residues in Samples of Plant-Origin, Soil, and Water. 3. Gas-Chromatographic Analysis and Confirmation. J. Assoc. Off. Anal. Chem. 1981, 64, 749–768. [Google Scholar] [CrossRef]
- Solomon, M.; Gedalovich, E.; Mayer, A.M.; Poljakoff-Mayber, A. Changes Induced by Salinity to the Anatomy and Morphology of Excised Pea Roots in Culture. Ann. Bot. 1986, 57, 811–818. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Sweeney, E.M.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Sayfzadeh, S.; Jabbari, H.; Valadabadi, S.A.; Hadidi Masouleh, E. Alleviation of Drought Stress Effects on Safflower Yield by Foliar Application of Zinc. Int. J. Plant Prod. 2019, 13, 297–308. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrao, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Anwar, S.; Khalilzadeh, R.; Khan, S.; Zaib-un, N.; Bashir, R.; Pirzad, A.; Malik, A. Mitigation of Drought Stress and Yield Improvement in Wheat by Zinc Foliar Spray Relates to Enhanced Water Use Efficiency and Zinc Contents. Int. J. Plant Prod. 2021, 15, 377–389. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, J.; Jiang, S.; Ning, S.; Liu, L. Quantitative Response of Soybean Development and Yield to Drought Stress during Different Growth Stages in the Huaibei Plain, China. Agronomy 2018, 8, 97. [Google Scholar] [CrossRef]
- Bijanzadeh, E.; Moosavi, S.M.; Bahadori, F. Quantifying water stress of safflower (Carthamus tinctorius L.) cultivars by crop water stress index under different irrigation regimes. Heliyon 2022, 8, e09010. [Google Scholar] [CrossRef] [PubMed]
- Frantová, N.; Rábek, M.; Elzner, P.; Středa, T.; Jovanović, I.; Holková, L.; Martinek, P.; Smutná, P.; Prášil, I.T. Different Drought Tolerance Strategy of Wheat Varieties in Spike Architecture. Agronomy 2022, 12, 2328. [Google Scholar] [CrossRef]
- Javadipour, Z.; Balouchi, H.; Movahhedi Dehnavi, M.; Yadavi, A. Roles of methyl jasmonate in improving growth and yield of two varieties of bread wheat (Triticum aestivum) under different irrigation regimes. Agric. Water Manag. 2019, 222, 336–345. [Google Scholar] [CrossRef]
- Hatzig, S.V.; Nuppenau, J.-N.; Snowdon, R.J.; Schießl, S.V. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018, 18, 297. [Google Scholar] [CrossRef]
- Ghaffari, M.; Gholizadeh, A.; Rauf, S.; Shariati, F. Drought-stress induced changes of fatty acid composition affecting sunflower grain yield and oil quality. Food Sci. Nutr. 2023, 11, 7718–7731. [Google Scholar] [CrossRef]
- Weisany, W.; Mohammadi, M.; Tahir, N.A.-r.; Aslanian, N.; Omer, D.A. Changes in Growth and Nutrient Status of Maize (Zea mays L.) in Response to Two Zinc Sources Under Drought Stress. J. Soil Sci. Plant Nutr. 2021, 21, 3367–3377. [Google Scholar] [CrossRef]
- Etienne, P.; Diquelou, S.; Prudent, M.; Salon, C.; Maillard, A.; Ourry, A. Macro and Micronutrient Storage in Plants and Their Remobilization When Facing Scarcity: The Case of Drought. Agriculture 2018, 8, 14. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological Responses and Yield of Wheat Plants in Zinc-Mediated Alleviation of Drought Stress. Front. Plant Sci. 2017, 8, 860. [Google Scholar] [CrossRef]
- Maqbool, R.; Alawadi, H.F.N.; Khan, B.A.; Nadeem, M.A.; Mahmood, A.; Javaid, M.M.; Syed, A.; Ahmad, W.; Ali, B. Exploring the effect of zinc and boron application on oil contents, protein contents, growth and yield of sunflower. Semin.-Cienc. Agrar. 2023, 44, 1353–1374. [Google Scholar] [CrossRef]
- Ghiyasi, M.; Rezaee Danesh, Y.; Amirnia, R.; Najafi, S.; Mulet, J.M.; Porcel, R. Foliar Applications of ZnO and Its Nanoparticles Increase Safflower (Carthamus tinctorius L.) Growth and Yield under Water Stress. Agronomy 2023, 13, 192. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Farroh, K.Y.; Amin, H.E.; Atta, A.M. Green Synthesis of Zinc Oxide Nanoparticles: Fortification for Rice Grain Yield and Nutrients Uptake Enhancement. Molecules 2021, 26, 584. [Google Scholar] [CrossRef] [PubMed]
- Movahhedy-Dehnavy, M.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Foliar application of zinc and manganese improves seed yield and quality of safflower (Carthamus tinctorius L.) grown under water deficit stress. Ind. Crops Prod. 2009, 30, 82–92. [Google Scholar] [CrossRef]
- Hao, B.; Ma, J.; Jiang, L.; Wang, X.; Bai, Y.; Zhou, C.; Ren, S.; Li, C.; Wang, Z. Effects of foliar application of micronutrients on concentration and bioavailability of zinc and iron in wheat landraces and cultivars. Sci. Rep. 2021, 11, 22782. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Zhang, W.; Pang, L.-L.; Zhang, Y.-Q.; Wang, X.-Z.; Liu, Y.-M.; Chen, X.-P.; Zhang, F.-S.; Zou, C.-Q. Effects of zinc application rate and zinc distribution relative to root distribution on grain yield and grain Zn concentration in wheat. Plant Soil 2016, 411, 167–178. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Mahmood, A.; Chattha, M.U.; Nawaz, M.; Subhani, M.N.; Kharal, M.; Khan, S. Biofortification of Wheat Cultivars to Combat Zinc Deficiency. Front. Plant Sci. 2017, 8, 281. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Suzuki, M.; Kobayashi, T.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsZIP4, a novel zinc-regulated zinc transporter in rice. J. Exp. Bot. 2005, 56, 3207–3214. [Google Scholar] [CrossRef]
- Yin, H.; Gao, X.; Stomph, T.; Li, L.; Zhang, F.; Zou, C. Zinc Concentration in Rice (Oryza sativa L.) Grains and Allocation in Plants as Affected by Different Zinc Fertilization Strategies. Commun. Soil Sci. Plant Anal. 2016, 47, 761–768. [Google Scholar] [CrossRef]

| Year | Soil Texture | Depth | pH | EC (dS m−1) | OM (g kg−1) | Ca (mg kg−1) | K (mg kg−1) | P (mg kg−1) | Zn (mg kg−1) | N (mg kg−1) | S (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | Clay loam | 0–30 | 8.85 | 1.4 | 9.45 | 380 | 280 | 9.0 | 0.54 | 920 | 280 |
| 2017 | Clay loam | 0–30 | 8.90 | 1.51 | 10.81 | 324 | 210 | 9.60 | 0.53 | 730 | - |
| Year | Month | Average Minimum Temperature (°C) | Average Maximum Temperature (°C) | Average Precipitation (mm) |
|---|---|---|---|---|
| 2016 | April | 3.7 | 15.3 | 62 |
| May | 8.6 | 24.4 | 28.1 | |
| June | 11.1 | 28.6 | 15.9 | |
| July | 16.5 | 32.9 | 1.6 | |
| August | 16.3 | 34.8 | 0 | |
| September | 13.9 | 32.3 | 0 | |
| 2017 | April | 4.0 | 15.8 | 54 |
| May | 9.3 | 25.2 | 22.1 | |
| June | 10.9 | 31.4 | 0 | |
| July | 16.9 | 34.1 | 1.3 | |
| August | 17.0 | 35.6 | 4.8 | |
| September | 14.0 | 33.8 | 0 |
| Irrigation | Zinc Sulfate | Chlorophyll Index | Leaf Area Index | RWC (%) | Height (cm) | Stem Diameter (mm) | Capitol Diameter (mm) |
|---|---|---|---|---|---|---|---|
| Normal | Control | 68.3 ± 6.11 d | 3.20 ± 0.38 de | 83.1 ± 3.20 d | 73.9 ± 19.3 d | 12.1 ± 0.31 bc | 33.0 ± 11.6 cd |
| 20 kg ha−1 S.A. | 72.9 ± 8.19 b | 3.81 ± 0.33 ab | 87.2 ± 2.06 b | 80.8 ± 13.2 a | 12.9 ± 0.26 b | 33.9 ± 13.8 c | |
| 40 kg ha−1 S.A. | 69.8 ± 9.26 cd | 3.90 ± 0.37 a | 86.7 ± 4.93 bc | 79.6 ± 15.8 a | 12.8 ± 0.38 b | 33.9 ± 11.8 c | |
| 60 kg ha−1 S.A. | 75.8 ± 7.13 a | 3.91 ± 0.48 a | 87.5 ± 2.40 b | 75.8 ± 17.4 c | 13.9 ± 0.11 a | 35.1 ± 13.2 b | |
| 2.5 gL−1 S.R. | 72.3 ± 4.65 b | 3.23 ± 0.53 de | 86.5 ± 3.04 bc | 79.2 ± 16.2 a | 12.8 ± 0.68 b | 36.9 ± 9.128 a | |
| 5 gL−1 S.R. | 72.4 ± 3.04 b | 3.37 ± 0.64 cd | 87.2 ± 5.11 b | 75.2 ± 15.7 c | 12.9 ± 0.32 ab | 34.7 ± 10.8 b | |
| 7.5 gL−1 S.R. | 75.4 ± 4.38 a | 3.90 ± 0.60 a | 89.9 ± 6.11 a | 79.5 ± 13.4 a | 12.6 ± 0.31 b | 34.8 ± 13.8 b | |
| 2.5 gL−1 S.F. | 72.8 ± 4.89 b | 3.41 ± 0.54 c | 86.9 ± 3.27 c | 78.0 ± 16.7 ab | 12.9 ± 0.22 b | 34.4 ± 13.0 b | |
| 5 gL−1 S.F. | 76.6 ± 5.37 a | 3.41 ± 0.43 c | 87.5 ± 4.13 b | 77.0 ± 19.9 bc | 13.3 ± 0.54 ab | 34.1 ± 12.2 bc | |
| 7.5 gL−1 S.F. | 69.1 ± 5.37 cd | 3.27 ± 0.45 de | 88.9 ± 5.87 a | 75.8 ± 12.6 c | 13.1 ± 0.31 ab | 34.3 ± 15.1 bc | |
| Stress | Control | 60.7 ± 4.11 f | 2.30 ± 0.68 i | 70.2 ± 4.31 h | 69.7 ± 14.0 f | 11.4 ± 0.41 e | 30.0 ± 9.48 f |
| 20 kg ha−1 S.A. | 69.6 ± 4.25 cd | 2.34 ± 0.63 i | 72.0 ± 5.45 gh | 73.7 ± 11.3 d | 12.3 ± 0.17 bc | 30.6 ± 12.7 f | |
| 40 kg ha−1 S.A. | 70.0 ± 5.04 c | 2.95 ± 0.59 f | 72.8 ± 6.00 g | 73.2 ± 14.2 d | 11.5 ± 0.23 d | 32.6 ± 16.4 d | |
| 60 kg ha−1 S.A. | 65.8 ± 4.08 eg | 2.86 ± 0.47 f | 73.8 ± 5.34 g | 76.2 ± 13.6 c | 11.7 ± 0.39 d | 30.1 ± 10.6 f | |
| 2.5 gL−1 S.R. | 68.3 ± 4.11 d | 2.93 ± 0.58 f | 72.6 ± 6.20 gh | 71.4 ± 12.4 e | 11.5 ± 0.58 d | 31.8 ± 14.1 e | |
| 5 gL−1 S.R. | 68.0 ± 3.48 de | 2.85 ± 0.54 h | 79.4 ± 3.88 e | 74.3 ± 15.6 cd | 11.9 ± 0.24 c | 31.8 ± 12.8 e | |
| 7.5 gL−1 S.R. | 71.8 ± 6.18 c | 2.53 ± 0.67 h | 79.8 ± 5.14 e | 75.8 ± 14.3 c | 11.9 ± 0.51 c | 31.1 ± 13.7 e | |
| 2.5 gL−1 S.F. | 68.2 ± 5.01 d | 2.55 ± 0.63 h | 77.1 ± 4.36 f | 70.3 ± 11.2 f | 12.1 ± 0.32 bc | 32.8 ± 12.3 d | |
| 5 gL−1 S.F. | 67.1 ± 5.84 de | 2.70 ± 0.60 g | 79.3 ± 5.49 ef | 72.0 ± 14.8 e | 11.8 ± 0.45 d | 32.5 ± 16.4 d | |
| 7.5 gL−1 S.F. | 68.6 ± 5.84 d | 2.27 ± 0.66 j | 80.2 ± 8.36 e | 74.2 ± 15.2 e | 11.9 ± 0.52 c | 32.7 ± 13.3 d | |
| Irrigation | Zinc sulfate | Number of capitol per plant | Number of seed per capitol | 1000-seed weight(g) | Oil content (%) | Harvest index (%) | |
| Normal | Control | 24.9 ± 4.01 e | 41.0 ± 6.94 i | 47.18 ± 4.11 bc | 29.4 ± 1.67 d | 29.4 ± 1.16 fg | |
| 20 kg ha−1 S.A. | 30.8 ± 6.11 c | 47.2 ± 7.47 de | 47.42 ± 3.05 bc | 30.7 ± 2.43 c | 33.7 ± 2.18 cd | ||
| 40 kg ha−1 S.A. | 28.8 ± 5.23 d | 47.9 ± 12.23 d | 47.31 ± 2.04 bc | 31.3 ± 4.25 c | 30.9 ± 1.40 f | ||
| 60 kg ha−1 S.A. | 30.1 ± 4.55 c | 55.2 ± 11.50 a | 48.75 ± 2.19 ab | 30.7 ± 2.13 c | 32.2 ± 2.29 de | ||
| 2.5 gL−1 S.R. | 33.1 ± 6.28 a | 51.7 ± 8.66 bc | 47.27 ± 2.84 bc | 30.1 ± 3.38 c | 39.6 ± 2.57 a | ||
| 5 gL−1 S.R. | 31.5 ± 5.40 b | 56.6 ± 12.11 a | 48.57 ± 3.77 ab | 31.4 ± 4.54 b | 38.5 ± 2.67 ab | ||
| 7.5 gL−1 S.R. | 31.9 ± 6.34 b | 46.2 ± 9.29 e | 48.7 ± 3.17 ab | 31.9 ± 3.29 b | 38.0 ± 2.44 b | ||
| 2.5 gL−1 S.F. | 31.7 ± 4.67 b | 51.0 ± 8.42 bc | 47.29 ± 2.29 c | 33.8 ± 4.19 a | 34.5 ± 3.11 c | ||
| 5 gL−1 S.F. | 31.7 ± 4.83 b | 47.3 ± 10.23 de | 49.89 ± 2,48 a | 31.1 ± 3.13 b | 32.8 ± 2.74 d | ||
| 7.5 gL−1 S.F. | 31.5 ± 4.98 b | 52.6 ± 12.2 b | 47.3 ± 3.64 bc | 31.4 ± 4.11 b | 30.3 ± 2.47 f | ||
| Stress | Control | 20.1 ± 2.85 f | 35.1 ± 7.91 j | 40.67 ± 1.77 g | 28.0 ± 4.44 e | 18.4 ± 2.27 j | |
| 20 kg ha−1 S.A. | 25.0 ± 4.39 e | 49.9 ± 8.32 c | 42.6 ± 2.94 ef | 29.5 ± 3.61 d | 18.3 ± 2.67 j | ||
| 40 kg ha−1 S.A. | 19.3 ± 3.65 f | 47.2 ± 9.12 d | 42.93 ± 3.81 e | 29.1 ± 4.83 d | 30.2 ± 2.46 f | ||
| 60 kg ha−1 S.A. | 18.1 ± 4.85 h | 53.8 ± 12.4 b | 43.05 ± 2.39 e | 30.7 ± 4.18 c | 28.1 ± 2.02 g | ||
| 2.5 gL−1 S.R. | 18.1 ± 4.54 h | 50.0 ± 12.8 cd | 40.91 ± 3.28 g | 30.7 ± 3.76 c | 28.4 ± 2.54 g | ||
| 5 gL−1 S.R. | 20.8 ± 4.29 f | 53.2 ± 13.7 b | 42.69 ± 2.55 ef | 29.6 ± 2.71 d | 29.5 ± 3.11 fg | ||
| 7.5 gL−1 S.R. | 20.6 ± 4.36 f | 47.2 ± 11.2 de | 41.95 ± 5.23 f | 29.9 ± 3.24 d | 26.1 ± 1.39 h | ||
| 2.5 gL−1 S.F. | 17.9 ± 3.18 h | 45.2 ± 12.3 f | 45.86 ± 2.13 d | 31.3 ± 4.70 c | 24.3 ± 2.52 i | ||
| 5 gL−1 S.F. | 18.1 ± 3.76 h | 41.5 ± 12.0 i | 44.96 ± 3.06 de | 30.8 ± 3.28 c | 23.9 ± 3.18 i | ||
| 7.5 gL−1 S.F. | 17.9 ± 3.77 h | 46.3 ± 12.2 e | 44.72 ± 2.59 de | 29.3 ± 3.43 d | 25.9 ± 2.34 h | ||
| Irrigation | Zinc Sulfate | Chlorophyll Index | Leaf Area Index | RWC (%) | Height (cm) | Stem Diameter (mm) | Capitul Diameter (mm) |
|---|---|---|---|---|---|---|---|
| Normal | Control | 51.8 ± 5.04 f | 2.75 ± 0.70 b | 61.9 ± 5.48 f | 51.3 ± 15.8 g | 9.5 ± 0.19 c | 30.1 ± 15.7 e |
| 20 kg ha−1 S.A. | 52.7 ± 6.43 f | 3.15 ± 0.84 a | 64.9 ± 6.07 e | 57.0 ± 17.1 d | 9.2 ± 0.38 c | 31.8 ± 9.39 cd | |
| 40 kg ha−1 S.A. | 57.5 ± 4.88 d | 3.26 ± 0.46 a | 67.9 ± 5.86 d | 60.5 ± 13.6 c | 10.4 ± 0.54 fg | 32.9 ± 16.1 bc | |
| 60 kg ha−1 S.A. | 72.1 ± 4.64 a | 3.15 ± 0.49 a | 68.8 ± 5.49 cd | 64.6 ± 17.3 a | 10.6 ± 0.22 b | 34.1 ± 11.4 a | |
| 2.5 gL−1 S.R. | 68.1 ± 4.28 b | 2.89 ± 0.59 b | 67.1 ± 6.39 d | 56.6 ± 11.9 de | 10.4 ± 0.49 b | 33.7 ± 12.1 b | |
| 5 gL−1 S.R. | 58.9 ± 9.30 cd | 2.97 ± 0.72 ab | 81.1 ± 4.39 a | 63.7 ± 12.6 ab | 11.1 ± 0.32 a | 34.8 ± 13.5 a | |
| 7.5 gL−1 S.R. | 60.9 ± 4.39 c | 2.88 ± 0.57 ab | 82.1 ± 5.69 a | 60.5 ± 14.1 c | 10.6 ± 0.64 b | 34.7 ± 11.9 a | |
| 2.5 gL−1 S.F. | 56.8 ± 7.46 d | 2.77 ± 0.53 b | 69.7 ± 7.94 c | 60.4 ± 13.4 c | 9.5 ± 0.21 c | 32.6 ± 16.0 bc | |
| 5 gL−1 S.F. | 72.4 ± 5.19 a | 2.88 ± 0.50 ab | 70.7 ± 4.38 b | 59.1 ± 13.1 c | 8.8 ± 0.44 cd | 32.4 ± 14.1 c | |
| 7.5 gL−1 S.F. | 49.9 ± 6.02 f | 2.76 ± 0.62 b | 71.2 ± 4.17 b | 62.2 ± 9.27 b | 9.4 ± 0.60 c | 33.6 ± 13.2 b | |
| Stress | Control | 39.0 ± 4.88 i | 1.77 ± 0.63 f | 57.9 ± 8.21 h | 50.1 ± 14.3 g | 8.3 ± 0.41 d | 28.9 ± 14.4 f |
| 20 kg ha−1 S.A. | 41.1 ± 6.48 h | 2.07 ± 0.57 e | 60.3 ± 8.15 g | 53.2 ± 11.8 f | 9.1 ± 0.52 c | 31.8 ± 14.4 cd | |
| 40 kg ha−1 S.A. | 50.3 ± 4.65 f | 2.62 ± 0.50 bc | 61.6 ± 6.56 f | 63.2 ± 12.0 ab | 10.2 ± 0.17 b | 33.5 ± 11.8 b | |
| 60 kg ha−1 S.A. | 42.9 ± 7.07 gh | 2.77 ± 0.68 b | 62.4 ± 10.68 ef | 64.1 ± 10.3 a | 10.5 ± 0.33 bf | 34.1 ± 16.2 a | |
| 2.5 gL−1 S.R. | 45.9 ± 4.94 g | 2.67 ± 0.48 bc | 57.3 ± 6.62 h | 61.3 ± 13.5 bc | 10.5 ± 0.41 b | 33.8 ± 11.8 b | |
| 5 gL−1 S.R. | 44.9 ± 5.39 g | 2.69 ± 0.41 bc | 69.9 ± 7.74 c | 56.5 ± 13.0 de | 11.4 ± 0.34 a | 34.0 ± 11.2 a | |
| 7.5 gL−1 S.R. | 56.9 ± 5.29 e | 2.55 ± 0.72 c | 67.4 ± 6.25 d | 59.9 ± 15.3 c | 9.6 ± 0.19 c | 32.5 ± 16.2 c | |
| 2.5 gL−1 S.F. | 42.1 ± 4.46 h | 2.49 ± 0.71 cd | 61.6 ± 4.94 f | 59.2 ± 11.9 c | 10.5 ± 0.56 b | 30.1 ± 14.1 ef | |
| 5 gL−1 S.F. | 44.1 ± 6.84 g | 2.29 ± 0.63 de | 61.5 ± 9.24 f | 52.9 ± 14.1 f | 9.3 ± 0.36 c | 31.1 ± 13.2 d | |
| 7.5 gL−1 S.F. | 48.4 ± 4.44 f | 2.09 ± 0.56 e | 60.0 ± 6.50 g | 52.7 ± 10.8 f | 8.8 ± 0.26 cd | 30.1 ± 11.7 e | |
| Irrigation | Zinc sulfate | Number of capitol per plant | Number of seed per capitol | 1000-seed weight (g) | Oil content (%) | Harvest index (%) | |
| Normal | Control | 16.5 ± 3.28 g | 37.1 ± 8.13 e | 45.57 ± 3.25 bc | 28.9 ± 4.23 de | 29.8 ± 2.03 e | |
| 20 kg ha−1 S.A. | 20.7 ± 4.12 e | 43.3 ± 11.4 c | 46.18 ± 3.60 b | 29.6 ± 4.33 d | 30.8 ± 2.87 d | ||
| 40 kg ha−1 S.A. | 22.1 ± 3.88 c | 44.0 ± 11.3 c | 46.89 ± 3.18 b | 30.8 ± 4.28 c | 28.5 ± 2.48 f | ||
| 60 kg ha−1 S.A. | 23.9 ± 4.03 b | 51.3 ± 9.39 a | 46.1 ± 3.48 b | 31.2 ± 2.14 a | 35.0 ± 2.33 c | ||
| 2.5 gL−1 S.R. | 24.6 ± 5.38 a | 47.8 ± 11.9 b | 46.73 ± 3.04 b | 29.7 ± 3.07 d | 37.5 ± 2.98 b | ||
| 5 gL−1 S.R. | 26.0 ± 4.91 b | 52.7 ± 11.2 a | 47.4 ± 3.93 ab | 29.9 ± 2.97 cd | 37.6 ± 3.40 b | ||
| 7.5 gL−1 S.R. | 24.7 ± 3.37 b | 42.3 ± 9.28 c | 48.62 ± 3.48 a | 30.6 ± 3.04 b | 39.1 ± 3.35 a | ||
| 2.5 gL−1 S.F. | 21.5 ± 3.67 d | 47.1 ± 9.18 b | 47.13 ± 3.29 ab | 31.1 ± 3.33 a | 30.2 ± 3.58 e | ||
| 5 gL−1 S.F. | 19.9 ± 3.41 f | 43.4 ± 11.1 c | 48.28 ± 3.24 a | 31.6 ± 4.04 a | 32.1 ± 3.01 d | ||
| 7.5 gL−1 S.F. | 21.4 ± 3.90 d | 48.7 ± 8.35 b | 46.41 ± 3.58 b | 31.1 ± 2.61 a | 29.6 ± 2.47 e | ||
| Stress | Control | 15.3 ± 2.31 h | 32.2 ± 15.2 f | 40.77 ± 2.68 e | 26.0 ± 3.41 g | 18.7 ± 2.98 k | |
| 20 kg ha−1 S.A. | 17.1 ± 3.22 fg | 46.0 ± 14.8 bc | 43.32 ± 2.94 d | 26.8 ± 3.32 f | 19.3 ± 2.07 jk | ||
| 40 kg ha−1 S.A. | 21.6 ± 3.59 d | 43.3 ± 13.6 c | 43.19 ± 3.33 d | 27.5 ± 3.36 ef | 29.2 ± 3.40 e | ||
| 60 kg ha−1 S.A. | 23.4 ± 3.79 bc | 46.1 ± 11.8 bc | 44.33 ± 3.49 c | 31.4 ± 3.65 a | 27.1 ± 2.46 fg | ||
| 2.5 gL−1 S.R. | 21.2 ± 3.24 d | 49.9 ± 16.4 ab | 44.32 ± 3.11 c | 28.4 ± 3.24 e | 27.6 ± 3.13 f | ||
| 5 gL−1 S.R. | 24.9 ± 3.49 b | 49.3 ± 11.4 ab | 43.97 ± 4.32 cd | 28.1 ± 3.18 e | 22.3 ± 1.43 i | ||
| 7.5 gL−1 S.R. | 23.1 ± 4.14 bc | 43.3 ± 9.47 c | 46.11 ± 4.38 b | 29.3 ± 3.00 d | 21.1 ± 2.50 i | ||
| 2.5 gL−1 S.F. | 21.8 ± 3.14 d | 41.3 ± 13.6 d | 46.84 ± 3.37 b | 30.4 ± 3.47 b | 26.3 ± 2.28 g | ||
| 5 gL−1 S.F. | 18.8 ± 3.27 f | 37.6 ± 5.61 e | 42.66 ± 4.56 d | 30.7 ± 3.63 b | 26.2 ± 2.38 g | ||
| 7.5 gL−1 S.F. | 19.0 ± 3.84 ef | 42.4 ± 11.3 c | 43.05 ± 2.28 d | 28.7±3.04 e | 24.7 ± 2.23 gh | ||
| Irrigation | Zinc Sulfate | Seed N (mg g−1) | Seed P (mg g−1) | Seed K (mg g−1) | Seed Zn (mg g−1) | Seed Fe (mg g−1) |
|---|---|---|---|---|---|---|
| Normal | Control | 48.8 ± 1.18 c | 8.6 ± 0.78 d | 6.03 ± 0.97 f | 0.079 ± 0.011 d | 0.070 ± 0.008 f |
| 20 kg ha−1 S.A. | 51.0 ± 1.45 b | 9.0 ± 0.88 c | 6.35 ± 0.82 de | 0.083 ± 0.010 b | 0.083 ± 0.011 d | |
| 40 kg ha−1 S.A. | 53.8 ± 2.01 a | 9.6 ± 0.97 a | 6.59 ± 0.62 c | 0.084 ± 0.014 b | 0.096 ± 0.011 b | |
| 60 kg ha−1 S.A. | 49.9 ± 1.16 bc | 8.8 ± 0.64 cd | 6.33 ± 0.68 e | 0.083 ± 0.014 b | 0.071 ± 0.009 f | |
| 2.5 gL−1 S.R. | 51.5 ± 1.65 b | 9.2 ± 0.66 b | 6.59 ± 1.01 c | 0.080 ± 0.014 c | 0.091 ± 0.010 bc | |
| 5 gL−1 S.R. | 51.4 ± 1.40 b | 9.2 ± 0.80 b | 6.33 ± 0.61 e | 0.088 ± 0.014 a | 0.085 ± 0.011 c | |
| 7.5 gL−1 S.R. | 49.4 ± 1.44 c | 9.4 ± 0.84 ab | 6.06 ± 0.39 f | 0.083 ± 0.011 b | 0.085 ± 0.011 c | |
| 2.5 gL−1 S.F. | 53.5 ± 1.36 a | 9.1 ± 0.77 bc | 6.33 ± 0.37 e | 0.083 ± 0.012 b | 0.076 ± 0.014 e | |
| 5 gL−1 S.F. | 51.3 ± 1.58 b | 9.5 ± 1.01 a | 6.87 ± 0.87 a | 0.089 ± 0.011 a | 0.084 ± 0.013 c | |
| 7.5 gL−1 S.F. | 50.6 ± 1.55 b | 9.0 ± 0.68 c | 6.78 ± 0.64 b | 0.086 ± 0.011 ab | 0.11 ± 0.019 a | |
| Stress | Control | 48.3 ± 1.45 d | 7.2 ± 0.67 g | 5.54 ± 0.58 h | 0.070 ± 0.010 g | 0.062 ± 0.013 g |
| 20 kg ha−1 S.A. | 51.2 ± 1.33 b | 8.9 ± 0.68 c | 6.59 ± 0.43 c | 0.074 ± 0.015 e | 0.086 ± 0.016 bc | |
| 40 kg ha−1 S.A. | 52.2 ± 1.76 a | 8.8 ± 0.77 cd | 6.60 ± 0.41 c | 0.075 ± 0.013 e | 0.085 ± 0.011 c | |
| 60 kg ha−1 S.A. | 52.5 ± 1.68 a | 8.7 ± 0.59 cd | 6.43 ± 0.77 d | 0.072 ± 0.013 f | 0.078 ± 0.016 de | |
| 2.5 gL−1 S.R. | 50.4 ± 1.38 b | 8.8 ± 0.83 cd | 6.60 ± 0.41 c | 0.076 ± 0.014 e | 0.086 ± 0.008 bc | |
| 5 gL−1 S.R. | 50.5 ± 1.54 b | 8.1 ± 0.62 e | 6.06 ± 0.86 f | 0.082 ± 0.011 bc | 0.077 ± 0.014 de | |
| 7.5 gL−1 S.R. | 49.0 ± 1.69 c | 8.1 ± 0.81 e | 6.06 ± 0.72 f | 0.081 ± 0.011 c | 0.088 ± 0.014 bc | |
| 2.5 gL−1 S.F. | 50.1 ± 1.33 b | 8.2 ± 0.68 e | 6.06 ± 0.63 f | 0.072 ± 0.008 f | 0.079 ± 0.012 de | |
| 5 gL−1 S.F. | 51.3 ± 1.70 b | 7.6 ± 0.97 f | 5.80 ± 0.79 g | 0.075 ± 0.013 e | 0.076 ± 0.015 e | |
| 7.5 gL−1 S.F. | 51.9 ± 1.94 b | 8.1 ± 0.62 e | 5.80 ± 0.74 g | 0.078 ± 0.020 d | 0.080 ± 0.017 d |
| Irrigation | Zinc Sulfate | Seed N (mg g−1) | Seed P (mg g−1) | Seed K (mg g−1) | Seed Zn (mg g−1) | Seed Fe (mg g−1) |
|---|---|---|---|---|---|---|
| Normal | Control | 34.8 ± 1.60 b | 7.7 ± 0.44 b | 4.47 ± 0.42 e | 0.024 ± 0.018 h | 0.053 ± 0.010 c |
| 20 kg ha−1 S.A. | 35.3 ± 2.04 b | 8.3 ± 0.83 a | 5.61 ± 0.47 a | 0.031 ± 0.012 g | 0.062 ± 0.011 b | |
| 40 kg ha−1 S.A. | 40.1 ± 1.67 b | 7.3 ± 0.86 c | 5.07 ± 0.49 c | 0.057 ± 0.017 c | 0.077 ± 0.010 a | |
| 60 kg ha−1 S.A. | 47.1 ± 1.85 a | 6.9 ± 0.84 cd | 4.93 ± 0.62 c | 0.079 ± 0.0113 a | 0.064 ± 0.017 b | |
| 2.5 gL−1 S.R. | 37.8 ± 1.38 b | 7.7 ± 0.69 b | 5.23 ± 0.90 b | 0.038 ± 0.008 f | 0.051 ± 0.019 c | |
| 5 gL−1 S.R. | 38.2 ± 1.64 b | 7.8 ± 0.51 b | 4.87 ± 0.86 d | 0.046 ± 0.0011 e | 0.051 ± 0.016 c | |
| 7.5 gL−1 S.R. | 39.4 ± 1.26 b | 7.6 ± 0.66 b | 4.12 ± 0.77 f | 0.060 ± 0.008 c | 0.041 ± 0.012 d | |
| 2.5 gL−1 S.F. | 32.6 ± 1.27 c | 8.1 ± 0.44 a | 5.21 ± 0.56 b | 0.035 ± 0.012 f | 0.050 ± 0.019 c | |
| 5 gL−1 S.F. | 36.2 ± 1.69 b | 7.5 ± 0.81 bc | 5.18 ± 0.81 b | 0.051 ± 0.011 d | 0.047 ± 0.018 d | |
| 7.5 gL−1 S.F. | 35.4 ± 1.44 bc | 6.9 ± 0.84 cd | 5.25 ± 0.66 b | 0.069 ± 0.011 b | 0.048 ± 0.011 cd | |
| Stress | Control | 22.7 ± 1.94 d | 5.9 ± 0.48 f | 2.04 ± 0.32 k | 0.018 ± 0.007 i | 0.031 ± 0.009 e |
| 20 kg ha−1 S.A. | 23.0 ± 1.50 d | 6.4 ± 0.66 e | 3.76 ± 0.79 h | 0.029 ± 0.010 g | 0.036 ± 0.014 e | |
| 40 kg ha−1 S.A. | 26.2 ± 1.72 d | 6.4 ± 0.68 e | 3.85 ± 0.83 gh | 0.035 ± 0.011 f | 0.055 ± 0.013 c | |
| 60 kg ha−1 S.A. | 32.2 ± 1.94 c | 6.0 ± 0.79 f | 3.82 ± 0.64 n | 0.051 ± 0.011 d | 0.034 ± 0.011 e | |
| 2.5 gL−1 S.R. | 24.3 ± 1.36 d | 6.1 ± 0.65 f | 4.19 ± 0.48 f | 0.023 ± 0.011 h | 0.036 ± 0.012 e | |
| 5 gL−1 S.R. | 25.8 ± 1.31 b | 6.4 ± 0.87 e | 4.13 ± 0.82 f | 0.035 ± 0.011 f | 0.036 ± 0.011 e | |
| 7.5 gL−1 S.R. | 26.4 ± 1.95 b | 6.0 ± 0.69 f | 3.94 ± 0.60 g | 0.039 ± 0.012 f | 0.036 ± 0.011 e | |
| 2.5 gL−1 S.F. | 31.4 ± 1.67 c | 7.3 ± 0.61 c | 3.26 ± 0.65 i | 0.024 ± 0.014 h | 0.043 ± 0.013 d | |
| 5 gL−1 S.F. | 31.7 ± 1.67 c | 7.1 ± 097 c | 3.12 ± 0.43 j | 0.040 ± 0.011 f | 0.042 ± 0.013 d | |
| 7.5 gL−1 S.F. | 32.3 ± 1.59 c | 6.0 ± 0.83 f | 2.98 ± 0.76 j | 0.053 ± 0.011 d | 0.033 ± 0.016 se |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, R.; Mahmoudi, M.; Shekari, F.; Afsahi, K.; Shekari, K.; Saba, J.; Mastinu, A. Application Methods of Zinc Sulphate Increased Safflower Seed Yield and Quality under End-Season Drought Stress. Horticulturae 2024, 10, 963. https://doi.org/10.3390/horticulturae10090963
Ahmadi R, Mahmoudi M, Shekari F, Afsahi K, Shekari K, Saba J, Mastinu A. Application Methods of Zinc Sulphate Increased Safflower Seed Yield and Quality under End-Season Drought Stress. Horticulturae. 2024; 10(9):963. https://doi.org/10.3390/horticulturae10090963
Chicago/Turabian StyleAhmadi, Reza, Mohammad Mahmoudi, Farid Shekari, Kamran Afsahi, Kiana Shekari, Jalal Saba, and Andrea Mastinu. 2024. "Application Methods of Zinc Sulphate Increased Safflower Seed Yield and Quality under End-Season Drought Stress" Horticulturae 10, no. 9: 963. https://doi.org/10.3390/horticulturae10090963
APA StyleAhmadi, R., Mahmoudi, M., Shekari, F., Afsahi, K., Shekari, K., Saba, J., & Mastinu, A. (2024). Application Methods of Zinc Sulphate Increased Safflower Seed Yield and Quality under End-Season Drought Stress. Horticulturae, 10(9), 963. https://doi.org/10.3390/horticulturae10090963








